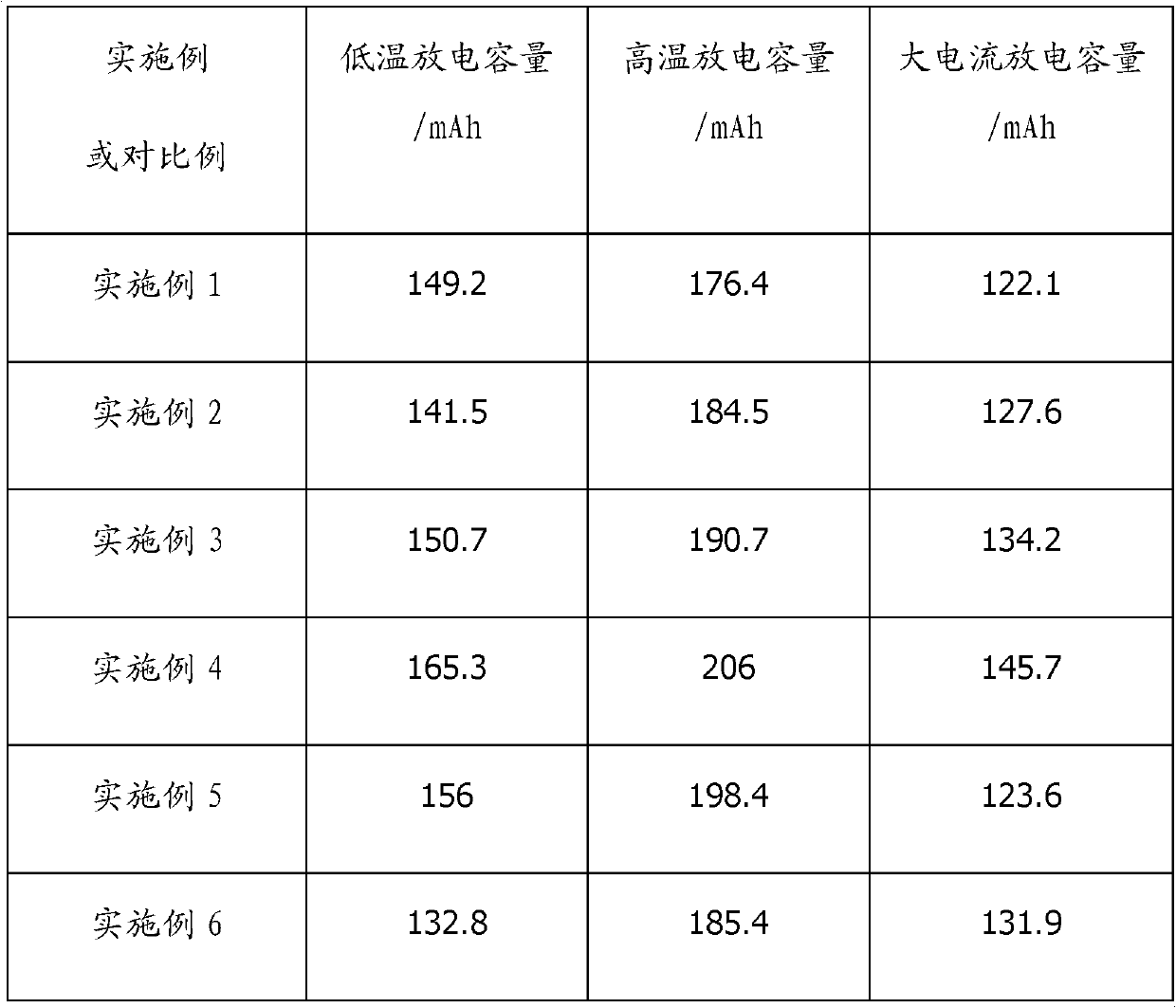
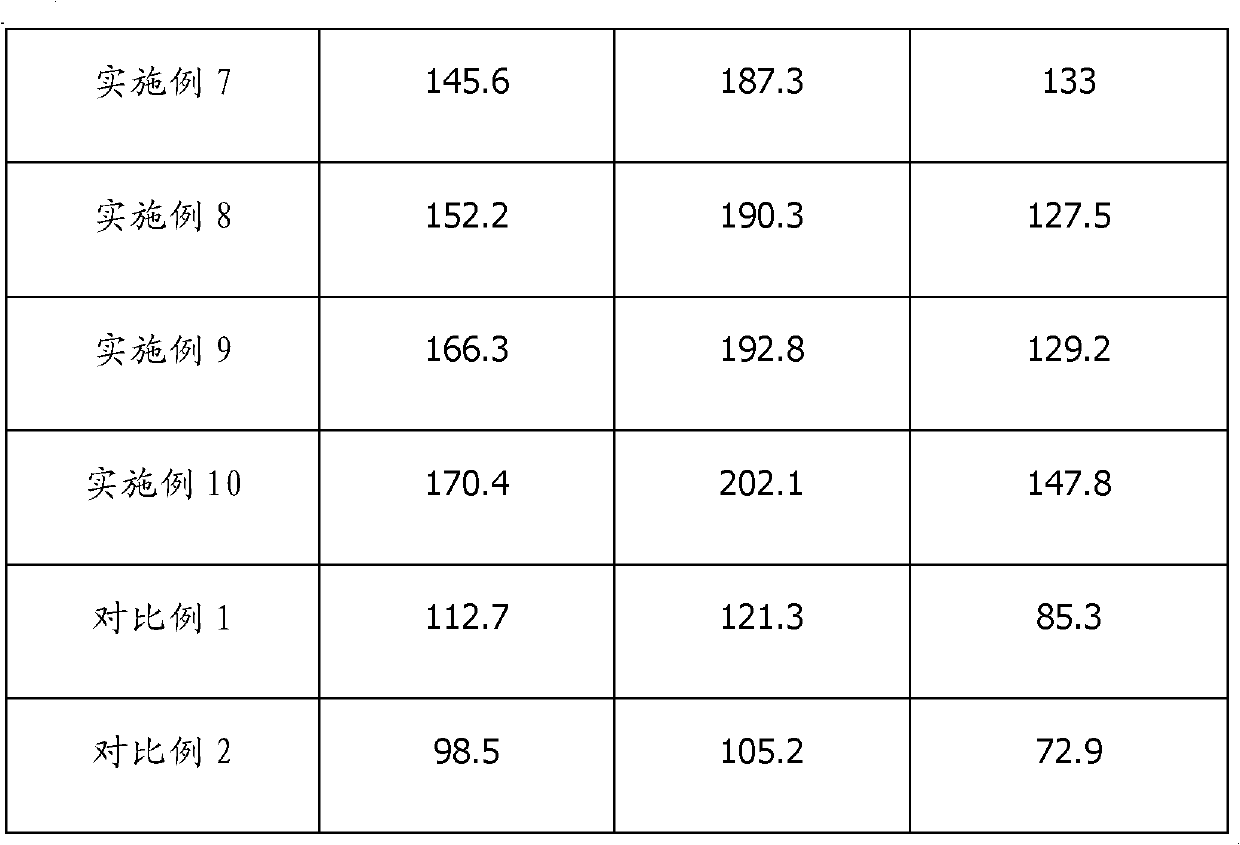
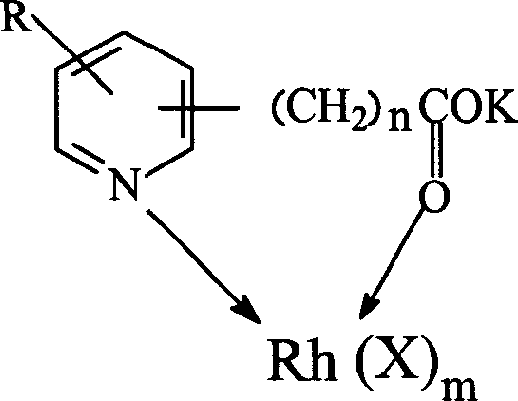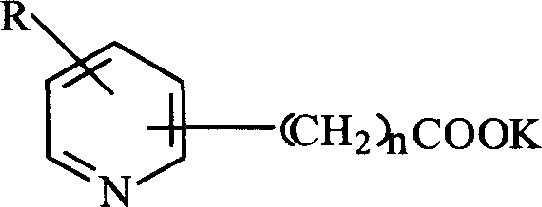Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
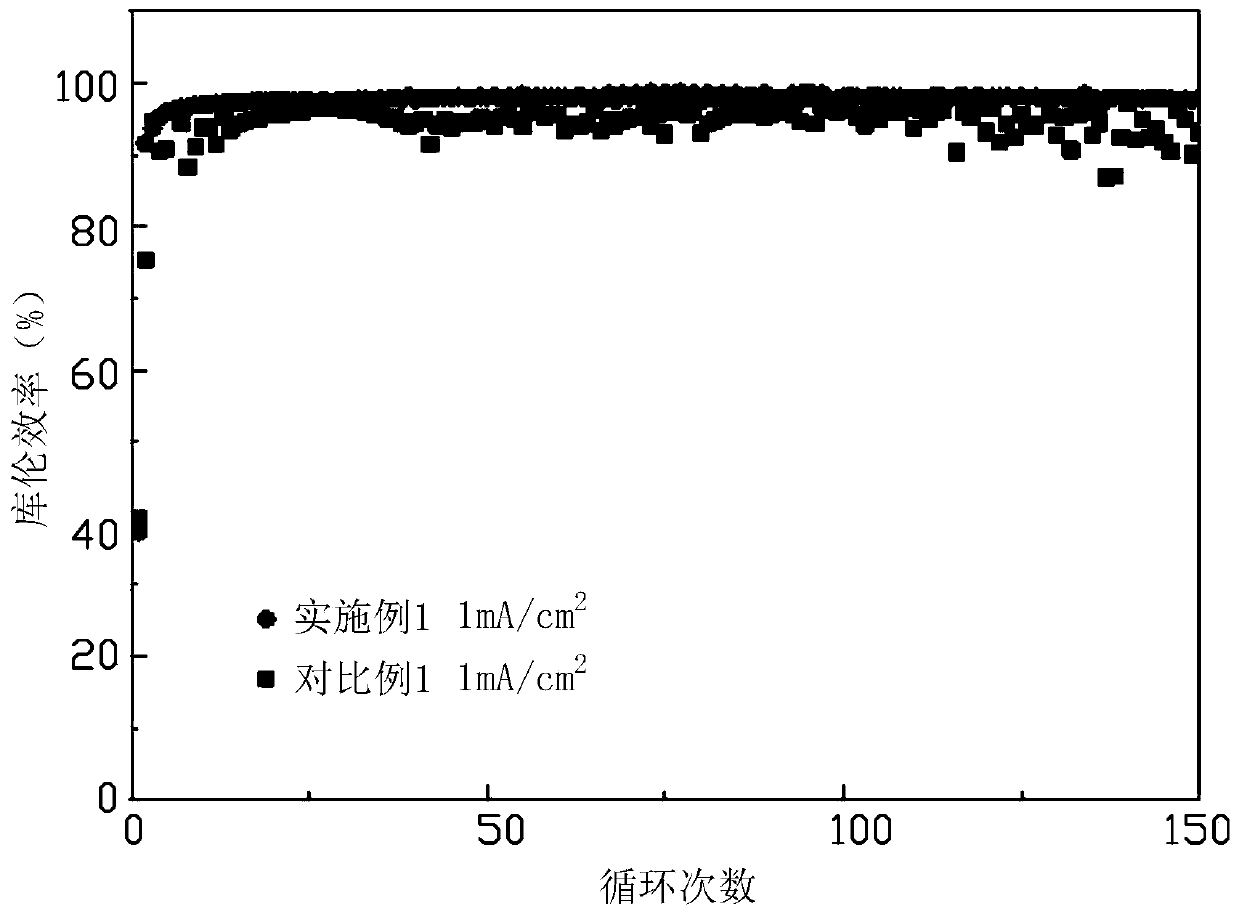
179 results about "Lithium iodide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lithium iodide, or LiI, is a compound of lithium and iodine. When exposed to air, it becomes yellow in color, due to the oxidation of iodide to iodine. It crystallizes in the NaCl motif. It can participate in various hydrates.
