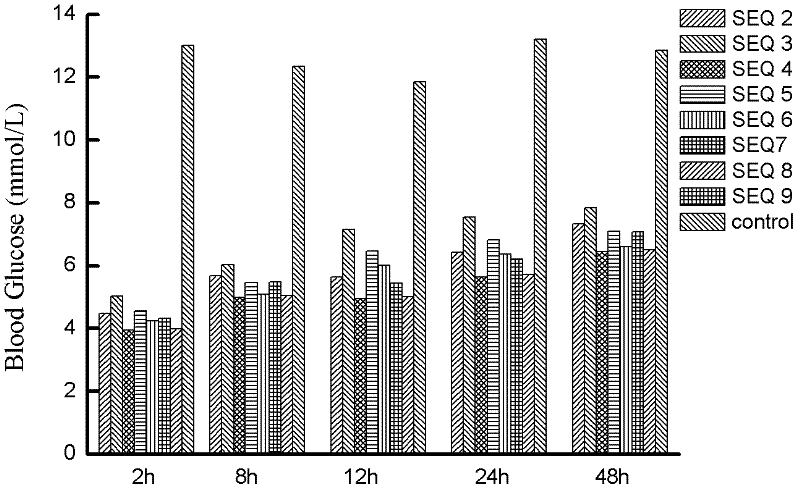
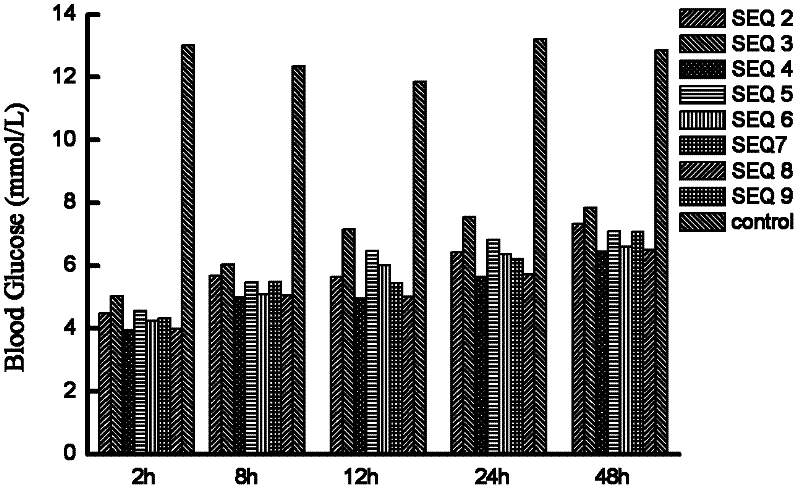
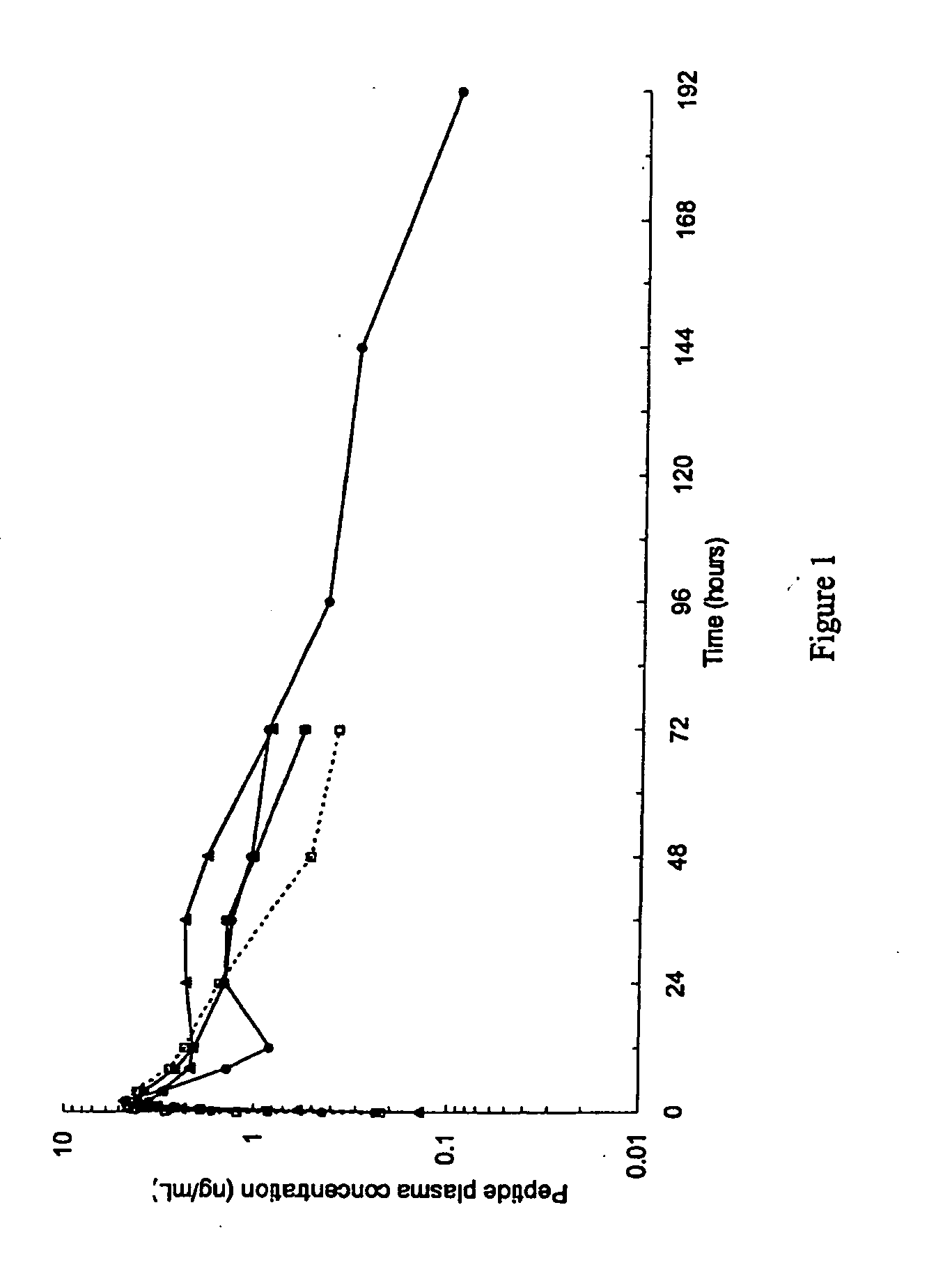
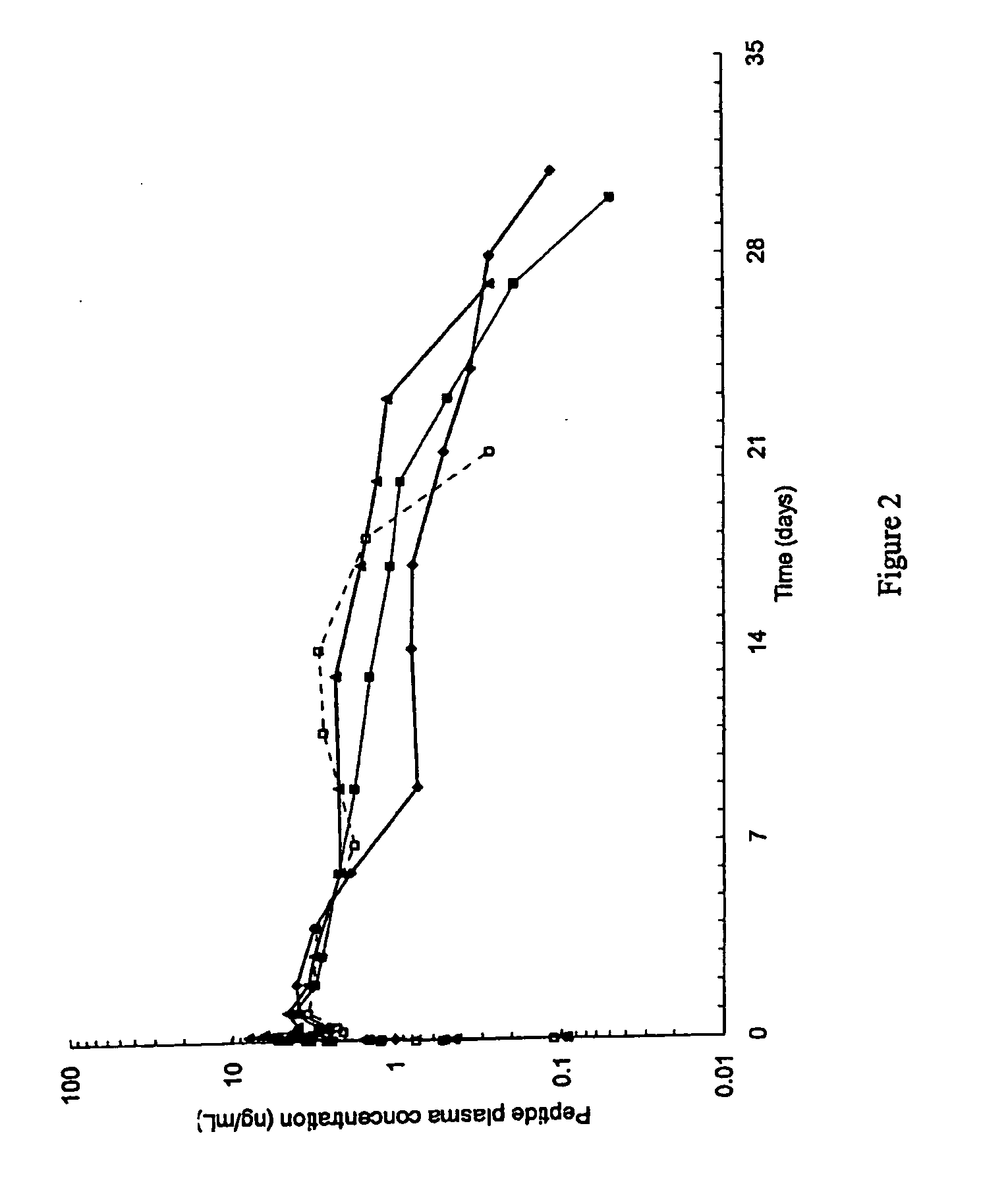
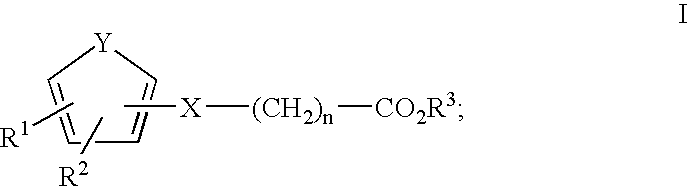Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
223 results about "Glucagon-Like Peptides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Peptides derived from proglucagon which is also the precursor of pancreatic GLUCAGON. Despite expression of proglucagon in multiple tissues, the major production site of glucagon-like peptides (GLPs) is the INTESTINAL L CELLS. GLPs include glucagon-like peptide 1, glucagon-like peptide 2, and the various truncated forms.
Long lasting synthetic glucagon-like peptide {GLP-1}
Modified insulinotropic peptides are disclosed. The modified insulinotropic peptides are capable of forming a peptidase stabilized insulinotropic peptide. The modified insulinotropic peptides are capable of forming covalent bonds with one or more blood components to form a conjugate. The conjugates may be formed in vivo or ex vivo. The modified peptides are administered to treat humans with diabetes and other related diseases.
Owner:CONJUCHEM
Use of GLP-1 analogs and derivatives administered peripherally in regulation of obesity
This invention relates the use of glucagon-like peptides such as GLP-1, a GLP-1 analog, or a GLP-1 derivative in methods and compositions for reducing body weight.
Owner:ELI LILLY & CO
Method for the production of polypeptides
The present invention relates to a novel method for the production of short chain polypeptides, including polypeptides having up to 3 disulfide bonds and / or structures rich in basic amino acid residues, and open structured short chain polypeptides, e.g. glucagon, glucagon like peptides and their functional analogues, in genetically modified yeast cells, said genetically modified yeast cells, and a method for the preparation of said yeast cells.
Owner:NOVO NORDISK AS
Combination drug
InactiveUS20060094722A1Enhance pharmacological effectsPrevent degradationAntibacterial agentsBiocideDipeptidyl peptidasePharmacology
Owner:EISIA R&D MANAGEMENT CO LTD
Use of glp-1 analogs and derivatives administered peripherally in regulation of obesity
This invention relates the use of glucagon-like peptides such as GLP-1, a GLP-1 analog, or a GLP-1 derivative in methods and compositions for reducing body weight.
Owner:DIMARCHI RICHARD +1
Stabilized pharmaceutical peptide compositions
ActiveUS20060178304A1Improve stabilityExtended shelf lifePeptide/protein ingredientsMetabolism disorderNeutral phMedicine
Method for increasing the shelf-life of a pharmaceutical composition for parenteral administration comprising a glucagon-like peptide which is prepared from a peptide product that has been subjected to treatment at a pH above neutral pH.
Owner:NOVO NORDISK AS
Glp-1 and methods for treating diabetes
InactiveUS20060057137A1Prevent and treat diseasePrevent and treat and delay onset of and reduce symptomPeptide/protein ingredientsMetabolism disorderDiseaseDiabetes mellitus
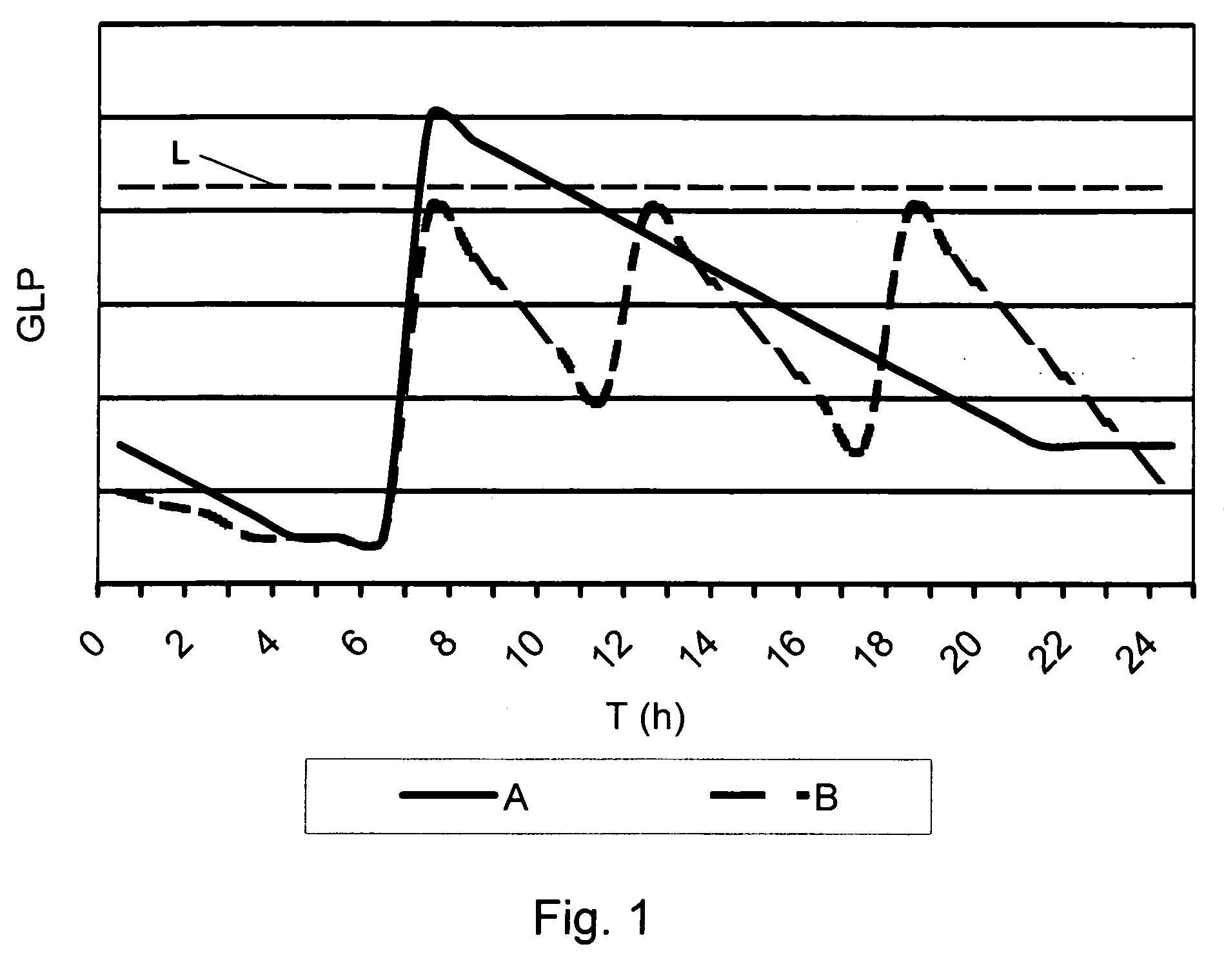
The present invention relates to use of GLP-1 or a related molecule having GLP-effect for the manufacture of a medicament for preventing or treating diabetes in a mammal. The amount and timing of administration of said medicament are subsequently reduced to produce a “drug holiday”. Practice of the invention achieves effective therapy without continuous drug exposure and without continuous presence of therapeutic levels of the drug. The invention also discloses a method of treating diabetes and related disorders in a mammal by administering glucagons like peptide (GLP-1) or a related molecule having GLP-1 like effect and thereby providing a therapeutically effective amount of endogenous insulin.
