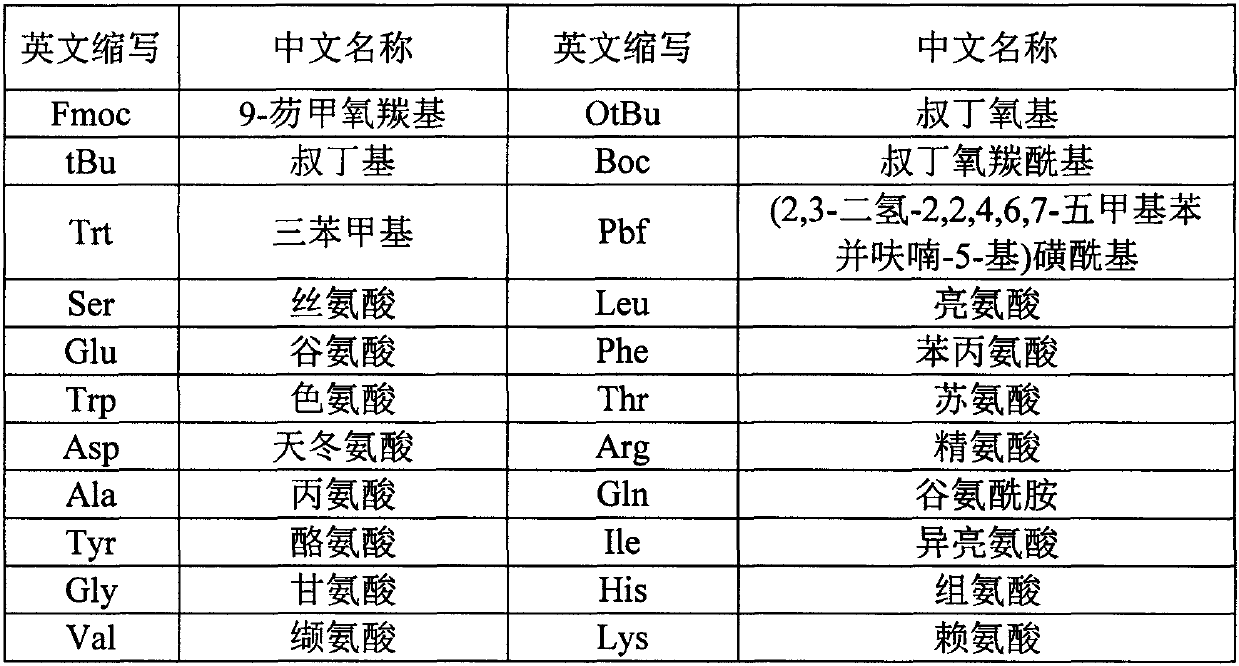
Preparation method of Tirzepatide
A technology of resin and peptide resin, which is applied in the field of peptide drug preparation, can solve the problems of low product purity, reduce the difficulty of purification, improve the purity of crude products, and achieve the effects of wide practical value and application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The synthesis of embodiment 1 Tirzepatide peptide resin
[0032] Tirzepatide Peptide Resin:
[0033] Boc-Tyr(tBu)-Aib-Glu(OtBu)-Gly-Thr(tBu)-Phe-Thr(tBu)-Ser(tBu)-Asp(OtBu)-Tyr(tBu)-Ser(tBu)-Ile- Aib-Leu-Asp(OtBu)-Lys(Boc)-Ile-Ala-Gln(Trt)-Lys(AEEA-AEEA-γGlu-Eicosanedioic acid(mon-tBu))-Ala-Phe-Val-Gln(Trt) -Trp(Boc)-Leu-Ile-Ala-Gly-Gly-Pro-Ser(tBu)-Ser(tBu)-Gly-Ala-Pro-Pro-Pro-Pro-Ser(tBu)-aminoresin
[0034]Using Rink Amide MBHA resin as the starting resin, Tirzepatide peptide resin was prepared by de-Fmoc protection and coupling reaction, followed by coupling with the protected amino acids shown in Table 2. The protected amino acids or fragments corresponding to the protected amino acids used in this example are as follows:
[0035] Table 2
[0036] The peptide sequence n= Protected Amino Acids 1 Fmoc-Ser(tBu) 2 Fmoc-Pro 3 Fmoc-Pro 4 Fmoc-Pro 5 Fmoc-Ala 6 Fmoc-Gly 7 Fmoc-Ser(tBu) 8 Fmoc-Ser(tBu) 9 F...
Embodiment 2
[0043] The preparation of embodiment 2 Tirzepatide crude product
[0044] Take the Tirzepatide peptide resin prepared in Example 1, add a cleaving reagent with a volume ratio of TFA:water:EDT=95:5:5 (cracking reagent 10mL / gram of resin), stir evenly, stir at room temperature for 3 hours, and use the reaction mixture Filter with sand core funnel, collect the filtrate, wash the resin 3 times with a small amount of TFA, combine the filtrates and concentrate under reduced pressure, add anhydrous ether to precipitate, then wash the precipitate with anhydrous ether 3 times, and drain to obtain off-white powder which is the crude product of Tirzepatide , the crude product purity is 72.7%.
Embodiment 3
[0045] Embodiment 3 The purification of Tirzepatide crude product
[0046] Take the crude Tirzepatide obtained in Example 2, add water and stir, adjust the pH to 8.5 with ammonia water until completely dissolved, filter the solution with a 0.45 μm mixed microporous membrane, and purify it for later use;
[0047] High-performance liquid chromatography was used for purification, and the chromatographic filler for purification was 10 μm reversed-phase C18. Two mobile phase systems were used for purification alternately. The first mobile phase system was 0.1% TFA / water solution-0.1% TFA / acetonitrile solution, and the second mobile phase system was 0.1% TFA / water solution-0.1% TFA / acetonitrile solution. The two mobile phase systems are 50mmol ammonium acetate / water solution-acetonitrile. The flow rate of the 77mm*250mm chromatographic column is 90mL / min, the gradient system is used for elution, and the sample is injected and purified in a circular manner. The crude product solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com