Rifaximin-containing in-situ gel for breast injection and preparation method of rifaximin-containing in-situ gel
An in-situ gel, rifaximin technology, applied in the directions of medical preparations containing active ingredients, medical preparations with non-active ingredients, pharmaceutical formulas, etc. Gel application, long residence time and other problems, to achieve the effect of strong adhesion, reducing bacterial resistance and slowing gel corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
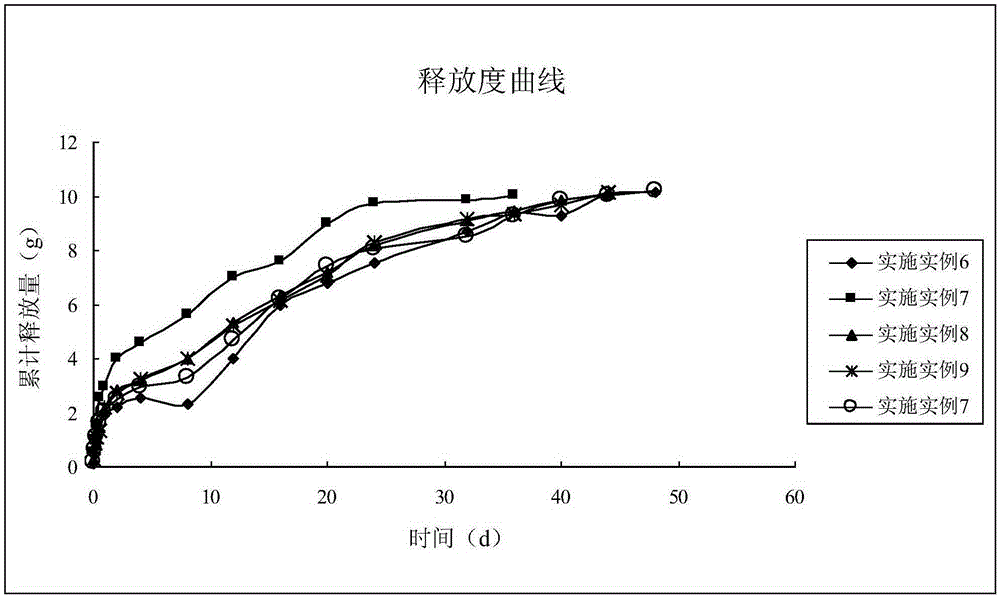
Image
Examples
Embodiment 1
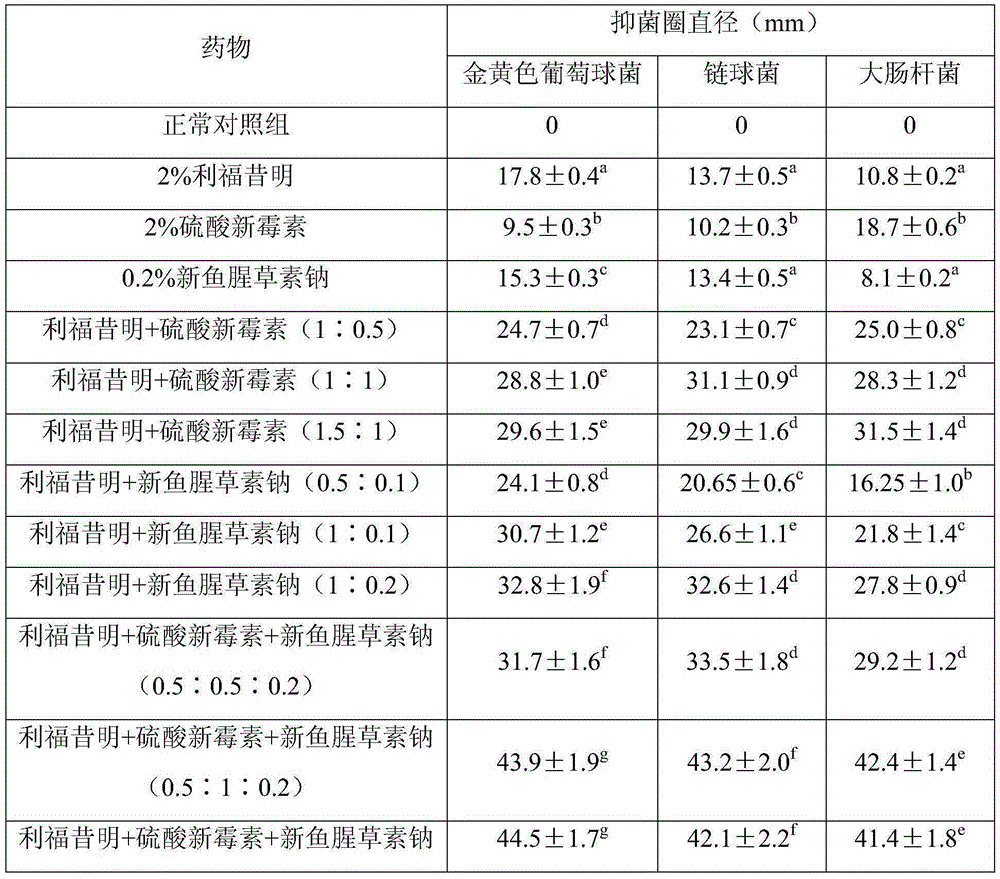
[0032] Embodiment 1: in vitro antibacterial test
[0033] (1) Test material
[0034] Drugs: rifaximin, neomycin sulfate, lysozyme, neohouttuydin sodium
[0035] Bacterial strains: clinically isolated drug-resistant strains of Staphylococcus aureus, Escherichia coli and Streptococcus agalactiae, common agar medium (self-made), common broth medium (self-made).
[0036] (2) Test method
[0037] 2% rifaximin solution and 0.2% neohouttuydin sodium solution were prepared with DMSO as solvent, and 2% neomycin sulfate solution was prepared with water for injection as solvent.
[0038] Inoculate the suspension of Staphylococcus aureus, Escherichia coli and Streptococcus on the agar medium, place it in a 37°C incubator and cultivate it for 24-48h, pick the grown colony and place it in a medium containing 2 mL of sterilized common broth medium In the test tube, dilute it properly with sterilized physiological saline to a bacterial content of 1.5×10 8 individual / mL. For aseptic opera...
Embodiment 2
[0045] Cyclodextrin inclusion complex of rifaximin
[0046] prescription
[0047] Rifaximin 100g