Owner:ZEALAND PHARM AS
Glp-1 and methods for treating diabetes
InactiveUS20090088369A1Reduce the amount requiredPrevent and treat diseasePeptide/protein ingredientsMetabolism disorderDiabetes mellitusTreatment level
The present invention relates to use of GLP-1 or a related molecule having a GLP-effect for the manufacture of a medicament for preventing or treating diabetes in a mammal. The amount and timing of administration of said medicament are subsequently reduced to produce a “drug holiday.” Practice of the invention achieves effective therapy without continuous drug exposure and without continuous presence of therapeutic levels of the drug. The invention also discloses a method of treating diabetes and related disorders in a mammal by administering glucagon like peptide (GLP-1) or a related molecule having GLP-1 like effect and thereby providing a therapeutically effective amount of endogenous insulin.
Owner:ZEALAND PHARM AS
Therapeutic formulations for transmucosal administration that increase glucagon-like peptide-1 bioavailability
What is described is a pharmaceutical formulation for intranasal delivery of glucagon-like protein-1 (GLP-1), comprising an aqueous mixture of GLP-1, a solubilizing agent, a chelator, and a surface active agent.
Owner:AMYLIN PHARMA INC +1
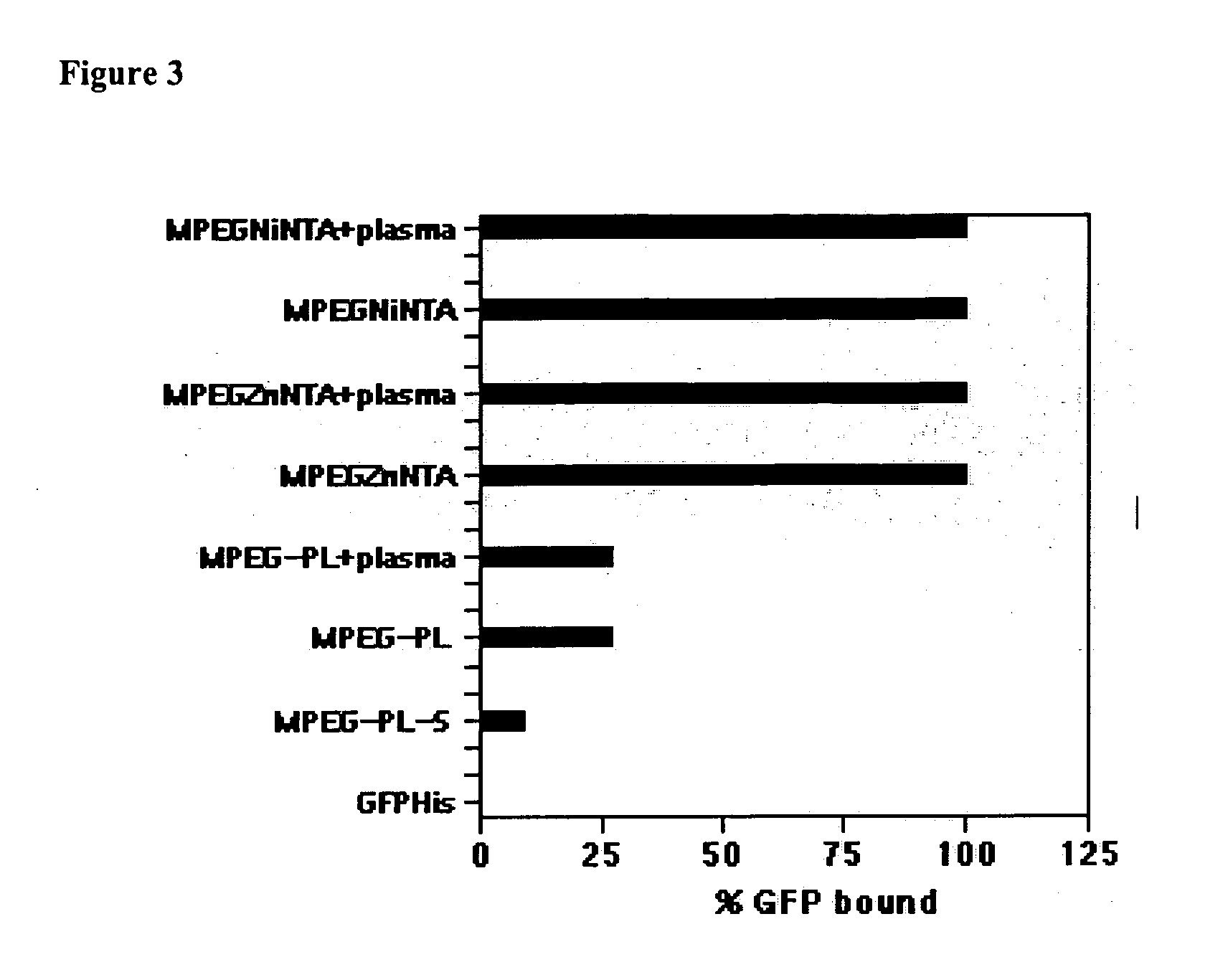
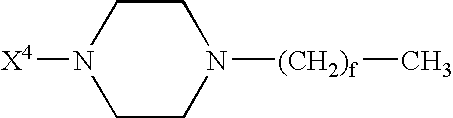
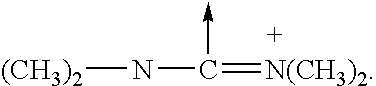
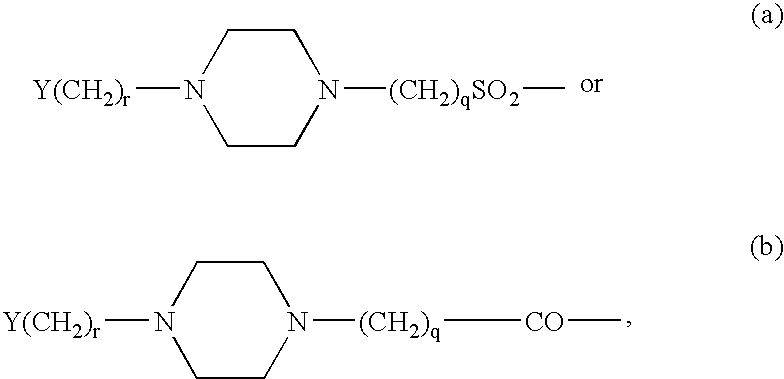
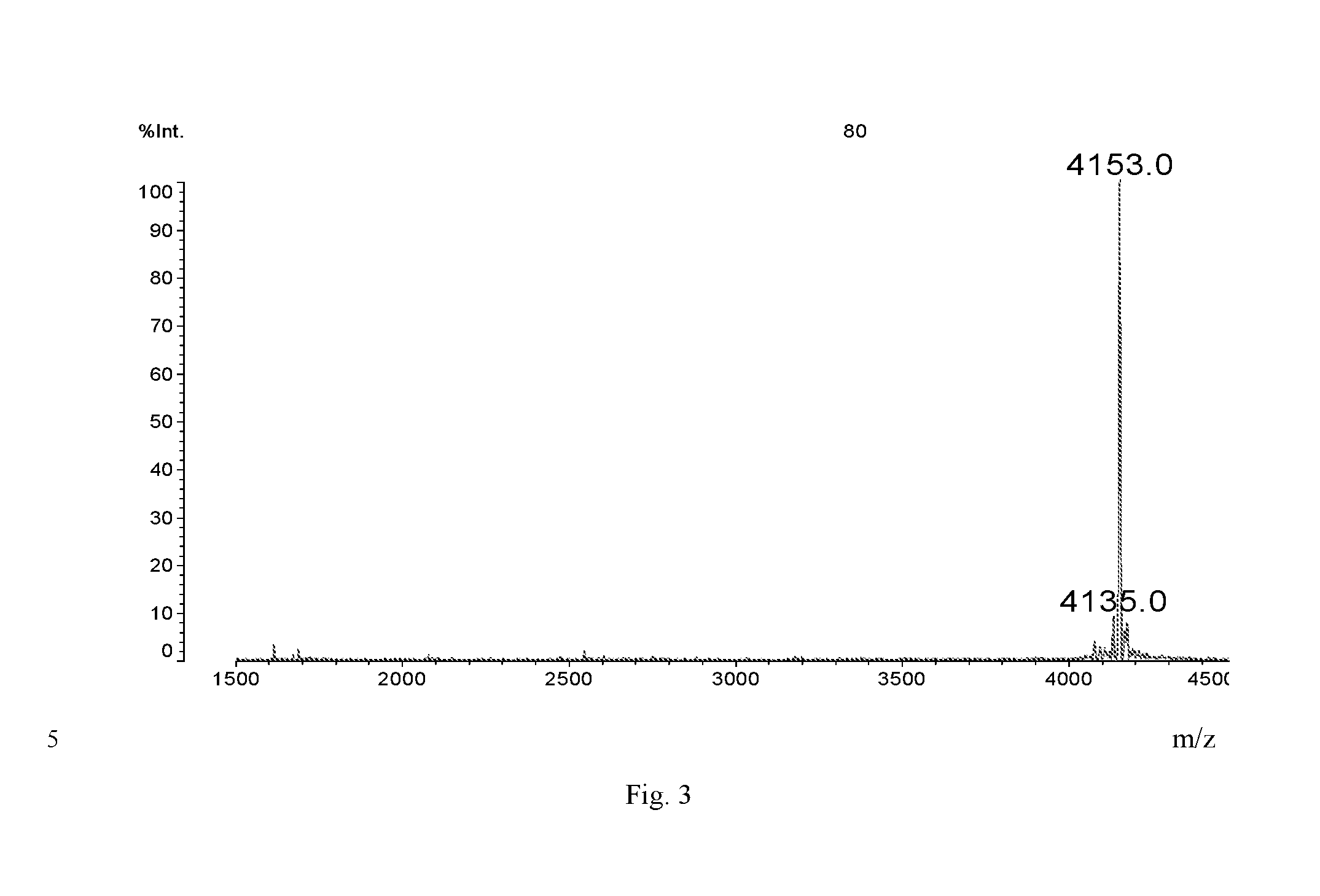
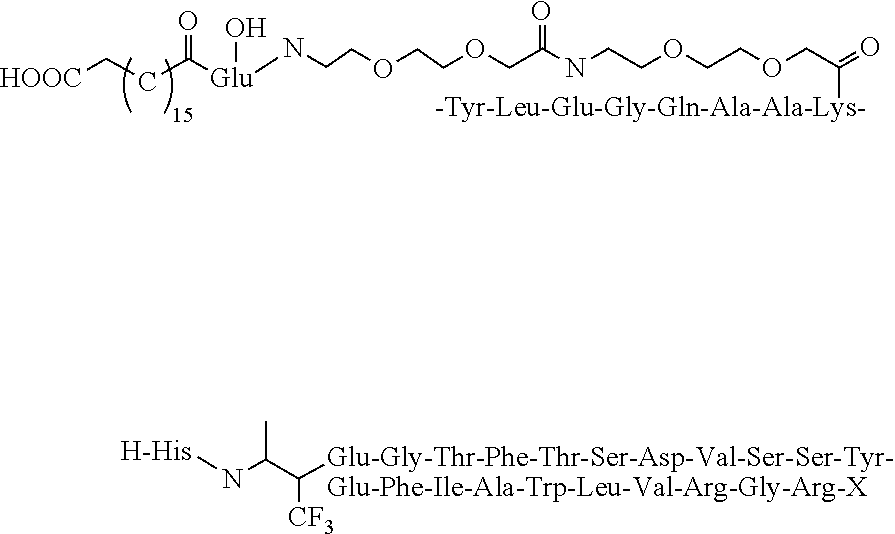
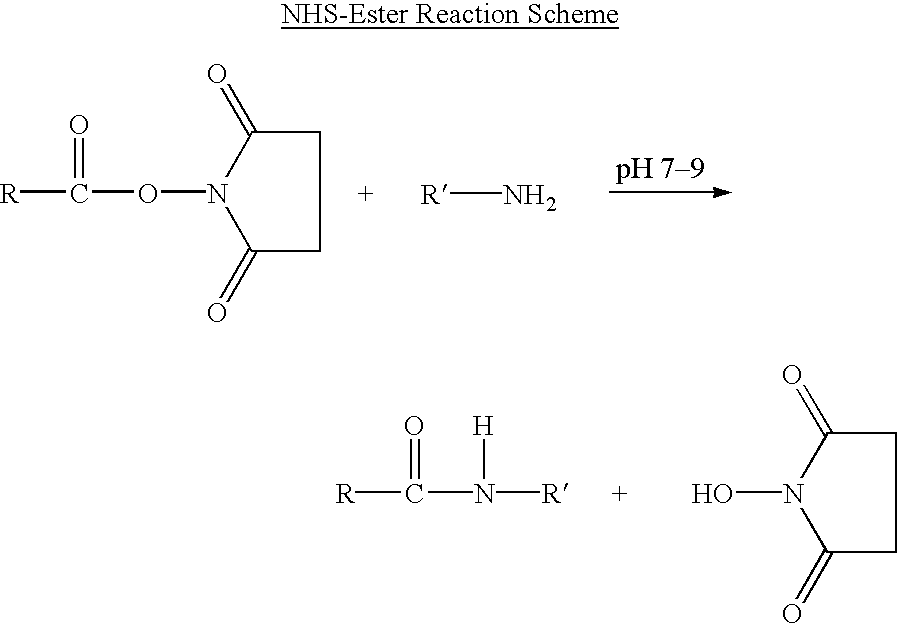
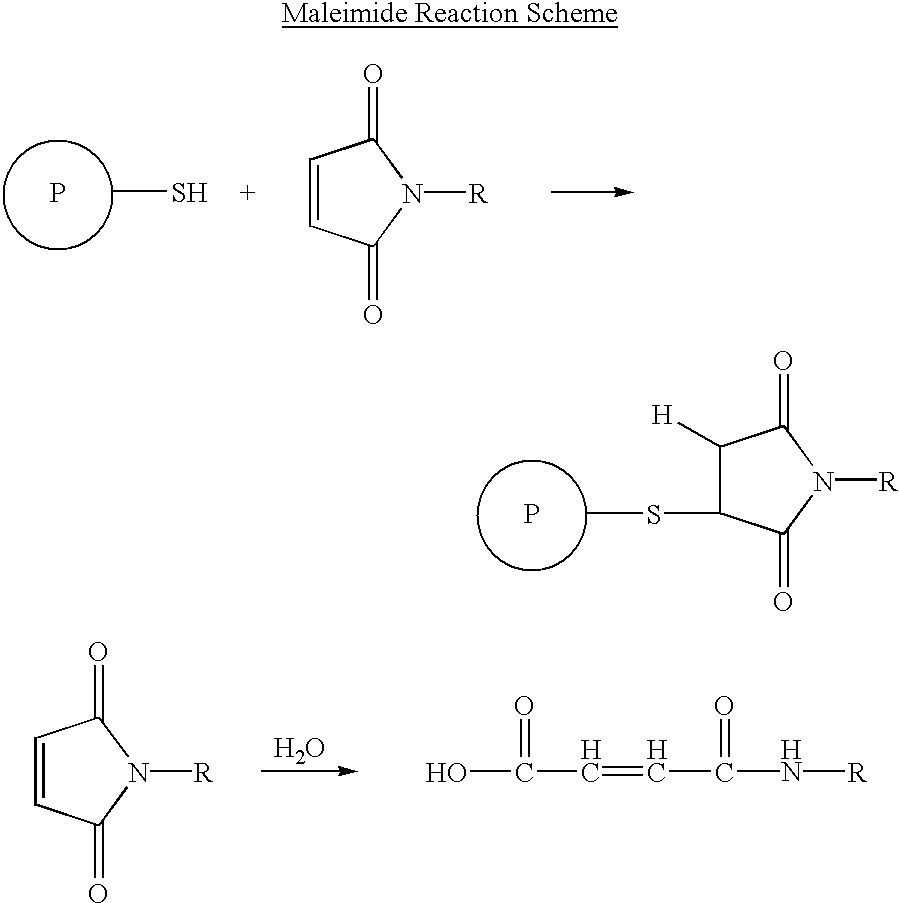
Branched Peg Remodeling and Glycosylation of Glucagon-Like Peptides-1 [Glp-1]
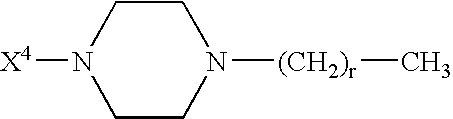
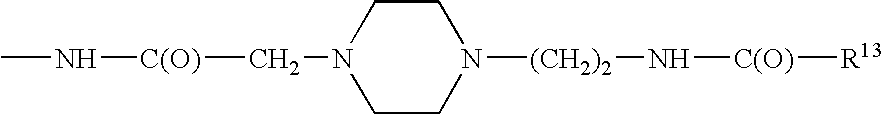
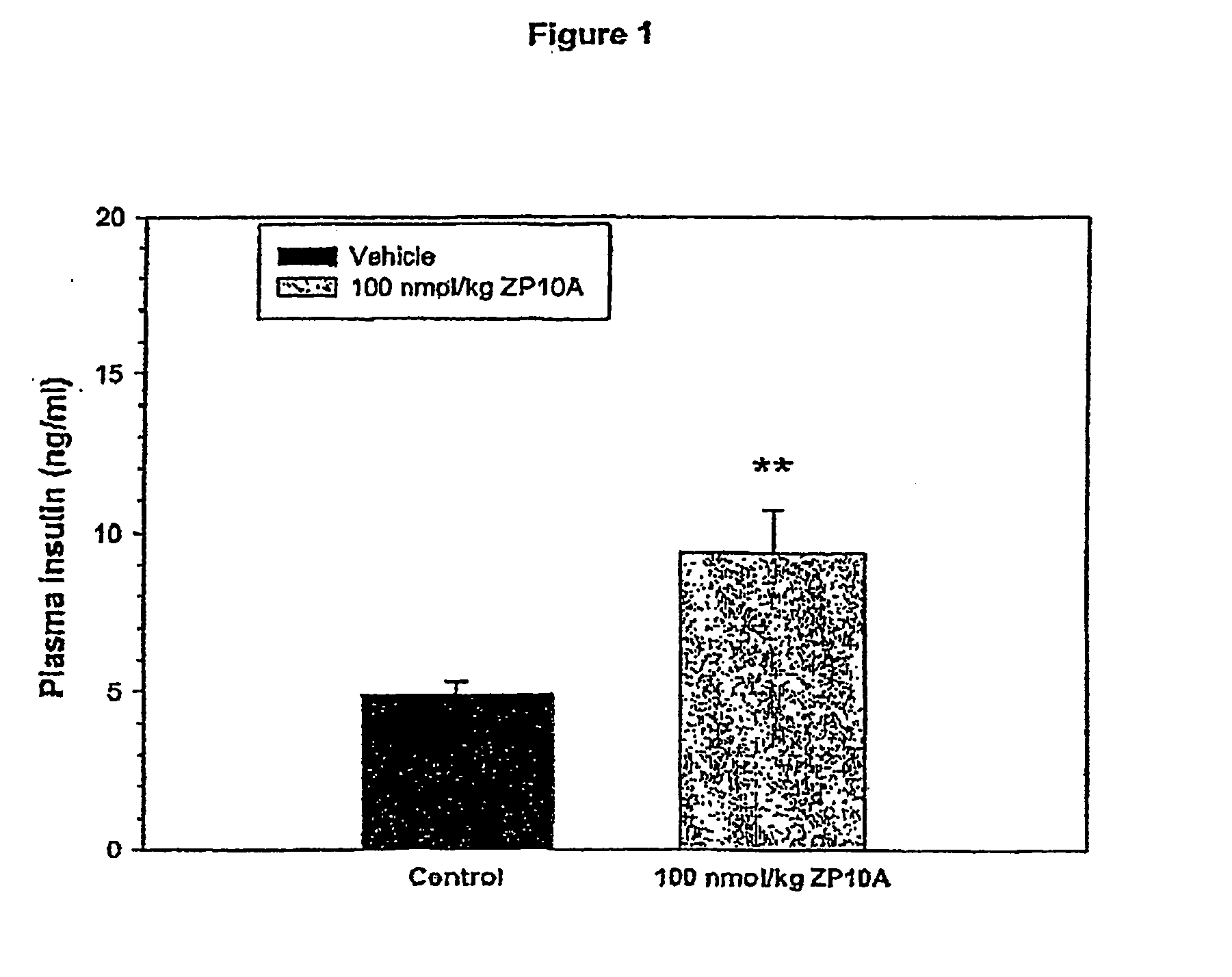
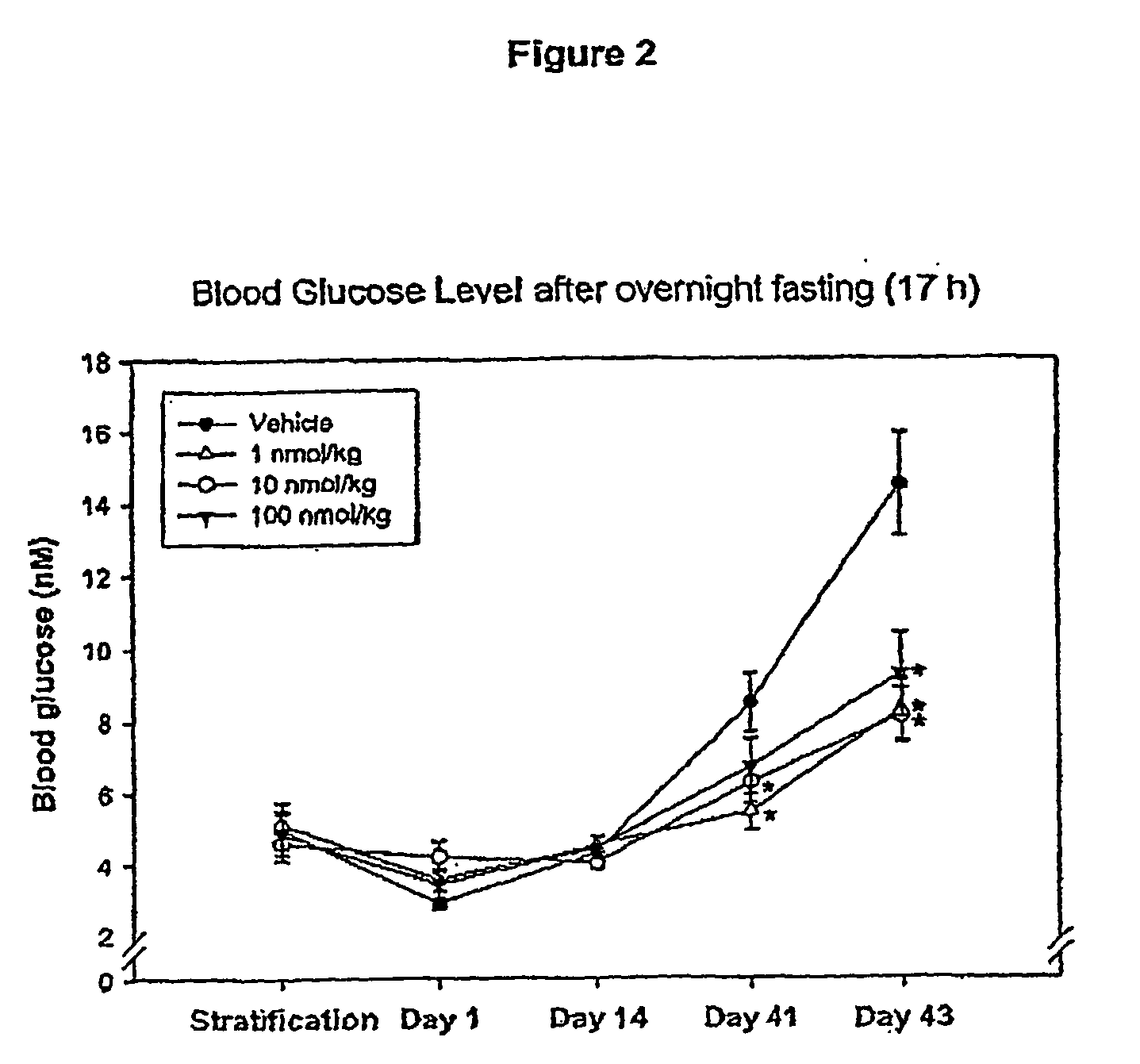
InactiveUS20080300173A1Improve blood sugar controlControlled more and lessSugar derivativesBacteriaMammalWild type
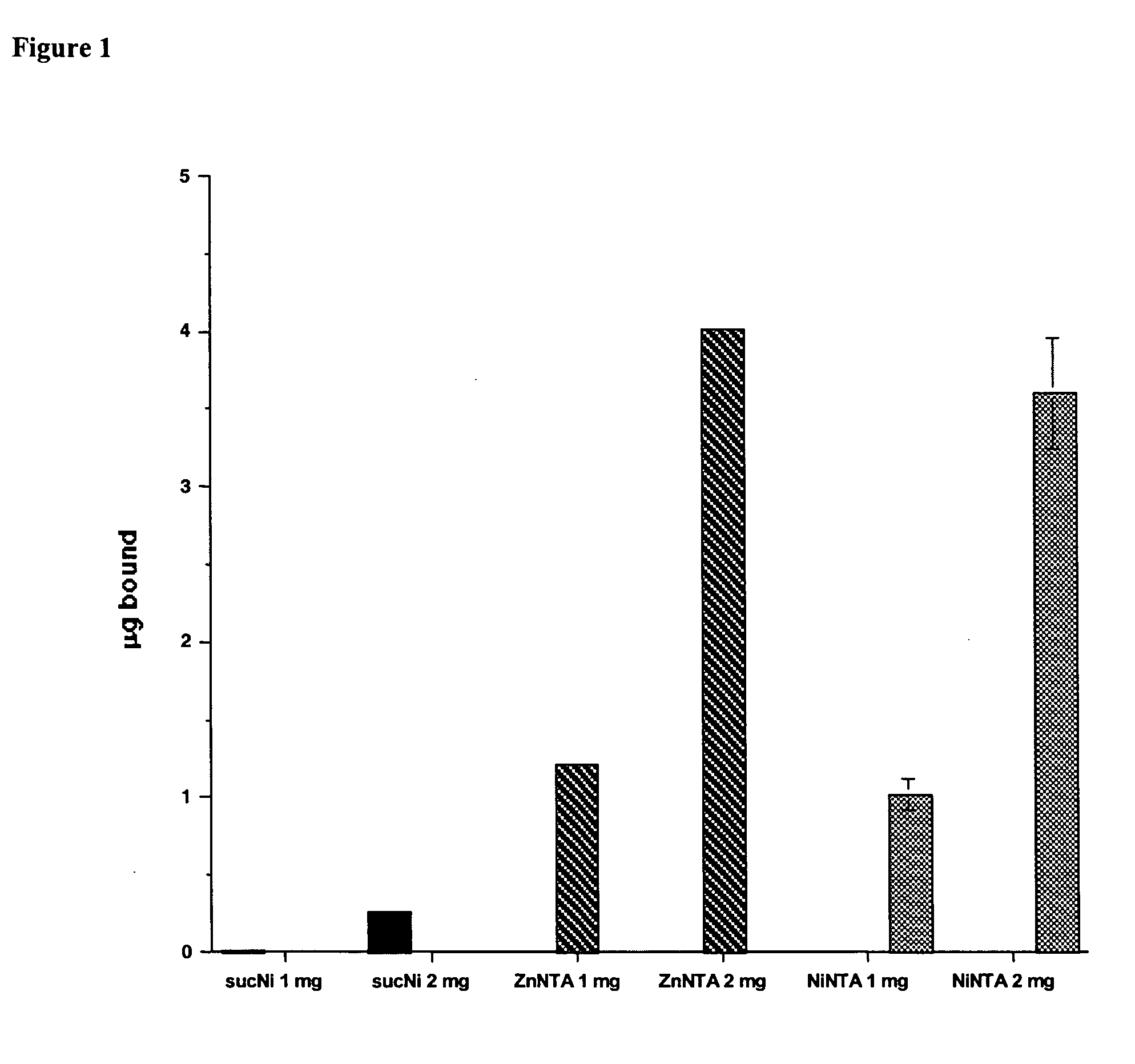
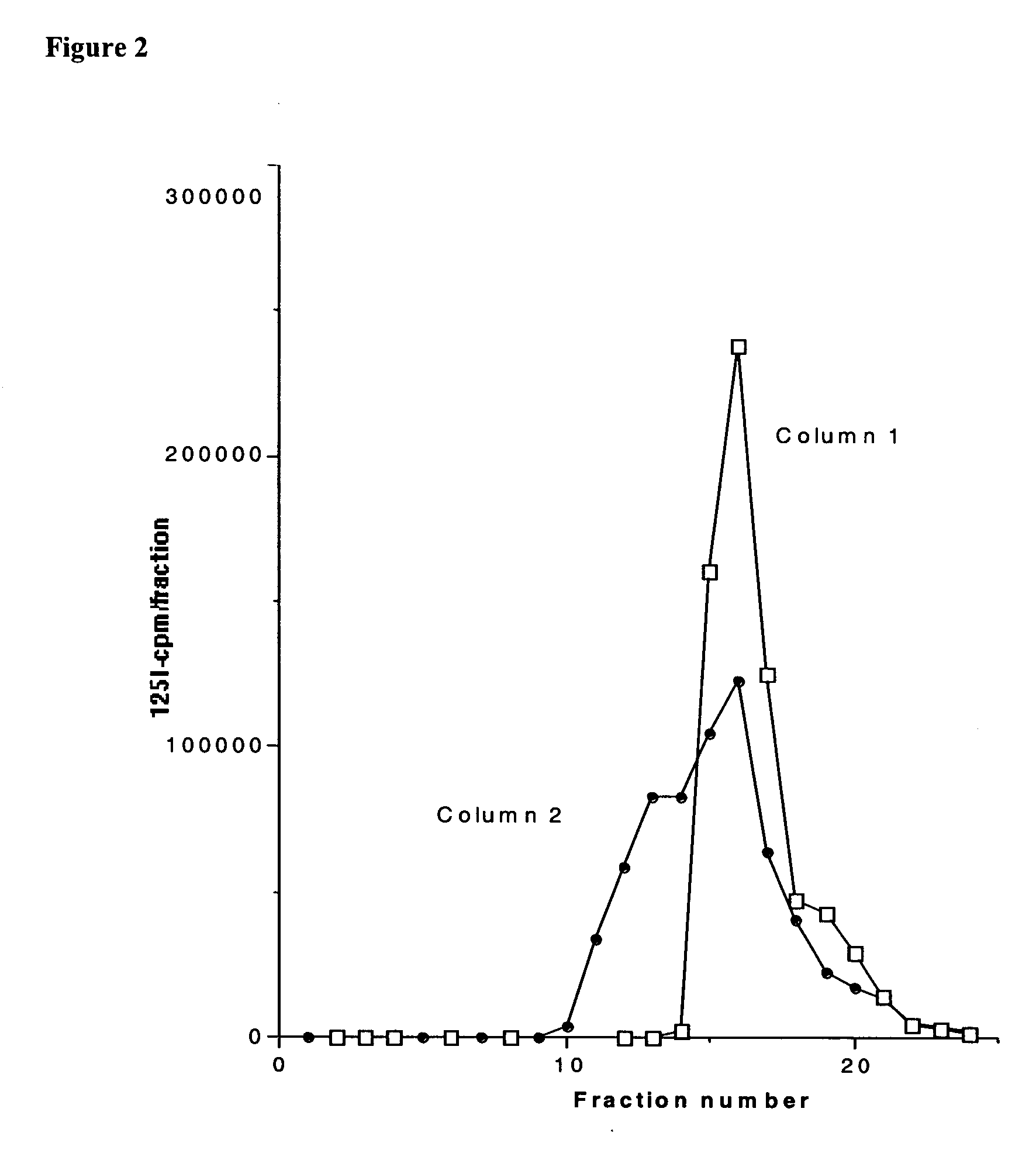
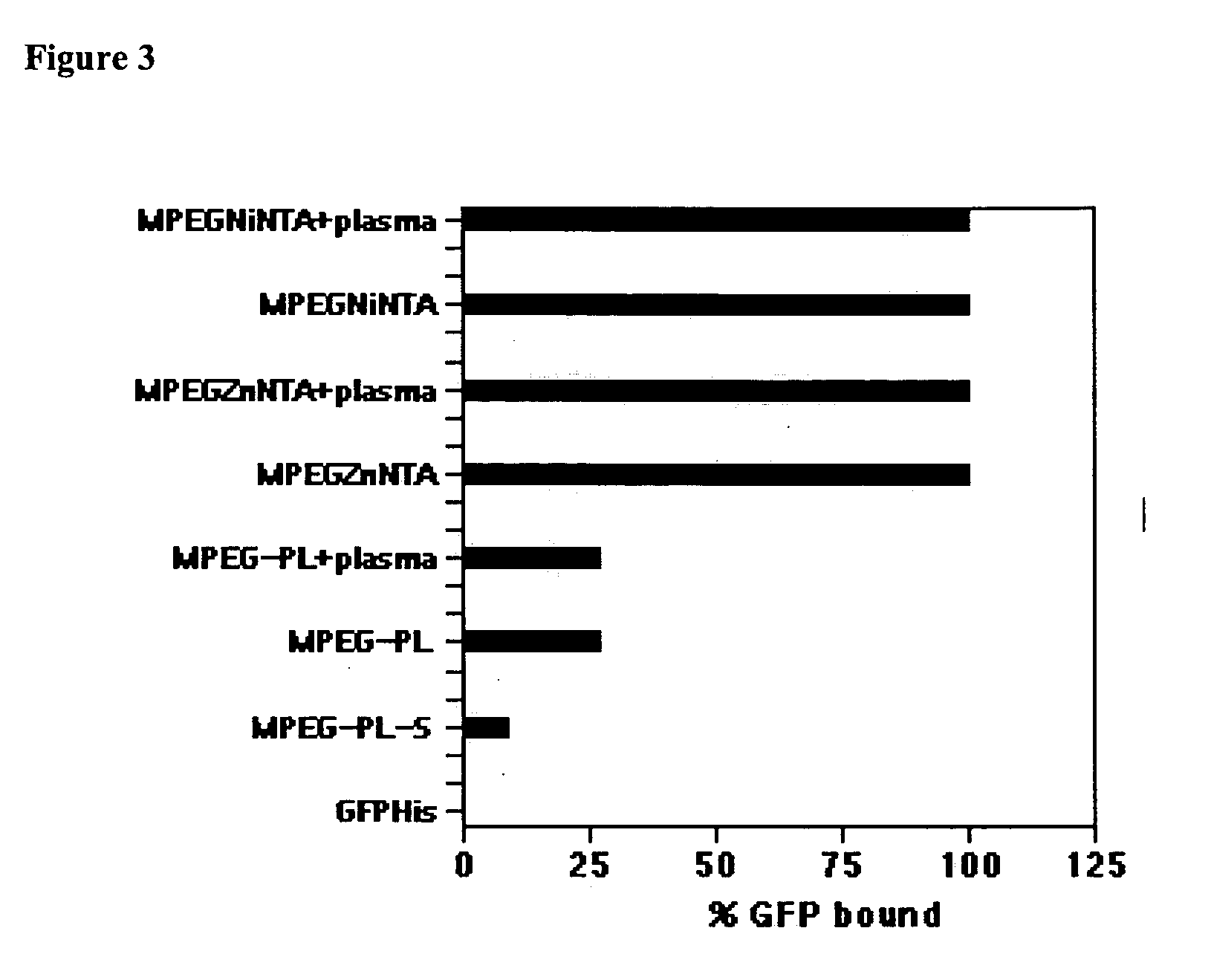
