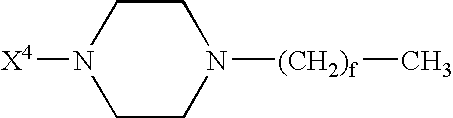
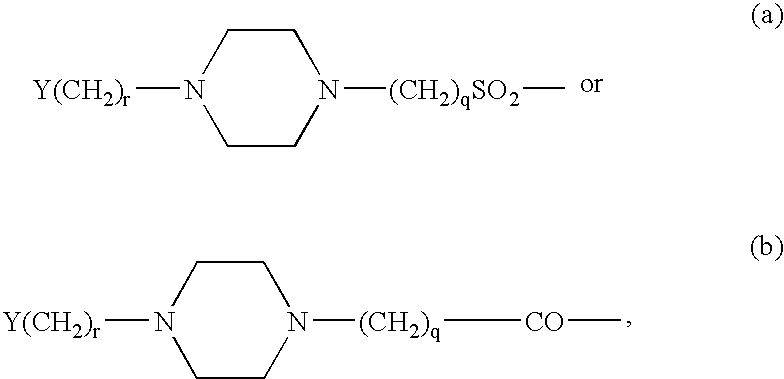
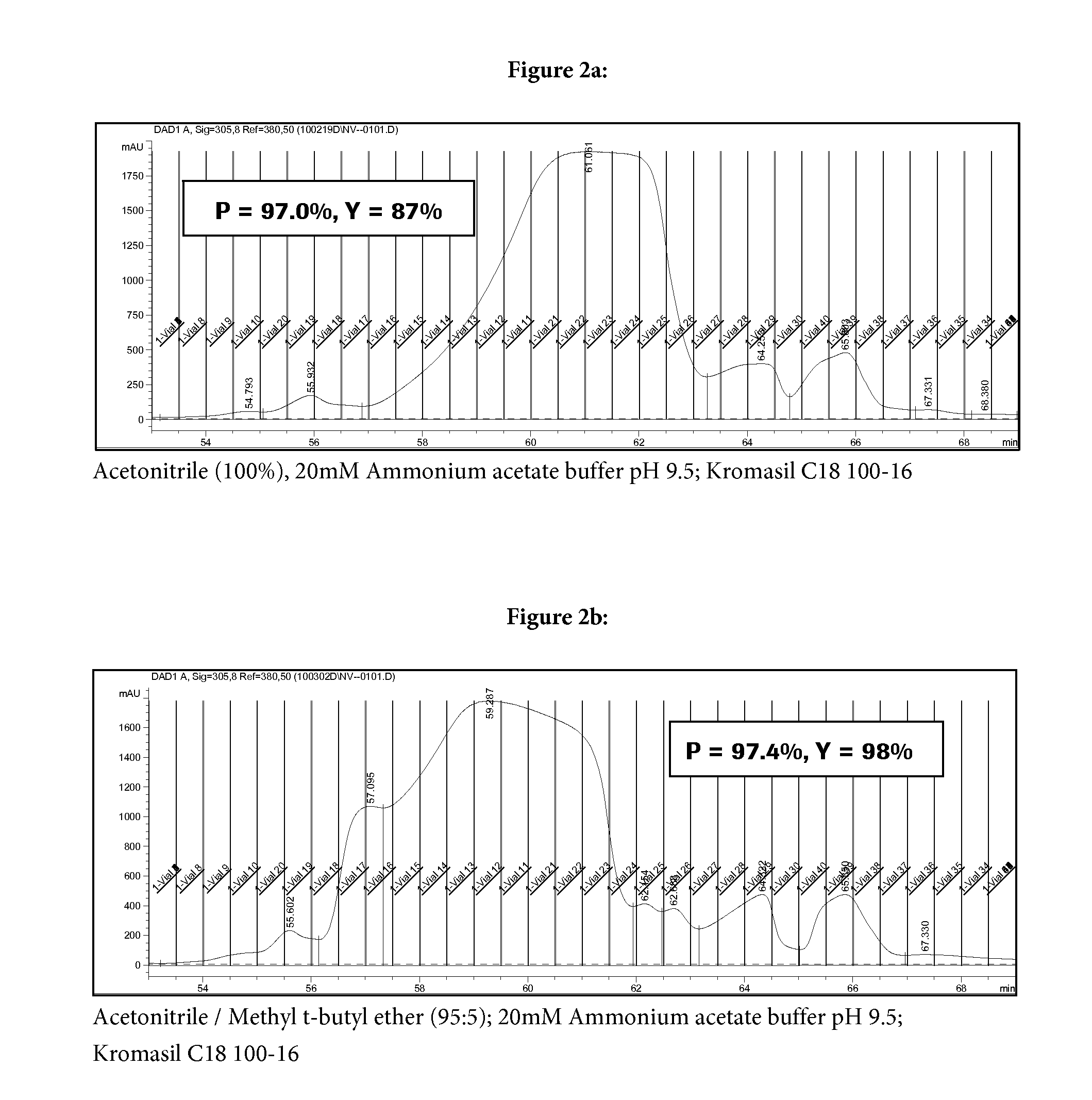
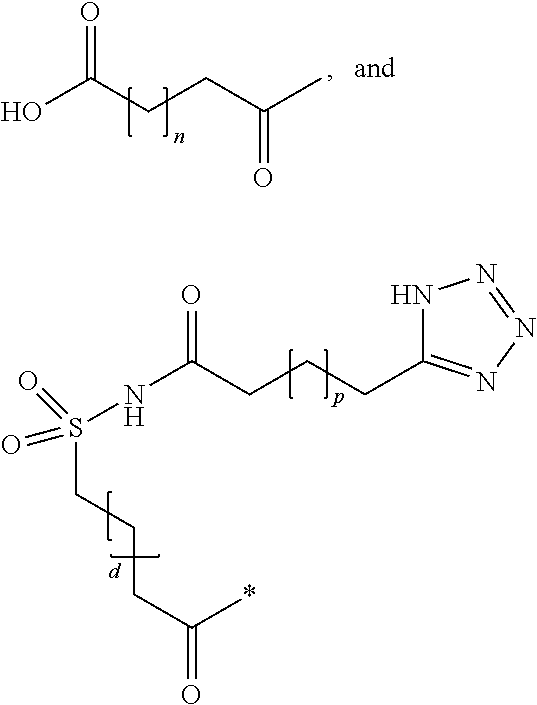
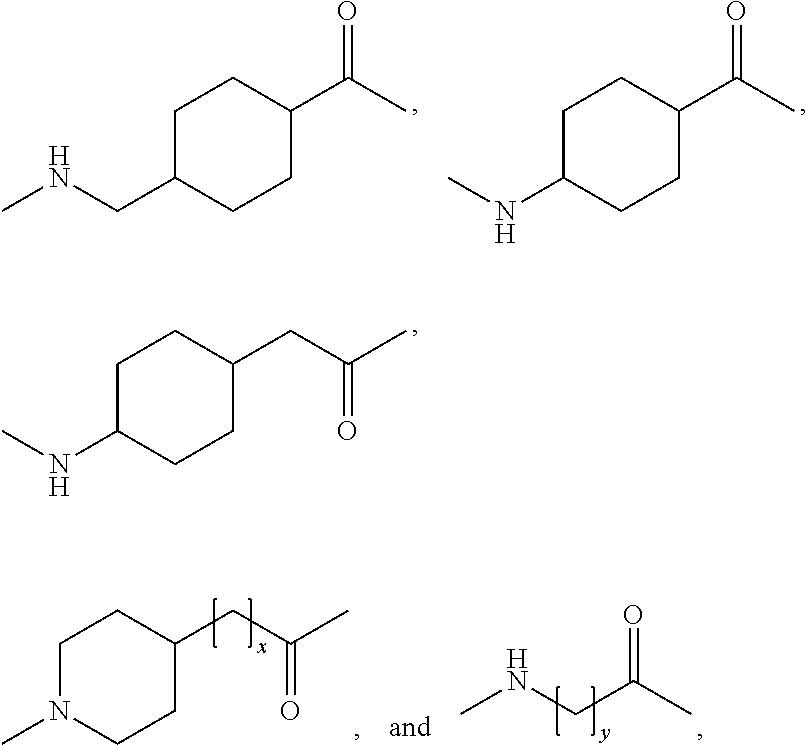
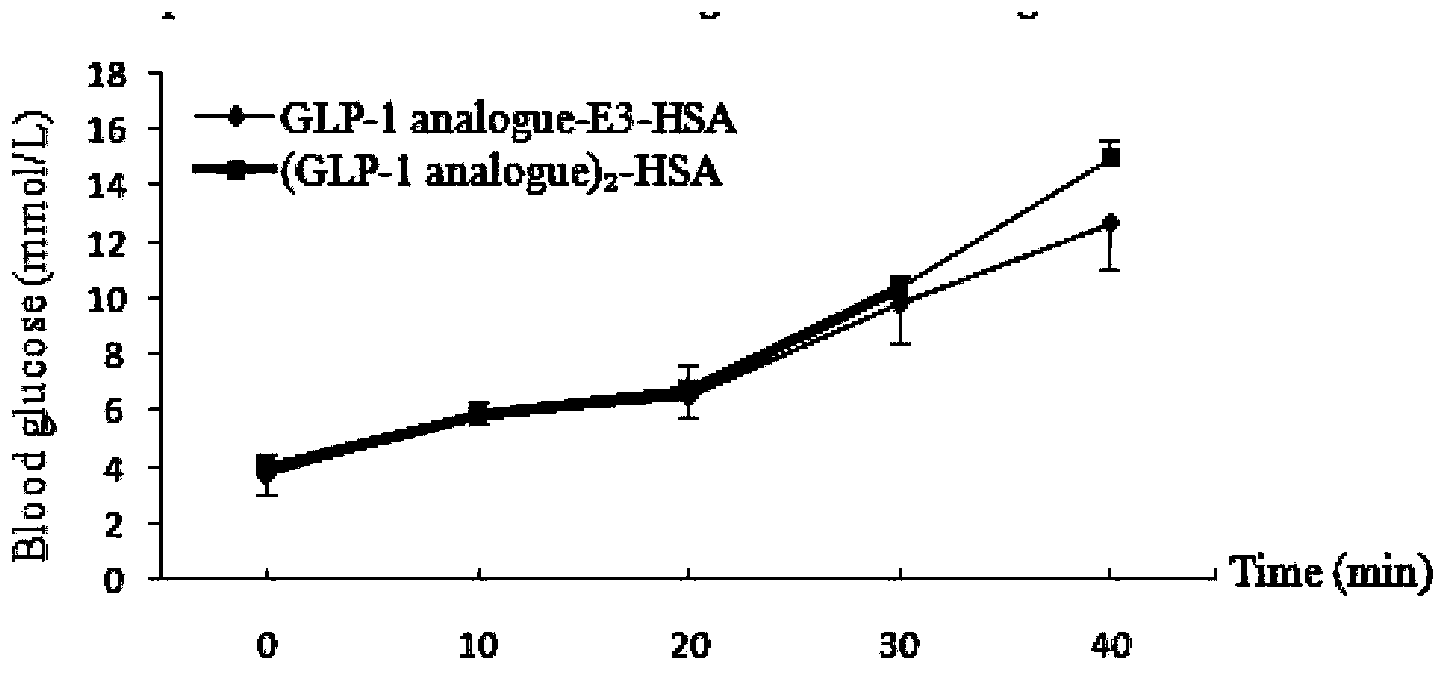
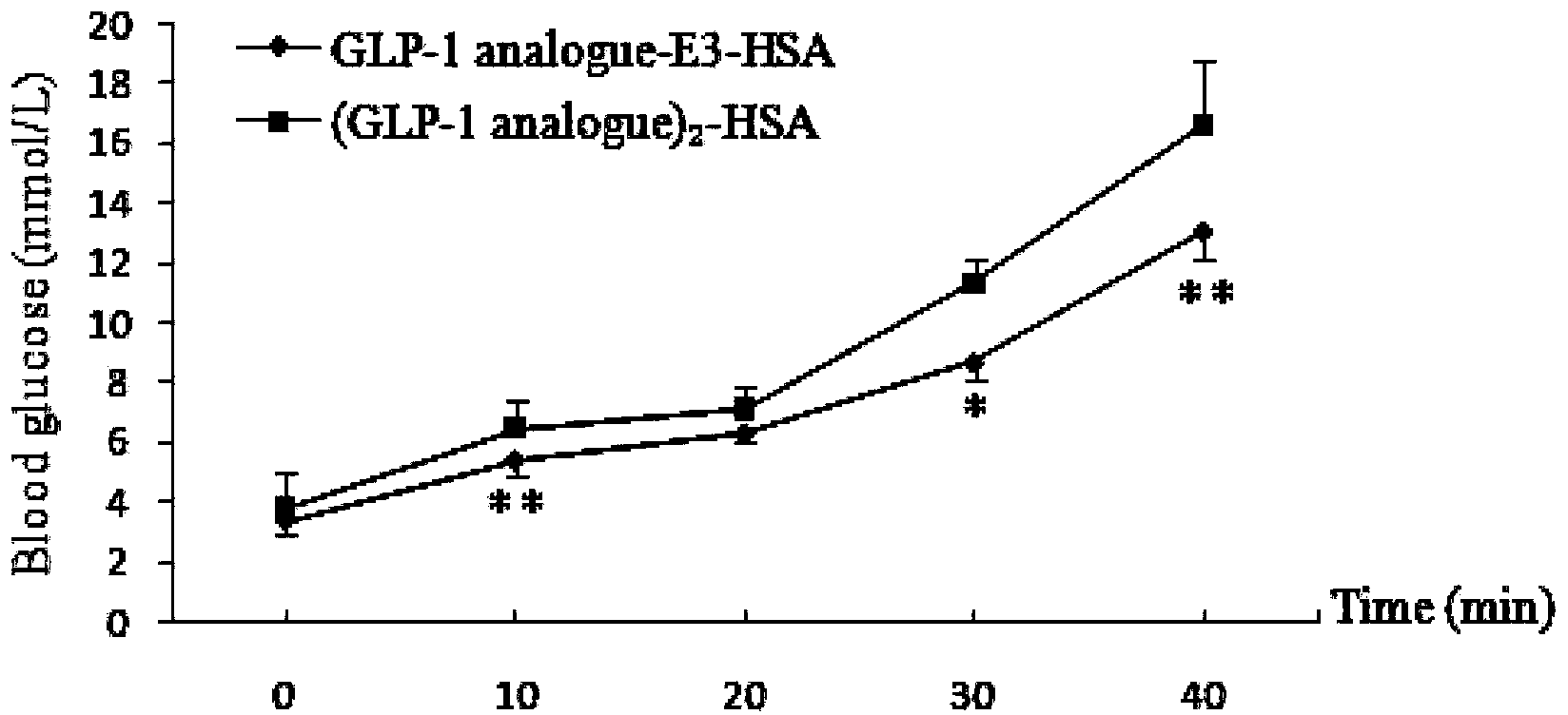
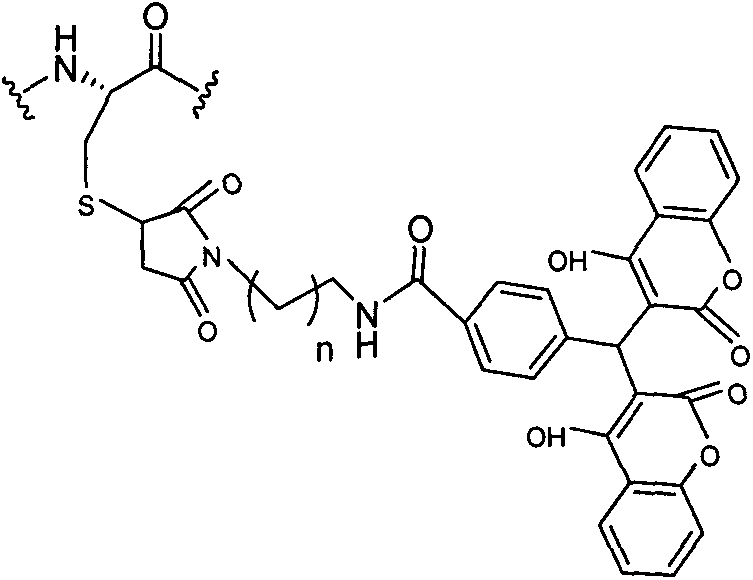
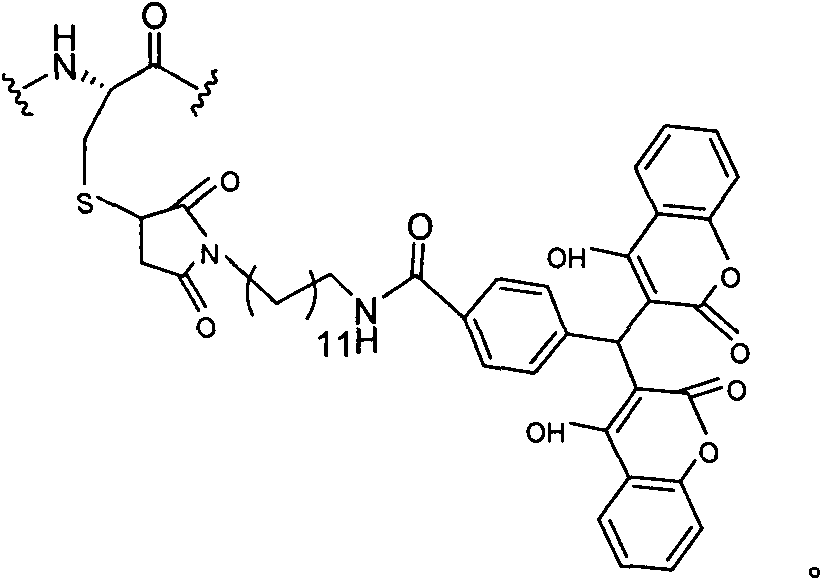
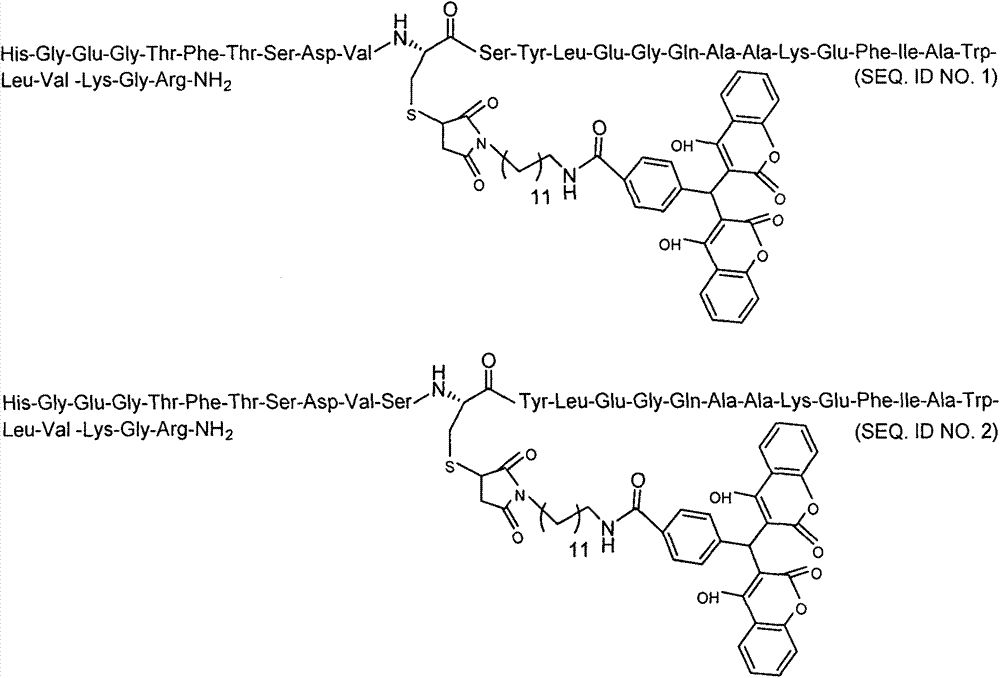
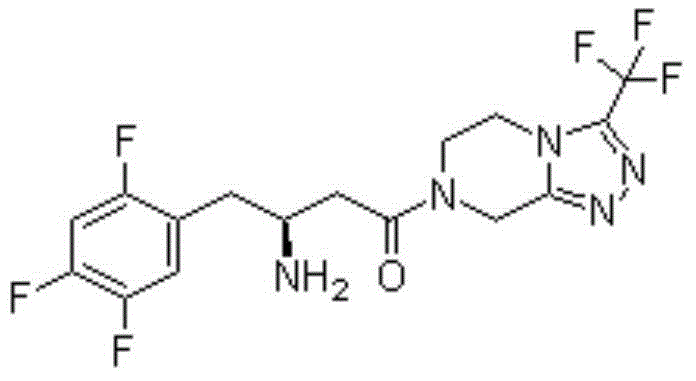
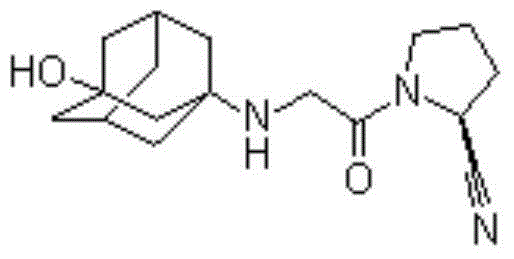
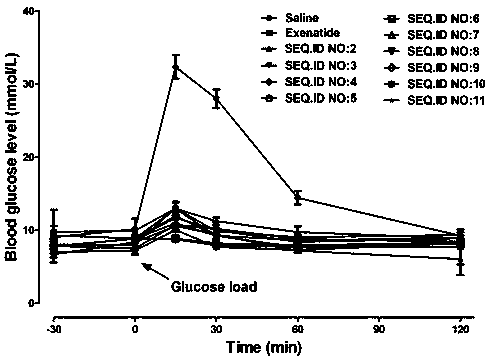
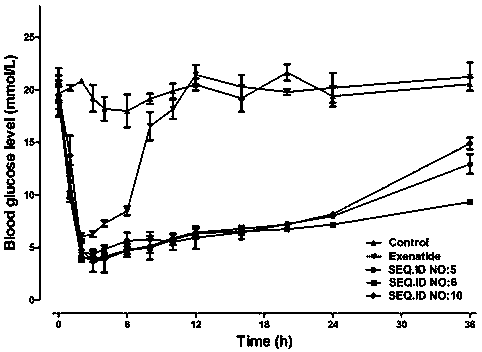
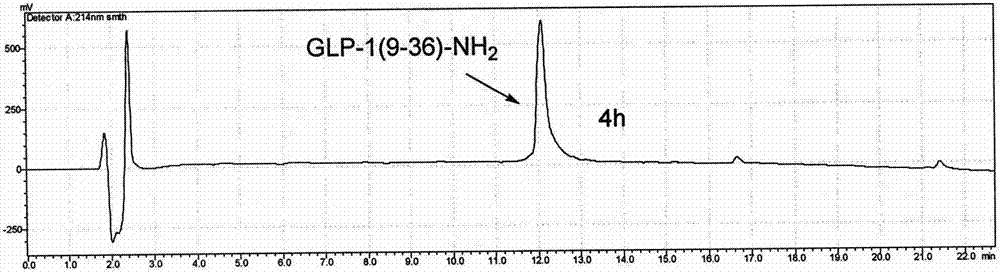
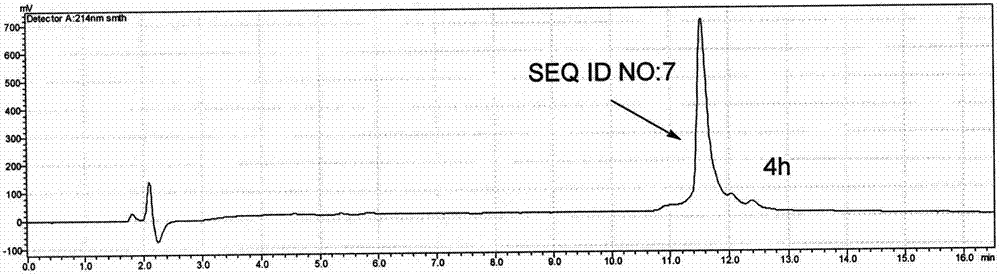