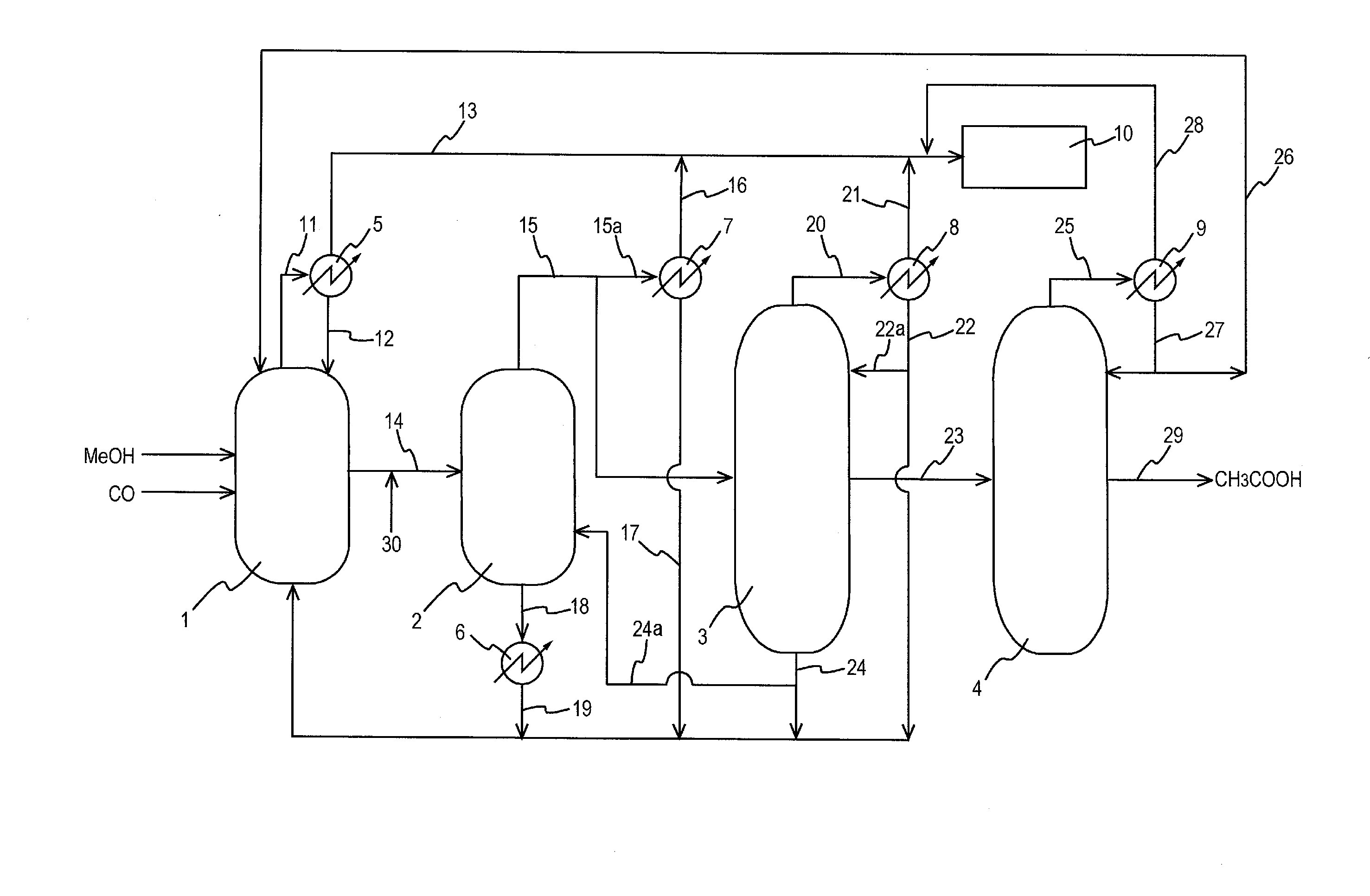
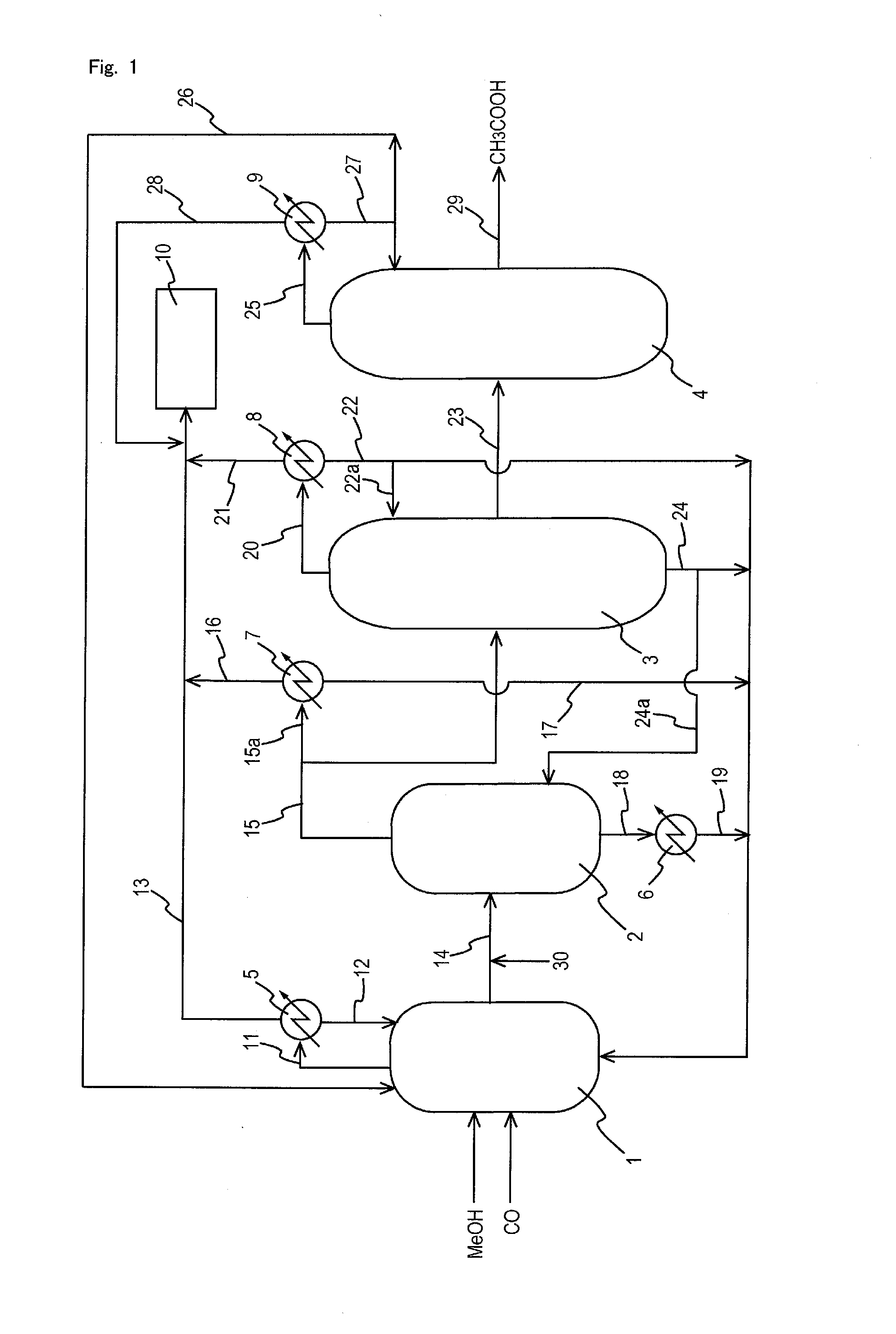
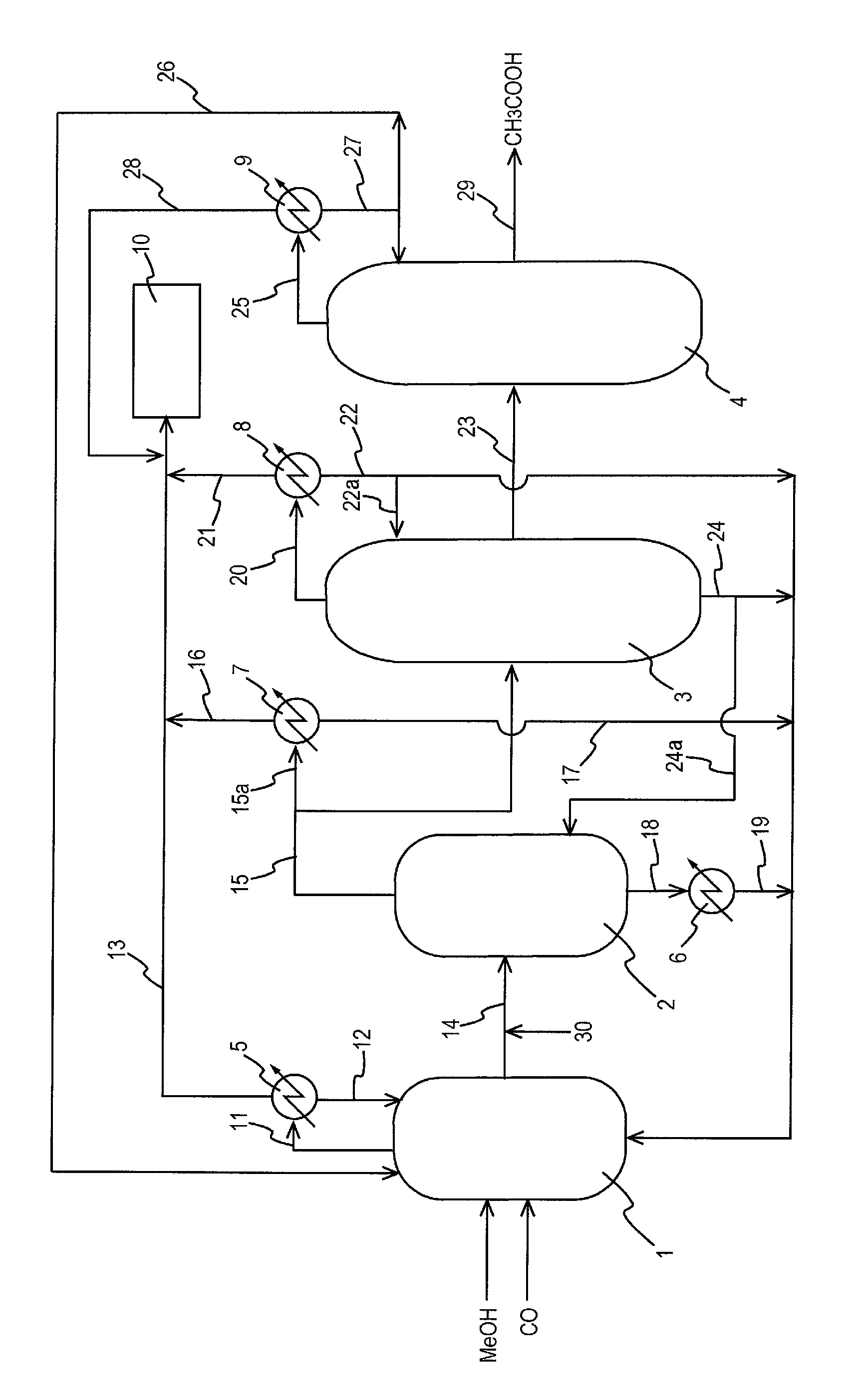
Process for producing acetic acid
ActiveUS20130310603A1Efficient productionEasy to save energyOrganic compound preparationOrganic chemistry methodsIodideReaction step
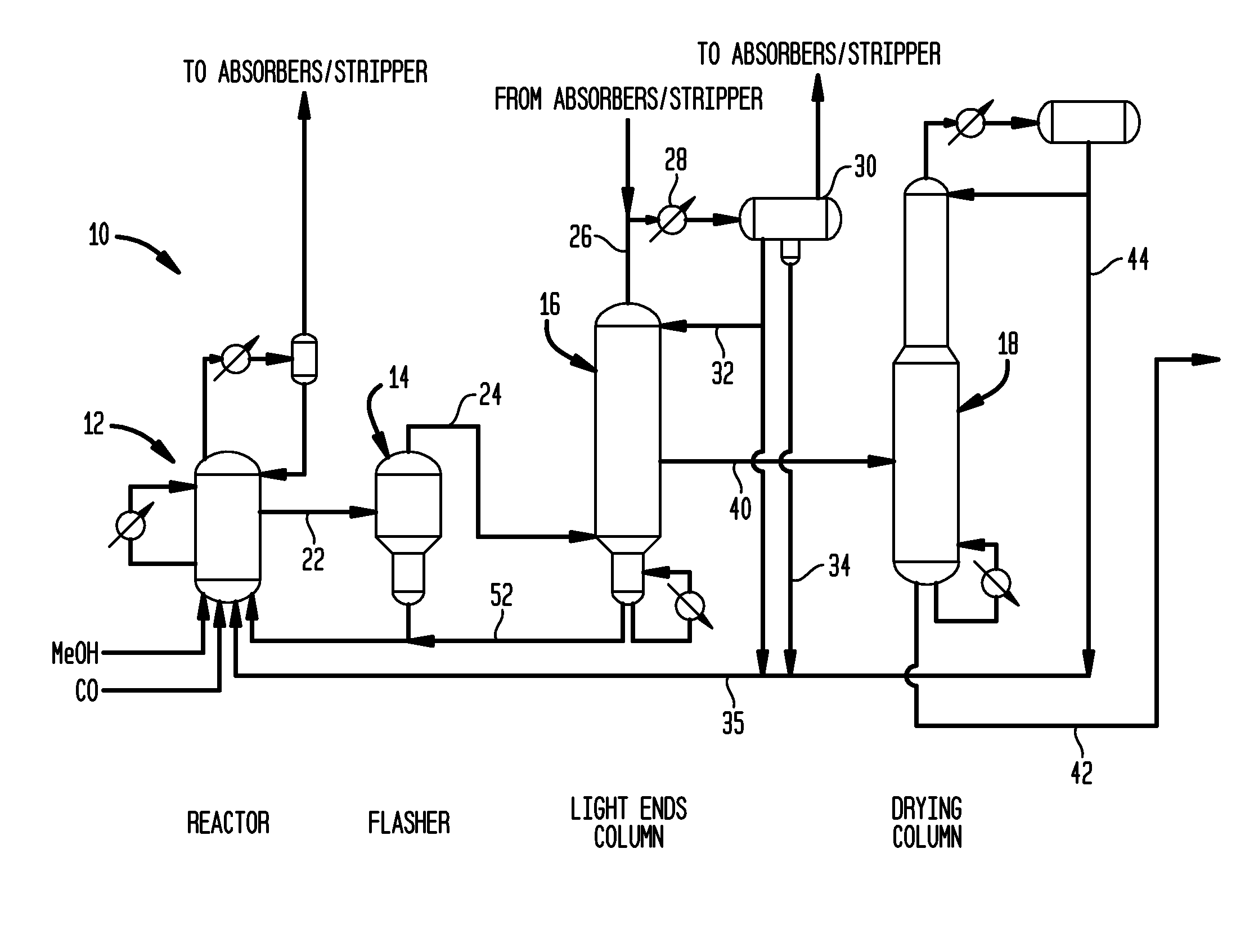
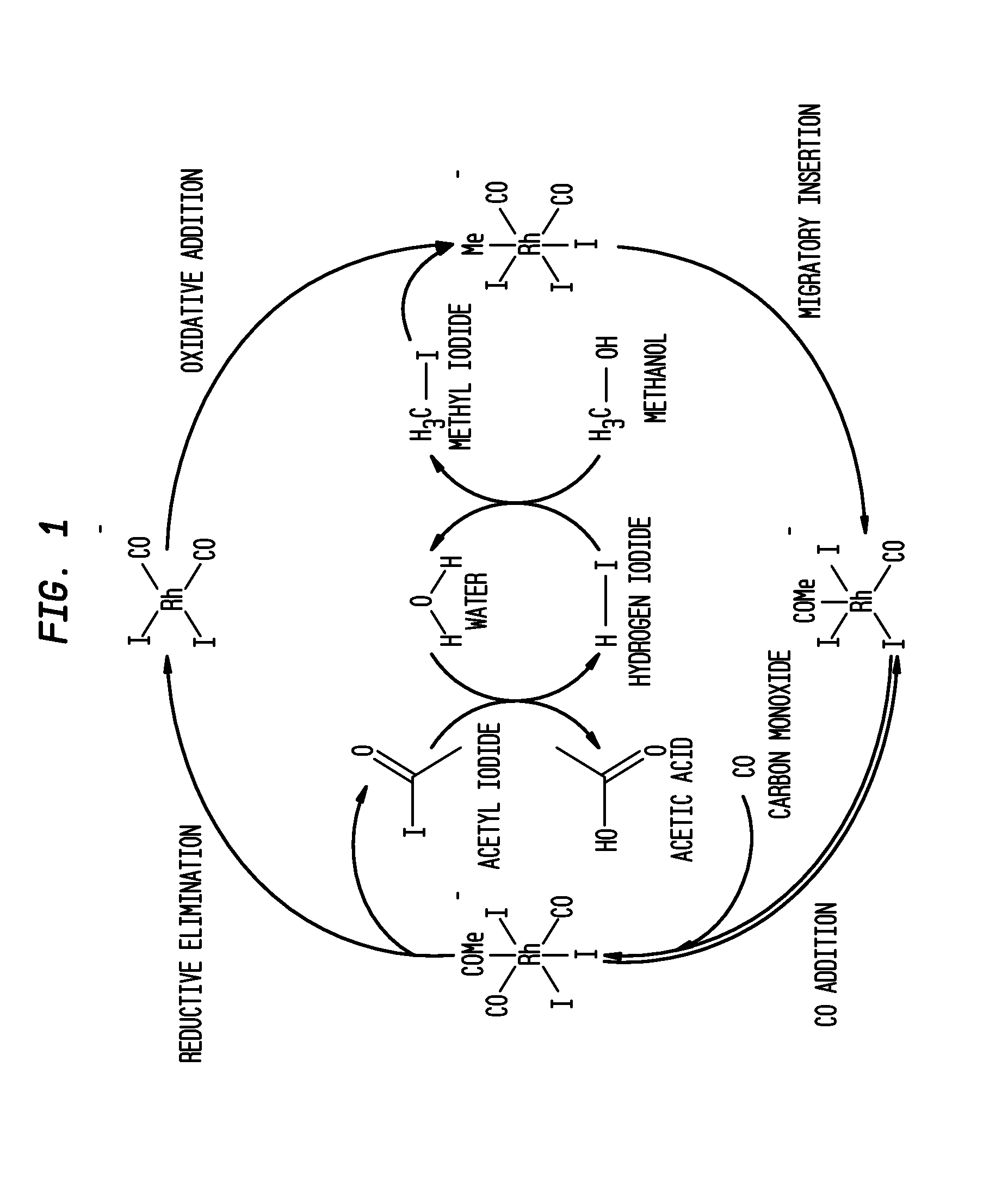
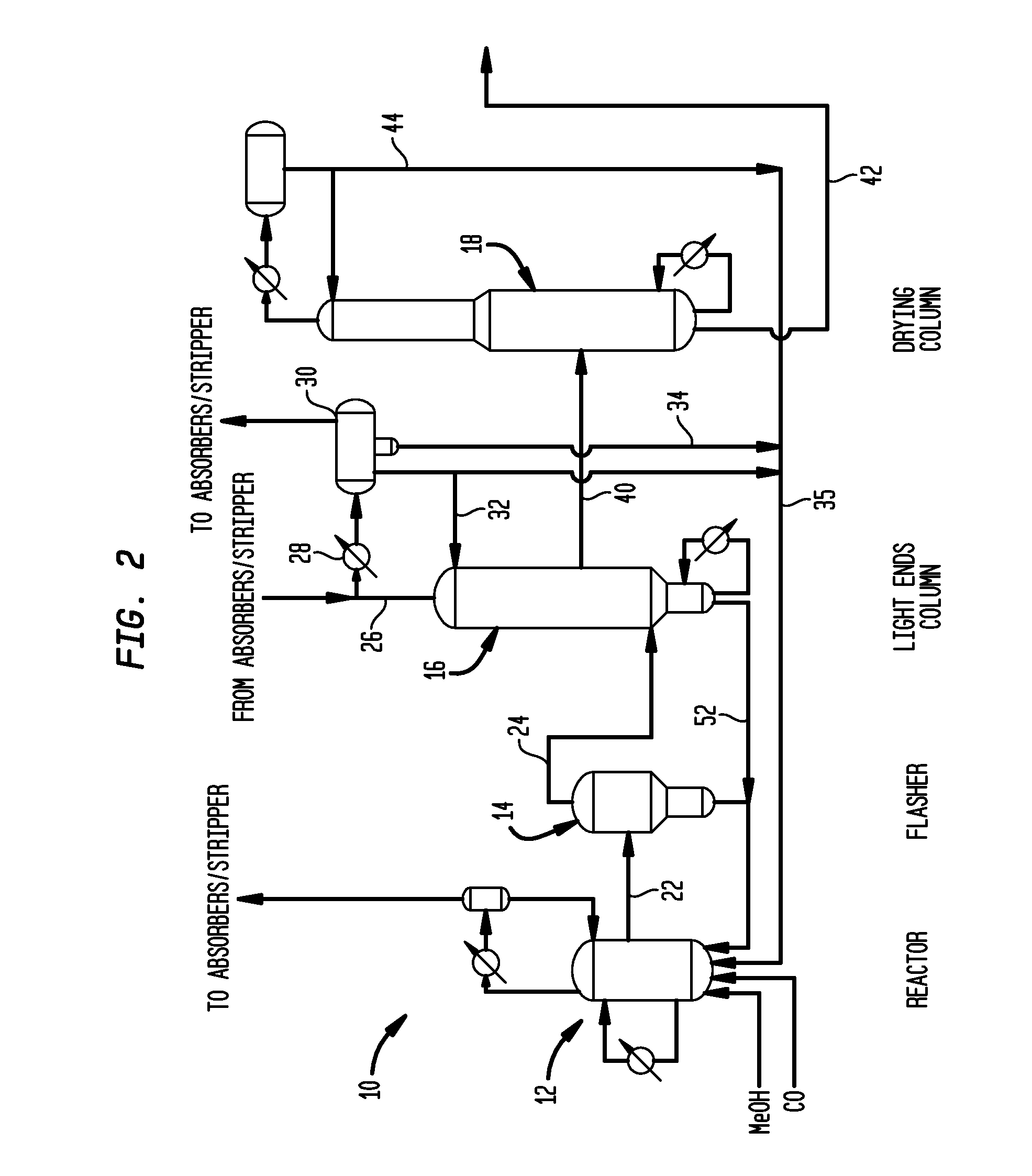
Acetic acid is produced while inhibiting an increased concentration or production of hydrogen iodide in a carbonylation reactor or corrosion of the carbonylation reactor.A production process of acetic acid comprises a reaction step for continuously allowing methanol to react with carbon monoxide in the presence of a catalyst system comprising a metal catalyst (e.g., a rhodium catalyst), an ionic iodide (e.g., lithium iodide), and methyl iodide in a carbonylation reactor; and in the process, (i) the concentration of the metal catalyst is maintained at not less than 860 ppm on the basis of weight, the concentration of water is maintained at 0.8 to 15% by weight, the concentration of methyl iodide is maintained at not more than 13.9% by weight, and the concentration of methyl acetate is maintained at not less than 0.1% by weight, in a whole liquid phase in the reactor, and / or (ii) the concentration of the metal catalyst is maintained at not less than 660 ppm on the basis of weight, the concentration of water is maintained at 0.8 to 3.9% by weight, the concentration of the ionic iodide is maintained at not more than 13% by weight, the concentration of methyl iodide is maintained at not more than 13.9% by weight, and the concentration of methyl acetate is maintained at not less than 0.1% by weight, in a whole liquid phase in the reactor.
