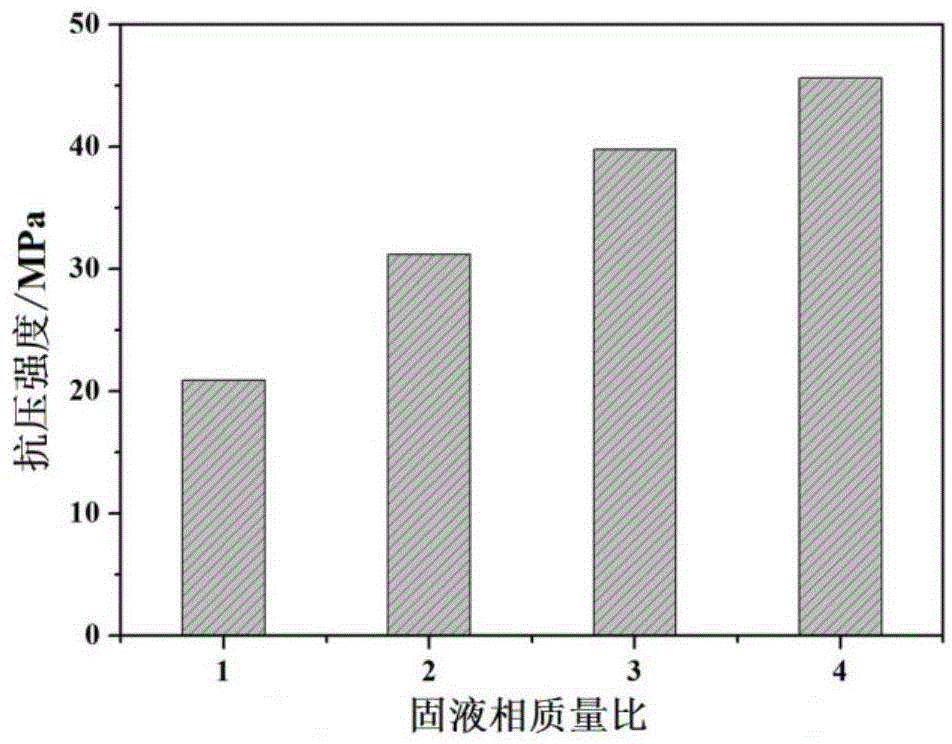
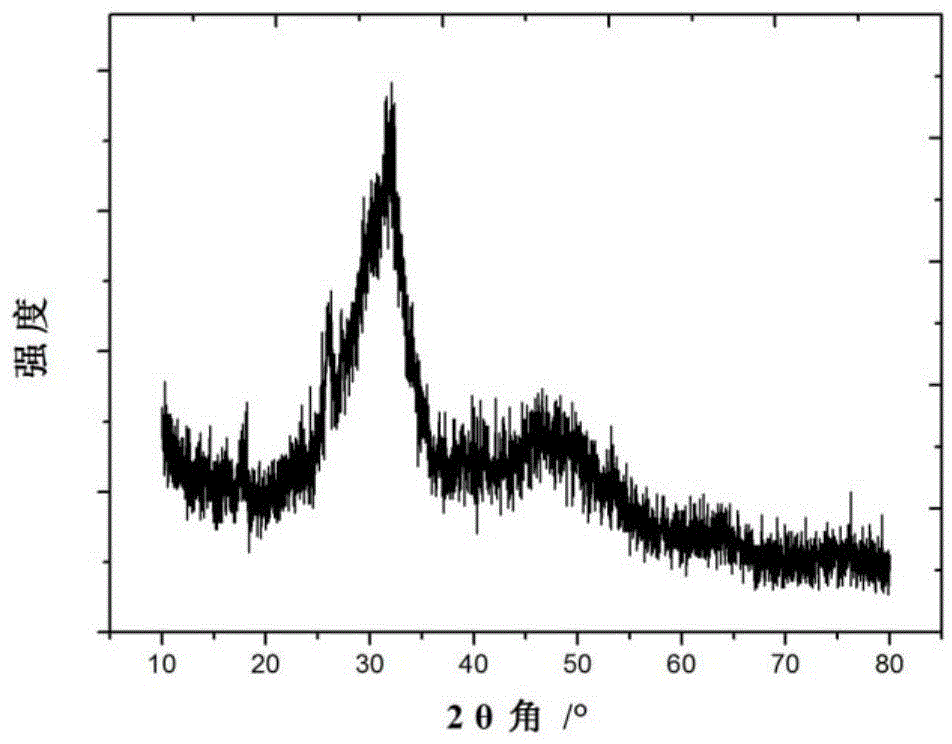
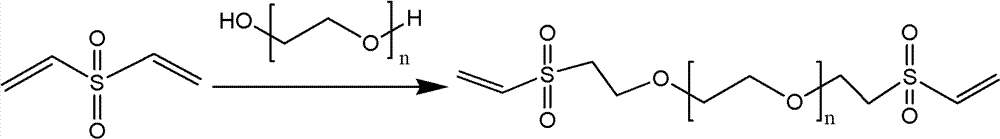
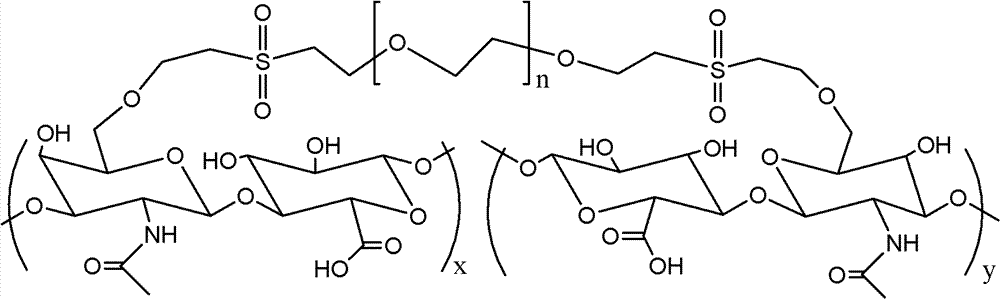
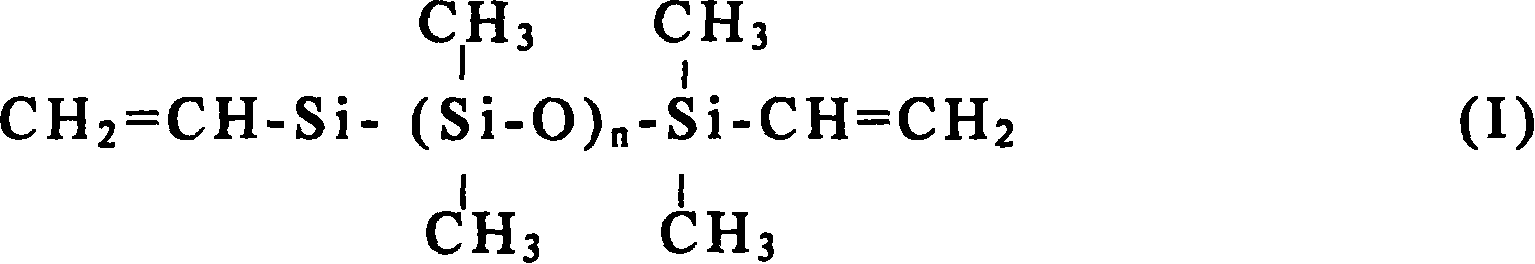Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32results about How to "Suitable for injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing crosslinking hyaluronic acid gel
The invention provides a crosslinking hyaluronic acid gel and its preparation method. The method is characterized in that divinylsulfone is combined with polyethylene glycol for generating a novel cross-linking agent at first, the novel cross-linking agent is reacted with a hyaluronic acid molecule for producing a crosslinking hyaluronic acid gel. The crosslinking sodium hyaluronate gel obtained in the invention has good biological compatibility and longer half life period, and the particle is small and uniform, and is suitable for beautifying shaping, tissue filling, bone articular lubrication or medicine sustained-release preparation and the like.
