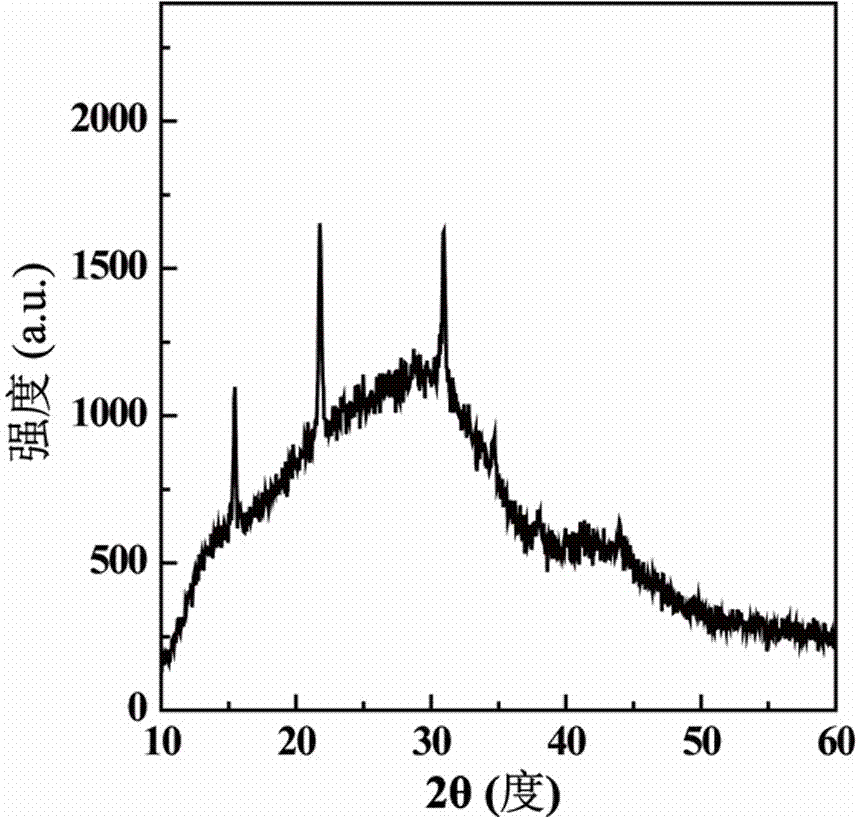
