Preparation method for liraglutide
A technology of liraglutide and liraglutide, which is applied in the field of polypeptide drug preparation, can solve the problems of low total yield and purity of the product, difficulty in separation and purification of the preparation method, etc., and achieve low purification difficulty, wide practical value and application Prospects, the effect of improving product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
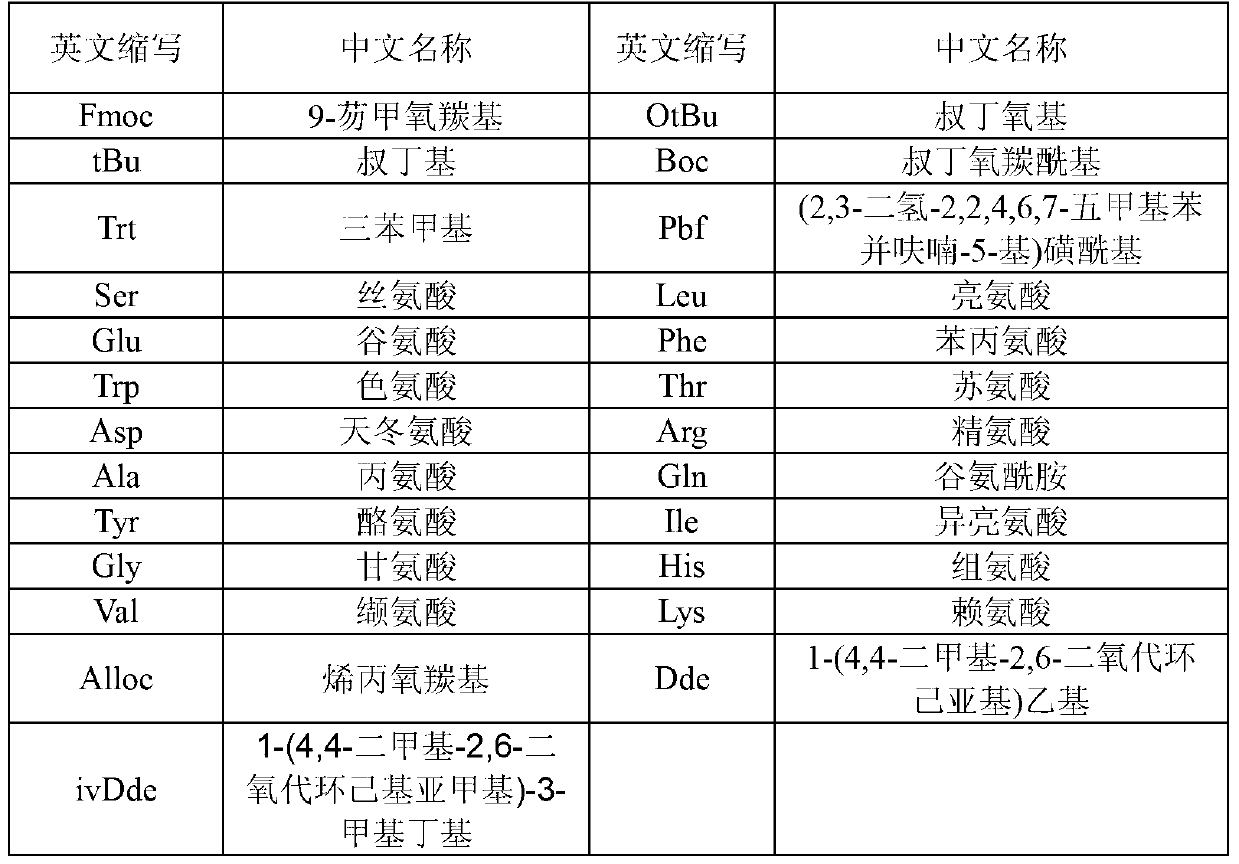
[0045] The preparation method of liraglutide comprises: adopting solid-phase polypeptide synthesis method to prepare liraglutide peptide resin, liraglutide peptide resin is subjected to acid hydrolysis to obtain liraglutide crude product, and finally liraglutide crude product is purified to obtain liraglutide peptide resin Laglutide pure product; wherein the steps of preparing liraglutide peptide resin by solid-phase peptide synthesis method are: on Fmoc-Gly-carrier resin, sequentially insert the corresponding protected amino acids in the following sequences or Fragment, preparation of liraglutide peptide resin:
[0046] Boc-W(Trt)-X(OtBu)-Thr(tBu)-Phe-Thr(tBu)-Ser(tBu)-
[0047] Asp(OtBu)-Val-Ser(tBu)-Ser(tBu)-Tyr(tBu)-Leu-X(OtBu)-
[0048] Gln(Trt)-Ala-Ala-Lys[Y(α-OtBu)]-Glu(OtBu)-Phe-Ile-Ala-
[0049] Trp(Boc)-Leu-Val-Z(Pbf)-Arg(Pbf)-Gly-resin;
[0050] Wherein, W is His-Ala, X is Glu-Gly, Y is Nα-PAL-Glu, and Z is Arg-Gly.
[0051] When accessing W, one-step access met...
Embodiment 1
[0078] The synthesis of embodiment 1 liraglutide peptide resin
[0079] Liraglutide peptide resin is:
[0080] Boc-W(Trt)-X(OtBu)-Thr(tBu)-Phe-Thr(tBu)-Ser(tBu)-
[0081] Asp(OtBu)-Val-Ser(tBu)-Ser(tBu)-Tyr(tBu)-Leu-X(OtBu)-
[0082] Gln(Trt)-Ala-Ala-Lys[Y(α-OtBu)]-Glu(OtBu)-Phe-Ile-Ala-
[0083]Trp(Boc)-Leu-Val-Z(Pbf)-Arg(Pbf)-Gly-resin;
[0084] Wherein, W is His-Ala, X is Glu-Gly, Y is Nα-PAL-Glu, and Z is Arg-Gly.
[0085] When inserting W, the corresponding protected amino acid is Boc-His(Trt)-Ala-OH; when inserting X, the corresponding protected amino acid is Fmoc-Glu(OtBu)-Ala-OH; when inserting Y, adopt two-step access method, the corresponding protected amino acids when Y is inserted in two steps are Fmoc-Glu(α-OtBu)-OH and PAL (palmitic acid); when Z is inserted, the corresponding protected amino acids are Fmoc-Arg(Pbf)-Gly-OH; The protected amino acid used when inserting Lys is Fmoc-Lys (Alloc).
[0086] Using Fmoc-Gly-resin as the initial carrier, the liraglu...
Embodiment 2
[0102] Example 2 Preparation of Liraglutide Crude Product
[0103] Take the liraglutide peptide resin prepared in Example 1, add a lysis reagent with a volume ratio of TFA:water:EDT=95:5:5 (lysis reagent 10mL / g resin), stir evenly, and stir at room temperature for 3 hours, The reaction mixture was filtered with a sand core funnel, the filtrate was collected, the resin was washed 3 times with a small amount of TFA, the combined filtrates were concentrated under reduced pressure, anhydrous ether was added to precipitate, and anhydrous ether was used to wash the precipitate 3 times, and the off-white powder was obtained by draining. It is the crude product of liraglutide, and the purity of the crude product is 45.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com