Owner:XIAN LIBANG PHARMA
Preparation method of injectable polypeptide hydrogel
InactiveCN102408575AImprove hydrophobicityPromote formationAerosol deliverySurgeryCrystallographyBiocompatibility Testing
The invention relates to a preparation method of injectable polypeptide hydrogel. The preparation method comprises the steps of: dissolving ion compensation polypeptide in 3-20 mM MX aqueous solution under assistance of ultrasonic vibration, and carrying out self-assembly on the obtained solution to obtain the injectable polypeptide hydrogel, wherein the ion compensation polypeptide chain is composed of alternately arranged hydrophobic and hydrophilic amino acids, the hydrophilic amino acid is in charge periodic complementation arrangement, the hydrophobic amino acid is methionine, in the ioncompensation polypeptide aqueous solution, the concentration of the ion compensation polypeptide is 5-30 mg / mL, M is Na or K, and X is Cl, Br or I. The obtained hydrogel has good biocompatibility anddegradability, can be changed into fluid under the action of mechanical force, can rapidly restore after mechanical damage, is especially suitable for injection by an injector, and is convenient for use.
Owner:NANJING UNIV
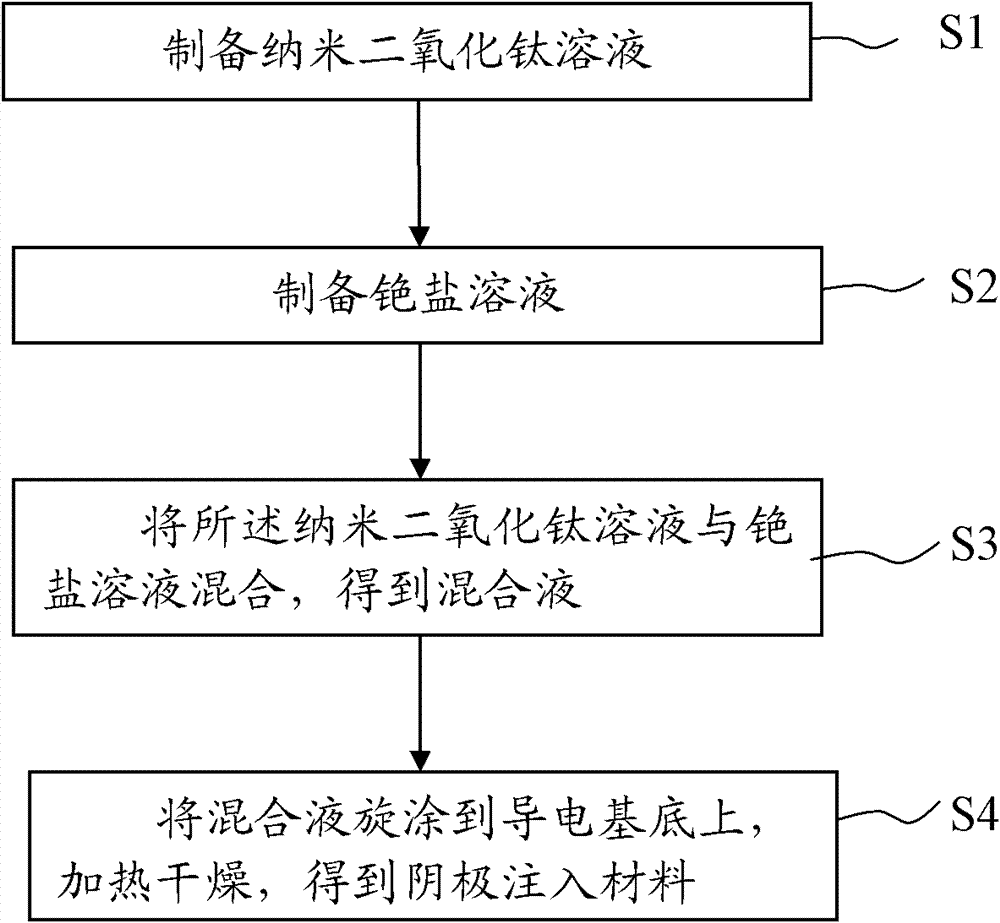
Cathode injection material, and manufacturing method and application thereof
ActiveCN102468446AAvoid leakage currentReduce lossSolid-state devicesSemiconductor/solid-state device manufacturingOrganic electroluminescenceChemistry
The invention discloses a cathode injection material, and a manufacturing method and an application thereof. The cathode injection material comprises nano-titanium dioxide and a cesium salt doped mutually. The manufacturing method comprises the following steps of: preparing a nano-titanium dioxide solution and a cesium salt solution respectively; mixing a nano-titanium dioxide solution dispersion system with the cesium salt solution to obtain a mixed solution; and coating the mixed solution onto a conductive substrate in a spinning way, and heating and drying to obtain the cathode injection material. The cathode injection material disclosed by the invention has the advantages of low cost, capability of effectively enhancing the input and the transmission of charges, increase in the LUMO energy level of a cathode when applied to an organic electroluminescent device, matching with the energy level of an organic electroluminescent structure, reduction in a potential barrier between the cathode injection material and the organic electroluminescent structure, more suitability for electron injection, increase in the electron injection efficiency, preferable increase in the light extraction capability of the organic electroluminescent device, high luminance and long service life. The manufacturing method of the cathode injection material has a simple procedure and high production efficiency.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +1
Glycyrrhetinic acid solid lipid nanoparticles and preparation method for same
InactiveCN102512369AUniform particle sizeHigh encapsulation efficiencyOrganic active ingredientsDigestive systemDiseaseActive agent
The invention relates to glycyrrhetinic acid solid lipid nanoparticles and a preparation method for the same, belonging to the field of medicinal preparation. The main ingredients of the glycyrrhetinic acid solid lipid nanoparticles disclosed by the invention comprise active raw material glycyrrhetinic acid, medicinal phospholipid, a lipid material and a surfactant. The glycyrrhetinic acid solid lipid nanoparticle solution and the freeze-dried powder injection thereof prepared by the preparation method disclosed by the invention are small in particle diameter, high in entrapment efficiency, good in stability and capable of being used for a plurality of administration routes such as oral administration and injection administration. The glycyrrhetinic acid solid lipid nanoparticles disclosed by the invention can reduce dosage, enhance curative effect and reduce the toxic and side effects of medicine, as well as are suitable for treating a plurality of diseases such as hepatitis, liver cancer, lung cancer, ovarian cancer, gastritis, gastric cancer, leukaemia and aids.
Owner:WUHAN UNIV
Finasteride lyotropic liquid crystal gel preparation precursor and preparation method thereof
ActiveCN108272747AImprove liquidityUniform concentrationOrganic active ingredientsAerosol deliveryGel preparationSide effect
The invention belongs to the field of pharmaceutical preparations and discloses a finasteride lyotropic liquid crystal gel preparation precursor. The finasteride lyotropic liquid crystal gel preparation precursor comprises the following raw materials in percentage by mass: 45-80% of phospholipid, 10-50% of glyceride, 5-9% of a cosolvent and 0.1-6% of finasteride. The finasteride in the finasteridelyotropic liquid crystal gel preparation precursor disclosed by the invention is uniformly dispersed; the precursor is low in viscosity and can be used for injection; after entering a human body, theprecursor can act with water to form the lyotropic liquid crystal with an excellent drug sustained release effect; the biocompatibility is excellent, no systemic inflammatory response is caused, andtoxic and side effects of the medicine are avoided; the drug administration frequency can be decreased; the precursor is low in cost, simple in preparation method and capable of easily realizing industrialization; the precursor can be used for treating male hair loss and benign prostatic hyperplasia, so that an enlarged prostate is shrunk, and symptoms brought by urine flow and benign prostatic hyperplasia are improved.
Owner:武汉百纳礼康生物制药有限公司
Antalgic, drug addiction-stopping medication and its preparation method
InactiveCN1485039ANo drug dependenceEase patient painOrganic active ingredientsNervous disorderPopulationAddiction
The invention relates to a drug rehabilitation and analgetic medicine and method for making the same, which comprises spheroidine, citric acid and distilled water. The medicine is highly effective, no drug dependency will be produced, no repetition will occur after ceasing application, so its adaptation human populations are wide.