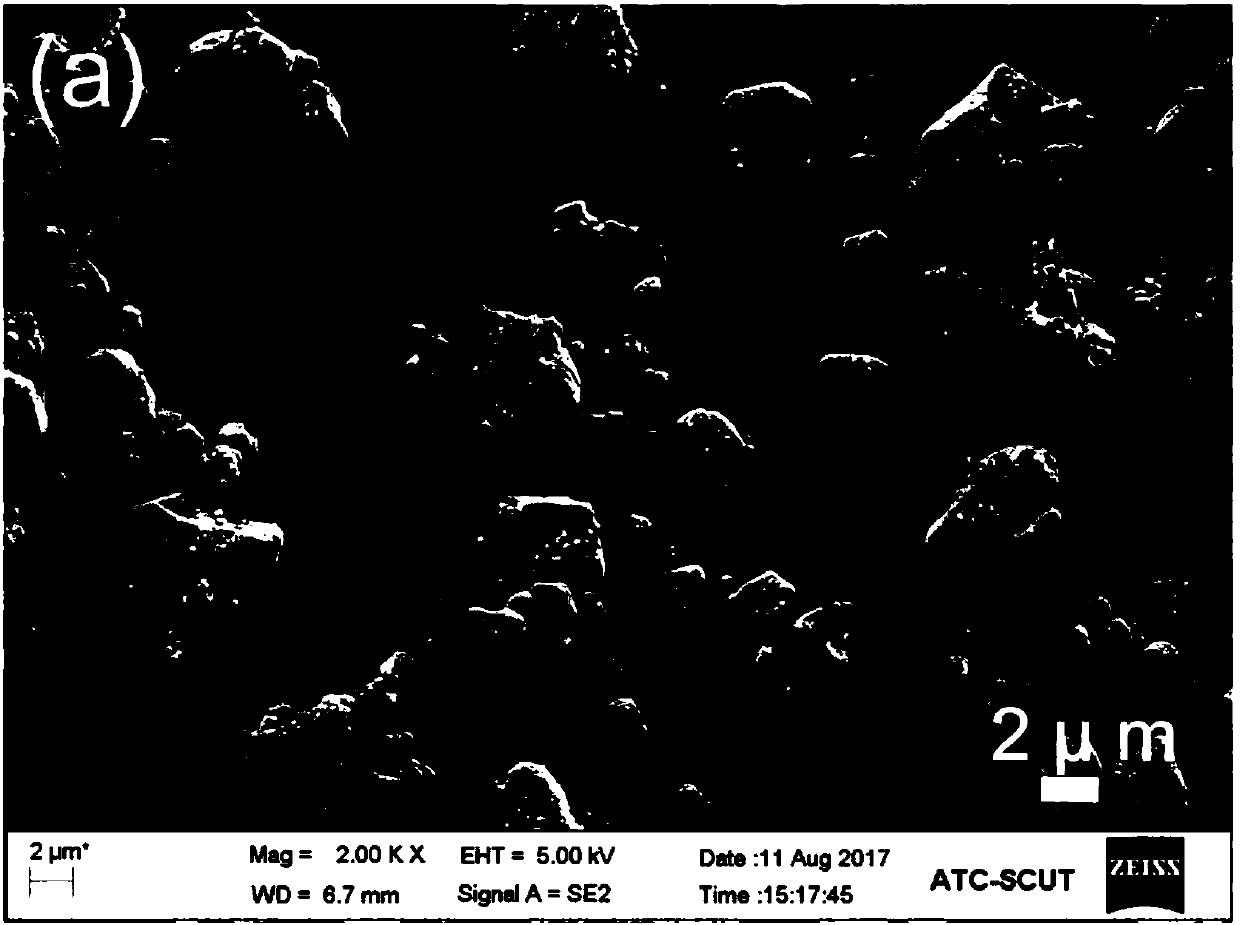
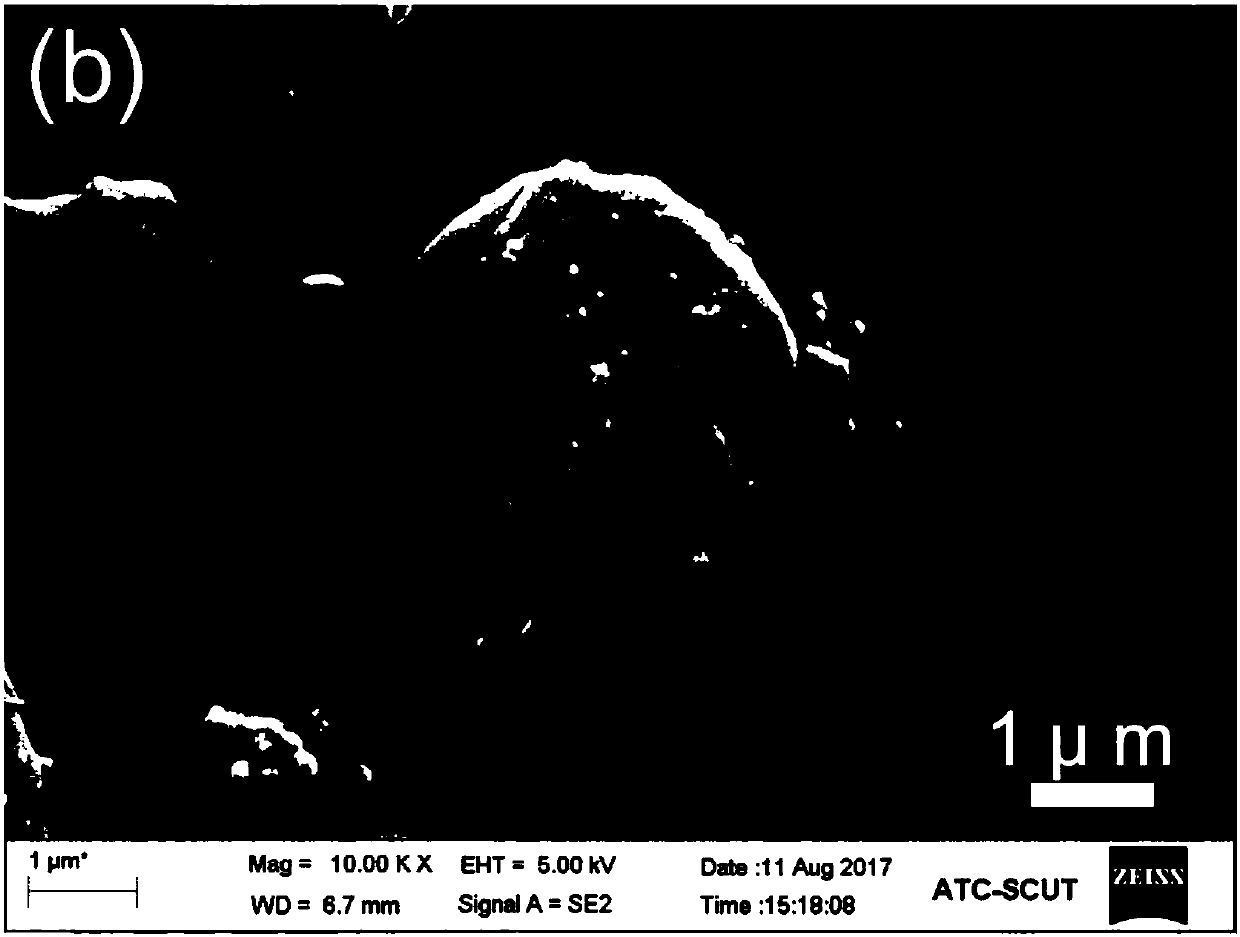