The present invention provides polypeptides that include an O-linked glycoconjugate in which a species such as a water-soluble polymer, a therapeutic agent of a biomolecule is covalently linked through an intact O-linked glycosyl residue to the polypeptide. The polypeptides of the invention include wild-type peptides and mutant peptides that include an O-linked glycosylation site that is not present in the wild-type peptide. Also provided are methods of making the peptides of the invention and methods, pharmaceutical compositions containing the peptides and methods of treating, ameliorating or preventing diseased in mammals by administering an amount of a peptide of the invention sufficient to achieve the desired response.
Owner:NOVO NORDISK AS
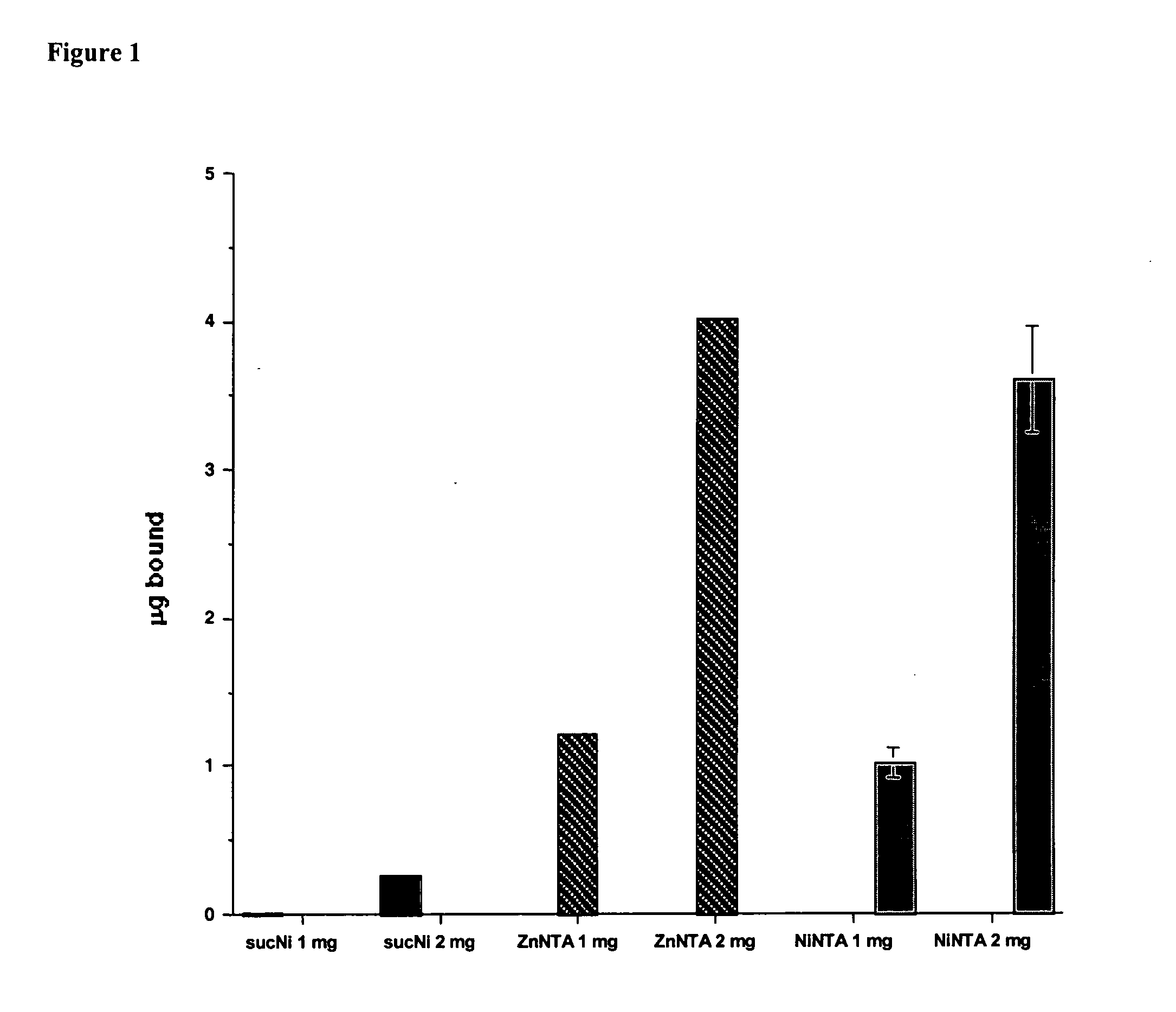
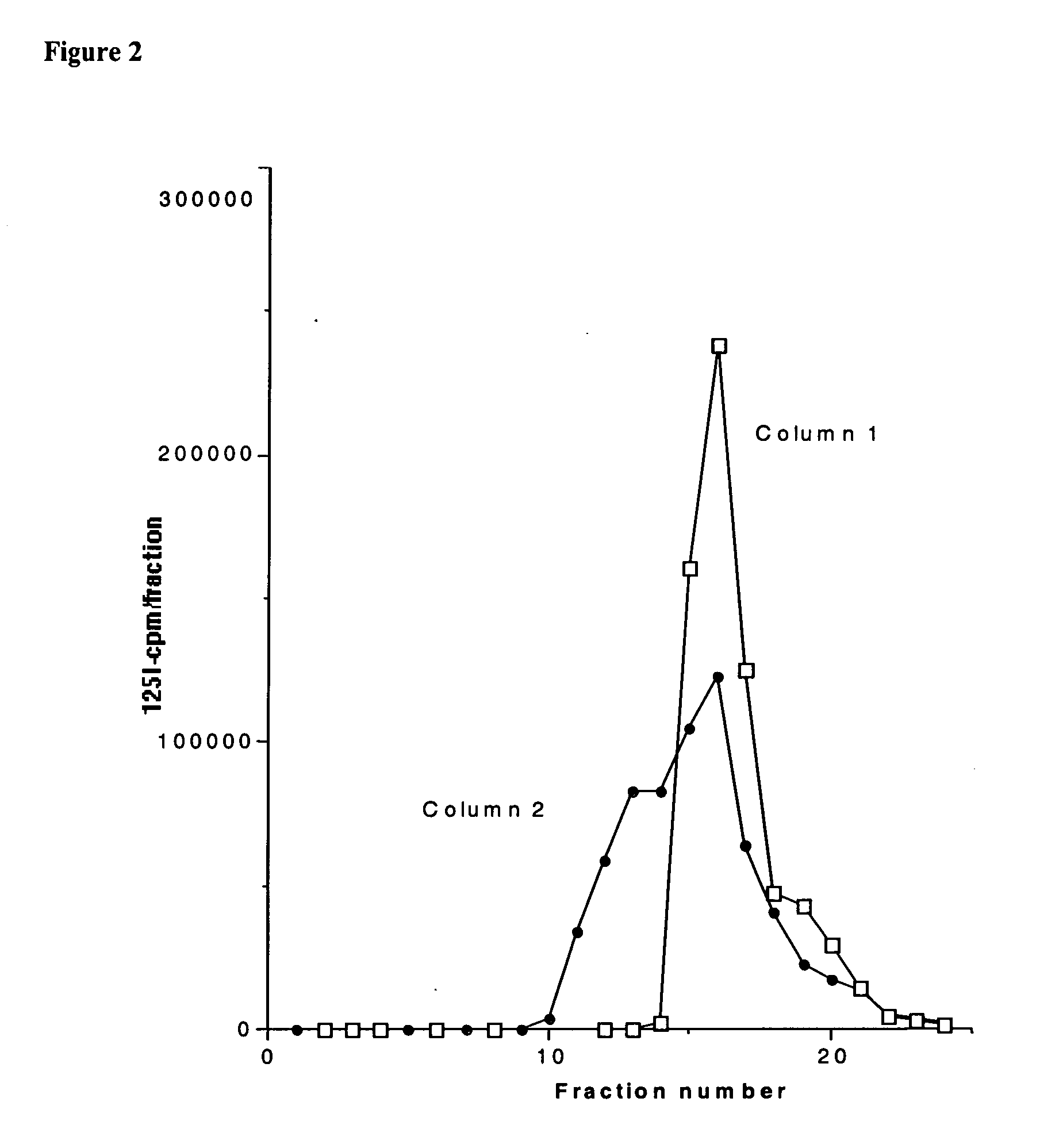
Compositions for treatment with glucagon-like peptide, and methods of making and using the same
InactiveUS20050260259A1Easy to introduceKind be easilyPeptide/protein ingredientsMetabolism disorderCrystallographyBinding domain
In part, the present invention is directed to compositions comprising a carrier with a metal binding domain, a metal ion, and GLP-1.
