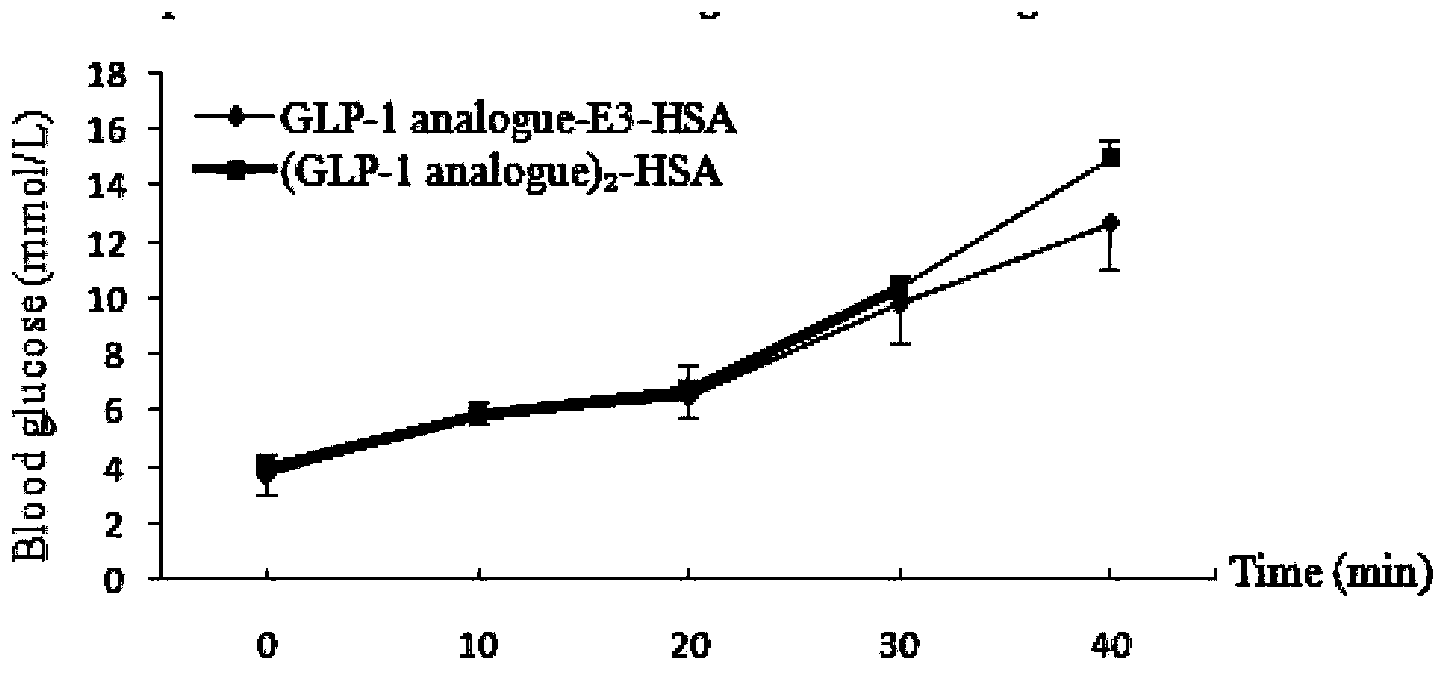
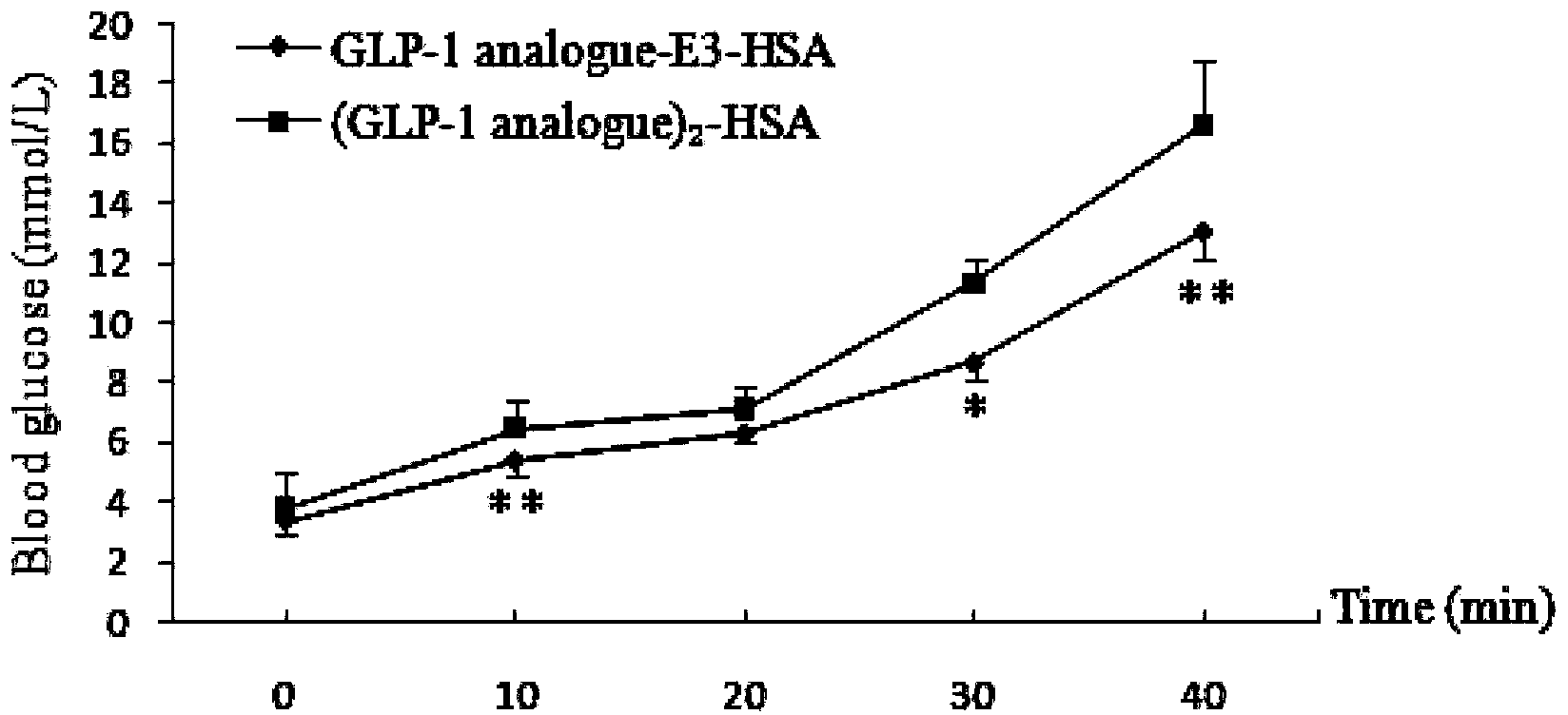
Fusion protein of GLP-1 analogue and preparation method and application thereof
A GLP-1 and fusion protein technology, applied in animal/human protein, drug combination, albumin peptide, etc., can solve the problems of increased risk of antibodies, increased risk of immunogenicity, instability of Albugon, etc., and achieve good protease resistance Stability, easy industrial preparation, excellent internal and external stability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0176] Embodiment 1 recombinant fusion protein expression plasmid construction
[0177] GLP-1 analogue nucleotide coding sequence (SEQ ID NO: 6):
[0178] CACGGCGAAGGGACCTTTACCAGTGATGTAAGTTTCTTATTTGGAAGAGCAAGCTGCCAAGGAATTCATTGCTTGGCTGGTGAAA
[0179] 1. HSA fusion fragment at the 13' end (GLP-1 analogue) 2 Gene fragment:
[0180] The following oligonucleotide sequence (SEQ ID NO: 17) was artificially synthesized:
[0181] CACGGCGAAGGGACCTTTACCAGTGATGTAAGTTCTTATTTGGAAGAGCAAGCTGCCAAGGAATTCATTGCTTGGC TGGTGAAACACGGCGAAGGGACCTTTACCAGTGATGTAAGTTCTTATTTGGAAGAGCAAGCTGCCAAGGAATTCATTGCT TGGCTGGTGAAA GATGCACACAAGAGTGAGG
[0182] Among them, the underlined part is the (GLP-1 analogue) 2 gene sequence, and the rest is the N-terminal coding sequence of HSA.
[0183] 1.23' GLP-1 analog with HSA fusion fragment-(Gly 4 Ser) 3 Gene fragment
[0184] The following oligonucleotide sequence (SEQ ID NO: 18) was artificially synthesized:
[0185] CACGGCGAAGGGACCTTTACCAGTGATGTAAGTTCT...
Embodiment 2
[0291] Embodiment 2 Construction of fusion protein expression engineering bacteria