Owner:DAICEL CHEM IND LTD
Graphene/phosphoric acid iron-lithium composite material with sandwich structure and preparation method thereof
InactiveCN102148371ASimple preparation processShort preparation cycleNon-aqueous electrolyte accumulator electrodesO-Phosphoric AcidLithium-ion battery
The invention relates to a graphene / phosphoric acid iron-lithium composite material with a 'sandwich' structure and a preparation method thereof. The structure characteristics of the graphene / phosphoric acid iron-lithium composite material are that: blocky particles are formed by a grapheme laminated sheet which is completely coated by a phosphoric acid iron-lithium shell; and the insides of the particles present a similar 'sandwich' structure overlapped by a plurality of layers of phosphoric acid iron-lithium and graphene one by one. The preparation method thereof adopts a 'two-step' method. The characteristic steps are as follows: a graphene / phosphoric acid iron precursor with a 'sandwich' structure is compounded by a liquid phase method during the first step; then lithium is embedded in the second step; lithium iodide liquid phase low temperature reaction is adopted for embedding the lithium; then the graphene / phosphoric acid iron-lithium composite material is obtained through high temperature calcination under reducing (inertia) atmospheres; moreover, the graphene / phosphoric acid iron-lithium composite material can also be formed by the embedding of the lithium through high temperature solid phase reaction. The graphene / phosphoric acid iron-lithium composite material prepared by the method has high capacity and good charging-discharging circulating performances, and is suitable to be used as an anode material of a lithium ion battery.
Owner:SHANGHAI UNIV
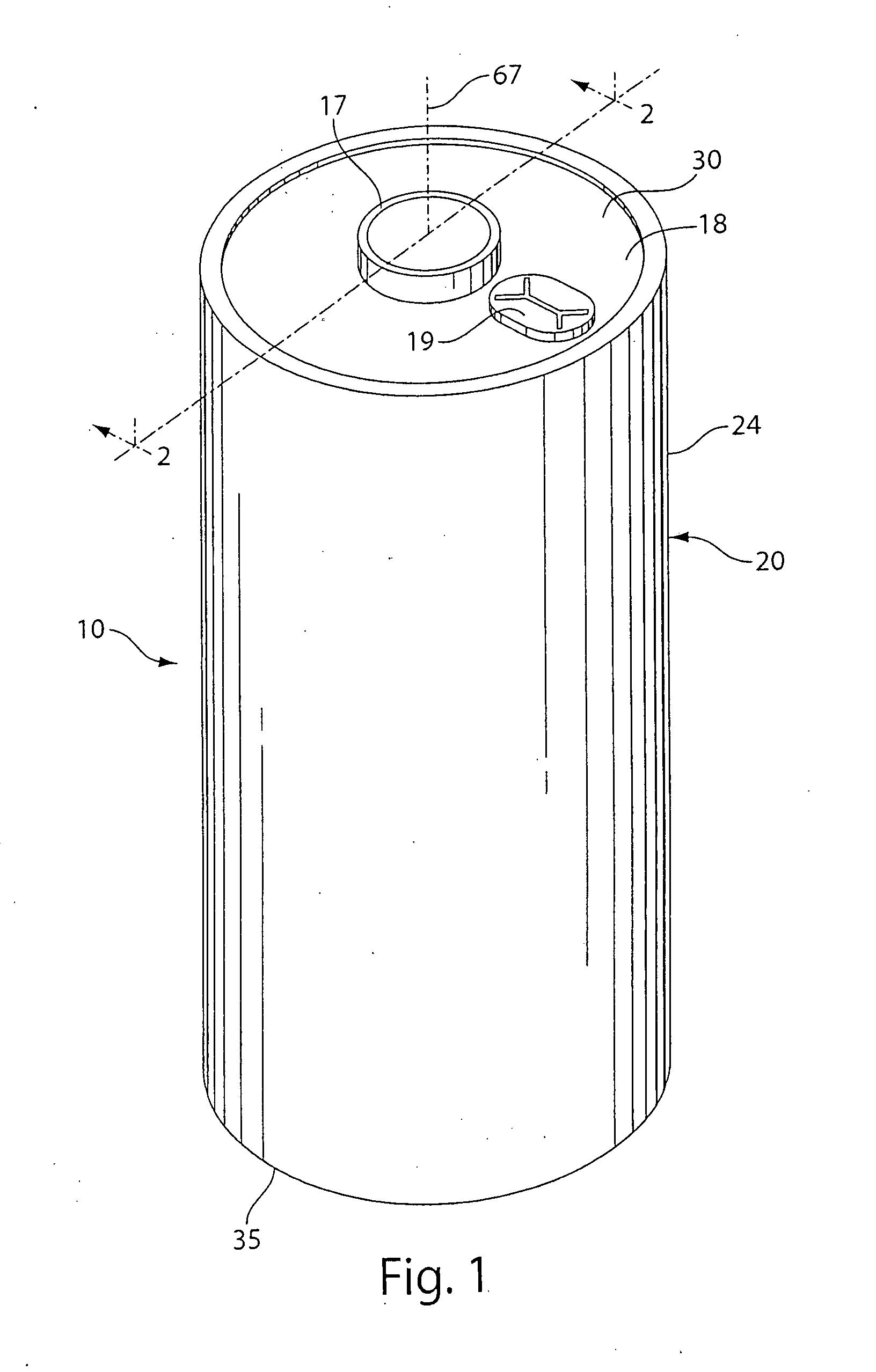
Lithium cell
ActiveUS20090208849A1Reduce passive layer resistanceSlow build ratesPrimary cell maintainance/servicingElectrode carriers/collectorsSulfolaneAlloy
A primary cell having an anode comprising lithium or lithium alloy and a cathode comprising iron disulfide (FeS2) and carbon particles. The electrolyte comprises a lithium salt preferably lithium iodide (LiI) dissolved in an organic solvent mixture. The solvent mixture preferably comprises dioxolane, dimethoxyethane and sulfolane. The electrolyte typically contains between about 100 and 2000 parts by weight water per million parts by weight (ppm) electrolyte therein. A cathode slurry is prepared comprising iron disulfide powder, carbon, binder, and a liquid solvent. The mixture is coated onto a conductive substrate and solvent evaporated leaving a dry cathode coating on the substrate. The anode and cathode can be spirally wound with separator therebetween and inserted into the cell casing with electrolyte then added.
Owner:DURACELL U S OPERATIONS
Nonaqueous electrolyte battery
InactiveUS20090061315A1Large capacityIncrease productionActive material electrodesSecondary cellsMetallic lithiumLithium hexafluorophosphate
A nonaqueous electrolyte battery includes a negative electrode composed of a metallic lithium foil and a positive electrode, the negative electrode and the positive electrode being arranged so as to face each other with an ion-conducting medium therebetween. The positive electrode is formed by a method in which a conductive agent and a binder are mixed, and then the mixture is press-formed onto a current collector. The ion-conducting medium contains, in addition to a lithium salt such as lithium hexafluorophosphate, a halogen such as iodine, and a halogen compound (e.g., lithium iodide). Furthermore, the positive electrode may contain a lithium halide.
Owner:TOYOTA CENT RES & DEV LAB INC
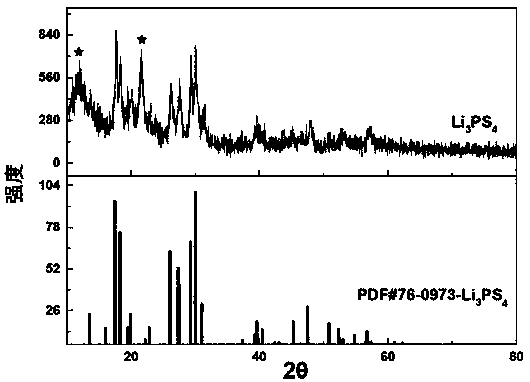
Sulfide solid electrolyte based on oxygen doping and preparation method of sulfide solid electrolyt
ActiveCN108493479AImprove stabilityImprove ionic conductivitySolid electrolytesLi-accumulatorsLithium chloridePhosphorus pentasulfide
The invention provides a sulfide solid electrolyte based on oxygen doping. The sulfide solid electrolyte is prepared from the chemical ingredients, in percentage by mass: 36-60% of lithium sulfide orlithium selenide, 18-48% of phosphorus pentasulfide or phosphorus selenide, 1-23% of metal oxide or specific nonmetallic oxide and 8-37% of lithium chloride, lithium bromide or lithium iodide. The preparation method of the sulfide solid electrolyte mainly comprises the steps that the raw materials are sufficiently blended and subjected to tabletting, then placed in a quartz tube to be burned and sealed, the product is placed in a muffle furnace and heated to be 400-600 DEG C at a slow heating rate, the optimal heating rate is 0.3 DEG C / minute, heat preservation is conducted for 12-48 hours, and then the product is cooled to reach the room temperature; and the product is ground to be powder, and the sulfide solid electrolyte based on oxygen doping is prepared. The sulfide solid electrolyteis easy to prepare and high in repeatability, the prepared solid electrolyte has the high ionic conductivity and good stability to the air and positive and negative electrodes.
Owner:YANSHAN UNIV
Lithium sulfur battery
ActiveCN104143614ASufficient conductivityThe charge and discharge reaction is smoothMaterial nanotechnologyLi-accumulatorsLithium chlorideLithium bromide
The invention belongs to the technical field of lithium sulfur batteries, and concretely relates to a lithium sulfur battery new system. The new system comprises an electrolyte and a diaphragm matched with the electrolyte. The electrolyte is a lithium salt solution with the concentration of 0.1-3mol / L, and a lithium salt solute in the lithium salt solution is one or a mixture comprising two or more of lithium fluoride, lithium chloride, lithium bromide or lithium iodide; a solvent is one of or a mixture comprising two or more of N,N-dimethyl formamide, N,N-dimethyl acetamide, dimethyl sulfoxide, tetramethyl sulfone, tetrahydrofuran, N-methyl-2-pyrrolidone and acetonitrile; and the diaphragm is a micro-porous membrane with the aperture of 0.5-10nm or a dense membrane containing anions. The micro-porous diaphragm subjected to surface modification and hot pressing can be fully infiltrated in the micro-molecular lithium salt electrolyte and has an appropriate dimension to make electrolyte ions freely go through in order to inhibit or prevent the migration of polysulfide. The lithium salt has a low price, so the battery has the cost advantage.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Novel thallium doped sodium, cesium or lithium iodide scintillators
InactiveUS20170355905A1Solve the low detection efficiencyShort decay timeX/gamma/cosmic radiation measurmentLuminescent compositionsSodium iodideThallium
The present invention provides for a composition comprising a crystal composition or inorganic scintillator comprising a thallium doped sodium iodide, cesium iodide, or lithium iodide scintillator useful for detecting nuclear material.