Owner:PHARMAIN CORP
Medical product for inhalation containing glucagon-like peptide-1 (GLP-1)
InactiveUS20060120969A1Reduce deliveryAccurate dosePowder deliverySpray deliveryPharmaceutical preservativesPulmonary inhalation
A medical product containing an accurately metered dose of a GLP-1 medicament intended for pulmonary inhalation put into a moisture-tight, high barrier seal container. The medical product optionally also contains a dose of insulin. The container is preferably adapted for application into a dry powder inhaler. The dose loaded in the container is intended for a prolonged delivery by inhalation to the deep lung where the active ingredients are absorbed into the system. Optionally the medical product also may comprise at least one biologically acceptable excipient.
Owner:MEDERIO AG
Synergistic use of thiazolidinediones with glucagon-like peptide-1 and agonists thereof to treat metabolic instability associated with non-insulin dependent diabetes
InactiveUS7223728B2Lower blood sugar levelsIncrease secretionPeptide/protein ingredientsMetabolism disorderInsulin dependent diabetesSide effect
Thiazolidinedione (TZD) and its pharmacologically active derivatives can be used, in combination with agonists of glucagon-like peptide-1 (GLP-1), to treat non-insulin dependent diabetes mellitus, optionally with other therapies, by improving glycemic control while minimizing side effects, such as heart hypertrophy and elevated fed-state plasma glucose, which are associate with both TZD and GLP-1 monotherapies. Thus, the co-administration of TZD and GLP-1 helps regulate glucose homeostasis in Type II diabetic patients.
Owner:ELI LILLY & CO
Treatment of diabetes
InactiveUS6899883B2Peptide/protein ingredientsMetabolism disorderDiabetes mellitusGlucagon-like peptide-1
Owner:LONDON HEALTH SCI CENT RES
Stable formulations of peptides
Method for increasing the shelf-life of a pharmaceutical formulation comprising a glucagon-like peptide.
Owner:NOVO NORDISK AS
Satiety inducing composition
InactiveUS20050238694A1Good effectIncrease satietyPeptide/protein ingredientsWhey manufactureBiotechnologyLactalbumin hydrolysate
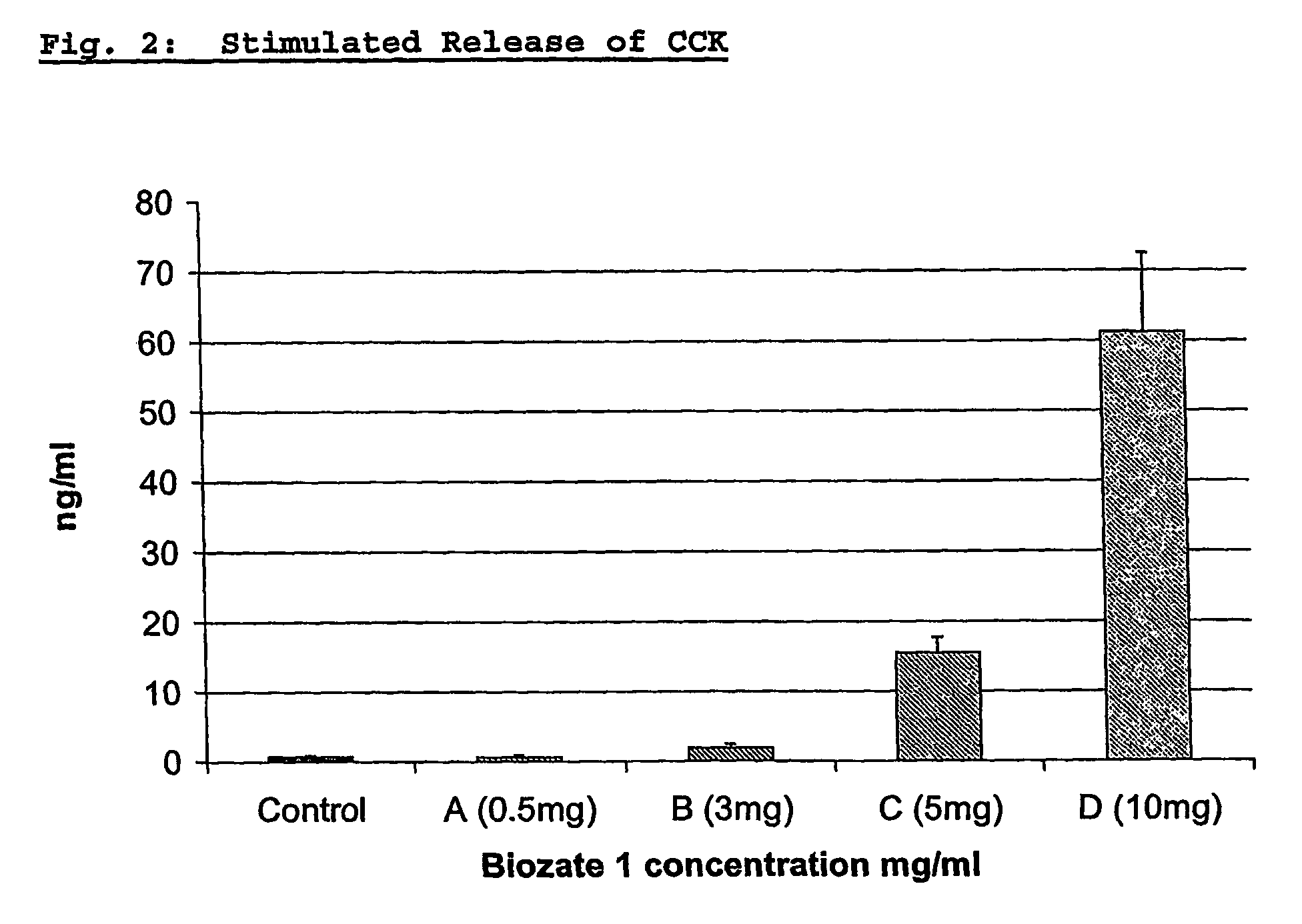
The invention provides the use of a whey protein and / or whey protein hydrolysate which stimulate the cellular release of the satiety peptides choleocystokinin and glucagon-like-peptide in the preparation of edible compositions. The edible compositions can be used to control body weight and have beneficial effects on satiety. Edible compositions are also provided.
Owner:KSF ACQUISITION
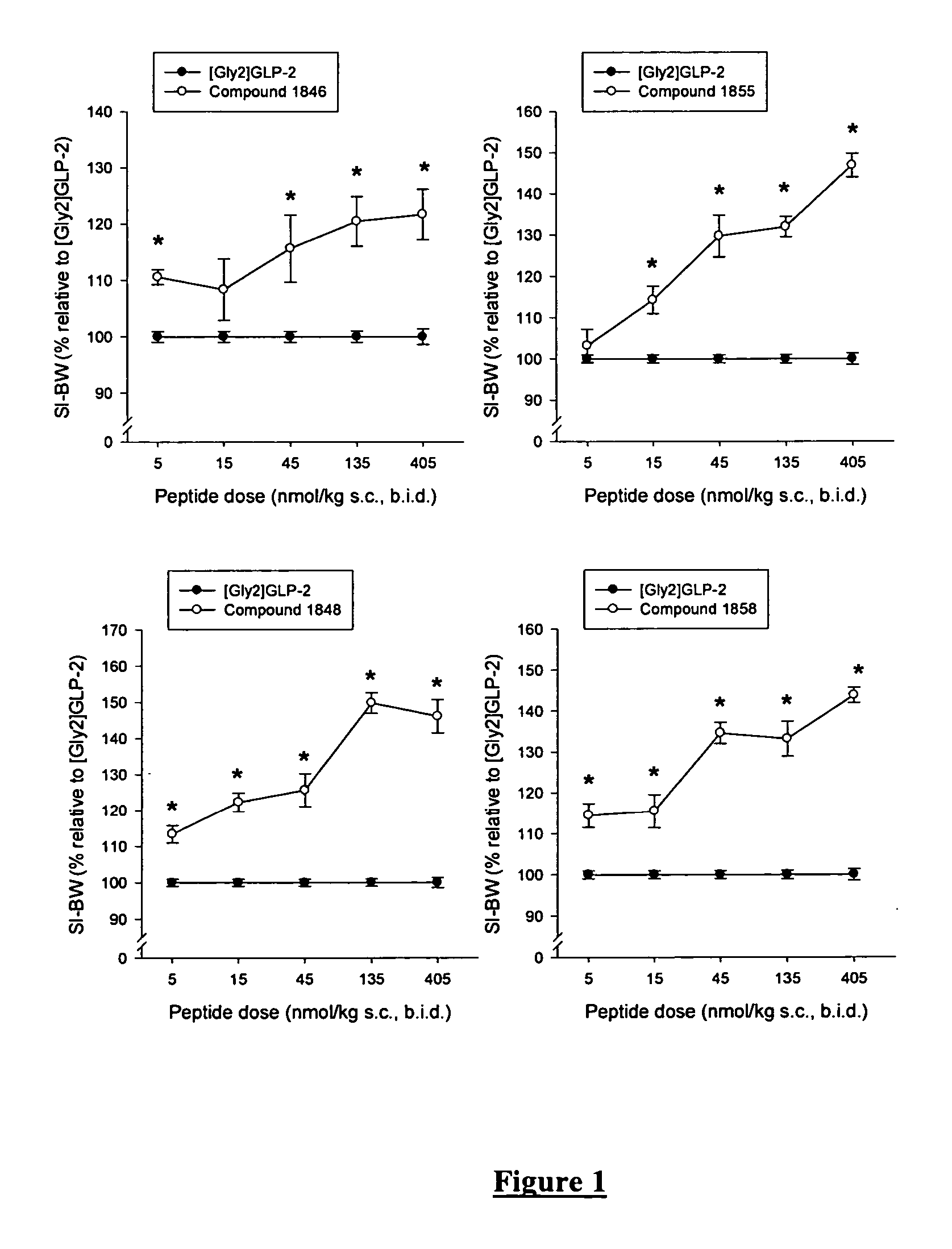
