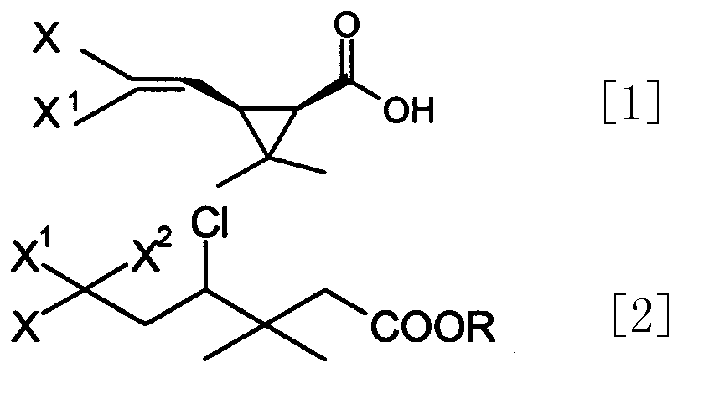
Process for preparing cis-halochrysanthemic acid
A production method, the technology of halochrysanthemic acid, which is applied in the production field of cis-halogenated chrysanthemic acid, can solve the problems of high energy consumption, long production cycle, and long reaction steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of Methyl 3,3-dimethyl-4,6,6-trichloro-7,7,7-trifluoroheptanoate
[0023] Under nitrogen protection, add 155 kg of methyl benicate, 400 kg of F113A and 900 kg of tert-butanol into a 2000-liter enamel reaction kettle, add 1.1 kg of cuprous chloride and 33 kg of ethanolamine in turn under stirring, and heat to reflux for 15 hours. The excess F113A and tert-butanol were recovered under normal pressure, and the fraction at 136-142° C. / 15 mmHg was collected by vacuum distillation, totaling 323.1 kg (content 92.24%), with a yield of 83%.
Embodiment 2
[0025] Preparation of Methyl cis, trans-3-(2,2-dichloro-3,3,3-trifluoropropyl)-2,2-dimethylCyclopropanecarboxylate
[0026] In a 500-liter enamel reaction kettle, under nitrogen protection, sequentially add petroleum ether, 6 kg of sodium amide and 13.7 kg of tert-butanol, and slowly heat up to reflux according to the gas release rate. GC traces that the content of tert-butanol no longer declines, about It takes 3-5 hours. Cool to -15°C, add 36.8kg of fluorochloroethene at one time, slowly heat up to -5°C, GC tracking (sampling 1ml, adding 1ml of water, sending organic layer sample) until the content of fluorochlorofluoroester is less than 2% (deducting solvent), Add 110 kg of saturated ammonium chloride solution, separate the organic layer, extract the water layer twice with 100 kg of petroleum ether, combine the organic layers, dry, and concentrate to obtain 29.6 kg of crude product (cis:trans=9:1), which is directly used in the next step hydrolysis.
Embodiment 3
[0028] Preparation of Methyl,trans-3-(2-chloro-3,3,3-trifluoropropenyl)-2,2-dimethylcyclopropanecarboxylate
[0029] In 500 liters of stainless steel reactors, add 50kg chlorofluorocyclic esters, 24kg potassium carbonate and 150kgDMF, heat up to 125 ℃ and react for 5 hours, GC tracks to raw material content
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com