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
56 results about "GLP-1 Analogue" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Derivative or pharmaceutically acceptable salt of GLP-1 analogue and application of derivative or pharmaceutically-acceptable salt of a GLP-1 analogue
ActiveCN101987868AReach the lengthPeptide/protein ingredientsMetabolism disorderInsulin dependent diabetesHalf-life
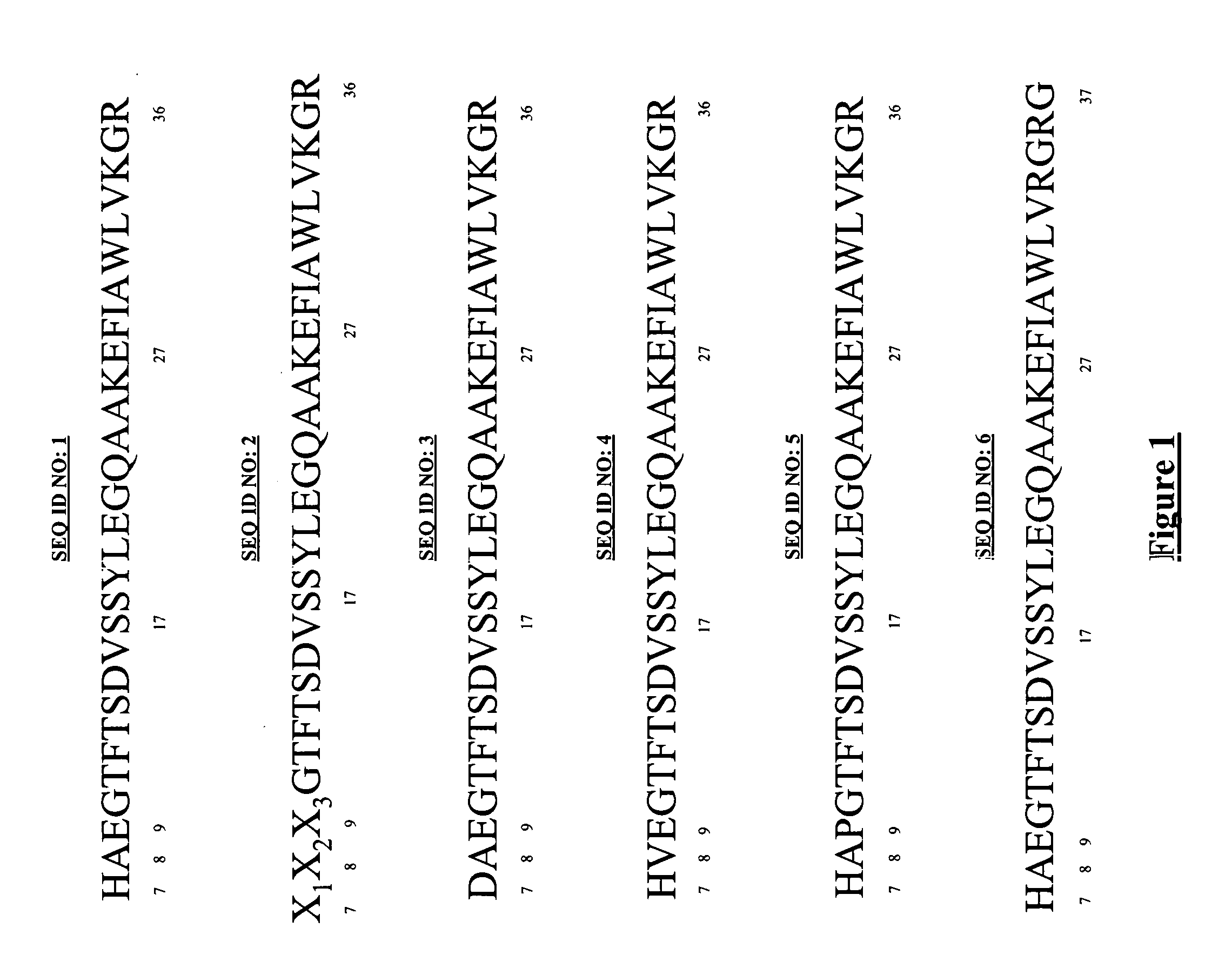
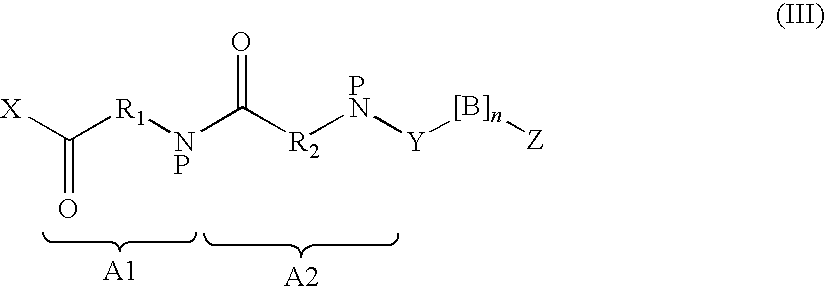
The invention relates to a derivative or pharmaceutically-acceptable salt of a GLP-1 analogue containing an amino acid sequence shown in a formula (I). The derivative of the GLP-1 analogue has the function of human GLP-1 analogue, and compared with the human GLP-1 analogue, the derivative has a longer half-life period in vivo. The derivative or pharmaceutically-acceptable salt of the GLP-1 analogue, or the pharmaceutical composition of derivatives containing the GLP-1 analogue or pharmaceutically-acceptable salts thereof can be widely used for treating non-insulin-dependent diabetes mellitus,insulin-dependent diabetes mellitus and obesity. The formula (1) is as follows: X1-X2-Glu-Gly-Thr-Phe-Thr-Ser-Asp-X10-Ser-X12-X13-X14-Glu-X16-X17-Ala-X19-X20-X21-Phe-Ile-X24-Trp-Leu-X27-X28-X29-X30-X31-X32-X33-X34-X35-X36-X37-X38-X39-Lys (I).