Owner:王开业
Aqueous suspension injection of cephalosporin medicines, and preparation method thereof
ActiveCN103191058ALow viscosityGood needle extractionAntibacterial agentsOrganic active ingredientsIrritationSuspending Agents
The invention provides an aqueous suspension injection of cephalosporin medicines, and a preparation method thereof. The aqueous suspension injection comprises the components of 5-20% W / V of a 7-aminocephalosporanic acid derivative, 10-40% W / V of an excipient, 5-25% W / V of a suspending agent, 0.1-0.6% W / V of a suspending aid, 0.1-0.5% W / V of a pH buffer, 0.1-1.0% of an addition amount of a surfactant, and balance of injection water. The 7-aminocephalosporanic acid derivative is micronized, wherein 7-aminocephalosporanic acid derivative particles with size smaller than 100mum account for no lower than 95% by volume of the total volume of the raw materials, and 7-aminocephalosporanic acid derivative particles with size smaller than 20mum and larger than 2mum account for no lower than 90% of the total volume of the raw materials. Compared with oily preparations or powdery preparations of cephalosporin medicines, the aqueous suspension injection of cephalosporin medicines provided by the invention has lower viscosity and better injection performance, such that cephalosporin medicine stability is improved. The viscosity of cephalosporin medicines is reduced, such that injection irritation is reduced.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Rifaximin-containing in-situ gel for breast injection and preparation method of rifaximin-containing in-situ gel
ActiveCN105232450AImprove complianceReduce corrosionAntibacterial agentsAerosol deliveryThermal stimulationIrritation
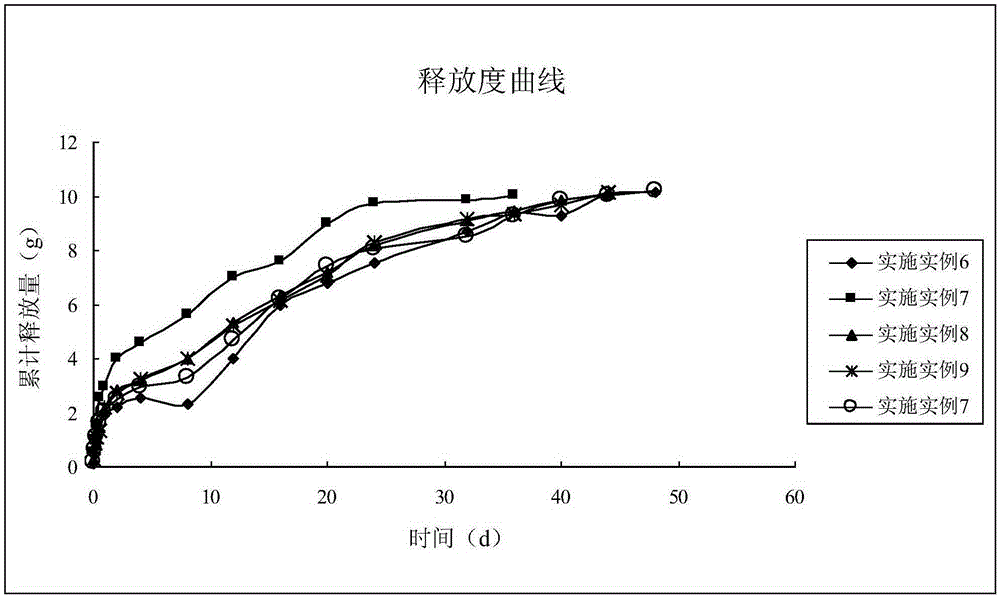
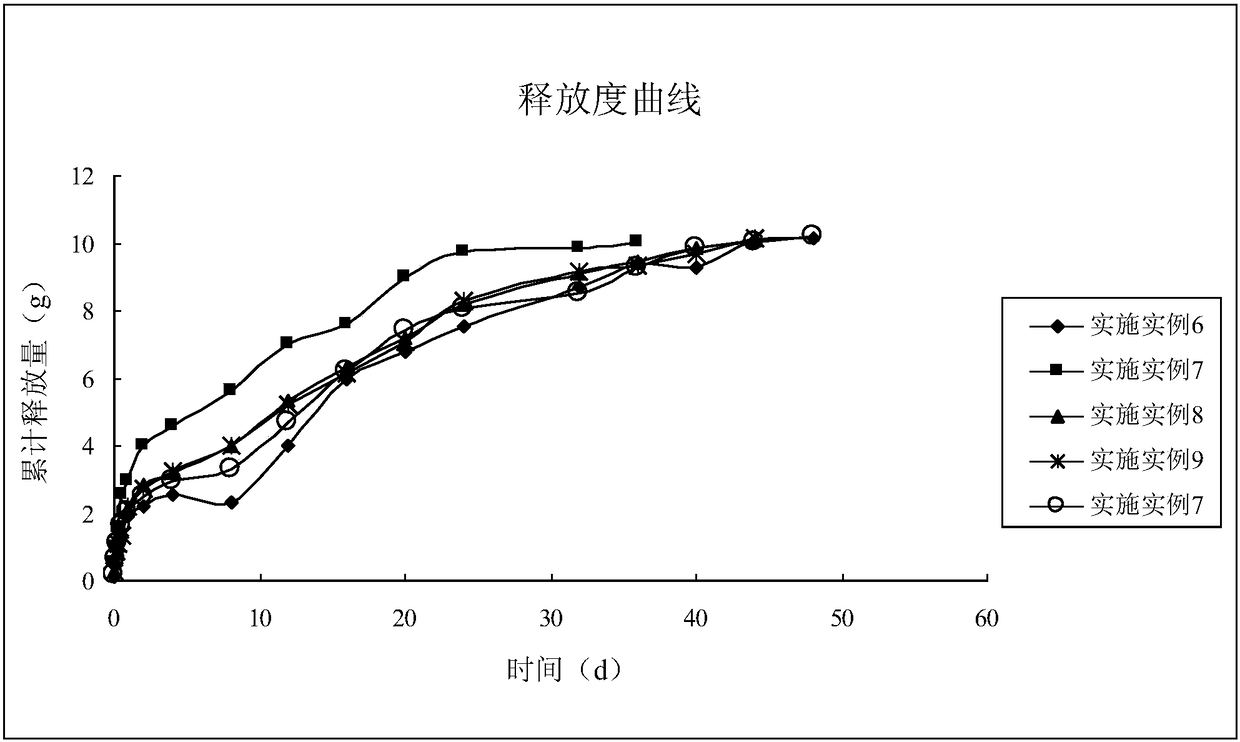
The invention discloses rifaximin-containing in-situ gel for breast injection. The rifaximin-containing in-situ gel comprises the following components in percentage by mass: 0.1-15% of an active drug component existing in a dispersed way, 0.25-20% of an active drug component existing in an inclusion compound form, 12-30% of poloxamer 407, 0.01-11% of poloxamer 188, 0.01-10% of a polymer drag reduction agent, 0.001-3% of a bacterial inhibitor, a pH regulator for regulating the pH value to 5.5-7.5 and the balance of injection water. The invention also discloses a preparation method of the rifaximin-containing in-situ gel for breast injection. Water is used as a solvent, so that the rifaximin-containing in-situ gel is good in biocompatibility, little in irritation and high in safety, exists in a liquid state under a storage condition, is completely formed within 4-15 seconds after being injected to the breast because of thermal stimulation inside the breast and can be used for effectively treating, preventing and controlling mastitis of milk cows within the whole dry cow period; and in addition, the sustained-release effect is remarkable, and the in-vitro drug release time is long and reaches up to 48 days.
Owner:NANJING DEPOT VETERINARY PHARMA R&D CO LTD
Preparation of aseptic vitamin C composition, product and application thereof
ActiveCN102631343AFor long-term storageAvoid degradationOrganic active ingredientsPowder deliveryActivated carbonVitamin C
The invention provides a preparation of aseptic vitamin C composition, which comprises the steps of: preparing aseptic vitamin C sodium, preparing aseptic vitamin C and mixing the preparing aseptic vitamin C sodium and the aseptic vitamin C for preparing the composition, wherein in the preparation process of the aseptic vitamin C sodium comprises the steps of: adding antioxidant, complexing agent and activated carbon into the aqueous solution of the vitamin C sodium, and the like. The invention provides the aseptic vitamin C composition, and the application of the aseptic vitamin C composition in preparation of vitamin C powder injection.