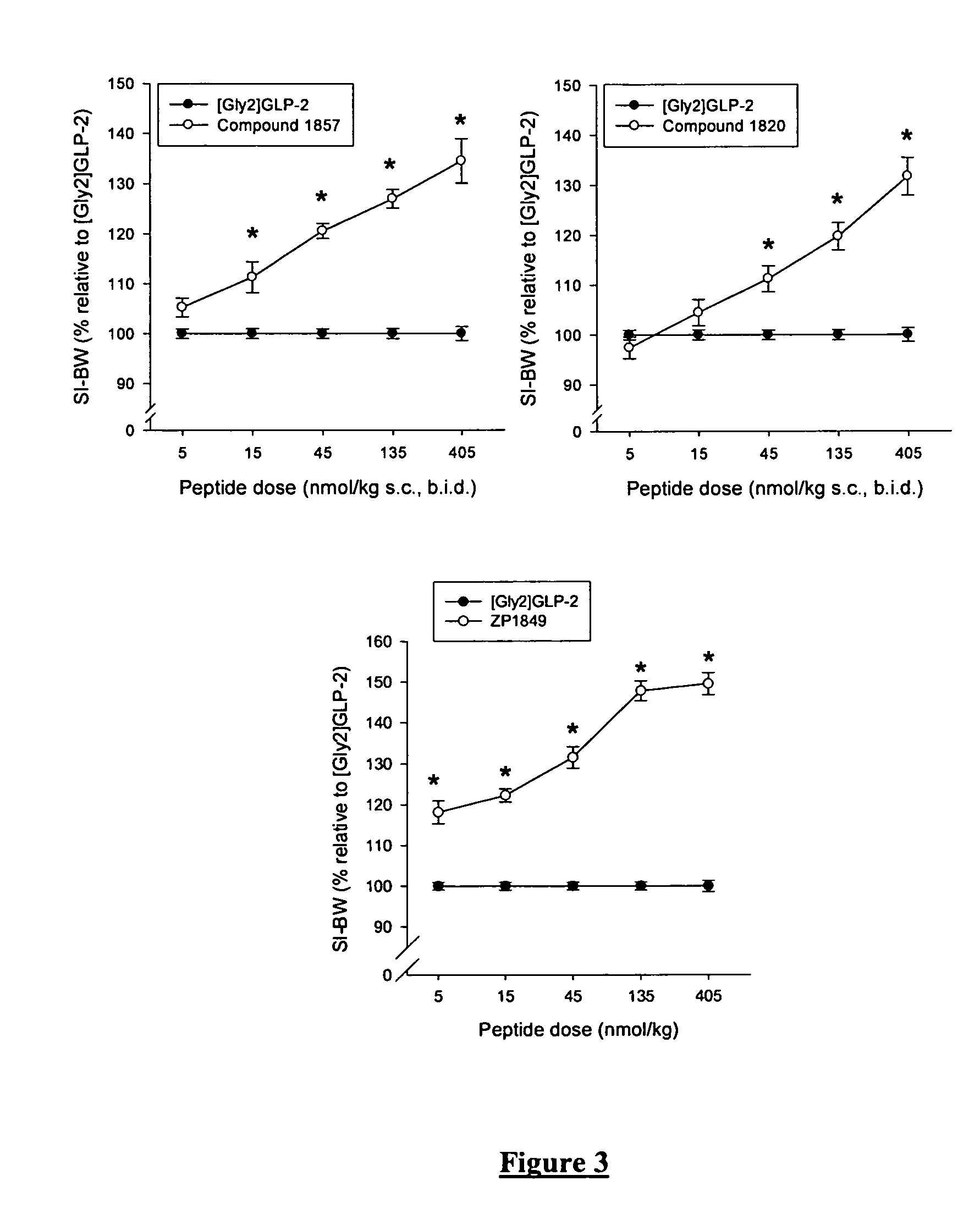
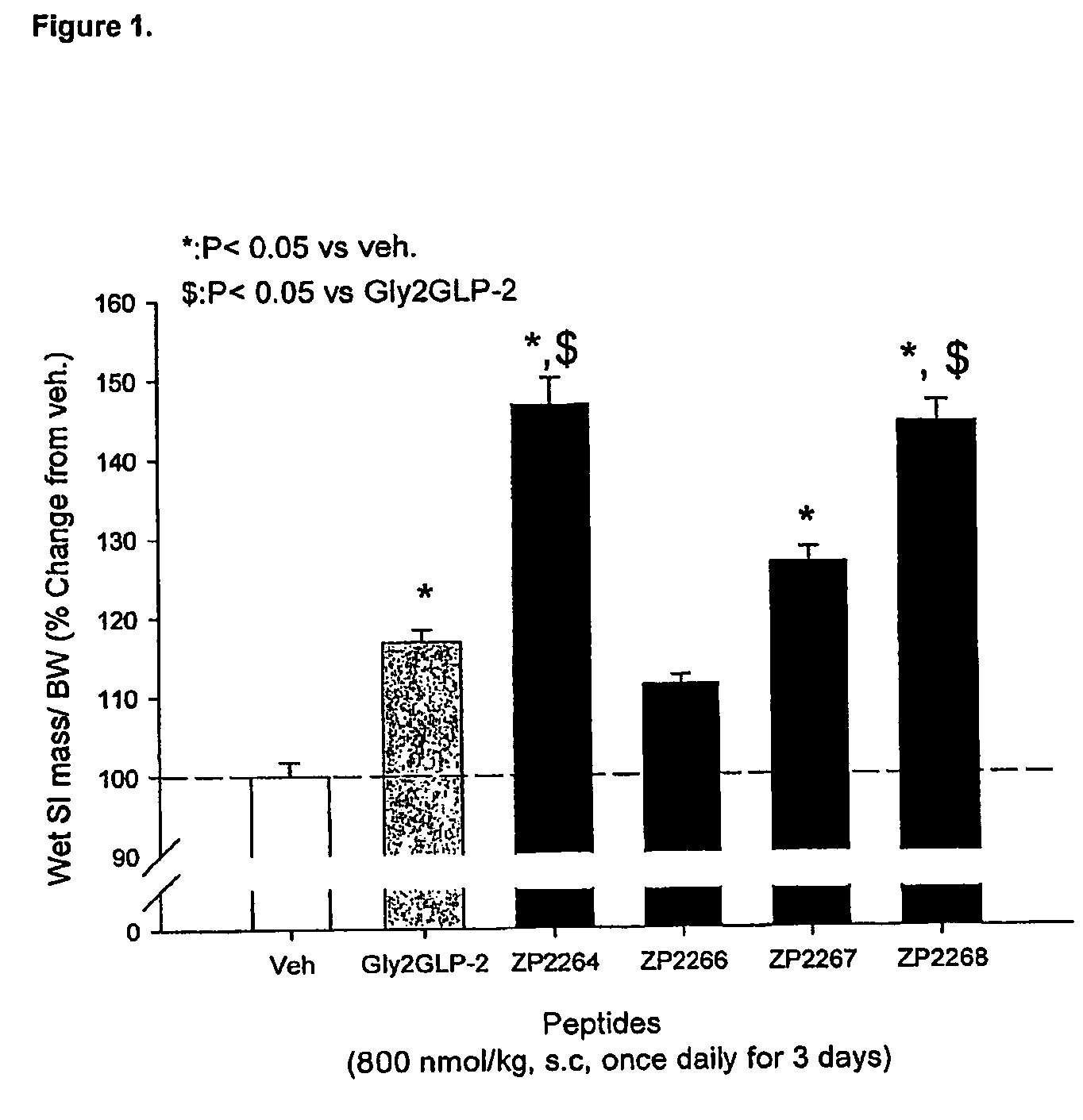
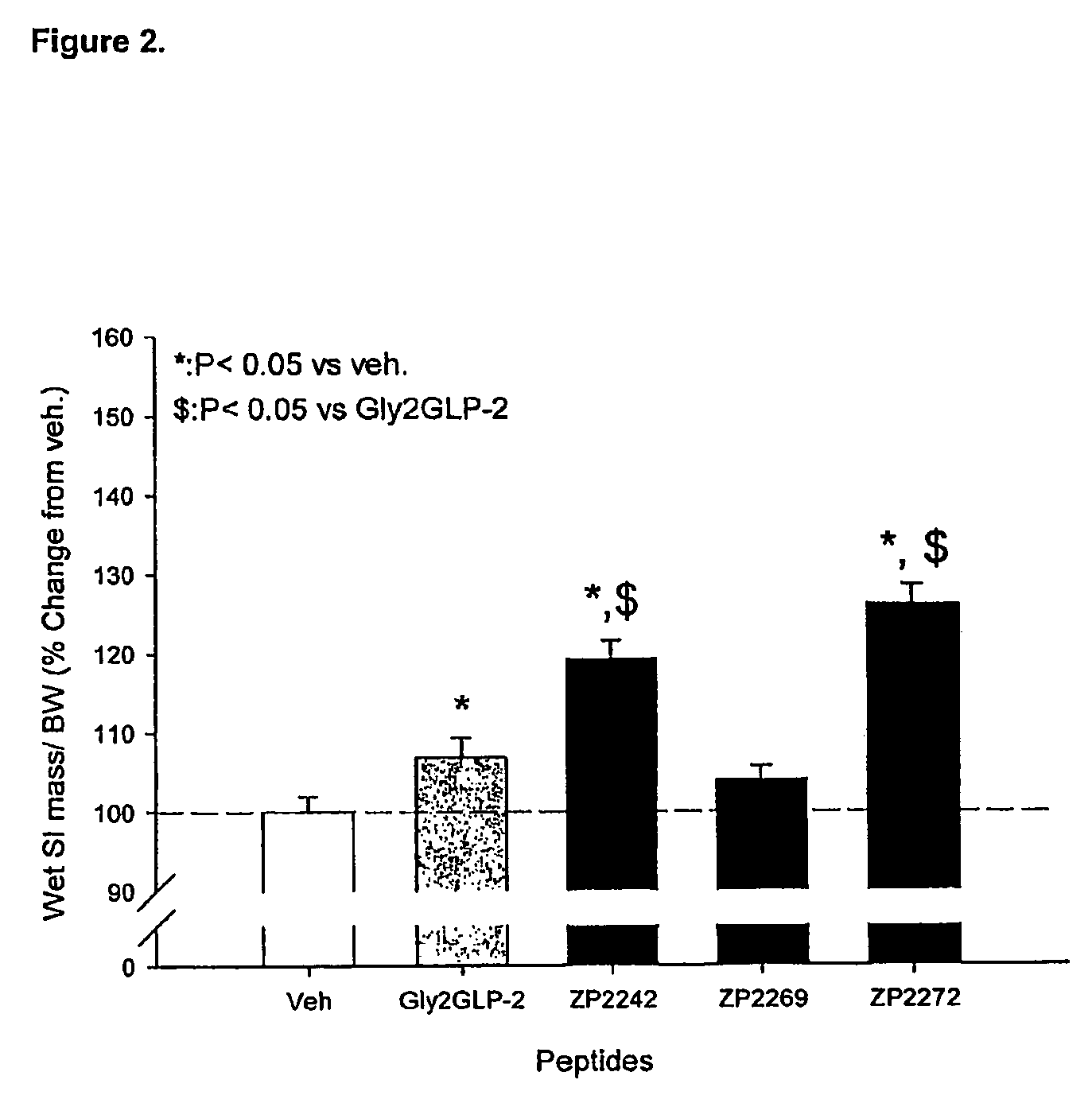
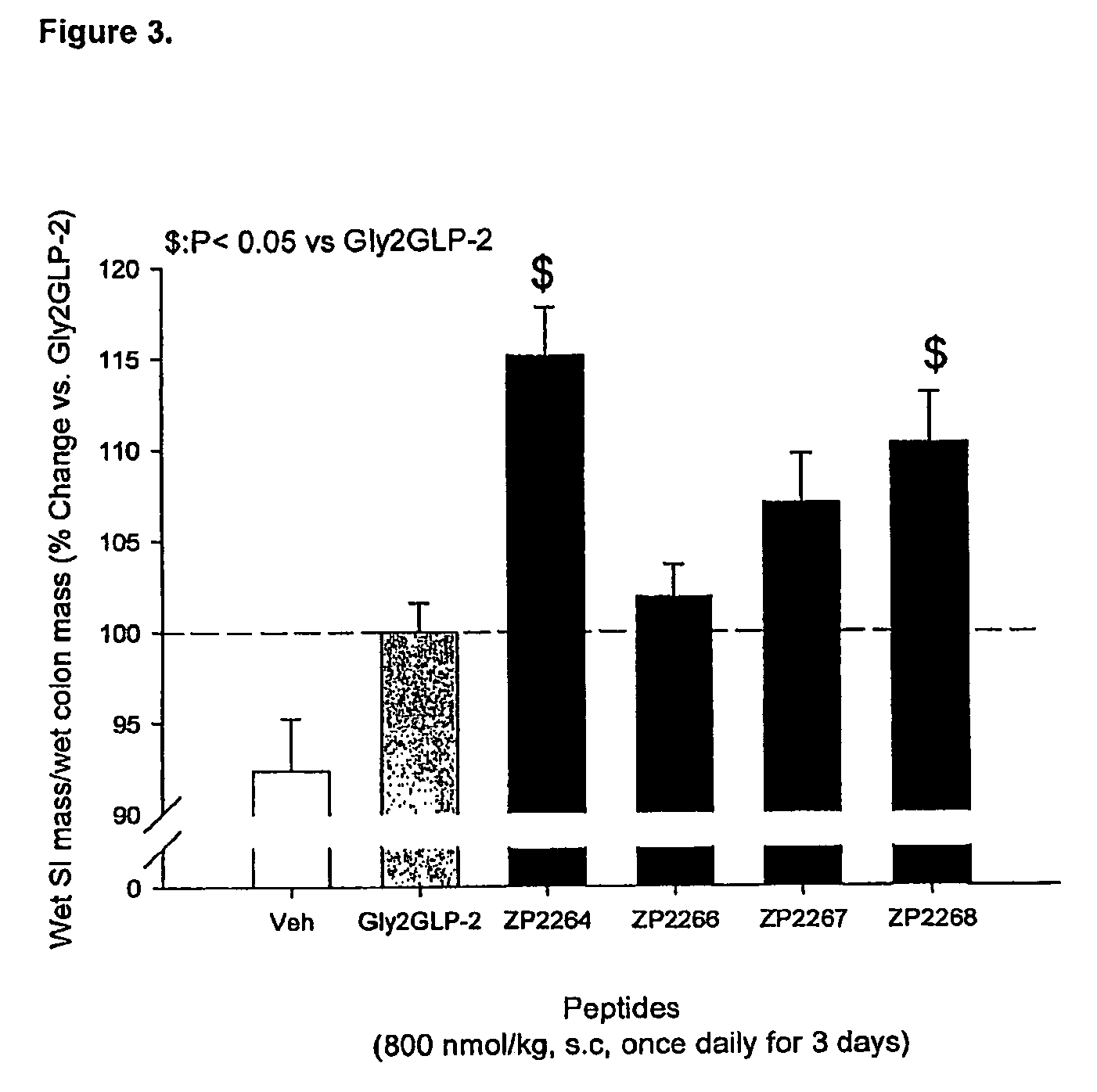
Glucagon-like-peptide-2 (GLP-2) analogues
ActiveUS20070117752A1Improve biological activityPreferential intestinal growth promoting activitySsRNA viruses negative-senseAntipyreticSide effectWild type
GLP-2 analogues are disclosed which comprise one of more substitutions as compared to [hGly2]GLP-2 and which improved biological activity in vivo and / or improved chemical stability, e.g., as assessed in in vitro stability assays. More particularly, preferred GLP-2 analogues disclosed herein comprise substitutions at one or more of positions 8, 16, 24 and / or 28 of the wild-type GLP-2 sequence, optionally in combination with further substitutions at position 2 (as mentioned in the introduction) and one or more of positions 3, 5, 7, 10 and 11, and / or a deletion of one or more of amino acids 31 to 33 and / or the addition of a N-terminal or C-terminal stabilizing peptide sequence. The analogues are particularly useful for the prophylaxis or treatment of stomach and bowel-related disorders and for ameliorating side effects of chemotherapy. Also disclosed are methods and kits for selecting a patient from populations suited for treatment with GLP-2 analogues.
Owner:ZEALAND PHARM AS
Stabilized Pharmaceutical Peptide Compositions
ActiveUS20080318865A1Improve stabilityPeptide/protein ingredientsMetabolism disorderNeutral phPeptide
Method for increasing the shelf-life of a pharmaceutical composition comprising a glucagon-like peptide which is prepared from a peptide product that has been dried at a pH above neutral pH.
Owner:NOVO NORDISK AS
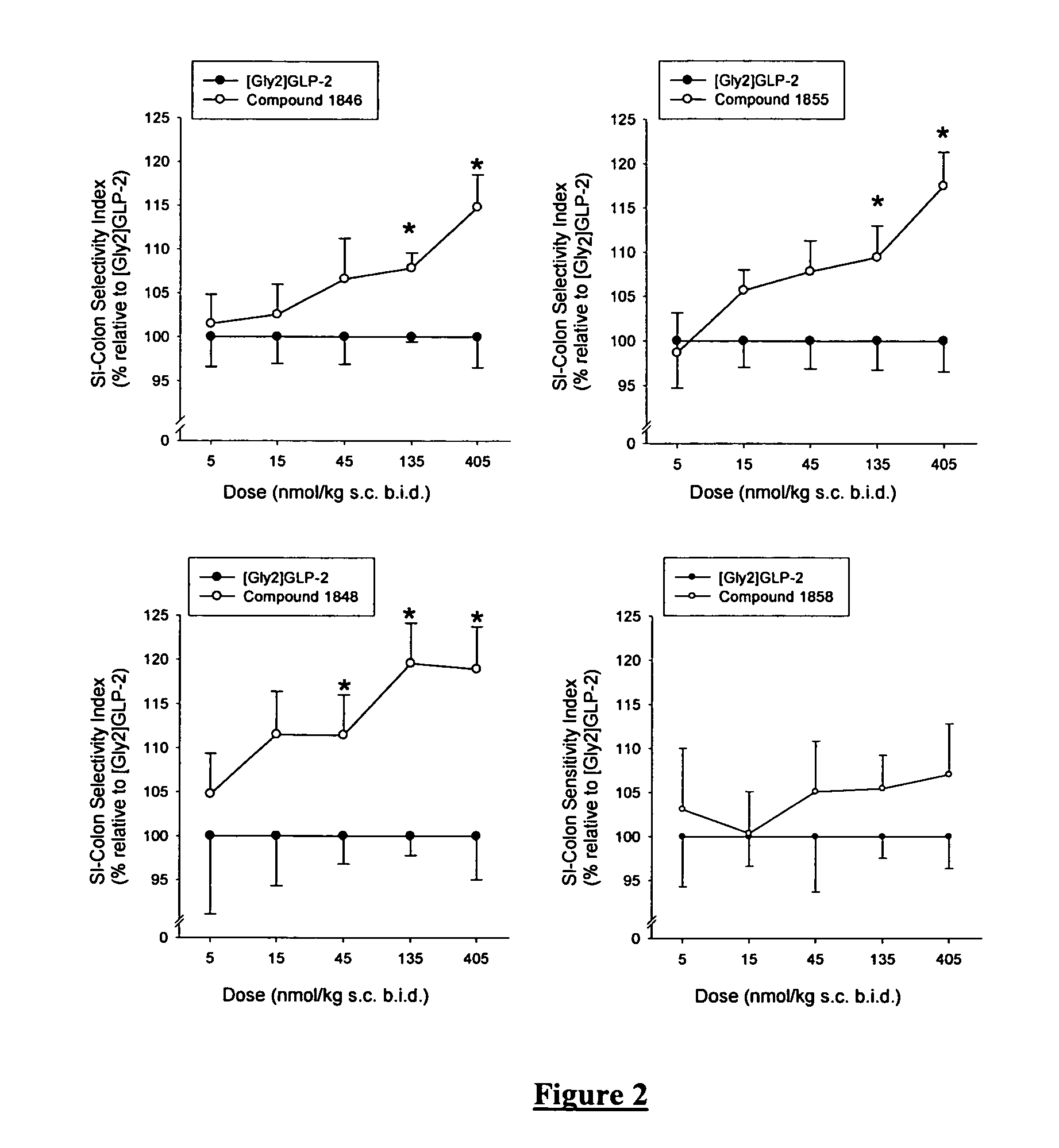
Selective glucagon-like-peptide-2 (GLP-2) analogues
GLP-2 analogues are disclosed which comprise one of more substitutions as compared to h[Gly2]GLP-2 and which may have the property of an increased small intestine / colon and stomach / colon selectivity. More particularly, preferred GLP-2 analogues disclosed herein comprise substitutions at one or more of positions 11, 16, 20, 24 and / or 28 of the wild-type GLP-2 sequence, optionally in combination with further substitutions at position 2 and one or more of positions 3, 5, 7, and 10, and / or a deletion of one or more of amino acids 31 to 33 and / or the addition of a N-terminal or C-terminal stabilizing peptide sequence. The analogues are particularly useful for the prophylaxis or treatment of stomach and bowel-related disorders and for ameliorating side effects of chemotherapy.