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Glucagon-like peptide-1 analogue monomer and dimer, preparation method therefor and application thereof
InactiveUS20150232527A1Conducive to clinical promotion and applicationPeptide/protein ingredientsMetabolism disorderHalf-lifeGlucagon-like peptide-1
Provided are a glucagon-like peptide-1 (GLP-1) analogue monomer and dimmer, a preparation method thereof, and an application thereof. The GLP-1 analogue monomer comprises one cysteine; and the dimer is formed by two monomer molecules connected via an intermolecular disulfide bond formed by the cysteine. The GLP-1 monomer comprising cysteine has the following general formula: 7HAEX10TFTSX15VSSYLEX22X23AAKEFIX30WLX33KGRG37, wherein X10 is glycine or cysteine, X15 is aspartate or cysteine, X22 is glycine or cysteine, X23 is glutamine or cysteine, X30 is alanine or cysteine, and X33 is valine or cysteine; and only one of X10, X15, X22, X23, X30, and X33 is cysteine. The glucagon-like peptide-1 analogue dimer of the present invention has an in vivo half-life of more than 8 to 96 hours, thus facilitating clinical promotion and application.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Use of GLP-1 Analogues for the Treatment of Disorders Associated with Dysfunctional Synaptic Transmission
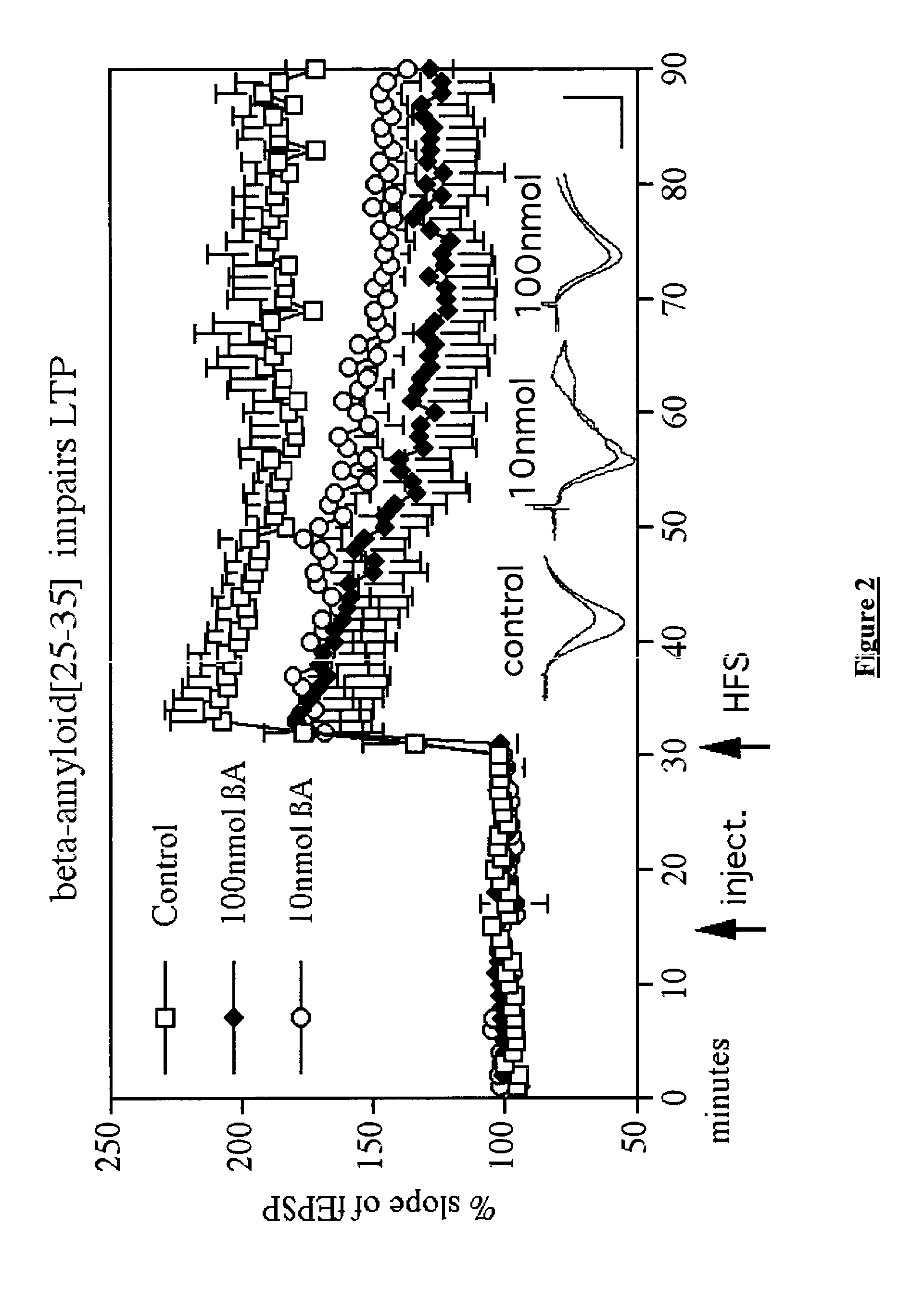
The present invention relates to a peptide analogue of glucagon-like peptide-1 (7-36), which is useful to prophylactically prevent, improve, or reverse the diminished cognitive function associated with these types of disorders, by increasing (or sustaining) the LTP of synaptic transmission. Moreover, sustaining LTP may find utility in the prophylaxis of neurological disease by delaying the onset of impaired cognitive processes, and could serve as a treatment, not only for the diminished cognitive function caused by neurodegeneration, but also for the dysfunctional cognitive processes associated with trauma or age.
Owner:INNOVATION ULSTER LTD
Micro-wave promoted solid-phase synthesis of glucagons-like peptide-1(GLP-1) analogue and uses thereof
InactiveCN101255191AImprove stabilityProlong the action timePeptide/protein ingredientsMetabolism disorderChemical synthesisMicrowave
The invention relates to new type GLP-1 analogues and a microwave promotive solid phase synthesis process of the same. GLP-1 analogues with longer pharmacological action time can be obtained by modifying 8, 9, 16, 22, 27 or 37 sites of natural GLP-1, the chemical synthesis is highly effectively and rapidly realized by microwave promotive solid phase synthesis process, the crude product is purified by highly effective liquid phase, and GLP-1 analogues is obtained after lyophilized.
Owner:CHINA PHARM UNIV
Glycosylated Form of Antigenic GLP-1 Analogue
InactiveUS20120264684A1Low antigenicityHigh blood stabilityMetabolism disorderDigestive systemD-GlucoseGlucagon-like peptide-1
Disclosed is a glucagon-like peptide-1 (GLP-1) analogue which is obtained by ameliorating a highly antigenic GLP-1 analogue so that the GLP-1 analogue has reduced antigenicity without being lowered in the blood glucose suppressing activity. Specifically disclosed is a glycosylated form of an antigenic GLP-1 analogue, which has GLP-1 activity and is obtained by substituting at least one amino acid of an antigenic GLP-1 analogue with a glycosylated amino acid.