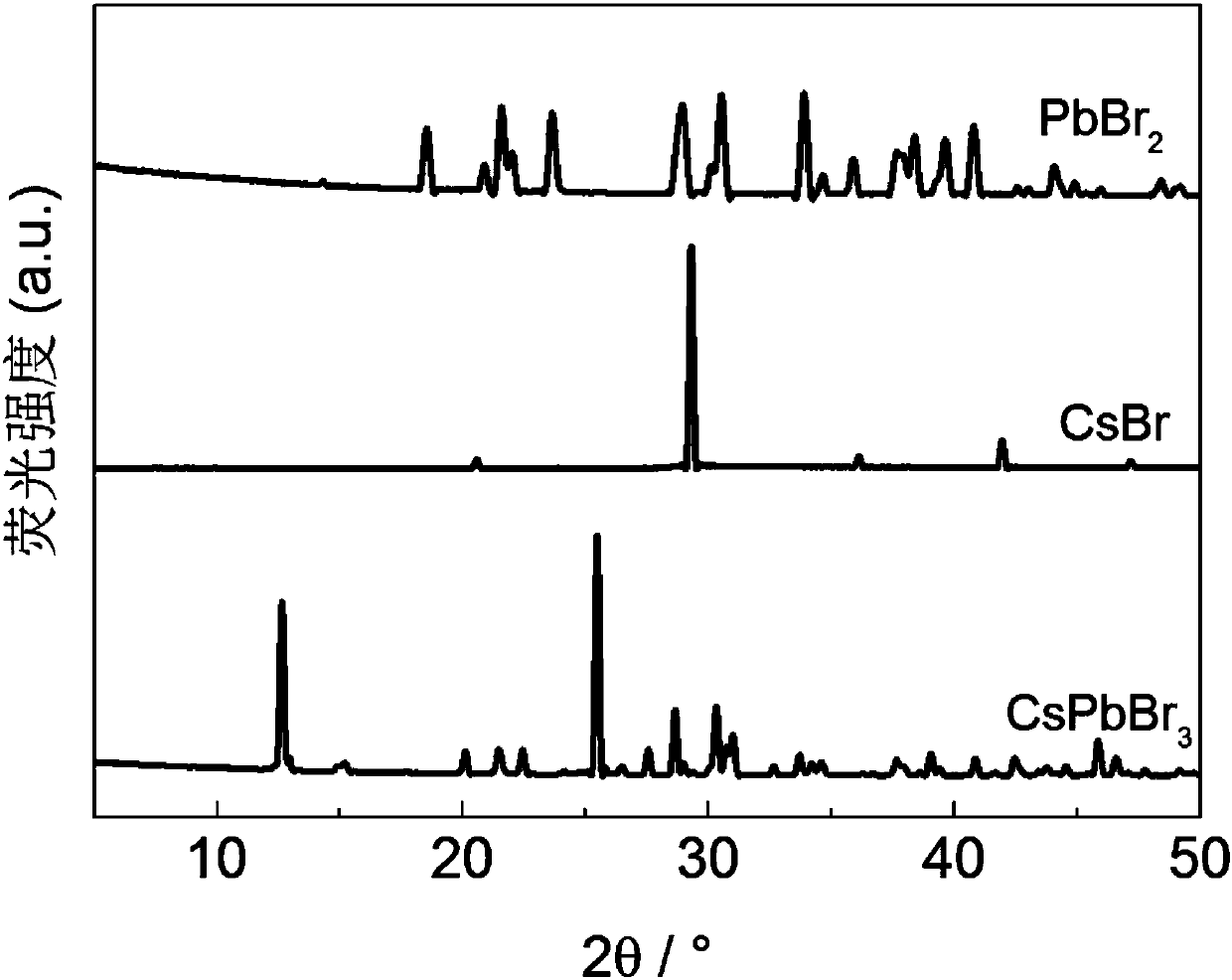