Owner:RGT UNIV OF CALIFORNIA
Electrolyte of lead-acid accumulator and preparation method thereof
InactiveCN101882694AExtended service lifeEnhanced cryogenic performanceFinal product manufactureLead-acid accumulators constructionLithium chlorideGas phase
The invention discloses an electrolyte of a lead-acid accumulator and a preparation method thereof. The electrolyte comprises the following raw materials in part by weight: 25 to 45 parts of deionized water, 60 to 70 parts of dilute sulfuric acid solution, 1.8 to 2.8 parts of gas-phase nanometer silicon dioxide and 2.5 to 3 parts of electrolyte activating agent, wherein the electrolyte activating agent is prepared from the following raw materials in part by weight: 1,000 parts of deionized water, 2 parts of nickel sulfate, 2 parts of cobalt sulfate, 25 parts of aluminum sulfate, 15 parts of sodium sulfate, 25 parts of magnesium sulfate, 20 parts of aluminium phosphate, 5 parts of lithium iodide, 5 parts of lithium chloride and 20 parts of lithium carbonate. Because the nickel sulfate, the cobalt sulfate, the lithium chloride and the lithium carbonate are added into the electrolyte activating agent, the large current charge and discharge and the ultra-low temperature performance of the accumulator are increased by 25 to 30 percent, and the service life of the accumulator is prolonged by 25 to 30 percent compared with the traditional accumulator with the traditional electrolyte. Because of the adoption of the nanometer silicon dioxide and the adoption of high-speed shearing way in the preparation method, the prepared electrolyte does not settle down within 30 days.
Owner:冯家齐
Electrolyte of lead-acid battery and preparation method thereof
ActiveCN101685884AGood dispersionImprove stabilityFinal product manufactureLead-acid accumulators constructionDispersityActive agent
The invention relates to an electrolyte of lead-acid battery and preparation method thereof. The electrolyte comprises the following raw materials by parts by weight: 20-40 parts of deionized water, 62-68 parts of dilute sulphuric acid, 1.5-2.5 parts of nano aerosol and 2-2.5 parts of electrolyte active agent; wherein the electrolyte active agent is formed by analytically pure potassium sulphate,zinc sulphate, ammonium sulphate, magnesium sulphate, aluminium sulphate, sodium sulphate, cobalt sulphate, aluminium phosphate and lithium iodide in certain ratio; and multiple micro elements are added into the electrolyte, so that the nano silicon oxide particles have favourable dispersity, the prepared electrolyte has no sedimentation, flocculation or layering for a long time, and meantime service life and environmental performance of battery can be effectively improved.
Owner:深圳市夺标环保技术有限公司
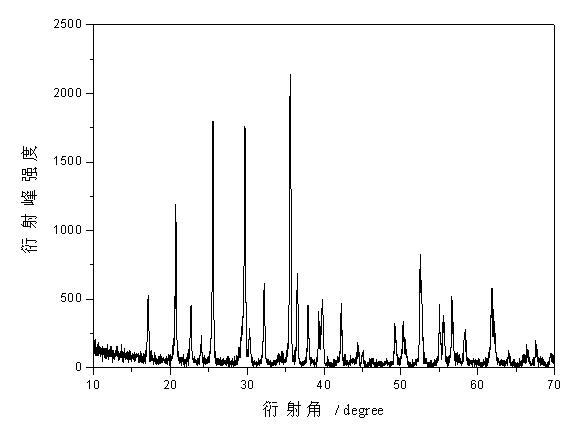
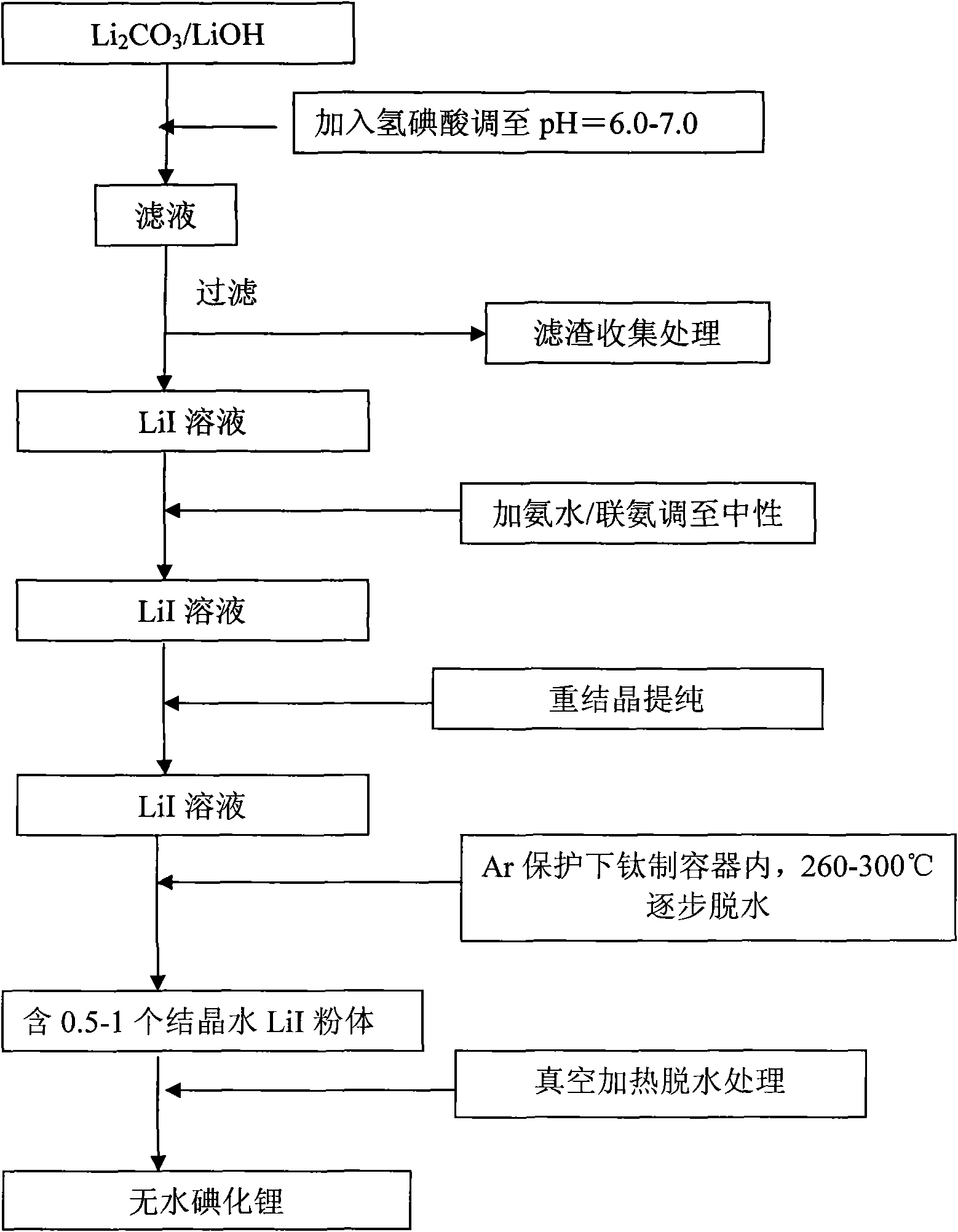
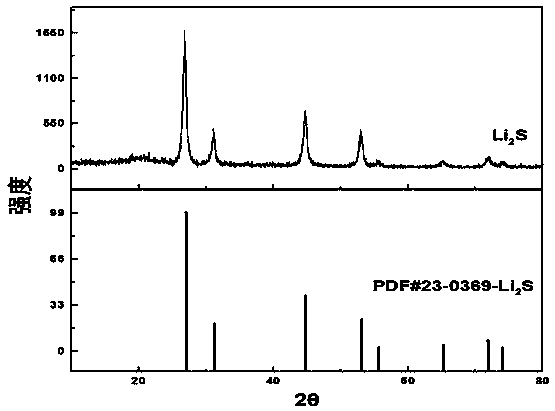
Methods for preparing anhydrous lithium iodide and scintillation crystal doped with lithium iodide
ActiveCN101565192AEasy to operateSimple processPolycrystalline material growthFrom frozen solutionsBridgman methodScintillation crystals
The invention discloses a method for preparing an anhydrous lithium iodide, comprising the following operation steps of: dehydrating the lithium iodide water solution to the lithium iodide powders containing 0.5-1 crystal water; and subsequently carrying out heating and dehydration treatment in vacuum to obtain the anhydrous lithium iodide. The invention also discloses a method for preparing a scintillation crystal doped with the lithium iodide, comprising the following operation steps of: dehydrating the lithium iodide water solution to the lithium iodide powders containing 0.5-1 crystal water; mixing the lithium iodide powder with the doped compound; subsequently carrying out heating and dehydration treatment in vacuum; and subsequently carrying out crystal growth under a vacuum state by adopting a Bridgman method, thus obtaining the scintillation crystal doped with the lithium iodide. The method for preparing the anhydrous lithium iodide has simple operation and no environmental pollution and is easy for large-scale industrial production; the method for preparing the scintillation crystal doped with the lithium iodide has simple process, is not easy to be oxidized at high temperature during the preparation process and can produce the high-quality scintillation crystal doped with the lithium iodide in batches.
Owner:上海新漫传感科技有限公司
Preparation method of cell-grade anhydrous lithium iodide
InactiveCN102030345AEffective removal of contentHigh purityLithium halidesPhysical chemistryOrganosolv
The invention discloses a preparation method of a cell-grade anhydrous lithium iodide. The preparation method is as follows: mixing and stirring lithium salt and hydroiodic acid; filtering, evaporating, crystallizing and separating, obtaining acicular crystal of lithium iodide trihydrate; then carrying out pre-drying and vacuum drying; and finally obtaining the cell-grade anhydrous lithium iodide. For the preparation method, the preparation process is simple, the material is cheap and is easy to obtain, the preparation cost is low, and in the whole synthetic process, no organic solvent is adopted, is non-toxic, and is green and environment-friendly. In the anhydrous lithium iodide prepared by the invention, the water content is lower than 0.02%, and the quality is equivalent to that of imported products.