Owner:HAIKOU PHARMA FACTORY
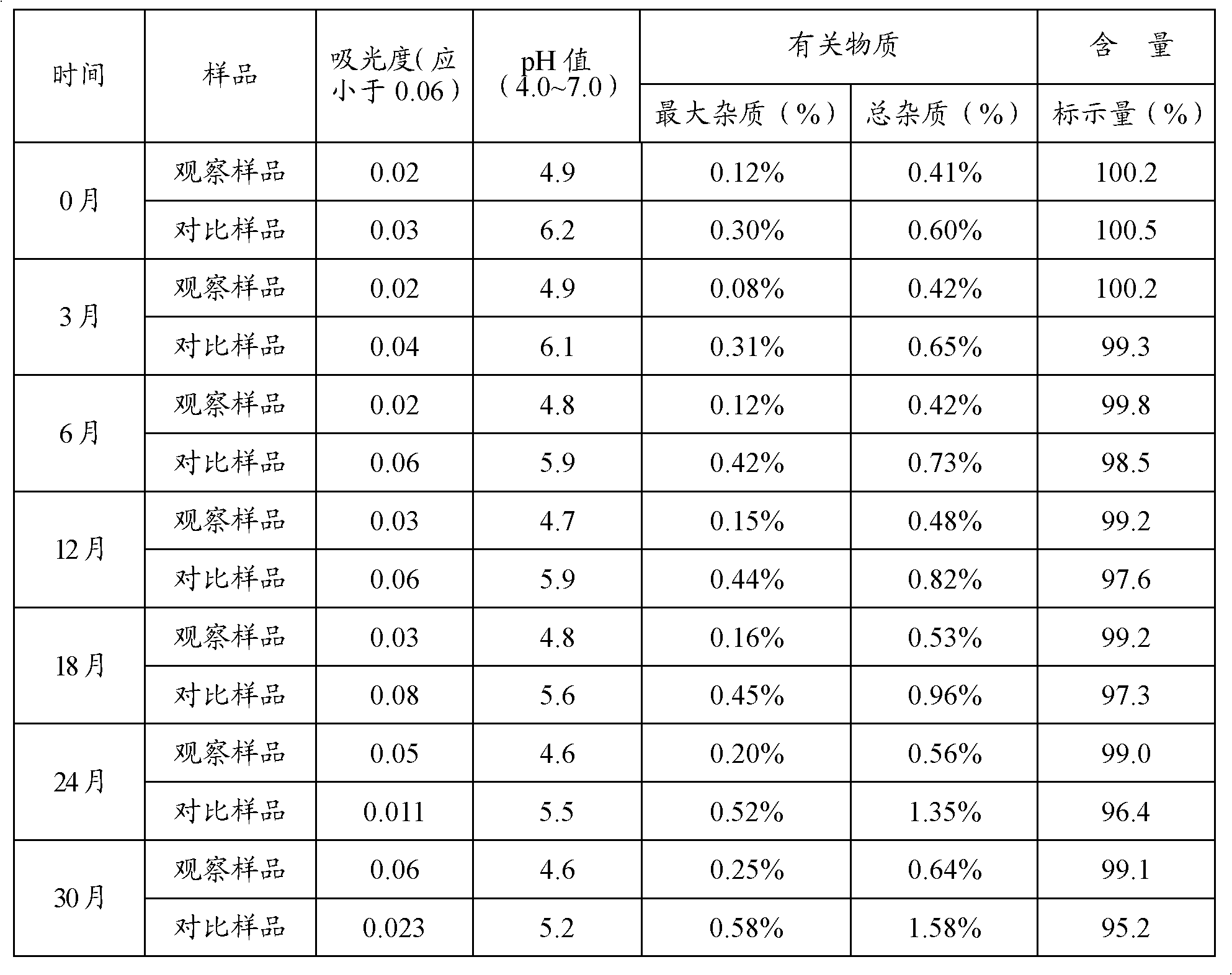
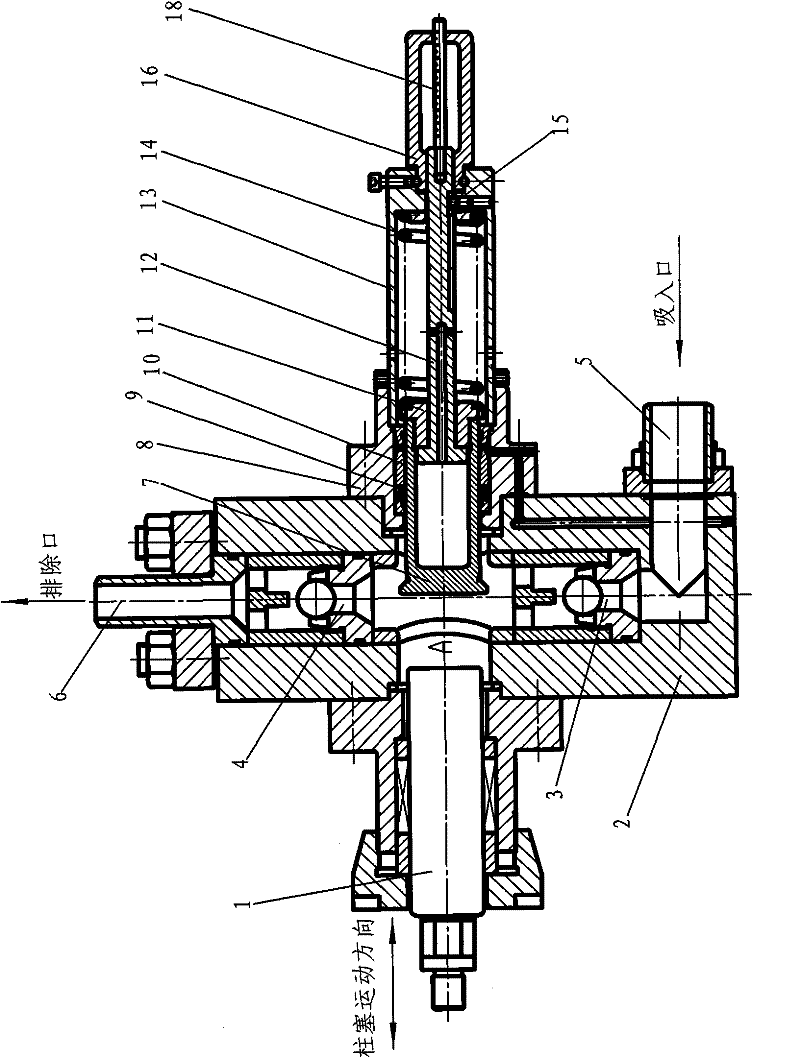
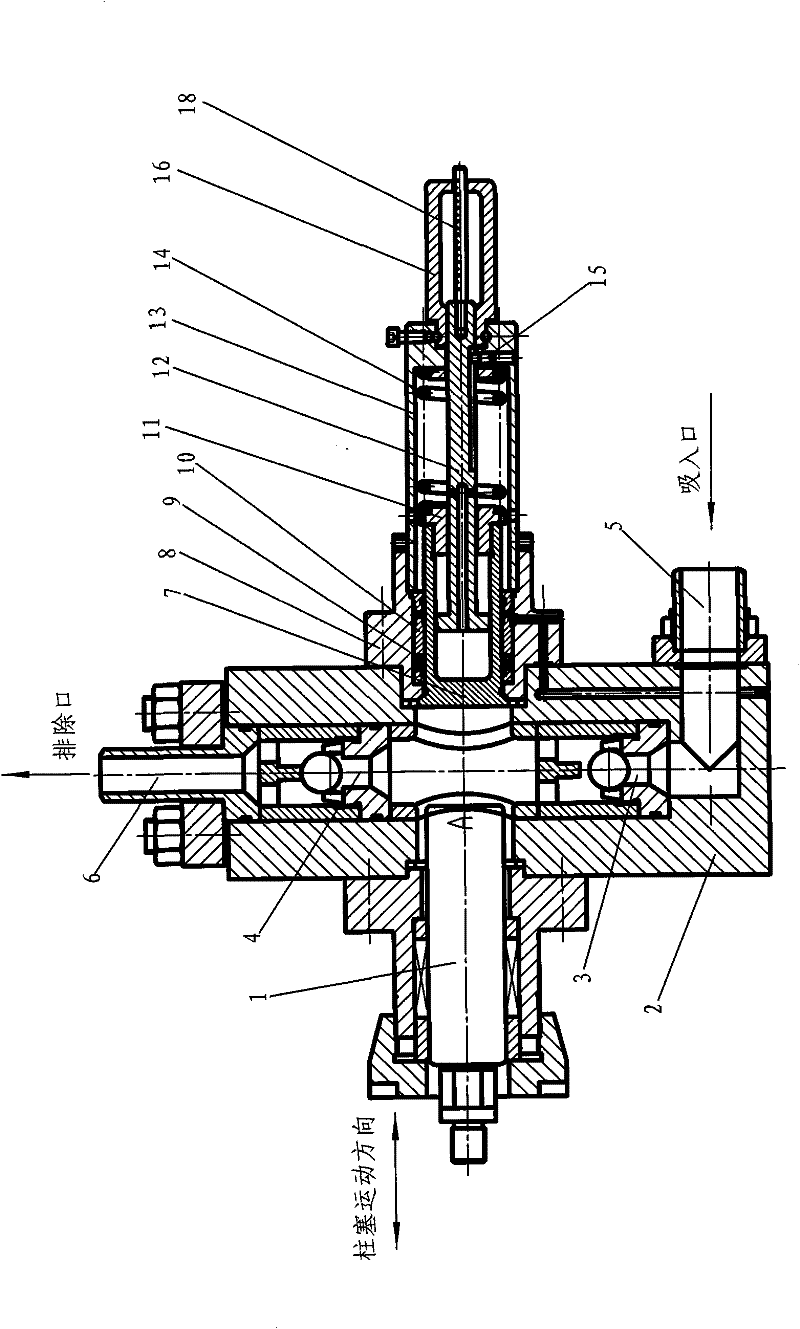
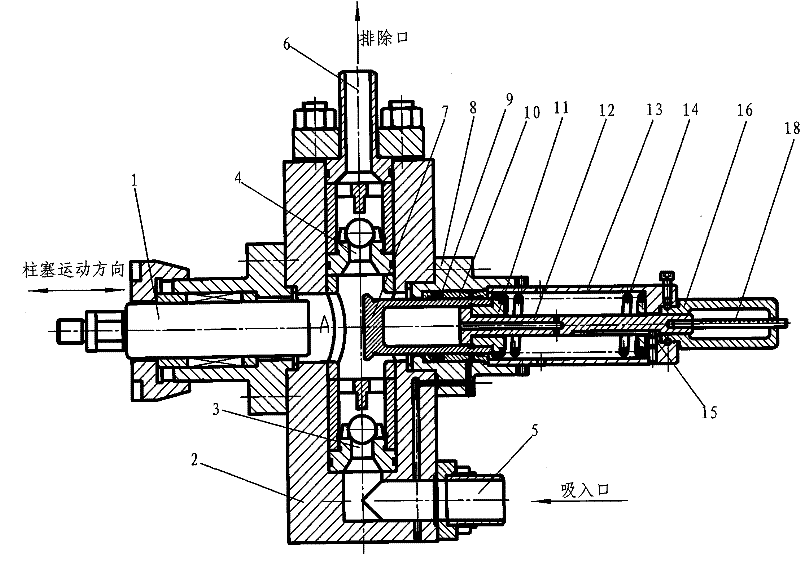
Plunger reciprocating pump
ActiveCN101994688BHigh adjustment accuracyLarge adjustment rangePositive displacement pump componentsLiquid fuel engine componentsExhaust valveInhalation Valve
The invention relates to a plunger reciprocating pump which comprises a pump body, a plunger, an inhalation valve bank and an exhaust valve bank. The plunger is glidingly matched in a plunger hole of the pump body, the inhalation valve bank and the exhaust valve bank are arranged in the pump body, and a pump cavity is formed among the plunger, the inhalation valve bank and the exhaust valve bank in the pump body. The plunger reciprocating pump is characterized in that an adjusting plunger component kept on the same central line as the plunger is mounted on the pump body and comprises a sealing packing box, an adjusting plunger, a screw, a spring and an adjusting handle, wherein the sealing packing box is mounted on the pump body and is provided with a hole with a valve seat in the center, a sealing component and a guiding sealing sleeve are mounted in the hole, the outer circumferential wall of the adjusting plunger is mounted in the sealing component and the guiding sealing sleeve, the valve surface of the front end of the adjusting plunger is arranged in the pump cavity and matched with the valve seat in the center of the packing sealing box, and the screw is provided with a lug boss at the front end, a cylinder in the middle and external threads at the tail. The invention has the advantages of great adjusting range, high adjusting accuracy and convenient adjustment and is suitable for adjusting the flow rates of a plurality of drain openings of a multi-cylinder reciprocating pump.
Owner:NINGBO HELI MECHANICAL PUMP CO LTD
Preparation method of injectable polypeptide hydrogel
InactiveCN102408575BImprove hydrophobicityPromote formationAerosol deliverySurgeryCrystallographyBiocompatibility Testing
The invention relates to a preparation method of injectable polypeptide hydrogel. The preparation method comprises the steps of: dissolving ion compensation polypeptide in 3-20 mM MX aqueous solution under assistance of ultrasonic vibration, and carrying out self-assembly on the obtained solution to obtain the injectable polypeptide hydrogel, wherein the ion compensation polypeptide chain is composed of alternately arranged hydrophobic and hydrophilic amino acids, the hydrophilic amino acid is in charge periodic complementation arrangement, the hydrophobic amino acid is methionine, in the ion compensation polypeptide aqueous solution, the concentration of the ion compensation polypeptide is 5-30 mg / mL, M is Na or K, and X is Cl, Br or I. The obtained hydrogel has good biocompatibility and degradability, can be changed into fluid under the action of mechanical force, can rapidly restore after mechanical damage, is especially suitable for injection by an injector, and is convenient for use.
