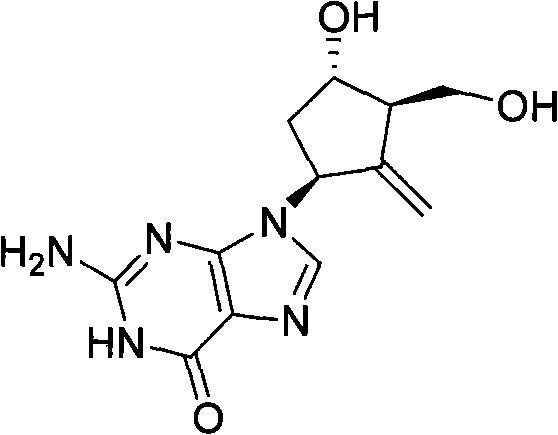
New synthesis process of antiviral drug entecavir
A compound and generative technology, which is applied in the field of new synthesis technology of entecavir, can solve the problems of expensive process route, rare raw materials, and high technical requirements of the method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] The preparation of embodiment 1 (1R, 5S)-5-[dimethyl (1-naphthyl) silane]-2-(hydroxymethyl)-2-cyclopentene-1-carboxylic acid methyl ester
[0117] This example is used to prepare (1R, 5S)-5-[dimethyl(1-naphthyl)silane]-2-(hydroxymethyl)-2-cyclopentene-1-carboxylic acid methyl ester, the substance As a raw material for the synthesis of Entecavir. The specific steps are as follows:
[0118] 1) 1-(dimethylchlorosilyl)-naphthalene (X, R 1 = naphthalene)
[0119] Under a nitrogen atmosphere, add magnesium powder (7.2 g, 0.3 mol) dried THF 60 ml and a grain of iodine into a 1000 ml three-necked flask. Add 1-bromonaphthalene (62.1 g, 0.3 mol) THF 180 ml solution dropwise. After triggering at 50° C., control the rate of addition to maintain reflux. After dropping, heat to 60° C. and reflux and stir for 1 hour.
[0120] A solution of dichlorodimethylsilane (51 g, 0.39 mol) in 180 ml of THF was added dropwise, and the mixture was heated under reflux and stirred for 15 ho...
Embodiment 2
[0132] The preparation of embodiment 2 entecavir
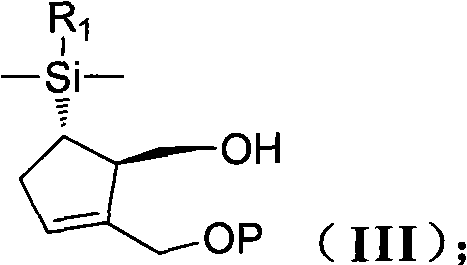
[0133] (1R,5S)-5-[Dimethyl(1-naphthyl)silane]-2-[(1-methoxy-1-methylethoxy)methyl] - Preparation of 2-cyclopentene-1-methanol (IIIa)
[0134] Add compound 5 (16 g, 0.05 mol) and 40 ml of toluene to a 500 ml three-necked round bottom flask equipped with a mechanical stirrer and a thermometer under a nitrogen atmosphere. The resulting solution was cooled to about 0°C in an ice-water bath, and 2-methoxypropene (70ml, 0.05mol) was added. 0.4 g of pyridinium p-toluenesulfonate was added at 0°C, and the resulting mixture was stirred at 0°C for 10 minutes. The cooling bath was removed and the reaction mixture was stirred at 120°C for 1.5 hours. After the reaction was complete, the reaction mixture was cooled to -78°C. Lithium aluminum hydride (2.0 g, 0.05 mol) was added. After 30 minutes, the cooling bath was removed, stirred at room temperature for 2 hours, and 20 ml of sodium hydroxide 2N solution was added. After the addi...
Embodiment 3
[0146] The preparation of embodiment 3 entecavir
[0147] [1R-(1α, 2α, 3β, 5α)]-3-[Dimethyl(1-naphthyl)silane]-6-oxabicyclo[3.1.0] Preparation of hexane-1,2-dimethanol (V')
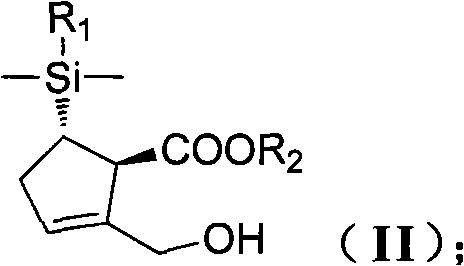
[0148] Under a nitrogen atmosphere, add 20 g of molecular sieves and 100 ml of DCM to a 500 ml three-neck flask equipped with a mechanical stirrer, a temperature probe and a nitrogen inlet. 2.3 g of DIPT and 2.3 ml of titanium isopropoxide were sequentially added to the mixture. The reaction was stirred at 30°C for 20 minutes. Compound (II) (10 g, 0.03 mol) in DCM 40 ml was added. Then add TBHP30ml. Stir at room temperature for 5 hours. Aqueous sodium sulfite solution quenched the reaction. Washed with saturated sodium bicarbonate and saturated brine. The organic layer was concentrated under reduced pressure to obtain a crude oil (10 g, 0.028 mol). Add 10 g of the newly obtained epoxide and 75 ml of isopropanol to a 500 ml three-necked flask equipped with a mechanical stirrer, a temperature pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com