Owner:XINJIANG RES INST OF NON FERROUS METALS
Metal lithium negative electrode and preparation method thereof
ActiveCN110660969AEasy to prepareImprove Coulombic efficiencyMaterial nanotechnologyElectrode carriers/collectorsMetallic lithiumLithium oxide
The invention provides a metal lithium negative electrode. The lithium ion battery negative electrode comprises a negative electrode current collector and a negative electrode active material layer deposited in the negative electrode current collector; the negative electrode active material layer is made of metal lithium; the negative electrode current collector comprises a current collector bodyand a gradient conductive ion layer coating the surfaces of pores in the current collector body and the outer surface of the current collector body; the ion and electron transmission of the current collector is ensured; the negative electrode active material layer is deposited on the surface of the gradient conductive ion layer; the current collector body is made of a porous conductive material; the porosity of the porous conductive material is 10%-95%; the gradient conductive ion layer is made of at least one material selected from lithium phosphide, lithium oxide, lithium nitride, lithium sulfide, lithium fluoride, lithium chloride, lithium bromide, lithium iodide and lithium phosphate. The invention further provides a preparation method of the metal lithium negative electrode.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
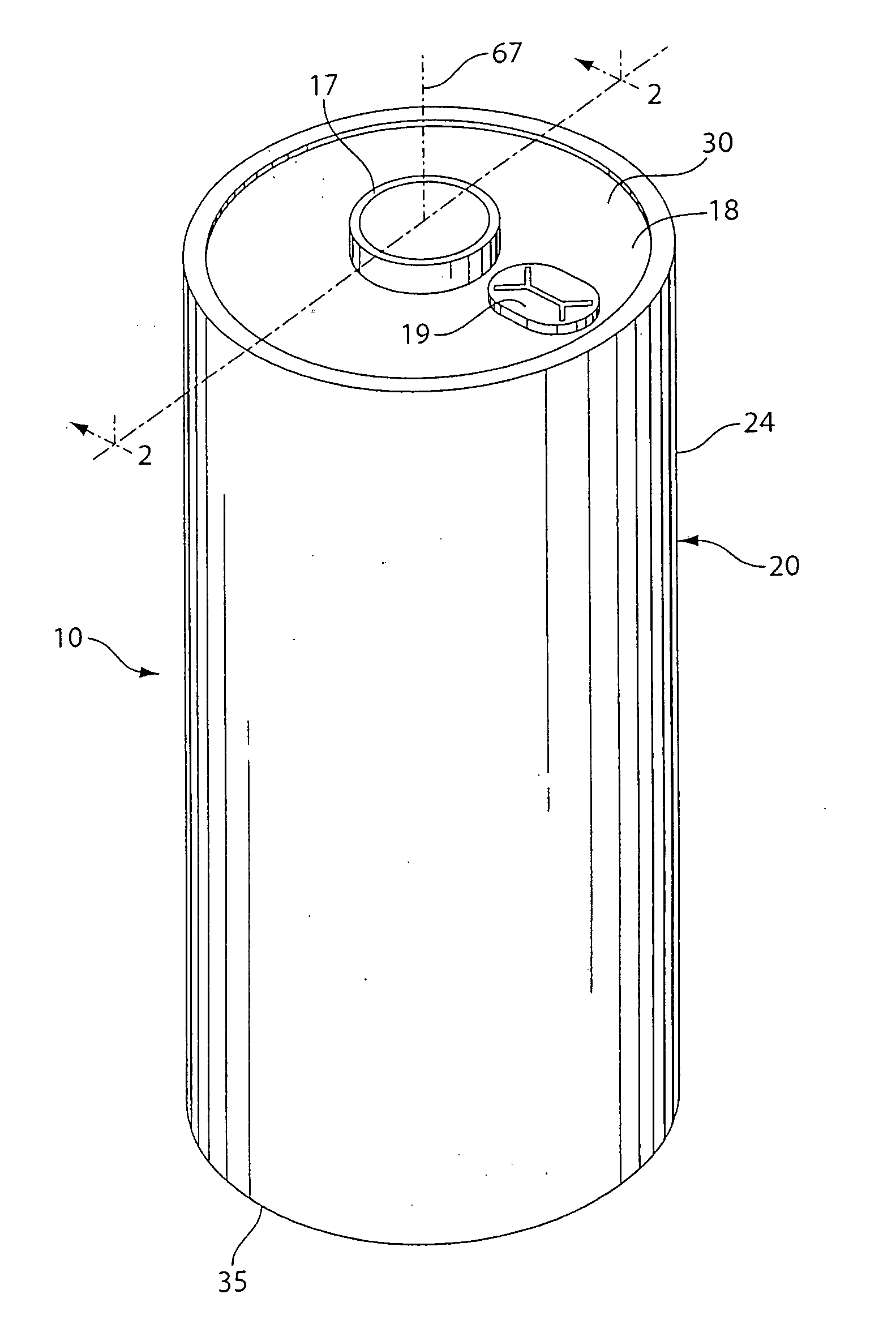
Lithium cell with iron disulfide cathode
InactiveUS20100203370A1Advantage in cell performanceAdvantages in cell performanceCell electrodesOrganic electrolyte cellsLithium metalAlloy
A primary cell having an anode comprising lithium or lithium alloy and a cathode comprising iron disulfide (FeS2) and carbon particles. The electrolyte comprises a lithium salt preferably lithium iodide (LiI) dissolved in an organic solvent mixture. The solvent mixture preferably comprises dioxolane and dimethoxyethane. The electrolyte typically contains between about 100 and 2000 parts by weight water per million parts by weight (ppm) electrolyte therein. The anode may be lithium metal or preferably is a lithium alloy. A cathode slurry is prepared comprising iron disulfide powder, carbon, binder, and a liquid solvent. The mixture is coated onto a conductive substrate and solvent evaporated leaving a dry cathode coating on the substrate. The anode and cathode can be spirally wound with separator therebetween and inserted into the cell casing with electrolyte then added.
Owner:THE GILLETTE CO
Method for synthesizing lithium sulfide
InactiveCN111517288ASynthetic raw materials are cheap and easy to obtainThe process steps are simpleHybrid capacitor electrodesPhosphorus sulfur/selenium/tellurium compoundsMetallic lithiumN-Butyllithium
The invention mainly relates to the field of lithium battery materials, and provides a method for synthesizing lithium sulfide by taking a lithiation solution and elemental sulfur as raw materials through direct reaction, which comprises the following steps: preparing the lithiation solution, an organic ether solution containing a metal lithium-aromatic compound, a lithium iodide solution and an n-butyl lithium solution; adding elemental sulfur into the lithiation solution for mixing; and sequentially carrying out a room-temperature reaction, separation and precipitation, drying and heat treatment on the mixed solution to obtain the lithium sulfide. The synthesis method of lithium sulfide has the advantages of cheap and easily available raw materials, simple process, no harmful gas generation, and mild and controllable synthesis process.
Owner:TIANMU LAKE INST OF ADVANCED ENERGY STORAGE TECH CO LTD +2
Process for producing acetic acid
ActiveUS9115071B2Inhibiting (or preventing) increaseEfficient productionOrganic compound preparationOrganic chemistry methodsIodideReaction step
Acetic acid is produced while inhibiting an increased concentration or production of hydrogen iodide in a carbonylation reactor or corrosion of the carbonylation reactor.A production process of acetic acid comprises a reaction step for continuously allowing methanol to react with carbon monoxide in the presence of a catalyst system comprising a metal catalyst (e.g., a rhodium catalyst), an ionic iodide (e.g., lithium iodide), and methyl iodide in a carbonylation reactor; and in the process, (i) the concentration of the metal catalyst is maintained at not less than 860 ppm on the basis of weight, the concentration of water is maintained at 0.8 to 15% by weight, the concentration of methyl iodide is maintained at not more than 13.9% by weight, and the concentration of methyl acetate is maintained at not less than 0.1% by weight, in a whole liquid phase in the reactor, and / or (ii) the concentration of the metal catalyst is maintained at not less than 660 ppm on the basis of weight, the concentration of water is maintained at 0.8 to 3.9% by weight, the concentration of the ionic iodide is maintained at not more than 13% by weight, the concentration of methyl iodide is maintained at not more than 13.9% by weight, and the concentration of methyl acetate is maintained at not less than 0.1% by weight, in a whole liquid phase in the reactor.
Owner:DAICEL CHEM IND LTD
Method for producing acetic acid by carbonylation of methanol as well as special catalyst and preparation method thereof
ActiveCN101182340AHigh selectivityImprove adaptabilityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 8/9/10/18 element organic compoundsPhosphateCarboxylic acid
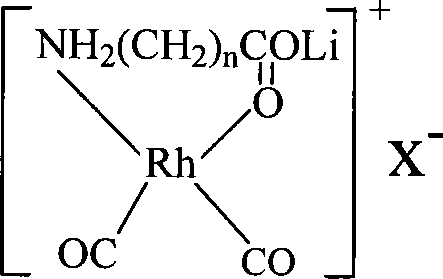
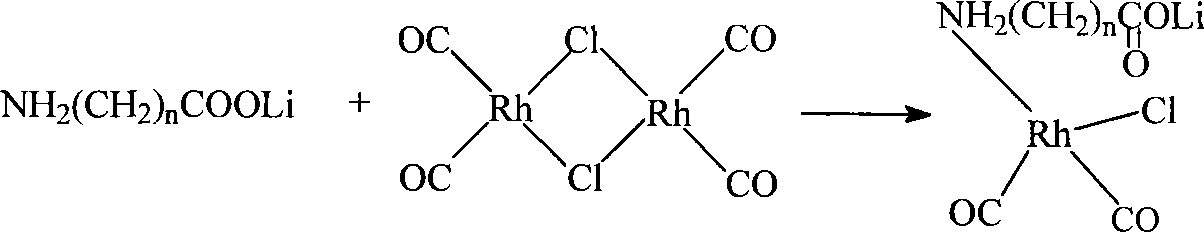
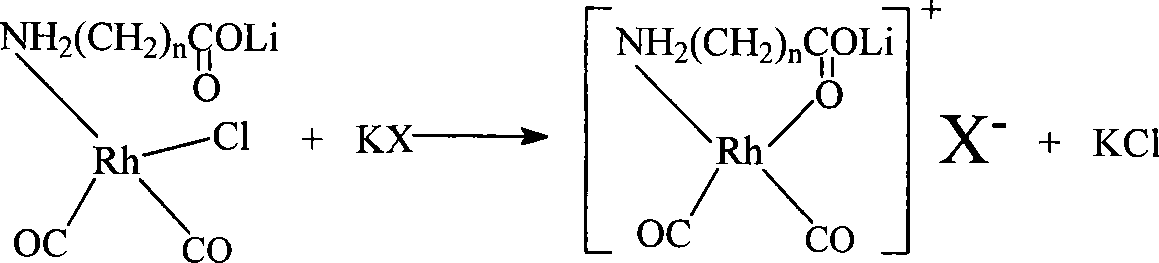
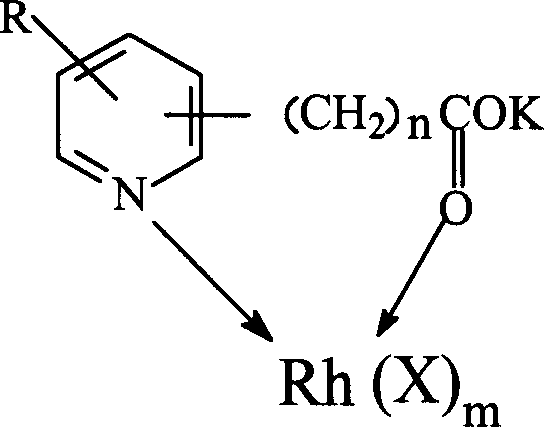
The invention discloses a method for producing acetic acid by carbonylation of methanol, a special catalyst and a preparation method thereof. The aminocarboxylate lithium rhodium complex of the present invention has a structure as shown in formula I, wherein, X=BPh4 or I; n=1, 2 or 3. In the present invention, lithium aminocarboxylate is used as a ligand and a rhodium complex to form a positive ion active center structure of a strong and weak coordination bond chelation type, and the positive ion part contains a strongly coordinated N→Rh bond and a weakly coordinated O→Rh bond, The stability and activity of the active center are guaranteed. Lithium metal and rhodium metal are co-located in the structure of the active center, which can form a synergistic catalytic effect; moreover, it can also be used in combination with other catalysts to improve its catalytic activity; The strong catalytic effect of the reaction provides a basis for the excellent performance of the catalyst; at the same time, the combination of lithium iodide, lithium acetate and phosphate is used as a promoter, so that the catalyst of the present invention can catalyze the carbonylation of methanol to produce acetic acid. Excellent overall performance.