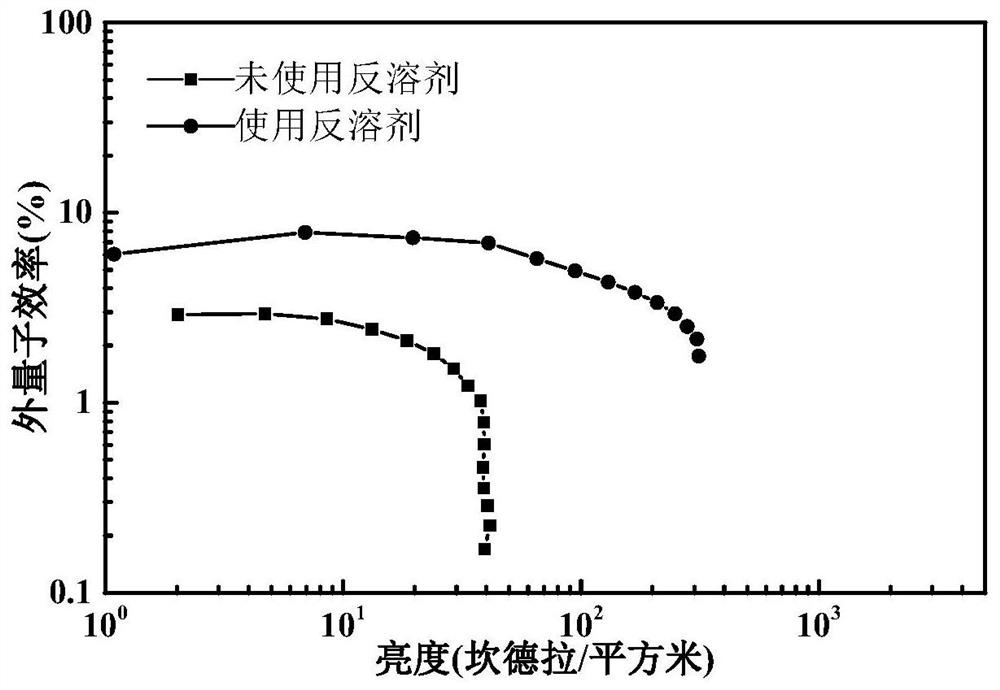
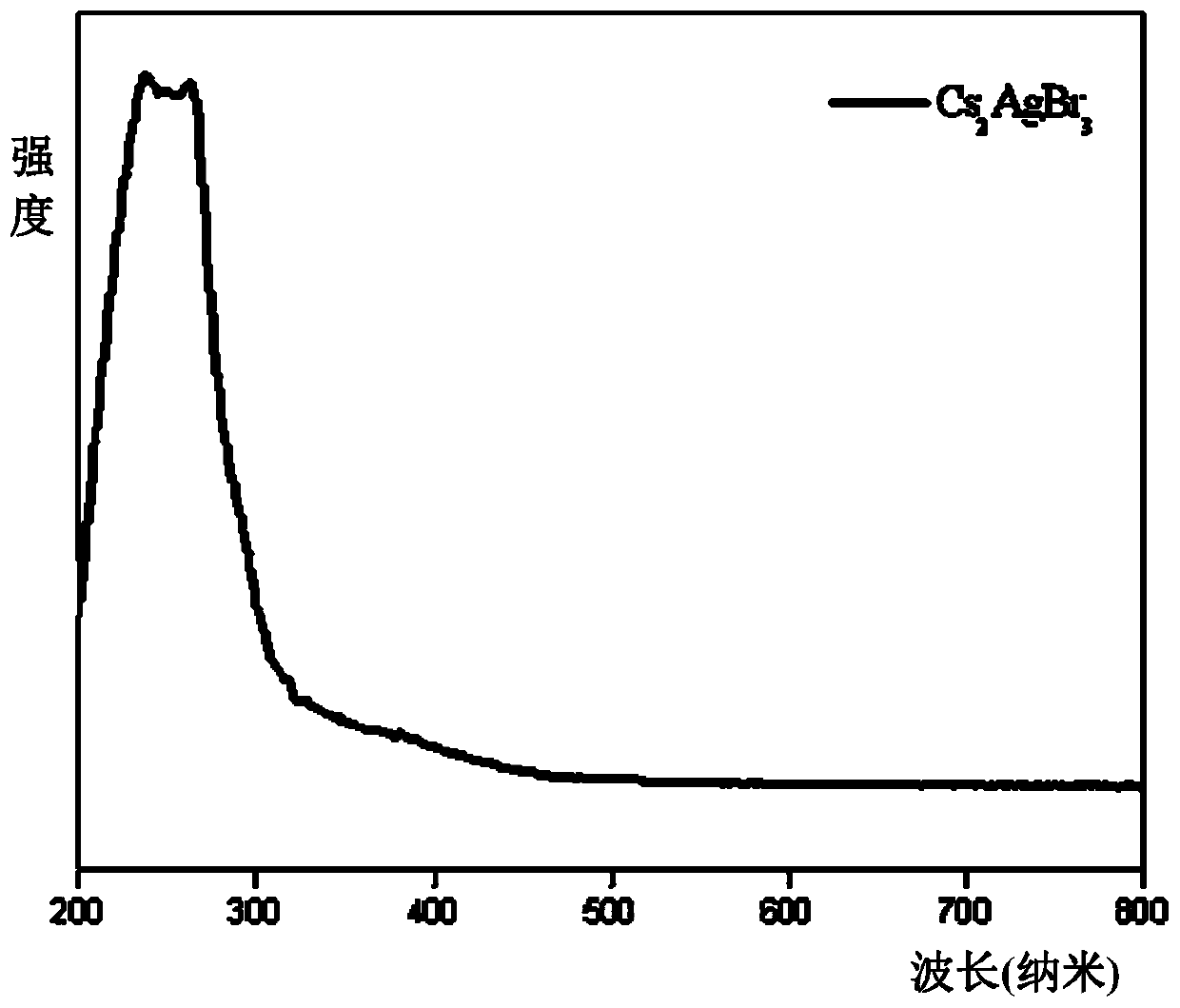