[0048] Ethyl-β-cyclodextrin 190g
[0049] 50% ethanol aqueous solution 1800mL.
[0050] Preparation method: (a), rifaximin is ultrafinely pulverized, and 90% of the particle size is controlled within 5 μm, and there must be no particles with a particle size larger than 10 μm; (b), 100 g of rifaximin and 190 g of ethyl- Put β-cyclodextrin into a grinder, add 900mL of 50% ethanol aqueous solution, and grind for 30 minutes; (c), add the remaining 900mL of 50% ethanol aqueous solution, stir for 4 hours, spray dry, and pass through a 300-mesh sieve to obtain rifaxime A cyclodextrin inclusion compound is disclosed, the particle size of the cyclodextrin inclusion compound is below 50 μm, and rifaximin exists in the form of inclusion compound in the cyclodextrin inclusion compound.
Embodiment 3
[0052] Rifaximin-Neomycin Sulfate Cyclodextrin Inclusion Complex
[0053] prescription
[0054]
[0055]
[0056] Preparation method: (a), rifaximin is ultrafinely pulverized, and 90% of the particle size is controlled within 5 μm, and there must be no particles with a particle size larger than 10 μm; (b), 100 g of rifaximin, 100 g of neosulfate Put mymycin and 400g of 2,6-diethyl-β-cyclodextrin into a grinder, add 2000mL of 50% ethanol aqueous solution, and grind for 30 minutes; (c), add the remaining 2000mL of 50% ethanol aqueous solution, stir for 5h, and spray dry through a 300-mesh sieve to obtain the rifaximin-neomycin sulfate cyclodextrin inclusion compound, the particle size of the cyclodextrin inclusion compound is below 50 μm, and the rifaximin and neomycin sulfate in the cyclodextrin inclusion compound Elements exist in the form of inclusion complexes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com