Owner:INST OF CHEM CHINESE ACAD OF SCI +1
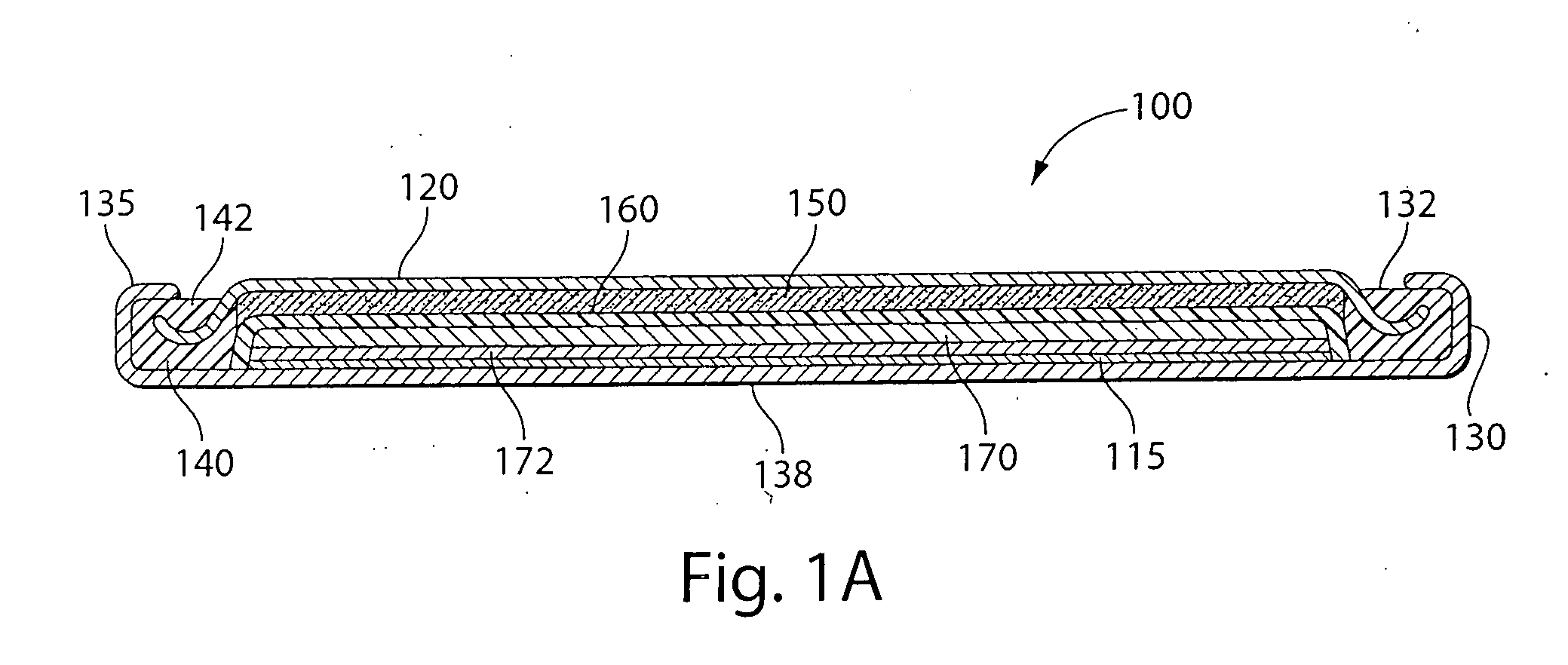
Lithium-ferrous disulfide battery and manufacturing method thereof
ActiveCN102751499AImprove discharge performanceImprove low temperature discharge performanceCell electrodesFinal product manufactureFiberCarbon fibers
The invention relates to a lithium-ferrous disulfide battery. The lithium-ferrous disulfide battery comprises a housing, an anode, a cathode, an isolating membrane for isolating the anode from the cathode, and an electrolytic solution, wherein the anode and the cathode are arranged in the housing, the anode contains 80-95% of ferrous disulfide (FeS2) by mass ratio, the cathode is a lithium tablet, solvents for the electrolytic solution comprise 1,3-dioxolane and glycol dimethyl ether, and electrolyte for the electrolytic solution comprises lithium iodide. A manufacturing method for the lithium-ferrous disulfide battery is as follows: adding a proper amount of carbon nanotube, vapor growth carbon fibers, conductive graphite and / or super conductive carbon into the anode, and adding a proper amount of additives such as N,N-dimethyl trifluoroacetamide and the like into the electrolytic solution. The lithium-ferrous disulfide battery manufactured by the manufacturing method is good in discharge performance in wider high and low temperature environments.
Owner:EVE ENERGY CO LTD
Ambient neutron dose equivalent meter
InactiveCN102043161ALightweight instrumentEasy to carry and useMeasurement with scintillation detectorsScintillation crystalsNeutron radiation
The invention aims to disclose an ambient neutron dose equivalent meter. The ambient neutron dose equivalent meter is characterized by comprising a neutron probe, a neutron energy response adjustor and a control system, wherein a neutron detection element is arranged in the neutron probe, the neutron detection element is europium-doped lithium iodide scintillation crystal, the signal output end of the neutron probe is connected with the signal input end of the control system, and the neutron probe is placed at the position of the geometric center of the neutron energy response adjustor. Because the photomultiplier with high sensitivity is used as a photoelectric converter, the meter has light weight, portability, sensitive neutron response and good angle response, can accurately measure the ambient neutron dose equivalent level, has sound and light alarm function, is used for ambient neutron radiation monitoring of large nuclear facilities such as reactors, high-energy particle accelerators and the like, ensures neutron radiation safety of the ambient and the public, and fulfills the purpose of the invention.
Owner:上海新漫传感科技有限公司
Method for preparation of lithium iodide organic electrolytic solution and lithium cell
InactiveCN101227002AEasy to produceIncrease productionOrganic electrolyte cellsSecondary cellsOrganic solventVacuum drying
The invention relates to a method for preparing a lithium iodide organic solvent electrolyte and a lithium battery, which belongs to the field of batteries. The method for preparing the lithium iodide organic solvent electrolyte is that lithium iodide salt with crystal water is put in a vacuum drying oven and is dried in the temperature of 100-180 DEG C, most of water is removed, the dried lithium iodide salt is dissolved in an organic solvent, a deicer with lithium is added and reacted in the temperature of 20-80 DEG C, the time is 1-200 hours, and the lithium iodide organic solvent electrolyte is obtained through filtering. The organic lithium iodide organic solvent electrolyte of the invention has simple process and high yield, production problems of the lithium iodide organic solvent electrolyte are solved, and the produce cost of the battery is reduced.
Owner:GUANGZHOU GREAT POWER ENERGY & TECH CO LTD
Preparation method of lithium battery electrolyte
InactiveCN103943882APrevent slow polymerizationLow viscosityFinal product manufacturePrimary cellsAlkalinityOrganic solvent
The invention discloses a preparation method of a lithium battery electrolyte, belonging to the technical field of lithium batteries. The preparation method is characterized by comprising the steps of adding an organic amine or metal oxide additive which accounts for 0.1-5 percent by weight of an organic solvent in the organic solvent forming an electrolyte; and reducing the temperature of the organic solvent to be 0 DEG C, then adding lithium salt in the organic solvent of the electrolyte under the stirring state, and controlling the temperature of the organic solvent to be 0-10 DEG C in an adding process. According to the preparation method, a defined amount of organic amine or metal oxide is added in a nonaqueous electrolyte, and has alkalinity and a certain inhibition effect; the temperature when lithium salt is added is strictly controlled, the electrolyte is effectively prevented from a polymerization reaction during production and transportation and after injection of a battery, and effects of stabilizing the viscosity of the lithium iodide nonaqueous electrolyte and reducing the internal resistance of a lithium-ferrous disulfide battery are realized.
Owner:BEIJING INST OF CHEM REAGENTS
Lithium iodide organic electrolyte for lithium iron battery and preparation method thereof
ActiveCN103378370AImprove low temperature dischargeImproved High Power Discharge PerformanceSecondary cellsMolecular sieveOrganic solvent
The invention discloses lithium iodide organic electrolyte for a lithium iron battery and a preparation method thereof. The organic electrolyte consists of an electrolyte lithium salt, an organic solvent and an additive, wherein the lithium salt is lithium iodide or the mixture of lithium iodide and other lithium salts, the organic solvent is the combination of organic solvents including ethers, sulfones and carbonates, and the additive is the mixture of an additive A and an additive B. The preparation method comprises the following steps of: (1) dehydrating the organic solvent and the additives in a drying environment, and stirring and mixing the organic solvent and the additives into a homogenous liquid; (2) dissolving the lithium salt into the liquid to obtain a semi-finished product; and (3) utilizing a lithium molecular sieve to adsorb and dehydrate the semi-finished product, and filtering the semi-finished product after the adsorption to obtain a finished product. The lithium iron battery which is made of the lithium iodide organic electrolyte not only can reduce the cost, but also can greatly improve the low-temperature discharging performance, high-temperature discharging performance and large-current discharging performance, and can meet the environment-friendly requirement.
Owner:ZHANGJIAGANG GUOTAI HUARONG NEW CHEM MATERIALS CO LTD
Methanol Carbonylation Process with Rhodium Catalyst, an Iodide Salt and a Metallic Co-Catalyst Selected from Transition Metals, Indium, Strontium, Barium, Zinc, Tin and Heteropoly Acids
ActiveUS20130102809A1Catalyst stabilityIncrease chanceOrganic compound preparationCarboxylic preparation from carbon monoxide reactionIndiumHeteropoly acid
A carbonylation process for making acetic acid uses a metallic co-catalyst composition effective as a rhodium stabilizer and rate promoter at molar ratios of metal / rhodium of from about 0.5 to 30. A preferred process includes: (a) reacting methanol with a carbon monoxide feedstock in a rhodium-based catalytic reaction mixture having: (i) a rhodium catalyst metal, (ii) methyl iodide maintained from about 1 to 20 weight percent, (iii) a lithium iodide co-catalyst, (iv) a metallic co-catalyst composition, (v) water maintained from 0.1 weight percent to less than 8 weight percent, (vi) methyl acetate maintained from about 0.5 to about 30 weight percent, and (vii) acetic acid; (b) flashing crude acetic acid from the reaction mixture; and (c) purifying the crude acetic acid. This process achieves stability and a STY greater than 10 mol / L / hr, with substantially less than a theoretically equivalent inorganic iodide content corresponding to the metallic co-catalyst and lithium iodide.