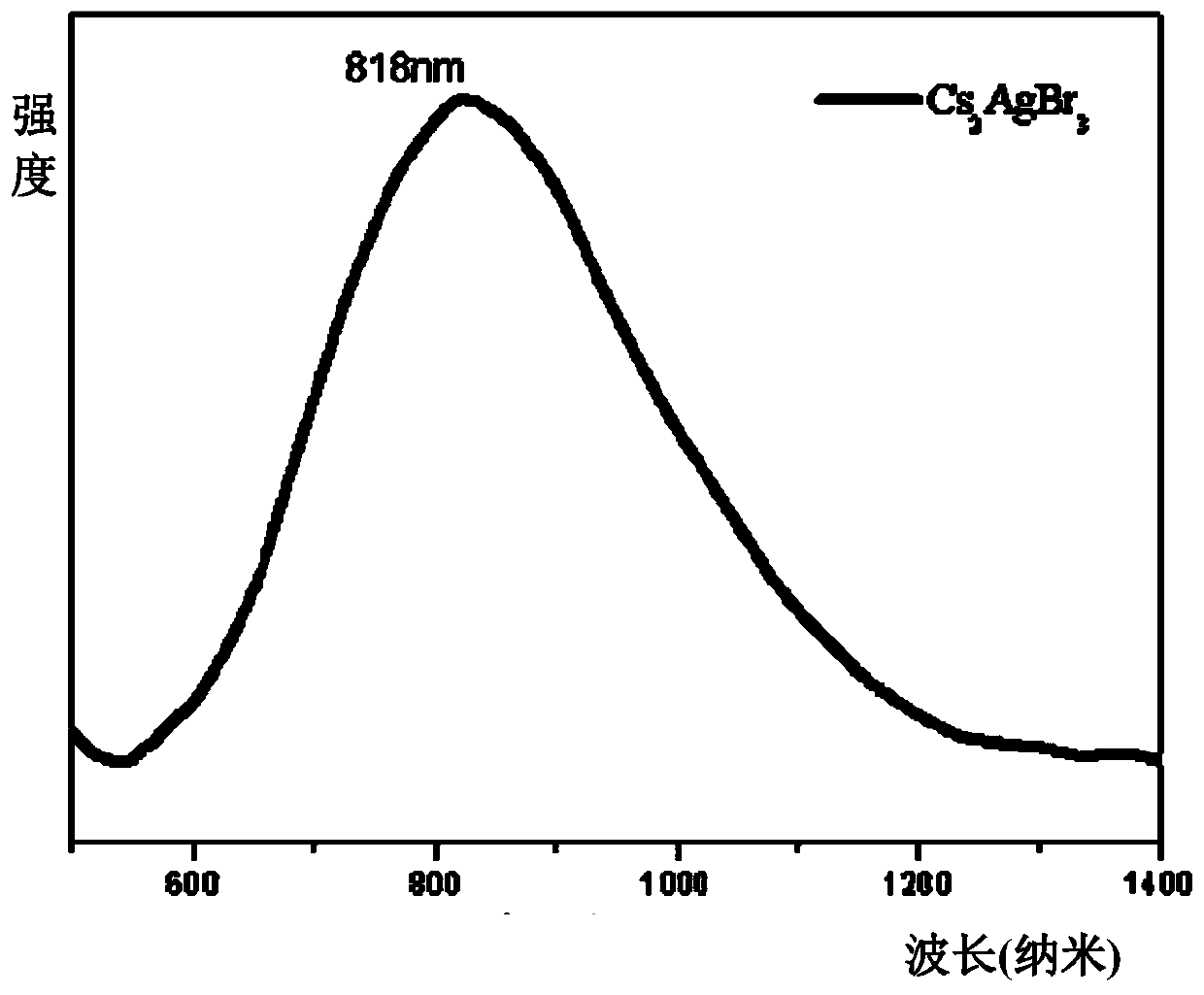