Owner:GLYTECH
Long-acting glucagon-like peptide 1 (GLP-1) analogues and application thereof
InactiveCN103087178AChemically stableEasy to degradePeptide/protein ingredientsMetabolism disorderMicrowaveSynthesis methods
The invention relates to long-acting glucagon-like peptide 1 (GLP-1) analogues and a synthesis method thereof. GLP-1 analogues with longer pharmacological action time are obtained through adding a modified 37th amino acid to natural GLP-1, the synthesis of target polypeptides is quickly realized through a microwave-promoted solid-phase synthesis method, and crude products are purified and freeze-dried to obtain the GLP-1 analogues.
Owner:CHINA PHARM UNIV
Truncated glp-1 derivaties and their therapeutical use
InactiveUS20100292133A1Nervous disorderPeptide/protein ingredientsAmino acid substitutionExtracellular Structure
The invention relates to truncated GLP-1 analogues, in particular a GLP-1 analogue which is a modified GLP-1(7-35) (SEQ ID No 1) having: i) a total of 2, 3, 4, 5 6, 7, 8, or 9 amino acid substitutions as compared to GLP-1(7-35), including a) a Glu residue at a position equivalent to position 22 of GLP-1(7-35), and b) an Arg residue at a position equivalent to position 26 of GLP-1(7-35); as well as derivatives thereof, and therapeutic uses and compositions. These analogues and derivatives are highly potent, have a good binding affinity to the GLP-1 receptor, also to the extracellular domain of the GLP-1 receptor, which is of potential relevance achieving long-acting, stable GLP-1 compounds with a potential for once weekly administration.
Owner:NOVO NORDISK AS
Novel glp-1 analogues linked to albumin-like agents
Owner:NOVO NORDISK AS
Method for synthesizing glucagon-like peptide (GLP)-1 analogue in solid-phase mode
InactiveCN102977204AReduce generationReduce the difficulty of purificationHormone peptidesPeptide preparation methodsSolid-phase synthesisGLP-1 Analogue
The invention relates to the field of polypeptide solid-phase synthesis and provides a method for synthesizing glucagon-like peptide (GLP)-1 analogue in a solid-phase mode. The method for synthesizing the GLP-1 analogue in the solid-phase mode solves the problems that in the process of synthesizing the GLP-1 analogue in the solid-phase mode, peptide deficiency products and by-products are generated because of difficult or incomplete amino acid sequence connection, and the by-products are resulted to be difficulty to separate in subsequent purification. The peptide deficiency products and by-products are quite same in properties. According to the method for synthesizing the GLP-1 analogue in the solid-phase mode, a segment convergent synthesis method and a substituent-introduced method are adopted in difficult-connection points and polypeptide synthesis yield coefficient is improved.
Owner:JILIN AOTENG BIOTECH
Reversed phase HPLC purification of a glp-1 analogue
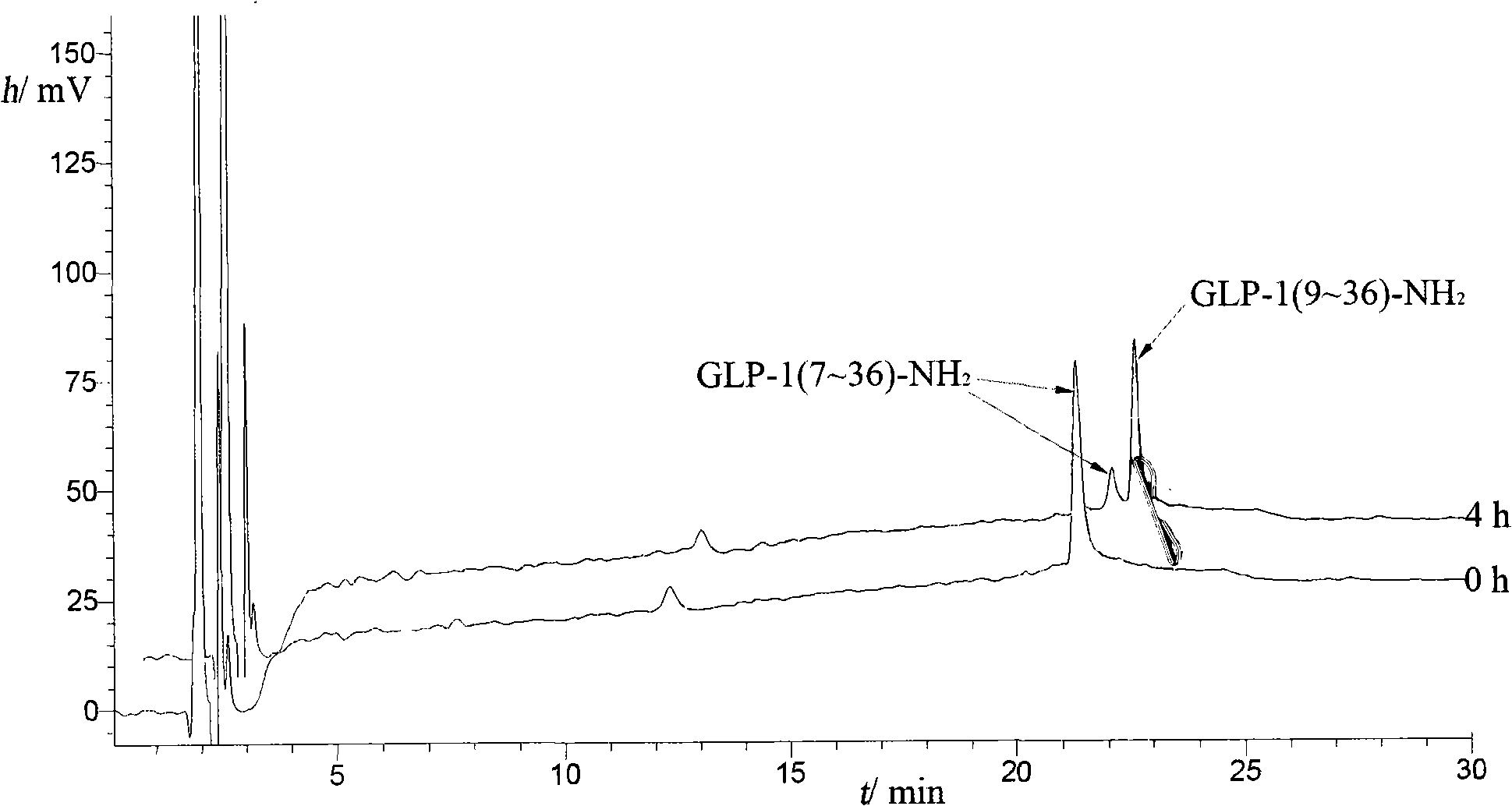
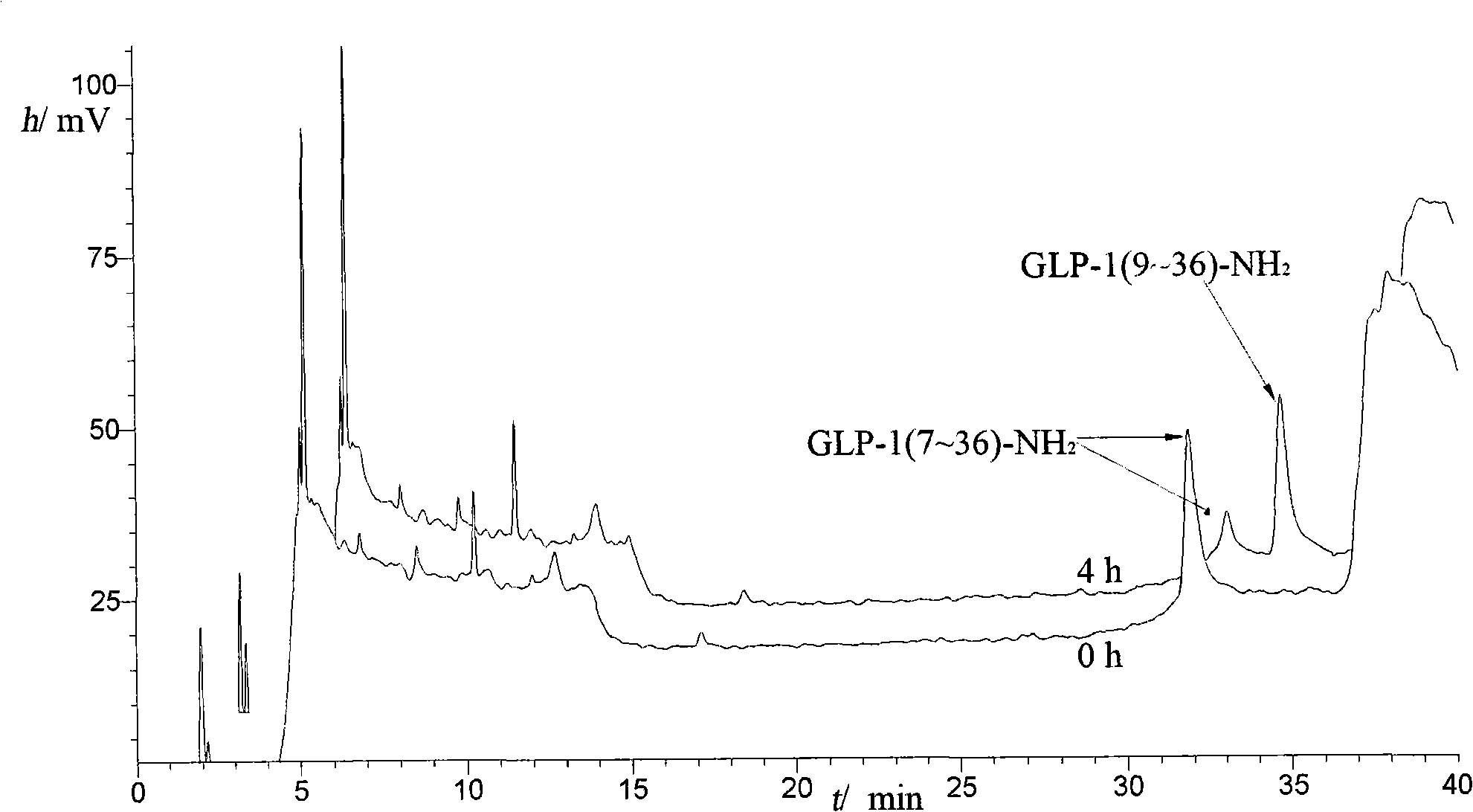
The invention comprises a process for the purification of a GLP-1 peptide analogue applying reversed phase high performance liquid chromatography (RP-HPLC).