Owner:ZEALAND PHARM AS
Compositions for treatment with glucagon-like peptide, and methods of making and using the same
InactiveUS20060093660A1Easy to introduceKind be easilyPowder deliveryPeptide/protein ingredientsCrystallographyBinding domain
Owner:PHARMAIN CORP
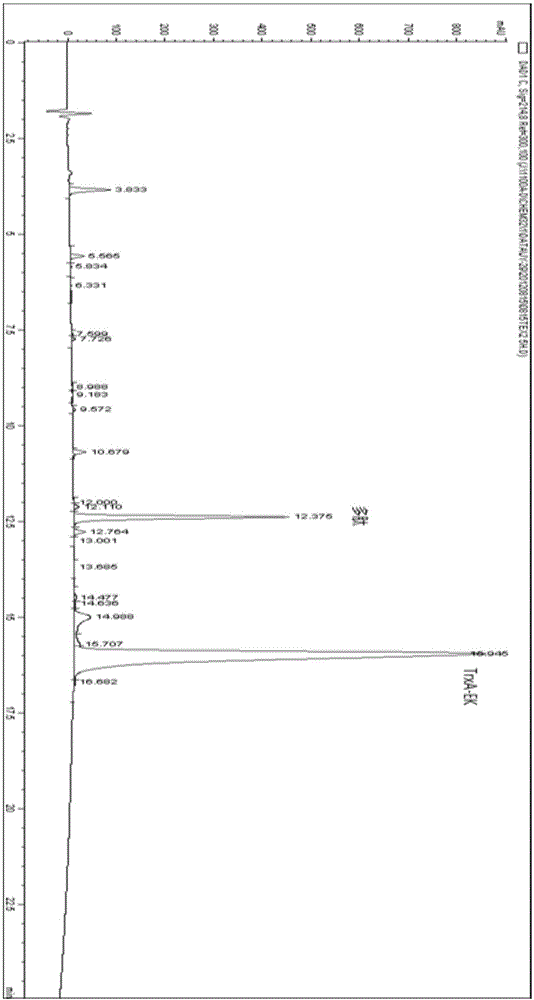
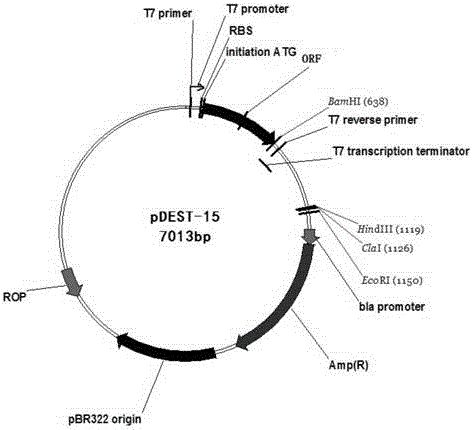
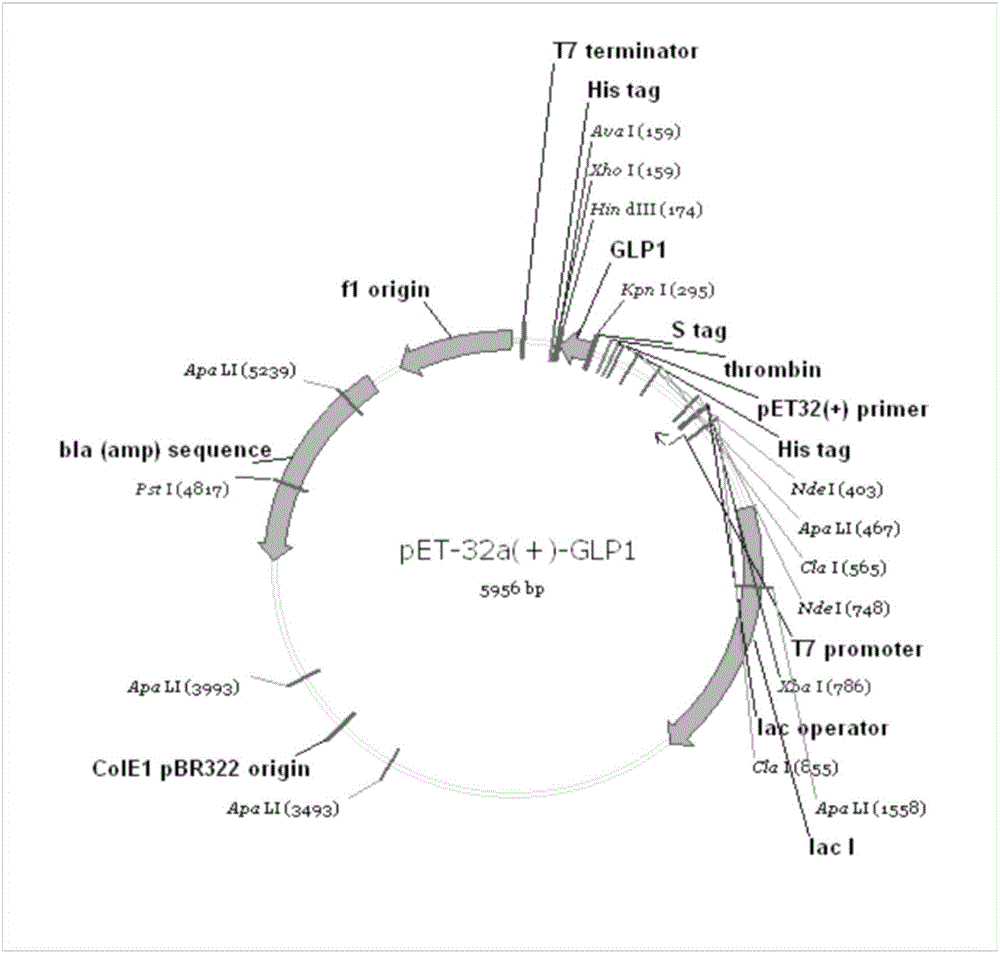
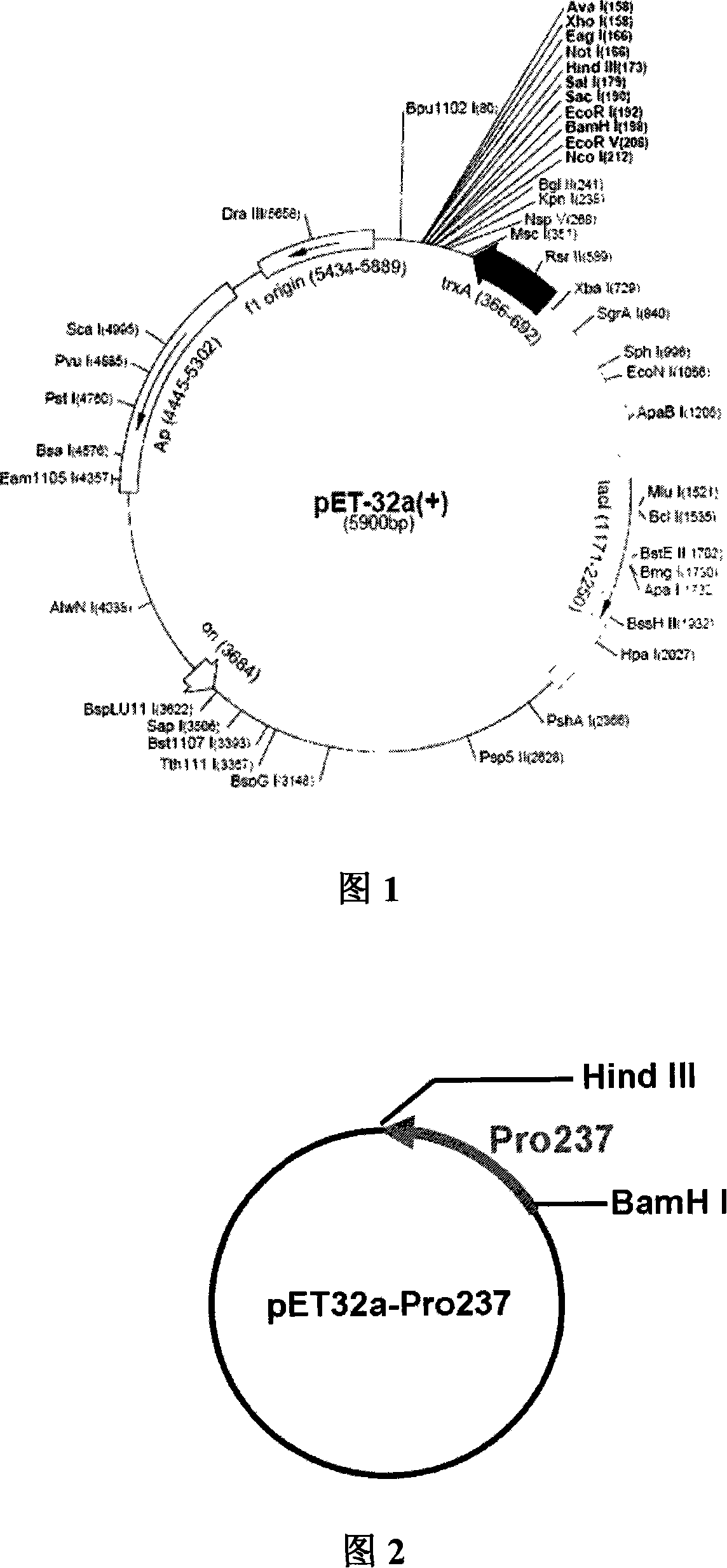
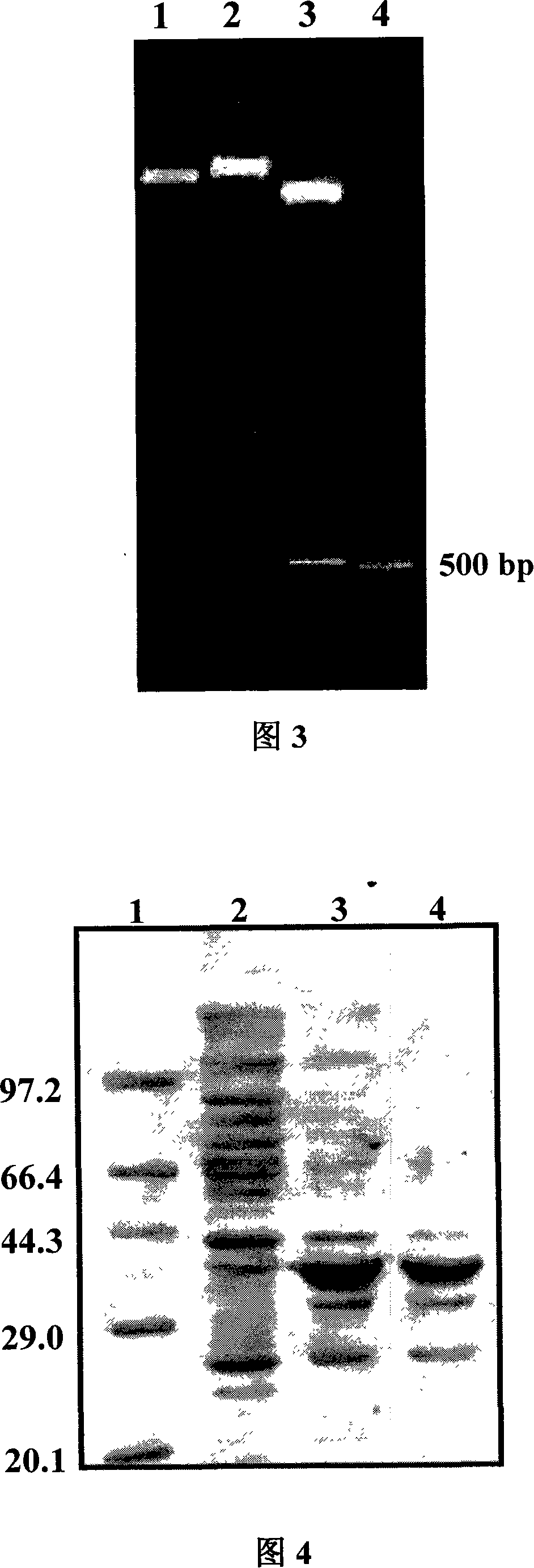
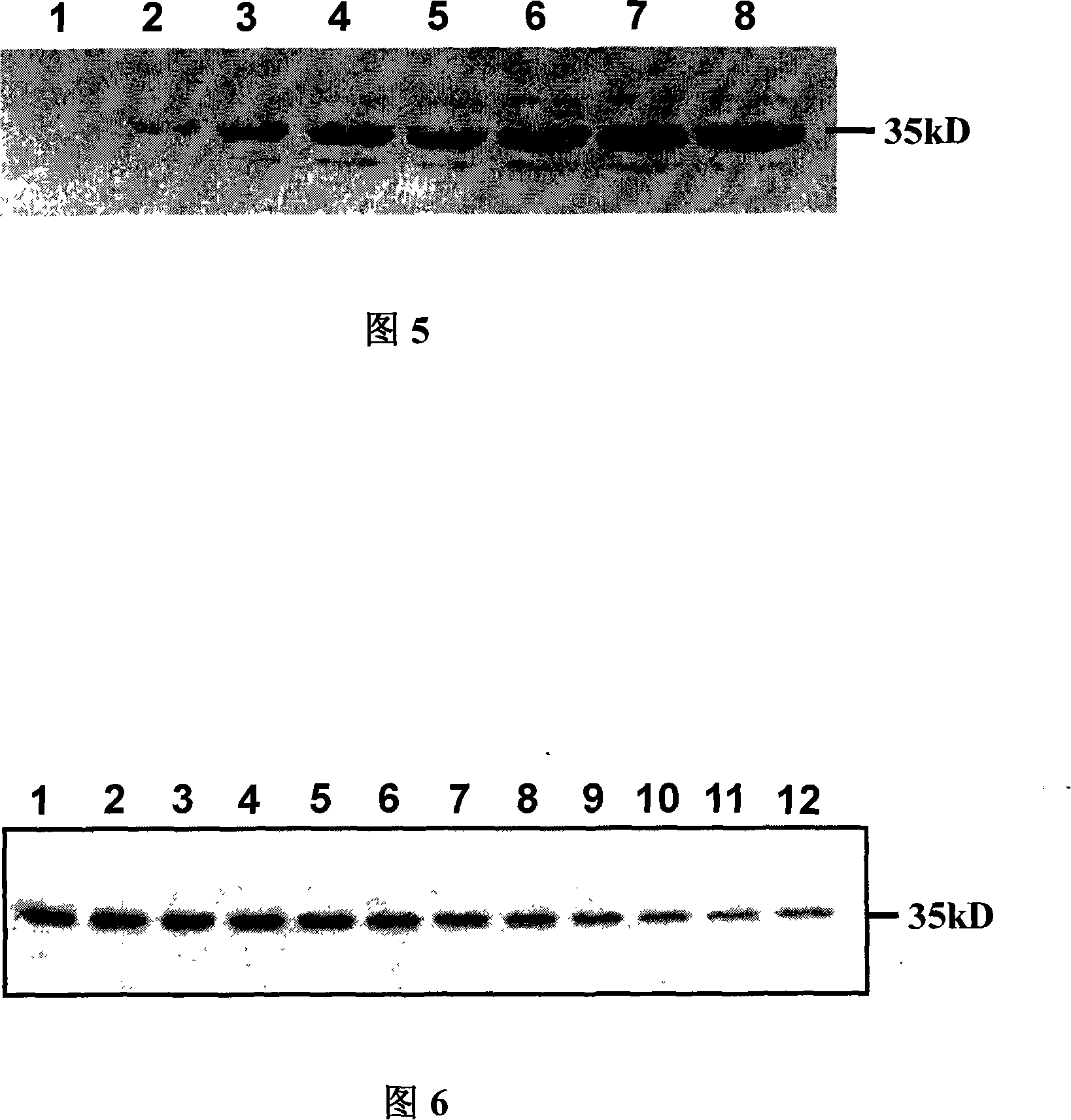
Method for biosynthesis preparation of human GLP-1 polypeptide or analogue thereof
InactiveCN106434717AEasy to separateHigh expressionNucleic acid vectorFusion with protease siteEscherichia coliEnzyme digestion
The invention relates to a method for biosynthesis preparation of human glucagon-like peptide-1 (GLP-1) and an analogue thereof. With adopting of a gene engineering technology, a recombinant escherichia coli expressed GLP-1 fusion protein is constructed, and a protein enzyme digestion site is designed in the fusion protein; a fusion gene has a gene sequence with a form of A-B-C structure, wherein A is a chaperonin gene, B is a nucleotide sequence encoding a connection peptide containing the enzyme digestion site, and C is a gene encoding the GLP-1 or the analogue thereof. After recombinant engineering bacteria is subjected to induced expression, the fusion protein is purified and subjected to enzyme digestion, and then the GLP-1 and the analogue thereof are obtained and are detected to have biological activity. The preparation method of the GLP-1 and the analogue thereof provided by the invention is simple and quick, the production conditions are mild, the product is convenient to separate and extract, the process is simple, and the industrialization prospect is good.