Owner:NANJING UNIV
Sugarcane peel fiber based blended PBS (polybutylene succinate) degradable plastic and preparation method thereof
The invention discloses sugarcane peel fiber based blended PBS (polybutylene succinate) degradable plastic and a preparation method of the degradable plastic. The degradable plastic is prepared from the following raw materials in parts by weight: 100 parts of PBS, 10-20 parts of silane coupling agent modified sugarcane peel fiber, 1-10 parts of polypropylene grafted maleic anhydride, 0.1-2 parts of vegetable oil and 0.1-1 part of antioxidant. The degradable plastic adopts melt blending of the silane coupling agent modified sugarcane peel fiber and PBS resin, and uses a waste biomass resource, namely sugarcane peel, so that the problems of environmental pollution and the like caused by discard of the sugarcane peel can be avoided, the sugarcane peel can be recycled, and the production cost is greatly lowered; and a degradation rate of the degradable plastic in natural soil is higher than that of pure PBS, so that the degradation time is saved.
Owner:CHIZHOU UNIV
Recombinant leukocyte inhibitory factor and leech peptide chimeric protein freeze-dried preparation for injection and preparation method thereof
PendingCN113368063AReduce degradationReduce aggregationPowder deliveryPeptide/protein ingredientsWhite blood cellChimera Protein
The invention belongs to the technical field of protein and polypeptide drugs, and particularly relates to a recombinant leukocyte inhibitory factor and leech peptide chimeric protein freeze-dried preparation and a preparation method thereof. The lyophilized powder for injection comprises the recombinant leukocyte inhibitory factor and leech peptide chimeric protein, an excipient, a cryoprotectant, and a buffer system. By researching different excipients, cryoprotectants, buffer systems and freeze-drying curves, the recombinant leukocyte inhibitory factor and leech peptide chimeric protein freeze-drying preparation for injection is provided, which is good in appearance, good in resolubility, high in activity, less in impurity, low in side effect, high in safety and stable in quality. The problems of unstable protein, easy aggregation and denaturation, reduced activity and the like are solved. The preparation is convenient to use, quick to absorb and convenient for storage and transport.