Owner:CELANESE INT CORP
Method for preparing amide compound
InactiveCN102746077AMild reaction conditionsMeet the requirementsOrganic compound preparationSulfonic acid esters preparationSodium iodidePotassium iodine
The invention discloses a method for preparing an amide compound, which comprises the steps of configuration of reaction system. Methyl ketone, amine, catalyst, an oxidizing agent and solvent are use, wherein the general formula of the methyl ketone is RCOCH3, and R is selected from one kind of C6-C14 aryl group, C2-C8 alkenyl group, and five-or six-membered heterocyclic group; the amine is primary amine; the catalyst is selected from one kind of potassium iodide, iodine, tetramethyl ammonium iodide, tetrabutyl ammonium iodide, tetrahexyl ammonium iodide, bismuth iodide, lithium iodide, phenyl trimethyl ammonium iodide, benzyl trimethyl ammonium iodide or sodium iodide; the oxidizing agent is tert-butyl hydroperoxide; the solvent is selected from one kind of water, dichloromethane, ethyl acetate, toluene, 1,2-dichloroethane, 1,1,1-trichloroethane, acetonitrile, and isopropanol. The methyl ketone, the catalyst and the amine are added into the reaction system under the temperature of 0 DEG C and stirred for 5 minutes, and then the oxidizing agent is added to react for 2-48 hours to obtain the amide compound. The method provided by the invention is green, moderate, high in selectivity and wide in application range.
Owner:云梦星联物流有限公司
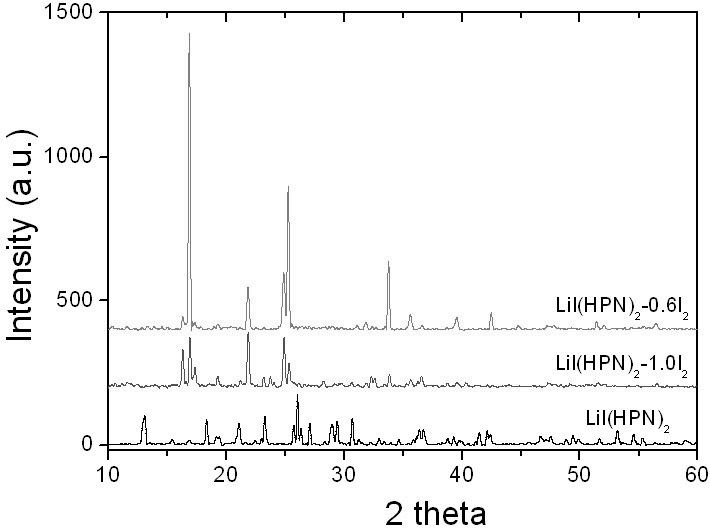
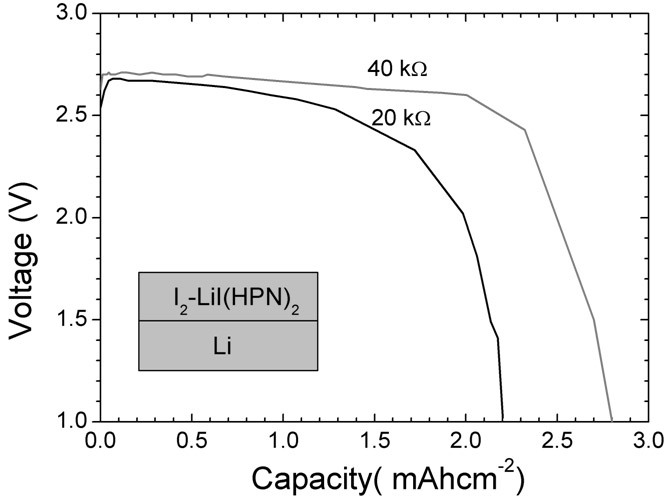
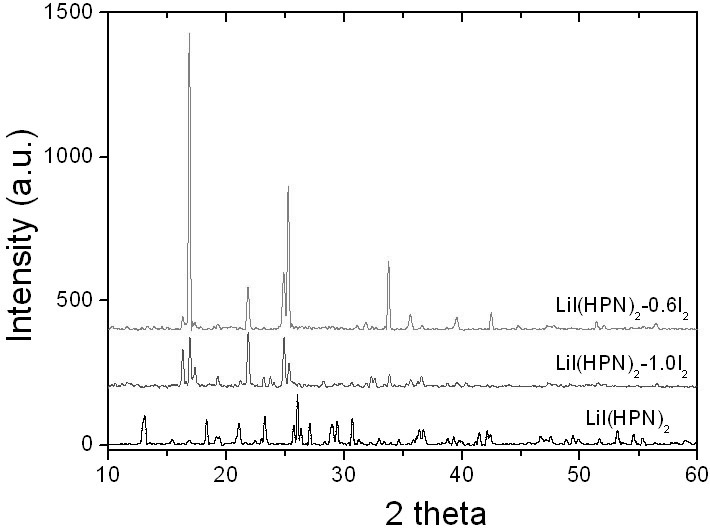
Anode material capable of being used for lithium-iodine cell and preparation method thereof
InactiveCN101950806ALow costEasy to prepareLithium organic compoundsCell electrodesMetallic lithiumAll solid state
The invention belongs to the technical field of electrochemistry, and in particular discloses an anode material capable of being used for a lithium-iodine cell and a preparation method thereof. The anode material is prepared by heating, melting and reacting iodine monomer and LiI(3-hydroxypropionitrile)2 (LiI(HPN)2) and recorded as LiI(HPN)2-xI2, wherein x is greater than or equal to 0.1 and less than or equal to 10. A battery is formed by directly contacting the material LiI(HPN)2-xI2 serving as an anode with metal lithium, and the material LiI(HPN)2-xI2 has high discharge performance, particularly the material with x greater than or equal to 0.6. The theoretical specific capacity of the LiI(HPN)2-I2 is 101mAh / g; the theoretical specific capacity of LiI(HPN)2-3I2 is 155mAh / g; and the capacity increases along with increase in proportion of the monomer iodine. The anode material has the advantages of high specific capacity, simple preparation method and low cost and is applicable to full solid-state lithium-iodine batteries.
Owner:FUDAN UNIV
Catalytic system used for homogeneous hydroxylation reaction and its manufacturing method and application
InactiveCN1517150AIncrease binding energyBinding energy stabilityOrganic-compounds/hydrides/coordination-complexes catalystsCarboxylic preparation from carbon monoxide reactionPotassiumPotassium hydroxide
A homologeous catalyst system for the hydroxylation reaction of methanol to obtain acetic acid or the carbonylating reaction of methyl acetate to obtain ethylanhydride is composed of the primary catalyst (rhodium acetate (or nitrate) as coordination center and potassium), cocatalyst (iodomethane), additive (lithium iodide, potassium iodide, or lithium acetate), and polar solvent (acetic acid, ethylanhydride, methyl acetate, or water). Its preparing process includes dissolving picolinic acid and hydrated potassium in methanol or water to obtain organic potassium ligand, dissolving in water or methanol solution, and dripping rhodium solution.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Gel poly ion liquid electrolyte for solar battery and method for manufacturing the same
InactiveCN101245186ALow costRich preparation methodLight-sensitive devicesFinal product manufactureSolid state electrolyteAlkane
The invention discloses a solar cell-used jellylike polyion liquid electrolyte and is characterized in that: the weight percentage of components are: poly-histidine-ester ion liquid: 98 to 96 percent; iodine: 1 to 2 percent and lithium iodide: 1 to 2 percent and the total amount is 100 percent; the monomer of the poly-histidine-ester ion liquid is (R1NHCOOHCH2C3H2NR2NR3)<+>X<->, wherein, R1 is benzoyl or Boc base; R2 and R3 are alkane base, and X is I<->, SeCN<-> or SCN<->. The electrolyte has the advantages of innocuity, environmental protection and low price; furthermore, the jellylike electrolyte of the histidine derivative series has excellent bonding property which can lead platinum electrodes to be adhered firmly, thus improving the filling factors of cells and forming solid electrolyte dye sensitization nanometer crystal solar cells with good stability.
Owner:WUHAN UNIV
All-solid-state organic alloy electrolyte for dye-sensitized solar battery
ActiveCN101719427AWell mixedImprove photoelectric conversion efficiencyLight-sensitive devicesSolid-state devicesPlastic crystalQuaternary ammonium cation
The invention discloses an all-solid-state organic alloy electrolyte for a dye-sensitized solar battery and a preparation method thereof. The electrolyte comprises an organic alloy material prepared from more than two or three plastic crystals selected from succinonitrite, neopentyl glycol, trimethylolethane, pentaerythritol, camphor, imidazole and the derivative quaternary ammonium salt thereof and pyridine and the derivative quaternary ammonium salt thereof, iodine accounting for 1-5 percent of the amount of substance of the organic alloy, lithium iodide or potassium iodide or sodium iodide accounting for 1- 10 percent of the amount of substance of the organic alloy and one or more of methyl ethyl imidazole iodine, tert-butyl pyridine, LiTFSI and LiBF4 conducting salt, which account for 1- 10 percent of the amount of substance of the organic alloy. The prepared electrolyte has a high melting point (more than 80 DEG C) and equivalent electrical conductivity with a liquid electrolyte, and the dye-sensitized solar battery (DSSCs) prepared from the electrolyte has high photoelectric transformation efficiency and stability.
Owner:常熟紫金知识产权服务有限公司
Thermal battery electrolyte with low melting point and high electric conductivity and preparation method thereof
InactiveCN102437345ALow melting pointWide working lower limit temperatureDeferred-action cellsInorganic saltsLithium bromide
The invention discloses a thermal battery electrolyte with low melting point and high electric conductivity and a preparation method thereof. The thermal battery electrolyte is composed of the following component in percentage by weight: 25%-35% of analytically pure lithium bromide (LiBr), 10%-22% of analytically purepatassium bromide (KBr), 45%-53% of analytically pure cesium bromide (CsBr) and 8%-13% of analytically pure lithium iodide (LiI). The thermal battery electrolyte with low melting point and high electric conductivity is prepared by sintering a eutectic salt composed by the four inorganic salts (LiBr, KBr, CsBr and LiI) according to a certain proportion under protection of an inert gas; the eutectic melting point is lower than 200 DEG C; and the electric conductivity is greater than 1.8S / cm at 500 DEG C. The thermal battery electrolyte with low melting point and high electric conductivity can obviously prolong working time of thermal cells, as well as reduce the surface temperature of thermal cells at work.
Owner:GUIZHOU MEILING POWER SUPPLY CO LTD
Ion-doped perovskite solar cell and manufacturing method thereof
ActiveCN106299139AHigh crystallinityImprove efficiencySolid-state devicesSemiconductor/solid-state device manufacturingSilver electrodePerovskite solar cell
The invention relates to an ion-doped perovskite solar cell and a preparation method thereof. The solar cell comprises a conductive glass layer, a compact titanium dioxide membrane, a methylamine lead iodine polycrystalline membrane, a hole-transporting material layer and an evaporation silver electrode layer, wherein the conductive glass layer, the compact titanium dioxide membrane, the methylamine lead iodine polycrystalline membrane, the hole-transporting material layer and the evaporation silver electrode layer are successively distributed, and the ion-doped perovskite solar cell is characterized in that the methylamine lead iodine polycrystalline membrane is doped with lithium iodide. The anhydrous lithium iodide is used for doping a perovskite precursor solution stock solution, the anhydrous lithium iodide is combined with the perovskite precursor solution stock solution, and the anhydrous lithium iodide can play a double efficacy. The manufacturing method is simple and easy to popularize.
Owner:NINGBO UNIV
Method for Producing Electrolyte Solution for Lithium Ion Battery and Battery Using Same
ActiveUS20090081559A1Easy to produceElectrolytic capacitorsOrganic electrolyte cellsHydrogen fluorideLithium chloride
There is provided a method for producing an electrolyte solution for lithium ion battery, which is characterized in that lithium fluoride, lithium chloride, lithium bromide, lithium iodide or a mixture of any of these is reacted with phosphorus pentachloride and hydrogen fluoride in a nonaqueous organic solvent, when an electrolyte solution for lithium ion battery, which contains lithium hexafluorophosphate as an electrolyte, is produced.
Owner:CENT GLASS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com