Glycyrrhetinic acid solid lipid nanoparticles and preparation method for same
A technology of solid lipid nanometer and glycyrrhetinic acid, which is applied in liposome delivery, pharmaceutical formula, oil/fat/wax non-effective ingredients, etc. It can solve the problems of large individual differences, low bioavailability, poor absorption, etc. , to achieve the effect of uniform particle size, good physiological compatibility and easy disinfection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Formula mass percentage (%)
[0036] Glycyrrhetinic acid 5
[0038] Stearic acid 30
[0039] mPEG-DSPE 25
[0040] Weigh glycyrrhetinic acid, egg yolk lecithin, stearic acid and mPEG-DSPE, dissolve them in 2ml of ethanol, and ultrasonically dissolve them to form a transparent solution, pour them into 50ml of distilled water at 50°C, keep stirring for 30 minutes, and place in a rotating Evaporate under reduced pressure on the evaporator to further volatilize the organic solvent, then circulate and homogenize for 3-5 times through a high-pressure homogenizer, let it cool at room temperature, and pass through a 0.45 μm filter membrane to obtain a colloidal solution of glycyrrhetinic acid solid lipid nanoparticles , the colloidal solution is translucent, blue opalescent. The drug content in the drug was determined by a reversed-phase high-performance liquid chromatography column. Column selection ODS C 18 Column (4.6*250mm, 5μm), the mobile p...
Embodiment 2
[0042] Formula mass percentage (%)
[0043] Glycyrrhetinic acid 1
[0045] Oleic acid 9
[0046] PEGCHS 10
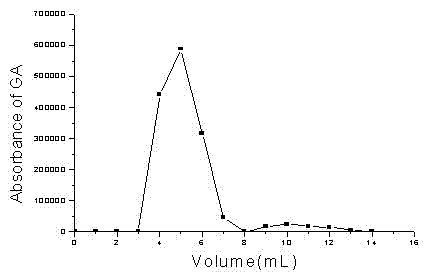
[0047] Weigh glycyrrhetinic acid, egg yolk lecithin, oleic acid and mPEG-DSPE into a 50ml round-bottomed flask, add 2ml chloroform to dissolve them, place a 40°C water bath on a rotary evaporator to recover the organic solvent under reduced pressure, and form A layer of film was further placed in a desiccator under reduced pressure to completely remove the organic solvent. Inject pH 8 phosphate buffer solution for hydration for 1 hour, place it in a probe ultrasonic breaker and sonicate for 10-30 minutes, pass through a 0.45 μm filter membrane, dilute the resulting liposome solution to volume, add 10% (g / ml) sucrose, and pass through Freeze-dry to obtain glycyrrhetinic acid solid lipid nanoparticle freeze-dried powder. After reconstitution, take 0.2ml to pass through the agarose CL-4B gel column, use pH 7.4 phosphate buffer as the eluent, ...
Embodiment 3
[0049] Formula mass percentage (%)
[0050] Glycyrrhetinic acid 4
[0051] Soy Lecithin 46
[0052] Glyceryl Palmitate 5
[0053] Poloxamer 50
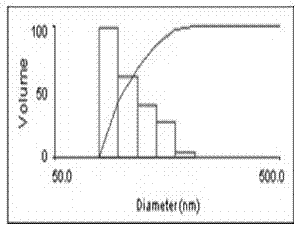
[0054] Weigh glycyrrhetinic acid, egg yolk lecithin, glyceryl palmitate, and poloxamer, melt and mix at 60°C, inject water at 60°C into the melt under stirring, continue stirring for 30 minutes, and circulate through a high-pressure homogenizer After massaging for 3-5 times, let cool at room temperature, pass through a 0.45 μm filter membrane, stabilize the volume of solid lipid nanoparticles obtained by ultrasound, add 5% (g / ml) mannitol, and freeze-dry to obtain glycyrrhetinic acid solid lipid nanoparticles. granules of freeze-dried powder. After adding distilled water to redissolve, take 0.2ml to pass through the agarose CL-4B gel column, use pH 7.4 phosphate buffer as the eluent, collect the effluent in an amount of 1ml per test tube, and calculate the encapsulation according to the elution curve The rate reached 98%. The pa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com