Owner:LUNAN PHARMA GROUP CORPORATION
Time-temperature double-sensitive degradable polymer hydrogel and preparation method thereof
InactiveCN105131302ASuitable for injectionEasy to useAerosol deliveryOintment deliveryControl releaseNanoparticle
The invention relates to a time-temperature double-sensitive degradable polymer hydrogel and a preparation method thereof. The time-temperature double-sensitive degradable polymer hydrogel is mainly composed of a degradable polymer and a medium; the degradable polymer is a triblock multipolymer, and is prepared via steps of material preparation, polymerization, refining, sol preparation, and gelatinization; the time-temperature double-sensitive degradable polymer hydrogel based on the degradable polymer has time and temperature double sensitivity because of a unique structure and a synthetic method, and is extremely suitable for injection; syringe needle blocking is not caused in injection processes; after injection, it is beneficial for free and full distribution of drug-containing hydrogel in infection cavities in the body, and is beneficial for improving adaptive capacity of the hydrogel on body local characteristics, so that drug controlled release effect and disease treatment effect are improved. The time-temperature double-sensitive degradable polymer hydrogel is suitable to be taken as a controlled release carrier of drugs, DNA, cells, functional nanoparticles, and tissue chips, and is capable of satisfying requirements on diagnosis, treatment, and health care in the field of biomedicine.
Owner:JIUJIANG UNIVERSITY
Silicon rubber composition and production thereof
A silicon rubber composition and its production are disclosed. The two-component liquid silicon rubber composition consists of A and B component, A component consists of base glue and platinum catalyst, B component consists of base glue, hydrogen-containing silicon oil and delay agent, base glue consists of basic polymer, filling and assistant. The basic polymer consists of tree constitutional formula as (I) polyorganosiloxane: viscosity of the first polymer eta=5000-6000mPa.s, n=200-250, viscosity of the second polymer eta=15000-30000mPa.s, n=500-600, viscosity of the third polymer eta=60000-80000mPa.s, n=900-1200. Its advantages include low viscosity, high structural resistant ability, good flow-ability, and high-temperature rapid solidification.
Owner:SHENZHEN CITY SQUARE SILICONE MATERIALS
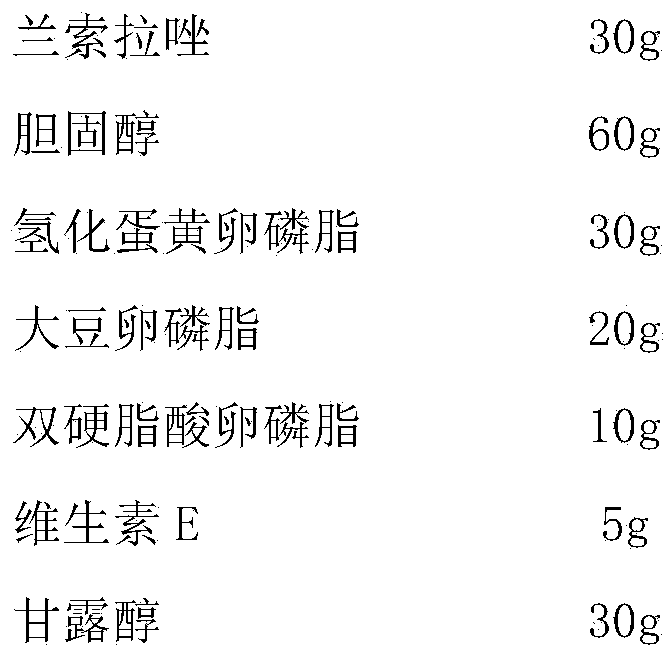
Lansoprazole lipidosome injection
ActiveCN105362227AHigh encapsulation efficiencyReduce leak rateOrganic active ingredientsPowder deliveryYolkLansoprazole
The invention discloses a lansoprazole lipidosome injection which comprises 3 parts of lansoprazole, 3 to 6 parts of cholesterol, 6 to 9 parts of phospholipid, 0.1 to 0.5 part of an antioxygen and 3 to 6 parts of an excipient. The phospholipid is composed of HEPC (hydrogenated egg phosphatidylcholine), soya bean lecithin and stearic acid lecithin of which the weight ratio is 3-2 to 3-2 to 1. The lipidosome injection has the advantages of high encapsulation efficiency, low leakage rate, stability and the like, and has a relatively high clinic applicable value.
Owner:福安药业集团庆余堂制药有限公司
Injectable and degradable bone cement, and preparation method and application thereof
Owner:TONGJI UNIV
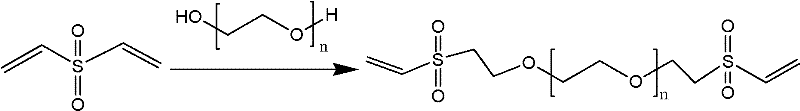
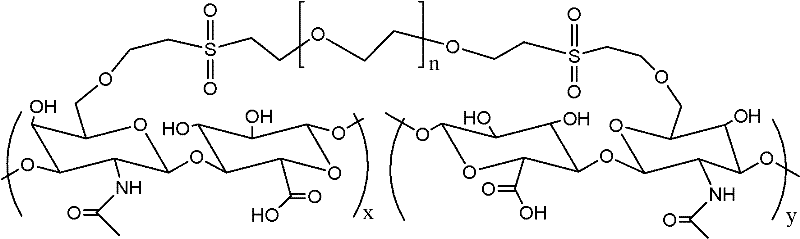
Method for preparing crosslinking hyaluronic acid gel
The invention provides a crosslinking hyaluronic acid gel and its preparation method. The method is characterized in that divinylsulfone is combined with polyethylene glycol for generating a novel cross-linking agent at first, the novel cross-linking agent is reacted with a hyaluronic acid molecule for producing a crosslinking hyaluronic acid gel. The crosslinking sodium hyaluronate gel obtained in the invention has good biological compatibility and longer half life period, and the particle is small and uniform, and is suitable for beautifying shaping, tissue filling, bone articular lubrication or medicine sustained-release preparation and the like.
Owner:XIAN LIBANG PHARMA
A high-concentration nimotuzumab preparation for subcutaneous or intramuscular injection and its preparation method and application
ActiveCN107898756BSuitable for injectionGood for dispersion and non-aggregationPharmaceutical delivery mechanismAntibody ingredientsHigh concentrationIntramuscular injection
The invention provides a high-concentration Nimotuzumab preparation for subcutaneous or intramuscular injection as well as a preparation method and application of the high-concentration Nimotuzumab preparation. The high-concentration Nimotuzumab preparation for subcutaneous or intramuscular injection is prepared from the following components: 15-150mg / mL of Nimotuzumab, 150-20,000IU / mL of hyaluronidase, 10-100mM of buffer agent, 5-200mM of stabilizer and 0.01-0.1% (w / v) nonionic surfactant. In the invention, the Nimotuzumab cooperates with the hyaluronidase, the nonionic surfactant, the bufferagent and the stabilizer to obtain the high-concentration Nimotuzumab preparation, and the obtained Nimotuzumab preparation is suitable for subcutaneous or intramuscular injection when high-concentration Nimotuzumab is contained.
Owner:TOT BIOPHARM CO LTD
Aqueous suspension injection of cephalosporins and preparation method thereof
ActiveCN103191058BLow viscosityGood needle extractionAntibacterial agentsOrganic active ingredientsSuspending AgentsExcipient
The invention provides an aqueous suspension injection of cephalosporin medicines, and a preparation method thereof. The aqueous suspension injection comprises the components of 5-20% W / V of a 7-aminocephalosporanic acid derivative, 10-40% W / V of an excipient, 5-25% W / V of a suspending agent, 0.1-0.6% W / V of a suspending aid, 0.1-0.5% W / V of a pH buffer, 0.1-1.0% of an addition amount of a surfactant, and balance of injection water. The 7-aminocephalosporanic acid derivative is micronized, wherein 7-aminocephalosporanic acid derivative particles with size smaller than 100mum account for no lower than 95% by volume of the total volume of the raw materials, and 7-aminocephalosporanic acid derivative particles with size smaller than 20mum and larger than 2mum account for no lower than 90% of the total volume of the raw materials. Compared with oily preparations or powdery preparations of cephalosporin medicines, the aqueous suspension injection of cephalosporin medicines provided by the invention has lower viscosity and better injection performance, such that cephalosporin medicine stability is improved. The viscosity of cephalosporin medicines is reduced, such that injection irritation is reduced.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
A kind of preparation method of aseptic vitamin C composition and its product and application
ActiveCN102631343BAvoid degradationLow costOrganic active ingredientsPowder deliveryActivated carbonVitamin C
The invention provides a preparation of aseptic vitamin C composition, which comprises the steps of: preparing aseptic vitamin C sodium, preparing aseptic vitamin C and mixing the preparing aseptic vitamin C sodium and the aseptic vitamin C for preparing the composition, wherein in the preparation process of the aseptic vitamin C sodium comprises the steps of: adding antioxidant, complexing agent and activated carbon into the aqueous solution of the vitamin C sodium, and the like. The invention provides the aseptic vitamin C composition, and the application of the aseptic vitamin C composition in preparation of vitamin C powder injection.
