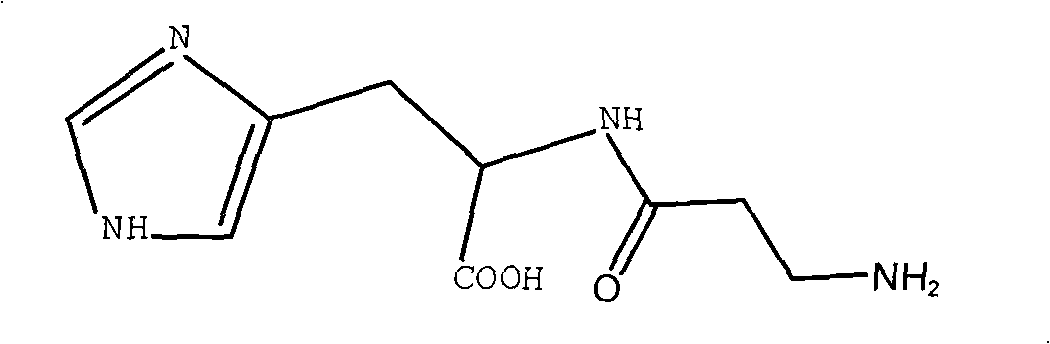
Synthetic method of L-carnosine
A synthesis method and carnosine technology, applied in the field of L-carnosine synthesis, can solve the problems that the quality cannot meet people's daily requirements, are not suitable for industrial production, and have high solvent consumption, achieve difficult separation and reuse, mild reaction conditions, and reduce raw materials. The effect of consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
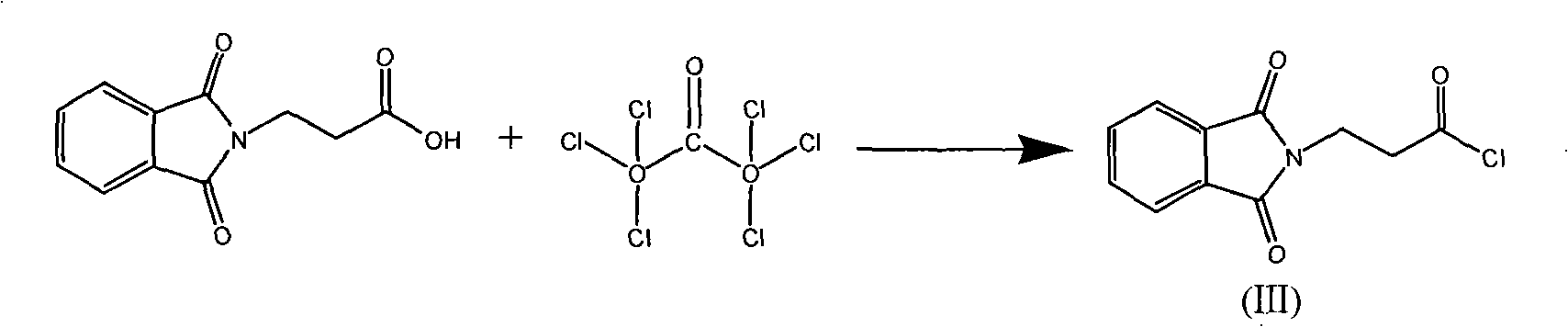
[0018] Preparation of phthaloyl-β-alanine (II).
[0019] In a 2000ml reaction flask, add 1000g toluene, 69.1g β-alanine (0.776mol), 114.9g phthalic anhydride (0.776mol) and 6.5g triethylamine (0.064mol), heat up to reflux, and separate the reaction For the generated moisture, when there is no obvious moisture in the water separator, the reaction is over, and the toluene is recovered under normal pressure. After a large amount of white solid appears, it is recovered under reduced pressure. The temperature of the material does not exceed 125 ° C. All dissolved, kept stirring for 15 min, then cooled to 10°C, filtered, and dried to obtain 167.5 g of product (II), with a yield of 98.5%, HPLC>99.5. The reaction formula of this embodiment is as follows, wherein (II) represents phthaloyl-β-alanine.
[0020]
Embodiment 2
[0022] Preparation of phthaloyl-β-alanine (II).
[0023] In a 2000ml reaction flask, add 1000g benzene, 69.1g β-alanine (0.776mol), 114.9g phthalic anhydride (0.776mol) and 6.5g triethylamine (0.064mol), heat up to reflux, and separate the reaction For the generated moisture, when there is no obvious moisture in the water separator, the reaction is over, and the toluene is recovered under normal pressure. After a large amount of white solid appears, it is recovered under reduced pressure. The temperature of the material does not exceed 125 ° C. All dissolved, kept stirring for 15 min, then cooled to 10°C, filtered, and dried to obtain 167 g of product (II), with a yield of 98.2%, HPLC>99.5.
[0024] The chemical reaction formula of this embodiment is the same as that of Embodiment 1.
Embodiment 3
[0026] Preparation of phthaloyl-β-alanine (II).
[0027] In a 2000ml reaction flask, add 1000g xylene, 69.1g β-alanine (0.776mol), 114.9g phthalic anhydride (0.776mol) and 6.5g triethylamine (0.064mol), heat up to reflux, separate The moisture generated by the reaction, when there is no obvious moisture in the water separator, the reaction is over, and the toluene is recovered under normal pressure. After a large amount of white solid appears, it is recovered under reduced pressure. The temperature of the material does not exceed 125°C, then add 800g of water, and heat to 90°C. All the solids were dissolved, kept stirring for 15 minutes, then cooled to 10°C, filtered, and dried to obtain 166.7 g of product (II), with a yield of 98%, HPLC>99.5. The chemical reaction formula of this embodiment is the same as that of Embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com