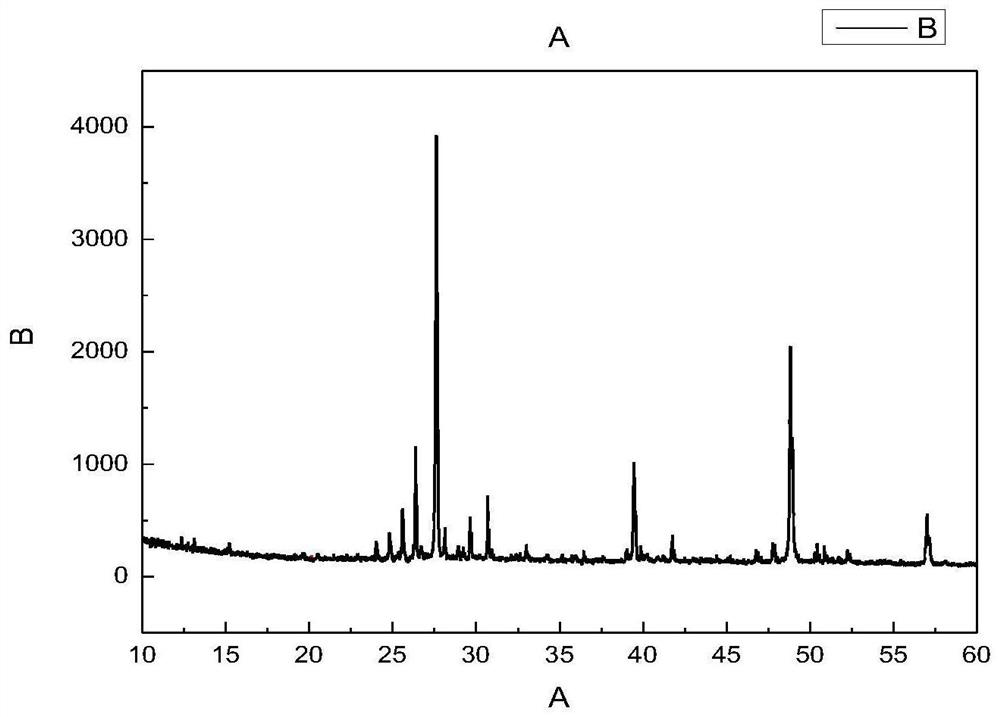