Owner:F HOFFMANN LA ROCHE & CO AG
Truncated GLP-1 Derivatives and Their Therapeutical Use
InactiveUS20140296131A1Nervous disorderPeptide/protein ingredientsAmino acid substitutionExtracellular Structure
The invention relates to truncated GLP-1 analogues, in particular a GLP-1 analogue which is a modified GLP-1(7-35) (SEQ ID No 1) having: i) a total of 2, 3, 4, 5 6, 7, 8, or 9 amino acid substitutions as compared to GLP-1(7-35), including a) a Glu residue at a position equivalent to position 22 of GLP-1(7-35), and b) an Arg residue at a position equivalent to position 26 of GLP-1(7-35); as well as derivatives thereof, and therapeutic uses and compositions. These analogues and derivatives are highly potent, have a good binding affinity to the GLP-1 receptor, also to the extracellular domain of the GLP-1 receptor, which is of potential relevance achieving long-acting, stable GLP-1 compounds with a potential for once weekly administration.
Owner:NOVO NORDISK AS
Fusion protein of GLP-1 analogue and preparation method and application thereof
Owner:JIANGSU T MAB BIOPHARMA
Class of long-acting glucagon-like peptide-1 (GLP-1) analog and application thereof
ActiveCN107056928AGood hypoglycemic effectChemically stablePeptide/protein ingredientsMetabolism disorderSynthesis methodsGlucagon-like peptide-1
The invention relates to a class of long-acting glucagon-like peptide-1 (GLP-1) analog and a synthesis method thereof. The GLP-1 analogue with longer pharmacological action time is obtained by modifying GLP-1. The synthesis of target polypeptide is quickly achieved by a orthogonal protection strategy solid phase synthesis method. The crude product is purified and lyophilized to obtain the GLP-1 analog.
Owner:CHINA PHARM UNIV
Preparing method and applications of long-acting GLP-1 analogues modified with side chains
ActiveCN104262481AOvercoming the problem of short half-lifeExtended half-lifePeptide/protein ingredientsMetabolism disorderGlycineHalf-life
GLP-1 analogues are disclosed. The general formula A of the GLP-1 analogues is shown as follows: <7>HAEGT FX<13>SDV SSY X<20>E X<22>QAA X<26>EFIAW LVKGR G<37>. In the general formula A, the X<13> is threonine or side-chain-modified lysine (K-nX), the X<20> is leucine or the side-chain-modified lysine (K-nX), the X<22> is glycine or the side-chain-modified lysine (K-nX), the X<26> is lysine (K-NH2) free of side chain modification or the side-chain-modified lysine (K-nX), just only one of the X<13>, the X<20>, the X<22> and the X<26> is the lysine (K-nX), the n is 4-10, and the X is the glycine, alanine or valine. The GLP-1 analogues effectively prolong the blood half-life period of GLP-1, overcome the current situation that the GLP-1 cannot be used in clinic due to the short half-life period of the GLP-1, and have an application prospect in the field of medicines treating diabetes and obesity. A preparing method of the GLP-1 analogues and applications of the GLP-1 analogues are also disclosed.
Owner:天津天诚新药评价有限公司
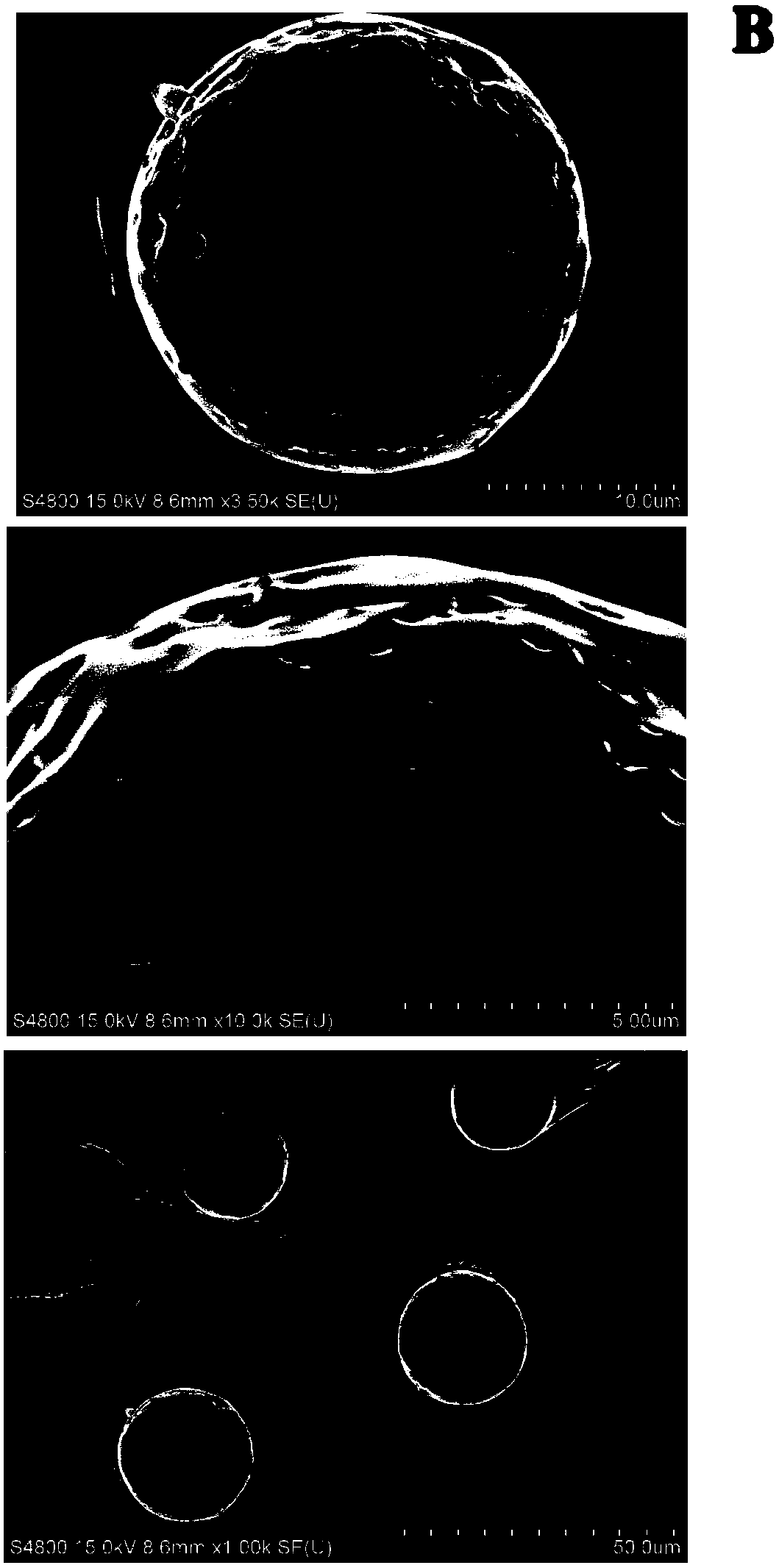
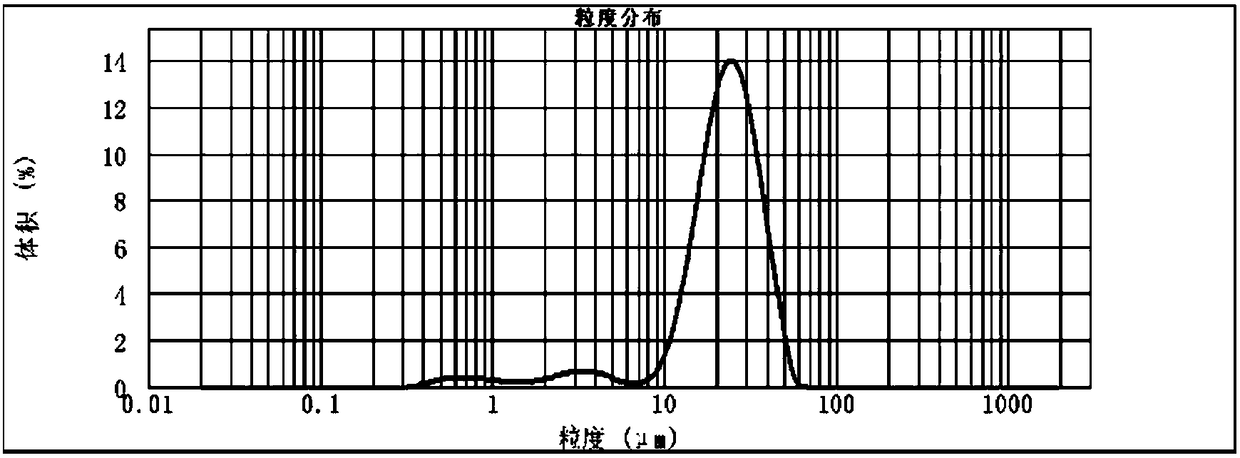
Glucagon-like peptide-1 analogue sustained release microsphere as well as preparation method thereof
InactiveCN108434118AReduce the number of dosesImprove compliancePeptide/protein ingredientsMetabolism disorderReduced doseMicrosphere
The invention discloses a glucagon-like peptide-1 analogue sustained release microsphere as well as a preparation method and an application thereof. The sustained release microsphere mainly includes 1-10 parts by weight of the glucagon-like peptide-1 analogue and 90-99 parts by weight of a polylactic acid-glycollic acid copolymer and is 0.5-100 [mu]m in range of average particle size. The invention also discloses the preparation method and the application of the sustained release microsphere and also inspects appearance, particle size, in-vitro releasing and in-vivo pharmacodynamic experimentof the microsphere. The sustained release microsphere supporting GLP-1 analogue has uniform particle size and has significant treatment effect on diabetes, can effectively control blood glucose for 14days and has the effect as a slow-effect preparation. The sustained release microsphere greatly reduces dosing frequency and increases compliance of patients, and also can inhibit food intake. The preparation method has simple process and gentle conditions, and is low in influence on activities of polypeptides.