Owner:HAIKOU PHARMA FACTORY
Nipple sealing agent for non-human animals and preparation method thereof
ActiveCN111840212AStrong compliancePrevent intrusionHydroxy compound active ingredientsAerosol deliveryBiocompatibilityBiology
The invention discloses a nipple sealing agent for non-human animals. The nipple sealing agent is prepared from the following components in percentage by mass: 11 to 40 percent of temperature-sensitive in-situ gel matrix, 0.001 to 2 percent of ion-sensitive in-situ gel matrix, 5 to 20 percent of carbomer gel, 0.02 to 8 percent of chitosan, 0.01 to 5 percent of polymer retardant, 0.001 to 2 percentof bacteriostatic agent and the balance of water for injection, wherein the temperature-sensitive in-situ gel matrix is poloxamer, and the ion-sensitive in-situ gel matrix is gellan gum. The nipple sealing agent is mainly based on an in-situ gel system, the sensitivity to the environment in the nipple is greatly improved, the environment in the nipple can be perceived in time, and gelling is rapidly generated; the chitosan is added so that the gel strength can be effectively improved, a compact barrier can be formed, and invasion of pathogenic bacteria is hindered. The gel matrix is good in biocompatibility, can be adhered to the inner wall of a nipple hole, and can be remained in the nipple hole for a long time to cover the whole dry period.
Owner:余祖功
Insulin injection site reminding machine
PendingCN107050583AGood choiceEasy to recordMedical devicesIntravenous devicesInjection siteInsulin injection
The invention discloses an insulin injection site reminding machine. The insulin injection site reminding machine comprises an analog machine body. The analog machine body at least comprises a simulated trunk, simulated upper limbs and simulated lower limbs, and button switches corresponding to insulin injection sites are arranged on the simulated trunk, the simulated upper limbs and the simulated lower limbs respectively. Every time injection is conducted, a button switch, corresponding to a certain injection site, on the analog machine body is pressed down; after injection of all sites, a new round of site selection is conducted, so as to help people choose injection sites more conveniently. For injections with a long time interval, injection positions can be recorded conveniently by means of the insulin injection site reminding machine, and confusion can be avoided. The insulin injection site reminding machine can be carried by a patient conveniently, and is suitable for self-injection of the patient.
Owner:沈霖
A hand-held glue injection booster device
ActiveCN103212524BSuitable for injectionCompact structureLiquid surface applicatorsCoatingsSyringe needleBiomedical engineering
The invention relates to a handheld glue solution injection reinforcing device. The handheld glue solution injection reinforcing device mainly comprises a reinforcing mechanism, a needle cylinder support, a baffle plate, a thread clamping sleeve, a needle cylinder, a needle head, a push rod and a pushing piece, wherein a traditional glass glue gun reinforcing mechanism is used as the reinforcing mechanism, the baffle plate is arranged at the front end of the needle cylinder support, the baffle plate and the needle cylinder support are welded together, a hole is formed in the middle of the thread clamping sleeve, and the thread clamping sleeve and the baffle plate are connected together through a thread; the needle cylinder is positioned in the needle cylinder support, the injection end of the needle cylinder is connected with the needle head, the needle head passes through the hole of the thread clamping sleeve, the push rod is a U-shaped rod, one end of the push rod is inserted the reinforcing mechanism of a glue gun, the other end of the push rod is connected with a piston through the pushing piece, and the section of the push rod is of an oblateness the two sides of which are scabbled; and a wafer with a round groove is used as the pushing piece, the pushing piece and the push rod are fixed through the thread, and a round head at the end part of the piston of the needle cylinder after compacting contacts the bottom of the groove of the pushing piece and is clamped in the groove. According to the handheld glue solution injection reinforcing device provided by the invention, a common medical needle cylinder containing glue solution and the glass glue gun are utilized as a reinforcing structure, the handheld glue solution injection reinforcing device is easily available, beneficial to recovery, energy-saving and environment-friendly.
Owner:BEIJING UNIV OF CHEM TECH
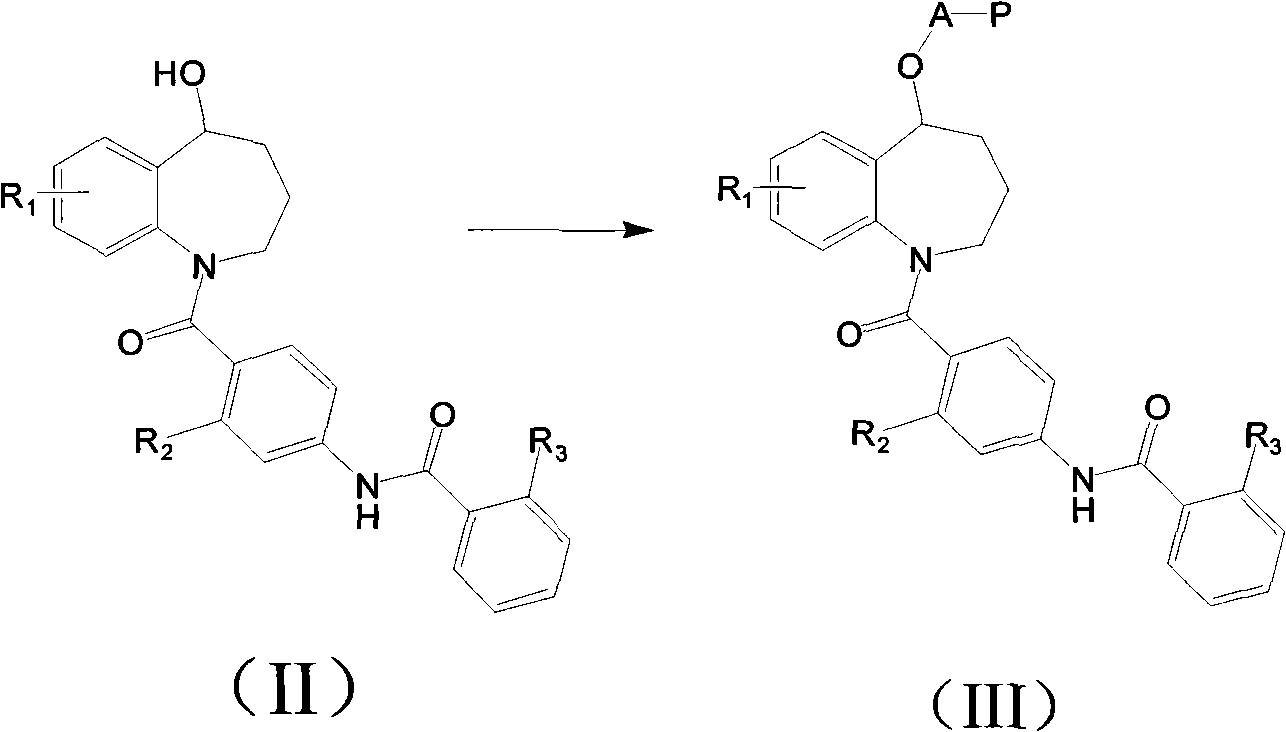
Benzazepines compounds serving as vasopressin receptor antagonism
InactiveCN102030709BGood water solubilitySuitable for injectionOrganic chemistryDigestive systemVasopressin AntagonistsVasopressin receptor
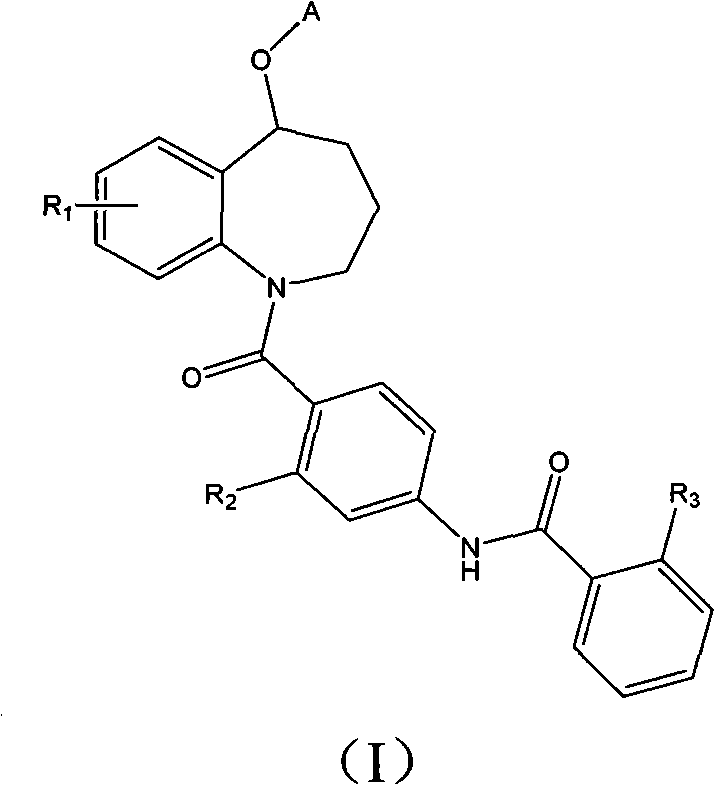
The invention relates to a benzazepines compounds serving as vasopressin receptor antagonism, in particular to a benzazepines compound shown in formula (I) or pharmaceutically acceptable salt thereof and a preparation method thereof, and a medicinal composition containing a compound containing curative dose of the compound or the pharmaceutically acceptable salt thereof, and application in preparing medicament for treating diseases related to the vasopressin thereof.