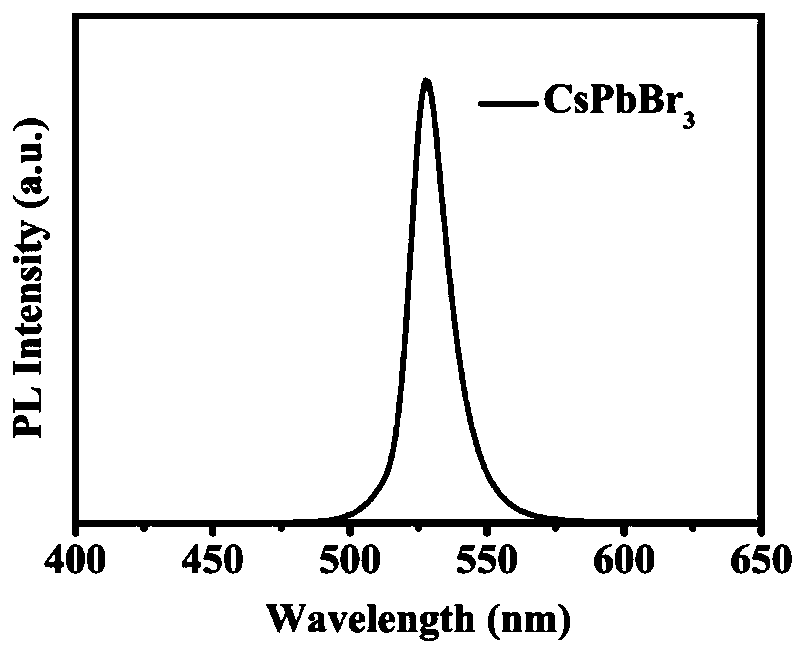
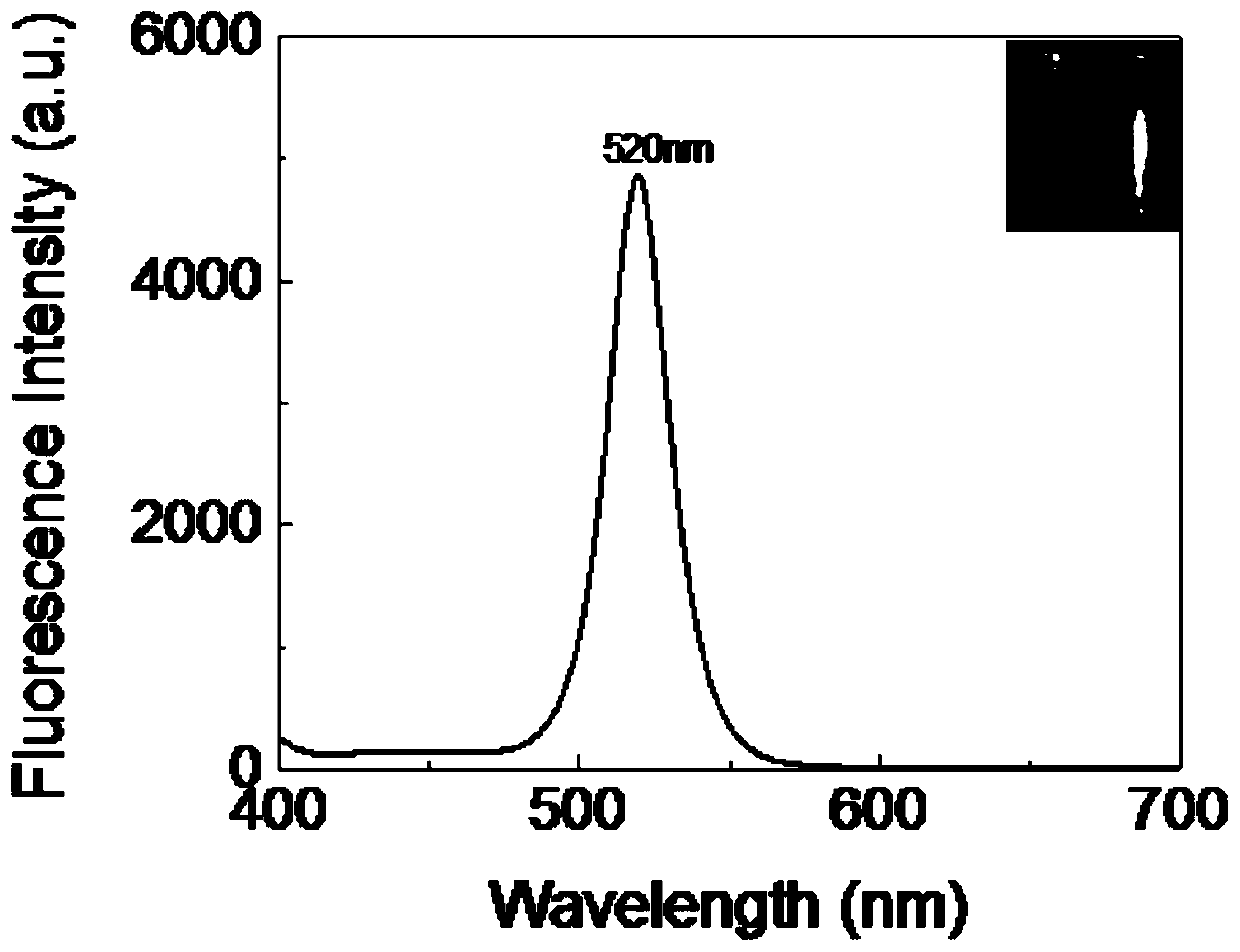