[0292] Separately select containing (GLP-1 analogue) 2 -HSA / pPIC9, GLP-1 analogs-(Gly 4 Ser) 3 -HSA / pPIC9, GLP-1 analogs-(Gly 4 Ser) 4 -HSA / pPIC9, GLP-1 analog-E1-HSA / pPIC9, GLP-1 analog-E2-HSA / pPIC9, GLP-1 analog-E3-HSA / pPIC9, GLP-1 analog-E4-HSA / pPIC9, GLP-1 analogue-E5-HSA / pPIC9, GLP-1 analogue-E6-HSA / pPIC9 bacterial clone of expression vector plasmid, extract each expression vector plasmid respectively, and then use SalI to linearize each plasmid respectively, pass Each linearized plasmid DNA was recovered by agarose gel electrophoresis, and finally each linearized plasmid was transformed into Pichia pastoris GS115 competent cells by electroporation. Immediately after the electric shock, add 1ml of 1M sorbitol solution to mix the cells, transfer them to a 1.5ml centrifuge tube, let stand at 30°C for 1.5 hours, and then spread the cell suspension on the RDB selective plate, one plat...
Embodiment 3
[0296] Preparation of embodiment 3 GLP-1 analog fusion protein
[0297] Referring to the Pichia expression manual (A Manual of Methods for Expression of Recombinant Proteins in Pichia pastoris. Invitrogen Corporation), the strains expressing each GLP-1 analog fusion protein obtained in Example 2 were inoculated in YPD medium at 30°C 220 ~280rpm shaking culture until the wet weight of the bacteria is about 50g / L, put it into the tank (Biostat C10, Sartorius) according to 10% inoculation amount, 30°C, pH5.0, 30% saturation dissolved oxygen culture for 20 hours, and then continuously add methanol to start induction , control 40% saturation of dissolved oxygen, reduce the temperature to 22° C. after 4 hours of induction, end the induction after 50 hours, and collect the fermentation supernatant by centrifugation at 10000 g for 15 minutes.
[0298] Purification adopts four-step chromatography of BLUE affinity, PHE hydrophobicity, DEAE ion exchange and gel exclusion. First, the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com