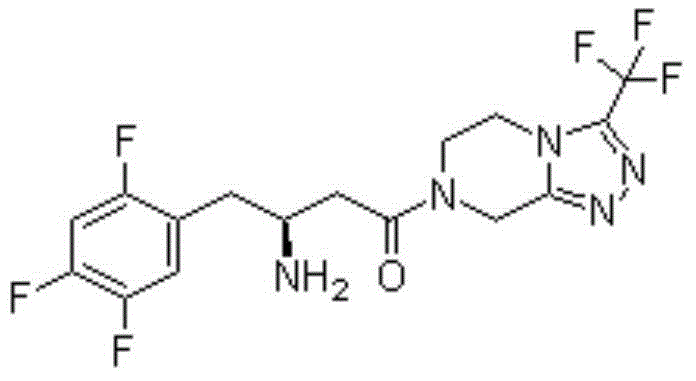
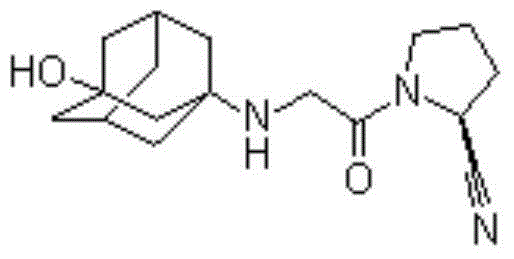
Pharmaceutical composition containing GLP-1 analogue and DPP-4 inhibitor and preparation method thereof
A GLP-1, DPP-4 technology, applied in the direction of drug combination, medical preparations containing active ingredients, drug delivery, etc., can solve the problems of elevated HbA1c, hypoglycemia, complicated patients, etc. Tolerability, the effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Preparation of liraglutide and sitagliptin injection
[0046] 1. Injection formula
[0047]
[0048] 2. Preparation process
[0049] Weigh 300mg of chlorobutanol and 1500mg of mannitol in a 10,000-class clean room and a 100-class ultra-clean workbench in a clean room, add 150mL of water for injection and stir to dissolve, then add to the batching tank; weigh 30mg of Liralu Peptide, 3000mg sitagliptin phosphate (calculated as sitagliptin), add 50mL water for injection to dissolve, add to the solution in the above liquid mixing tank, stir and dissolve until the solution is clear, add 0.1N sodium hydroxide, adjust the pH of the solution to 8.0, add the remaining amount of injection water to make up the volume, filter and sterilize with a 0.22 μm filter membrane; dispense into injection pens, each 3ml, 100 in total.
Embodiment 2
[0050] Example 2: Preparation of liraglutide and sitagliptin injection
[0051] 1. Injection formula
[0052]
[0053] 2. Preparation process
[0054] Weigh 3000mg of chlorobutanol and 2500mg of propylene glycol in a 10,000-class clean room and a 100-class ultra-clean workbench in a clean room, add 150mL of water for injection and stir to dissolve, then add to the batching tank; weigh 1080mg of liraglutide , 3000mg of sitagliptin phosphate (calculated as sitagliptin), dissolved in 50mL of water for injection, added to the solution in the above liquid mixing tank, stirred and dissolved until the solution was clear, added 0.1N sodium hydroxide, adjusted the pH of the solution to 8.4 , add the remaining amount of injection water to make up the volume, and use a 0.22μm filter membrane to filter and sterilize; dispense it into injection pens, each 3ml, 100 in total.
Embodiment 3
[0055] Example 3: Preparation of liraglutide and sitagliptin injection
[0056] 1. Injection formula
[0057]
[0058] 2. Preparation process
[0059] Weigh 3000mg of chlorobutanol and 2500mg of propylene glycol in a 10,000-class clean room and a 100-class ultra-clean workbench in a clean room, add 150mL of water for injection and stir to dissolve, then add to the batching tank; weigh 600mg of liraglutide , 600mg of sitagliptin phosphate (calculated as sitagliptin), dissolved in 50mL of water for injection, added to the solution in the above liquid mixing tank, stirred and dissolved until the solution was clear, added 0.1N sodium hydroxide, adjusted the pH of the solution to 8.4 , add the remaining amount of injection water to make up the volume, and use a 0.22μm filter membrane to filter and sterilize; dispense it into injection pens, each 3ml, 100 in total.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com