Owner:CHINA PHARM UNIV
GLP-1 analogue composition for microneedle devices
InactiveCN103391798AIncrease viscosityGood release performancePeptide/protein ingredientsMetabolism disorderPolyethylene glycolGlycerol
A GLP-1 analogue composition for microneedle devices, which contains a GLP-1 analogue and at least one kind of solvent that is selected from the group consisting of water, glycerol, propylene glycol, ethylene glycol, 1,3-butylene glycol and polyethylene glycol.
Owner:HISAMITSU PHARM CO INC
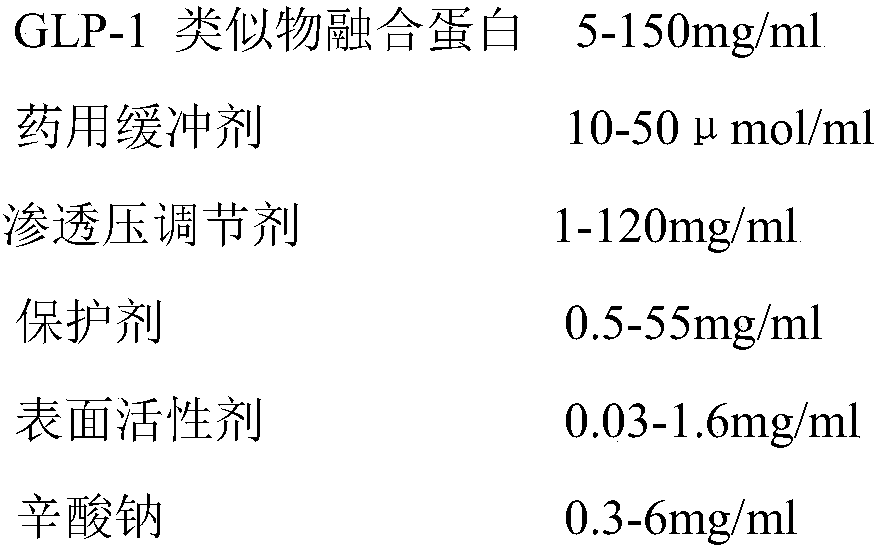
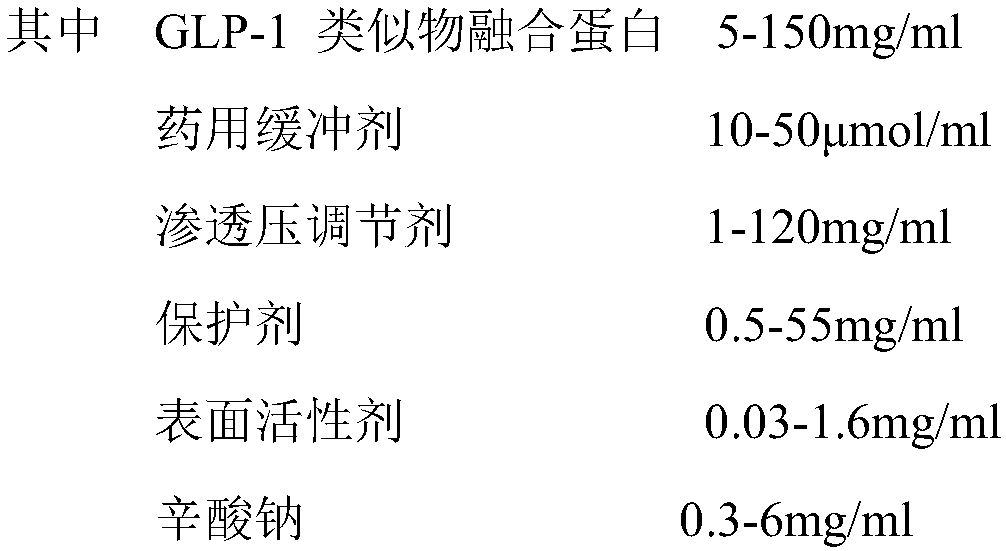
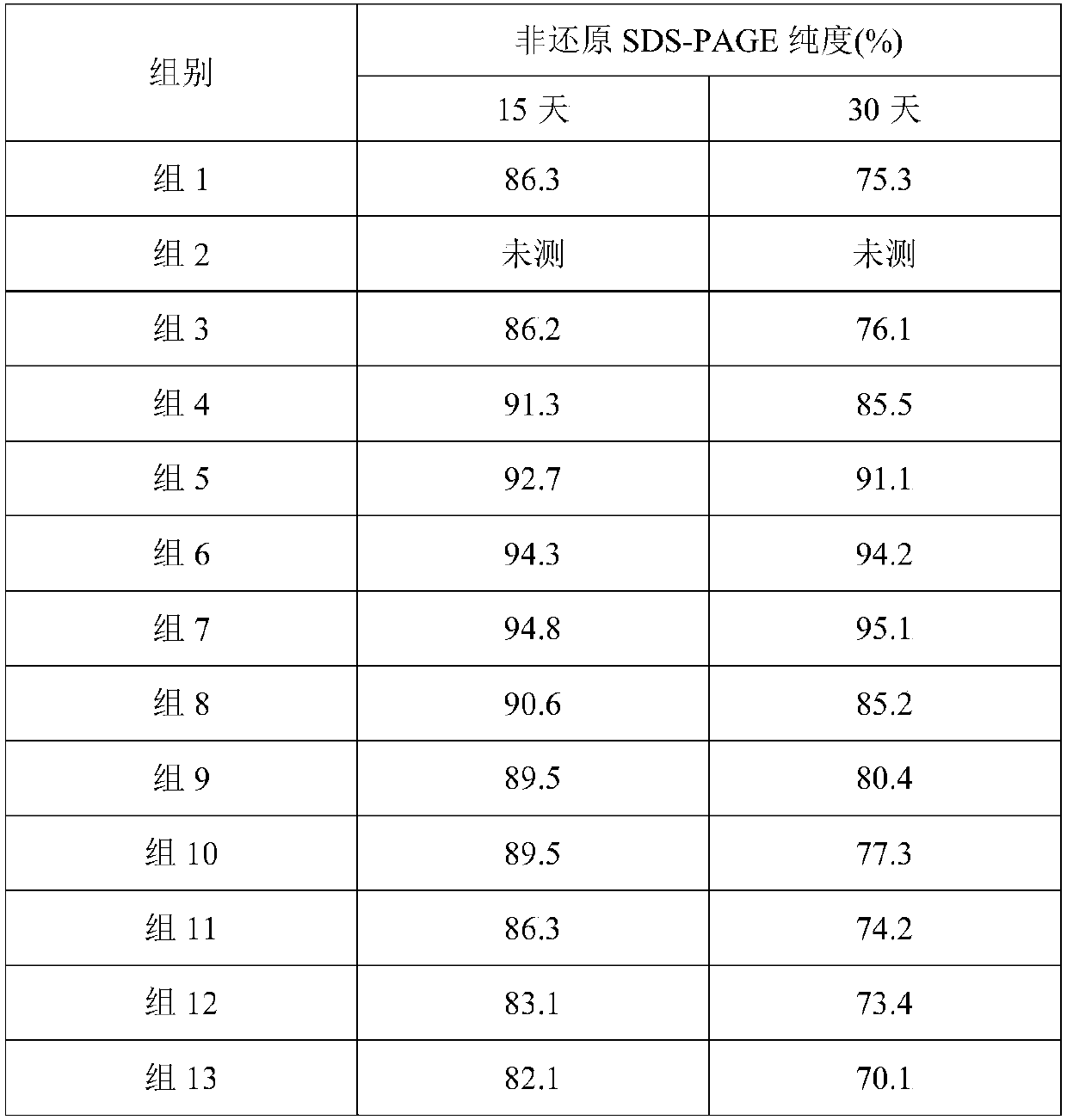
Stable liquid preparation containing GLP-1 analogue fusion protein and preparation thereof
InactiveCN107661288APeptide/protein ingredientsMetabolism disorderSurface-active agentsBuffering agent
The invention discloses a stable liquid preparation containing GLP-1 analogue fusion protein and preparation thereof. The stable liquid preparation is prepared from the effective components of: the GLP-1 analogue fusion protein, a pharmaceutical buffering agent, an osmotic-pressure regulating agent, a protective agent, a surface active agent, sodium caprylate and the like. The stable liquid preparation can be used for treating the diabetes and relevant diseases thereof.