Owner:SHANGHAI HANSOH BIOMEDICAL
A kind of finasteride lyotropic liquid crystal gel preparation precursor and preparation method thereof
ActiveCN108272747BImprove liquidityUniform concentrationOrganic active ingredientsAerosol deliveryGel preparationDrug administration
The invention belongs to the field of pharmaceutical preparations and discloses a finasteride lyotropic liquid crystal gel preparation precursor. The finasteride lyotropic liquid crystal gel preparation precursor comprises the following raw materials in percentage by mass: 45-80% of phospholipid, 10-50% of glyceride, 5-9% of a cosolvent and 0.1-6% of finasteride. The finasteride in the finasteridelyotropic liquid crystal gel preparation precursor disclosed by the invention is uniformly dispersed; the precursor is low in viscosity and can be used for injection; after entering a human body, theprecursor can act with water to form the lyotropic liquid crystal with an excellent drug sustained release effect; the biocompatibility is excellent, no systemic inflammatory response is caused, andtoxic and side effects of the medicine are avoided; the drug administration frequency can be decreased; the precursor is low in cost, simple in preparation method and capable of easily realizing industrialization; the precursor can be used for treating male hair loss and benign prostatic hyperplasia, so that an enlarged prostate is shrunk, and symptoms brought by urine flow and benign prostatic hyperplasia are improved.
Owner:武汉百纳礼康生物制药有限公司
A degradable polymer hydrogel sensitive to time and temperature and its preparation method
InactiveCN105131302BSuitable for injectionEasy to useAerosol deliveryOintment deliveryDiseaseBiomedicine
The invention relates to a time- and temperature-sensitive degradable polymer hydrogel and a preparation method thereof. The hydrogel is mainly composed of a degradable polymer and a medium. , polymerization, refining, sol preparation, gelation and other steps, the unique structure and synthesis method make the hydrogel based on the degradable polymer have both time and temperature sensitivity, very suitable for injection use, not There will be a problem of clogging the needle during the injection process. After injection, it is also conducive to the free and sufficient distribution of the drug-containing sol in the body lesion cavity, which is conducive to improving the adaptability of the gel to the local characteristics of the body, thereby improving the controlled release of drugs and disease treatment. Effect. The hydrogel of the present invention is suitable for use as a controlled release carrier for drugs, DNA, cells, functional nanoparticles, tissue debris, etc., and meets the needs of diagnosis, treatment, health care, etc. in the field of biomedicine.
Owner:JIUJIANG UNIV
A kind of cathode injection material and its preparation method and application
ActiveCN102468446BLower HOMO levelIncrease HOMO levelSolid-state devicesSemiconductor/solid-state device manufacturingElectron injectionInjected material
The invention discloses a cathode injection material, and a manufacturing method and an application thereof. The cathode injection material comprises nano-titanium dioxide and a cesium salt doped mutually. The manufacturing method comprises the following steps of: preparing a nano-titanium dioxide solution and a cesium salt solution respectively; mixing a nano-titanium dioxide solution dispersion system with the cesium salt solution to obtain a mixed solution; and coating the mixed solution onto a conductive substrate in a spinning way, and heating and drying to obtain the cathode injection material. The cathode injection material disclosed by the invention has the advantages of low cost, capability of effectively enhancing the input and the transmission of charges, increase in the LUMO energy level of a cathode when applied to an organic electroluminescent device, matching with the energy level of an organic electroluminescent structure, reduction in a potential barrier between the cathode injection material and the organic electroluminescent structure, more suitability for electron injection, increase in the electron injection efficiency, preferable increase in the light extraction capability of the organic electroluminescent device, high luminance and long service life. The manufacturing method of the cathode injection material has a simple procedure and high production efficiency.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +1
In situ gel for breast injection containing rifaximin and preparation method thereof
ActiveCN105232450BImprove complianceReduce corrosionAntibacterial agentsAerosol deliveryThermal stimulationIrritation
The invention discloses rifaximin-containing in-situ gel for breast injection. The rifaximin-containing in-situ gel comprises the following components in percentage by mass: 0.1-15% of an active drug component existing in a dispersed way, 0.25-20% of an active drug component existing in an inclusion compound form, 12-30% of poloxamer 407, 0.01-11% of poloxamer 188, 0.01-10% of a polymer drag reduction agent, 0.001-3% of a bacterial inhibitor, a pH regulator for regulating the pH value to 5.5-7.5 and the balance of injection water. The invention also discloses a preparation method of the rifaximin-containing in-situ gel for breast injection. Water is used as a solvent, so that the rifaximin-containing in-situ gel is good in biocompatibility, little in irritation and high in safety, exists in a liquid state under a storage condition, is completely formed within 4-15 seconds after being injected to the breast because of thermal stimulation inside the breast and can be used for effectively treating, preventing and controlling mastitis of milk cows within the whole dry cow period; and in addition, the sustained-release effect is remarkable, and the in-vitro drug release time is long and reaches up to 48 days.
Owner:NANJING DEPOT VETERINARY PHARMA R&D CO LTD
Artesunate preparation for injection, and application thereof
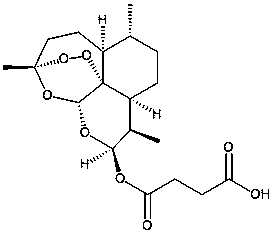
InactiveCN108245681AShorten the dissolution timeEasy clinical preparationOrganic active ingredientsDigestive systemDipotassium hydrogen phosphateSolubility
The invention discloses an artesunate preparation for injection. The artesunate preparation for injection includes sterile artesunate powder and an auxiliary material pH regulator powder for injection. The pH regulator for injection is one of disodium hydrogen phosphate, dipotassium hydrogen phosphate, sodium dihydrogen phosphate, potassium dihydrogen phosphate, sodium hydroxide and potassium hydroxide, or a mixture containing more of above substances according to an arbitrary ratio, and preferably one of the disodium hydrogen phosphate and dipotassium hydrogen phosphate or a mixture containing the disodium hydrogen phosphate and dipotassium hydrogen phosphate according to an arbitrary ratio. A molar ratio of the sterile artesunate to the pH regulator is 1:1 to 1:6, and preferably 1:1.0 to1:4.0. The above composition has obviously better solubility, solution stability and solid preparation stability than commercially available artesunate for injection, and is suitable for being clinically used.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com