Owner:HANGZHOU JIUYUAN GENE ENG +1
Stable pharmaceutical compositions
Pharmaceutical composition for parenteral administration comprising a glucagon-like peptide and human serum albumin or a variant thereof.
Owner:NOVO NORDISK AS
Compounds, Methods and Formulations for the Oral Delivery of a Glucagon-Like Peptide (Glp)-1 Compound or a Melanocortin-4 Receptor (Mc4) Agonist Peptide
Owner:EMISPHERE TECH INC
Amalgamation protein of human glucagons-like peptide-1and uses thereof
InactiveCN101220088ANon-immunogenicNot easy to dischargePeptide/protein ingredientsMetabolism disorderDiseaseChemical synthesis
The invention relates to a fusion protein of a human glucagon-like peptide -1(hGLP-1) analogues and the preparation method for the fusion protein, wherein, the fusion protein is a pro-drug with high biological stability and long half-life in vivo that releases active GLP-1 molecule after being degraded by enzyme in vivo and then brings into playing the pharmacological action. The scope of the invention extends to the treatment application of the products made by the production technique. The fusion protein can be used for treating and preventing the diseases or disorders relating the GLP-1 activity, in particular to the 2 Diabetes Mellitus. The fusion protein of a human glucagon-like peptide -1(hGLP-1) analogues and the preparation method for the fusion protein relates to the biotechnological field, with the fusion protein prepared by the gene engineering method, which has the advantages of much lower production cost than the chemical synthetic method, simple operation, and easy acquisition of raw material, and has possibility for commercial production.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Glucagon like peptide-1 mutant polypeptide and preparation method, medicinal composition and use thereof
InactiveCN102363633AImprove compliancePeptide/protein ingredientsMetabolism disorderHalf-lifeRetention time
The invention discloses a glucagon like peptide(GLP)-1 mutant polypeptide and a preparation method, a medicinal composition and use thereof. The mutant polypeptide is formed by bonding a Cys-containing elongation peptide to the N terminal of the glucagon like peptide-1 mutant, the mutant polypeptide itself folds to form a disulfide bond. The glucagon like peptide-1 mutant polypeptide is used for preparing a medicinal composition for treating diabetes and treating and preventing obesity. For overcoming the drawbacks of short retention time in body and need of daily injection administration of clinic GLP-1 analogue, the invention provides the GLP-1 analogue which has a longer half-life period. With the longer half-life period, the GLP-1 mutant polypeptide is not required to be injected into a patient every day, and can increase the obedience of the patient effectively.
Owner:天津拓飞科技有限公司
GLP-1 pharmaceutical compositions
InactiveUS20070021339A1Suppressing plasma blood levelPreventing beta-cell deteriorationBiocideNervous disorderGlucagon-like peptide-1Peptide
Owner:IPSEN PHARMA SAS
Blood glucose regulating composition
InactiveUS20060171992A1Aids in blood glucose regulationNervous disorderPeptide/protein ingredientsMedicineWhey protein
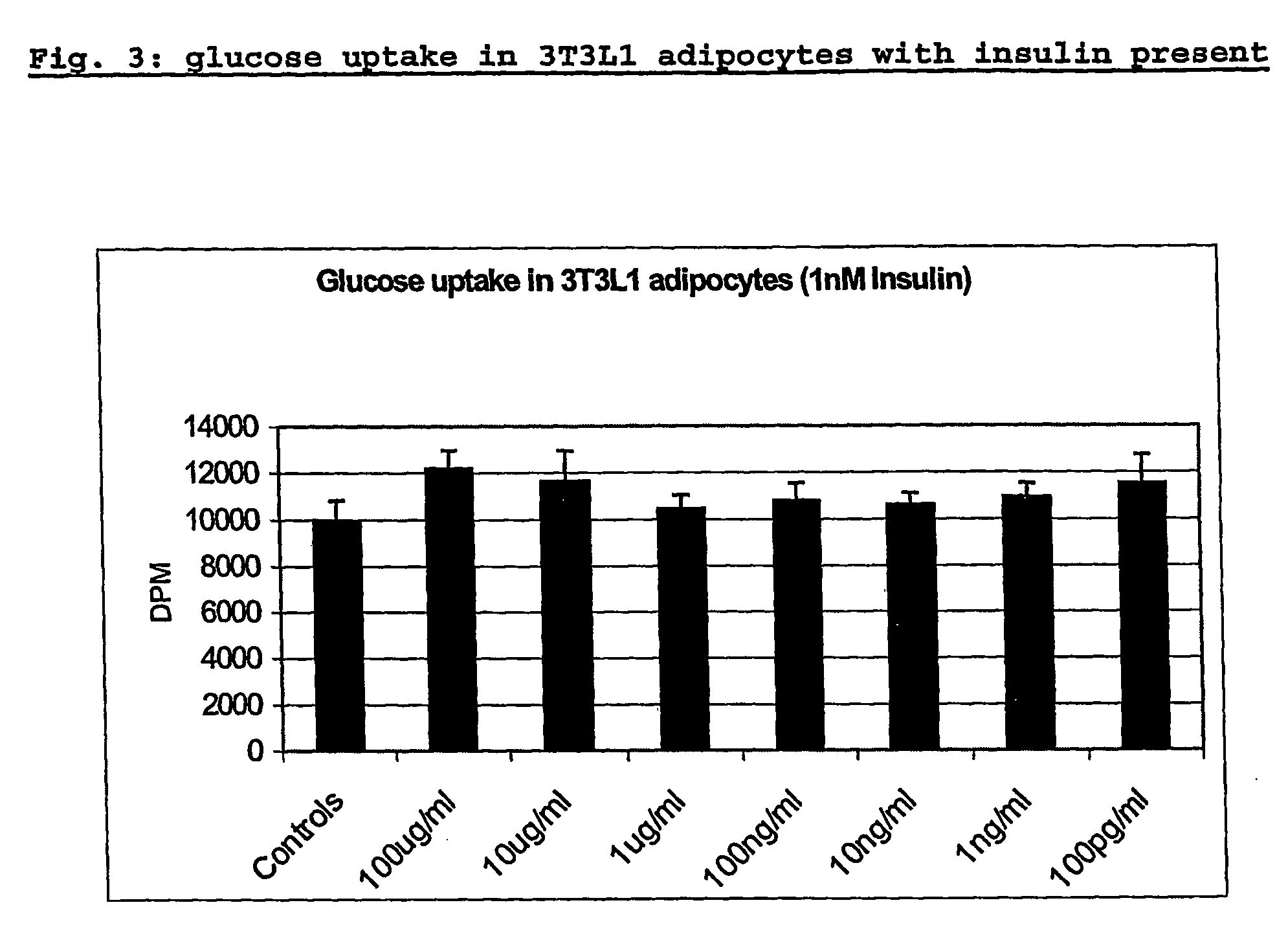
The invention provides the use of a whey protein hydrolysate in an edible composition the whey protein hydrolysate being able to induce the cellular release of glucagon-like-peptides and cholecystokinins and / or increasing glucose uptake in target tissues, wherein the whey protein hydrolysate regulates blood glucose levels or results in, or is used for, improving or preventing decline in mental performance and / or for providing a sustained feeling of energy and / or for maintaining or providing a feeling of well-being during the post-prandial period in a subject consuming the composition.
Owner:UNILEVER BESTFOODS NORTH AMERICA DIV OF CONOPCO
Glucagon-like peptide-1 analogues and uses thereof
ActiveUS20130053304A1Delaying and prevent deteriorationDecrease apoptosisNervous disorderPeptide/protein ingredientsLysine residueHalf-life
Provided is a glucagon-like peptide-1 (GLP-1) analogue shown as the following formula, wherein X is selected from glycine and glycinamide. The GLP-1 analogue has a non-proteogenic amino acid residue in position 8 relative to the sequence GLP-1, and is acylated with a moiety comprising two acidic groups to the lysine residue in position 26. The GLP-1 analogue is resistant to dipeptidyl peptidase IV so as to have an extended half-life in vivo. Also provided is a use of the GLP-1 analogue in conquering blood sugar.
Owner:BETTA PHARM CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
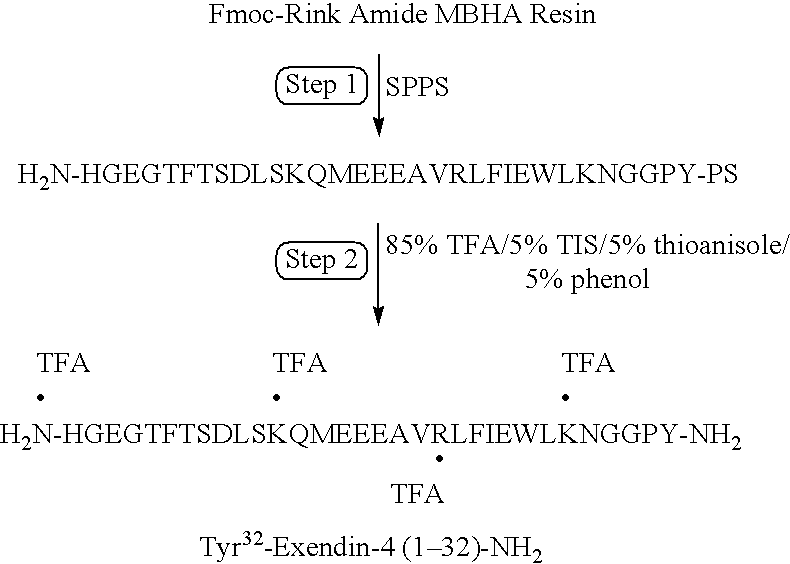


![Branched Peg Remodeling and Glycosylation of Glucagon-Like Peptides-1 [Glp-1] Branched Peg Remodeling and Glycosylation of Glucagon-Like Peptides-1 [Glp-1]](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4ee91e84-dc70-492d-8101-8e27c2003d31/US20080300173A1-20081204-D00001.png)
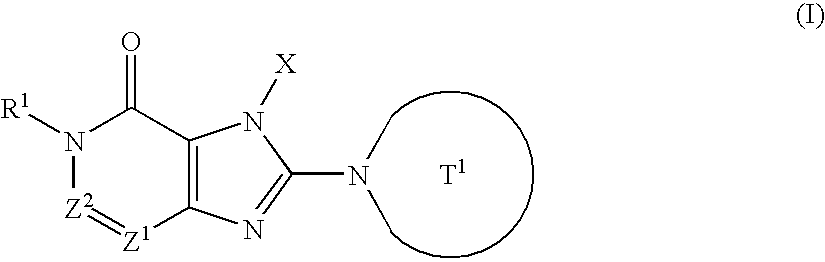
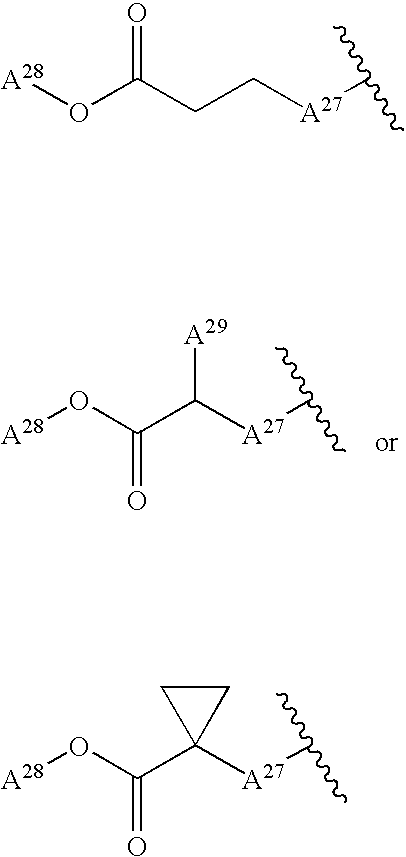
![Branched Peg Remodeling and Glycosylation of Glucagon-Like Peptides-1 [Glp-1] Branched Peg Remodeling and Glycosylation of Glucagon-Like Peptides-1 [Glp-1]](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4ee91e84-dc70-492d-8101-8e27c2003d31/US20080300173A1-20081204-C00001.png)
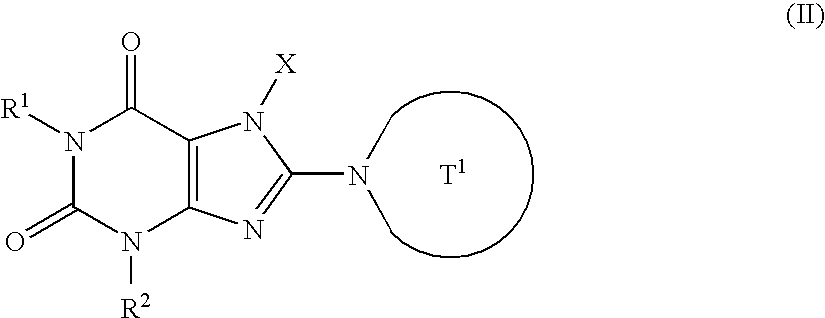
![Branched Peg Remodeling and Glycosylation of Glucagon-Like Peptides-1 [Glp-1] Branched Peg Remodeling and Glycosylation of Glucagon-Like Peptides-1 [Glp-1]](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4ee91e84-dc70-492d-8101-8e27c2003d31/US20080300173A1-20081204-C00002.png)