Owner:JIANGSU T MAB BIOPHARMA
Glp-1 analogues and their pharmaceutical salts and uses
ActiveUS20120196798A1Long half-lifePeptide/protein ingredientsMetabolism disorderInsulin dependent diabetesHalf-life
This invention discloses GLP-1 analogues and their pharmaceutical salts, wherein the GLP-1 analogue comprises an amino acid sequence of general formula (I), wherein Lys represents a modified lysine with a lipophilic acid. The GLP-1 analogues provided by this invention have the function of human GLP-1, and a longer half-life in vivo compared with the human GLP-1. Uses of such compounds and compositions include treating non-insulin-dependent diabetes, insulin-dependent diabetes, and obesity.(I) X1-X2-Glu-Gly-Thr-Phe-Thr-Ser-Asp-X10-Ser-X12-X13-X14-Glu-X16-X17-Ala-X19-X20-X21-Phe-Ile-X24-Trp-Leu-X27-X28-X29-X30-X31-X32-X33-X34-X35-X36-X37-X38-X39-Lys
Owner:JIANGSU HANSOH PHARMA CO LTD
Pharmaceutical composition containing GLP-1 analogue and DPP-4 inhibitor and preparation method thereof
InactiveCN104548096ALose weightImprove blood sugarPeptide/protein ingredientsMetabolism disorderDiseaseSide effect
The invention belongs to the technical field of medicine preparation, and discloses a pharmaceutical composition containing a therapeutically effective amount of a glucagon-like peptide-1 (GLP-1) analogue and a dipeptidyl peptidase-4 (DPP-4) inhibitor and a preparation method thereof. The composition can effectively prevent, retard and treat type II diabetes, especially can effectively control the blood glucose concentration, and also can be used for treating diseases related to rising of the blood glucose concentration. In addition, the composition can reduce the use dosage of the GLP-1 analogue and the DPP-4 inhibitor, so as to effectively reduce side effects of the GLP-1 analogue and the DPP-4 inhibitor and improve the patient tolerance level.
Owner:HYBIO PHARMA
Semi-recombinant preparation of GLP-1 analogues
A semi-recombinant method for the production of GLP-1 analogues and derivatives with non-proteogenic amino acids in the N-terminal part combining the use of recombinant expression techniques and chemical peptide synthesis.
Owner:NOVO NORDISK AS
Aggregable glp-1 analogue and sustained-release pharmaceutical composition
InactiveUS20090286724A1High activityAssociation-aggregability has been improvedSenses disorderNervous disorderAcute hyperglycaemiaDiabetic complication
The present invention provides a GLP-1 analogue having a high association-aggregability or a pharmaceutically acceptable salt thereof, and a pharmaceutical composition to be used for preventing or treating diabetes, hyperglycemia, a diabetic complication caused by diabetes or hyperglycemia, or obesity, using the same.
Owner:CHUGAI PHARMA CO LTD
Xenopus laevis GLP-1 analogue and uses thereof
InactiveCN108359005AExtensive treatmentImprove in vivo stabilityNervous disorderPeptide/protein ingredientsSynthesis methodsSide chain
The invention relates to an Xenopus laevis GLP-1 analogue, a synthesis method and applications thereof. According to the present invention, the side chain and the C terminal of Xenopus laevis GLP-1 are subjected to structure modification to obtain the Xenopus laevis GLP-1 analogue with advantages of high blood glucose lowering activity and long pharmacological action time; and the Xenopus laevis GLP-1 analogue has significantly improved biological activity, and further has effects of strong long-acting blood glucose lowering and strong weight reducing.
Owner:XUZHOU NORMAL UNIVERSITY
Insulinotropic compounds and uses thereof
The invention provides novel GLP-1 analogues and compositions comprising such analogues. The compounds of the invention are useful in treating diabetes mellitus and related disorders.
Owner:ACTIVOTEC SPP LTD
Long-acting GLP-1 (glucagon-like peptide-1) analogue dimer and medical application thereof
ActiveCN107266555AGood hypoglycemic effectOvercoming the problem of short half-lifeNervous disorderPeptide/protein ingredientsGlucagon-like peptide-1GLP-1 Analogue
The invention belongs to the technical field of medicine and particularly provides a long-acting GLP-1 (glucagon-like peptide-1) analogue dimer. The dimer is formed by two GLP-1 analogue monomers which are represented as the general formula HAX1GTFTSDVSSYLEGQAAKEFIX2WLVK X3RZ, wherein Z is NH2, G, GNH2, -GCG or -GCA; X1 is Leu, Pro, Phe or Tyr; X2 is Ala or Cys; X3 is Gly or Aib; when X2 is Ala, Z is -GCG or -GCA; when X2 is Cys, Z is NH2, G or GNH2. The long-acting GLP-1 analogue dimer has a long-acting hypoglycemic effect and is highly homologous with endogenous GLP-1(7-37), thereby being capable of avoiding safety risks, and clinical medication compliance can be improved.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
GLP-1 analogue and ziconotide composite slow-release microsphere preparation
The invention relates to a GLP-1 analogue and ziconotide composite slow-release microsphere preparation and a preparation method thereof. The slow-release microsphere preparation is prepared from a GLP-1 analogue, ziconotide, a biodegradable high polymer material with biocompatibility, a stabilizing agent and a freeze drying protection agent. The preparation method comprises the following steps of (1) adding water for preparing the GLP-1 analogue and the ziconotide into a medicine solution A; adding an organic solvent for preparing the biodegradable high polymer material with biocompatibility into a solution B; (2) mixing the solution A and the solution B; performing ultrasonic processing to form primary emulsion; adding the primary emulsion into a stabilizing agent water solution saturated by an organic mixed solvent; performing homogenizing emulsification to obtain secondary emulsion; (3) performing room-temperature stirring on the secondary emulsion for 1 hour; then, raising the temperature to 40 DEG C to 45 DEG C; maintaining the temperature for one hour; then, lowering the temperature to 10 DEG C; adding the freeze drying protection gent; screening and collecting particles; performing freeze drying; performing radiation sterilization. By using a related technology, the effect of treating diabetes and PDN (painful diabetic neuropathy) complications for a long time can be achieved; the pain of a patient is reduced; the medicine compliance of the patient is improved; the clinic practical significance is realized.
Owner:深圳市健翔生物制药有限公司
Long-acting African clawed frog glucagon-like peptide-1 (GLP-1) analogue and application thereof
PendingCN106699870AChemically stableLow immunogenicityPeptide/protein ingredientsMetabolism disorderHalf-lifeSynthesis methods
The invention relates to a long-acting African clawed frog glucagon-like peptide-1 (GLP-1) analogue as well as a synthesis method and application thereof. An African clawed frog glucagon-like peptide-1 is subjected to PEG (Polyethylene Glycol) modification to obtain the African clawed frog GLP-1 analogue which can keep blood glucose lowering activity and has longer pharmacological action time. The African clawed frog GLP-1 analogue provided by the invention is high in synthesis yield and low in cost; and the half-life period of the analogue is remarkably prolonged and the biological activity is remarkably improved.
Owner:XUZHOU NORMAL UNIVERSITY
Long-acting glucagon-like peptide 1 (GLP-1) analogues and application thereof
InactiveCN103087176AChemically stableEasy to degradePeptide/protein ingredientsMetabolism disorderMicrowaveSynthesis methods
The invention relates to long-acting glucagon-like peptide 1 (GLP-1) analogues and a synthesis method thereof. GLP-1 analogues with longer pharmacological action time are obtained through replacing the 34th amino acid of natural GLP-1, the synthesis of target polypeptides is quickly realized through a microwave-promoted solid-phase synthesis method, and crude products are purified and freeze-dried to obtain the GLP-1 analogues.
Owner:CHINA PHARM UNIV
Novel GLP-1 analogues linked to albumin-like agents
Owner:NOVO NORDISK AS
Methods and compositions for treating NAFLD, hepatic steatosis, and sequelae thereof
Provided herein are oral pharmaceutical compositions containing a GLP-1 analogue and / or insulin for treating and reducing the incidence of nonalcoholic fatty liver disease (NAFLD), hepatic steatosis, and sequelae thereof, and methods of utilizing same.
Owner:ORAMED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com