Fondaparinux sodium, intermediates thereof and preparation methods
A technology of fondaparinux sodium and dimethyl tert-butyl silicon trifluoromethanesulfonate, which is applied in the preparation of fondaparinux sodium and its intermediates, fondaparinux sodium and disaccharides, tetrasaccharides and The pentasaccharide intermediate can solve the problems of high purity of fondaparinux sodium, high product purity, and high production cost, and achieve the effects of reducing the difficulty of purification and production cost, the difficulty of synthesis, and the production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
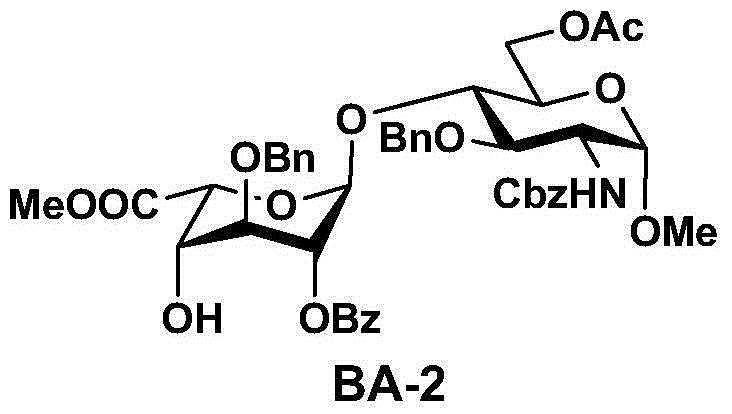
[0057] 2. Preparation route of disaccharide (BA-2) ;
[0058] Step 1, in the presence of a catalyst, monosaccharide B-1 and monosaccharide A-1 are coupled to obtain disaccharide BA-1; step 2, in the presence of an organic solvent and an organic base, disaccharide BA-1 is obtained by using Thiourea is deprotected to obtain the disaccharide BA-2. The synthetic route is as follows:
[0059]
[0060] 3. Preparation route of tetrasaccharide (DCBA-2) ;
[0061] Step 1, in the presence of an organic solvent and a catalyst, the disaccharide BA-2 and the disaccharide DC-3 are coupled to obtain the tetrasaccharide DCBA-1; Step 2, in the presence of an organic solvent and an organic base, the tetrasaccharide DCBA-1 -1 was deprotected with thiourea to give the tetrasaccharide DCBA-2. The synthetic route is as follows:
[0062]
[0063] 4. Preparation route of pentasaccharide (EDCBA-1) ;
[0064] In the presence of organic solvents and catalysts, tetrasaccharide DCBA-2 and...
Embodiment 1
[0069] Embodiment 1, the preparation of disaccharide (BA-2);
[0070] 0.3 kg of compound (A-1) and 0.41 kg of compound (B-1) were dissolved in 4.5 liters of dichloromethane, cooled to 0°C, added with 0.15 kg of silver trifluoromethanesulfonate, and stirred at 0°C for 2-3 hours. After filtration and concentration under reduced pressure, 0.7 kg of disaccharide compound (BA-1) was obtained.
[0071] 0.7 kg of disaccharide compound (BA-1) and 0.07 kg of thiourea were dissolved in 1.2 liters of ethanol and 1.8 liters of pyridine, and the reaction was heated to 70-80°C and stirred for 1 hour. After the reaction was completed, the temperature was lowered to room temperature, followed by post-treatment with water and dichloromethane, and the separated organic phase was successively washed with potassium bisulfate solution, sodium bicarbonate solution and saturated brine, and dried over anhydrous sodium sulfate. After concentration, the crude product was purified by column chromatog...
Embodiment 2
[0076] Embodiment 2, the preparation of disaccharide (BA-2);
[0077] 0.3 kg of compound (A-1) and 0.41 kg of compound (B-1) were dissolved in 4.5 liters of dichloromethane, cooled to -50 ° C, added 0.15 kg of silver trifluoromethanesulfonate, stirred at -50 ° C for 2-3 Hour. After filtration and concentration under reduced pressure, 0.7 kg of disaccharide compound (BA-1) was obtained.
[0078] 0.7 kg of disaccharide compound (BA-1) and 0.07 kg of thiourea were dissolved in 1.2 liters of ethanol and 1.8 liters of pyridine, and the reaction was heated to 60-70°C and stirred for 1 hour. After the reaction was completed, the temperature was lowered to room temperature, followed by post-treatment with water and dichloromethane, and the separated organic phase was successively washed with potassium bisulfate solution, sodium bicarbonate solution and saturated brine, and dried over anhydrous sodium sulfate. After concentration, the crude product was purified by column chromatogr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com