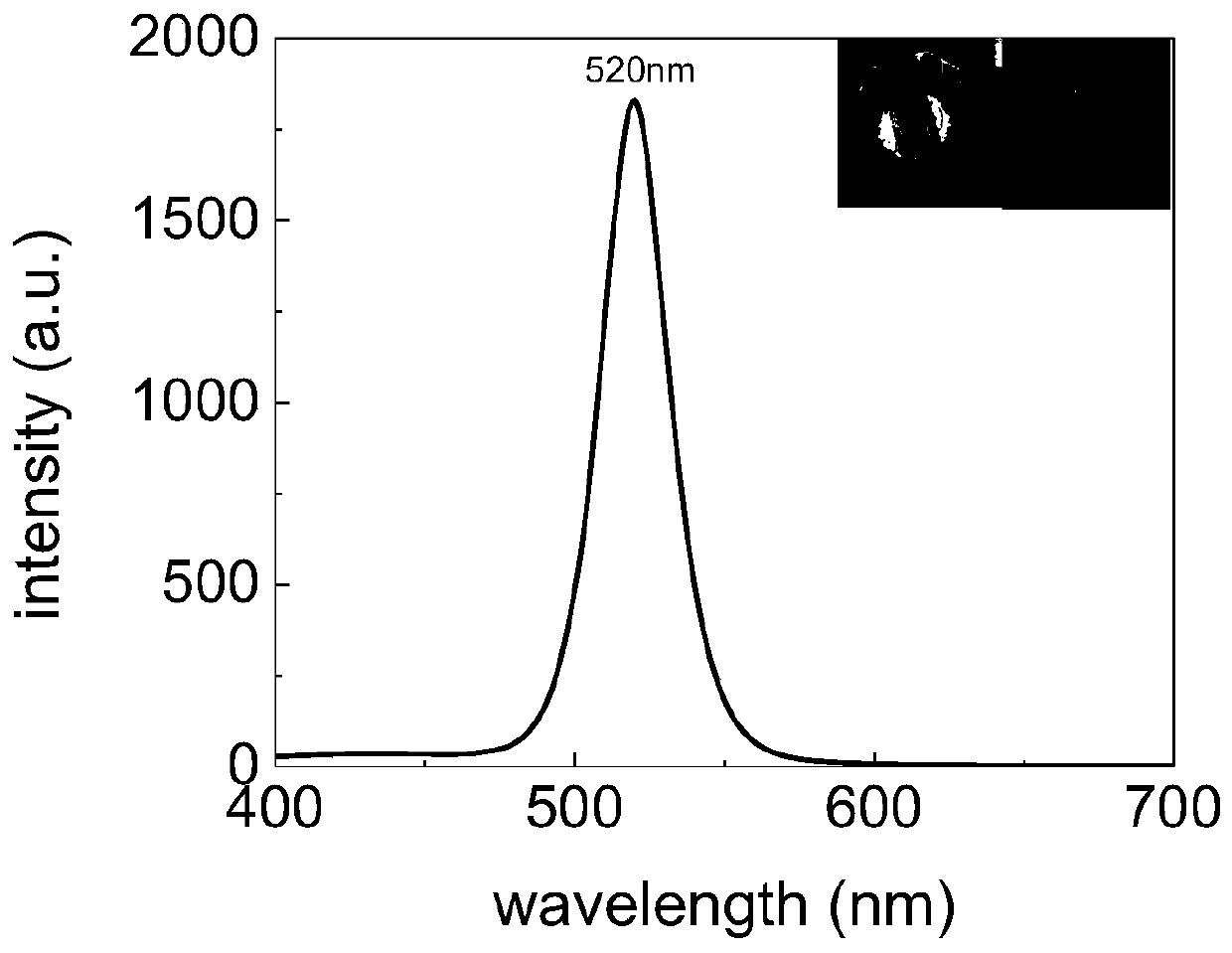
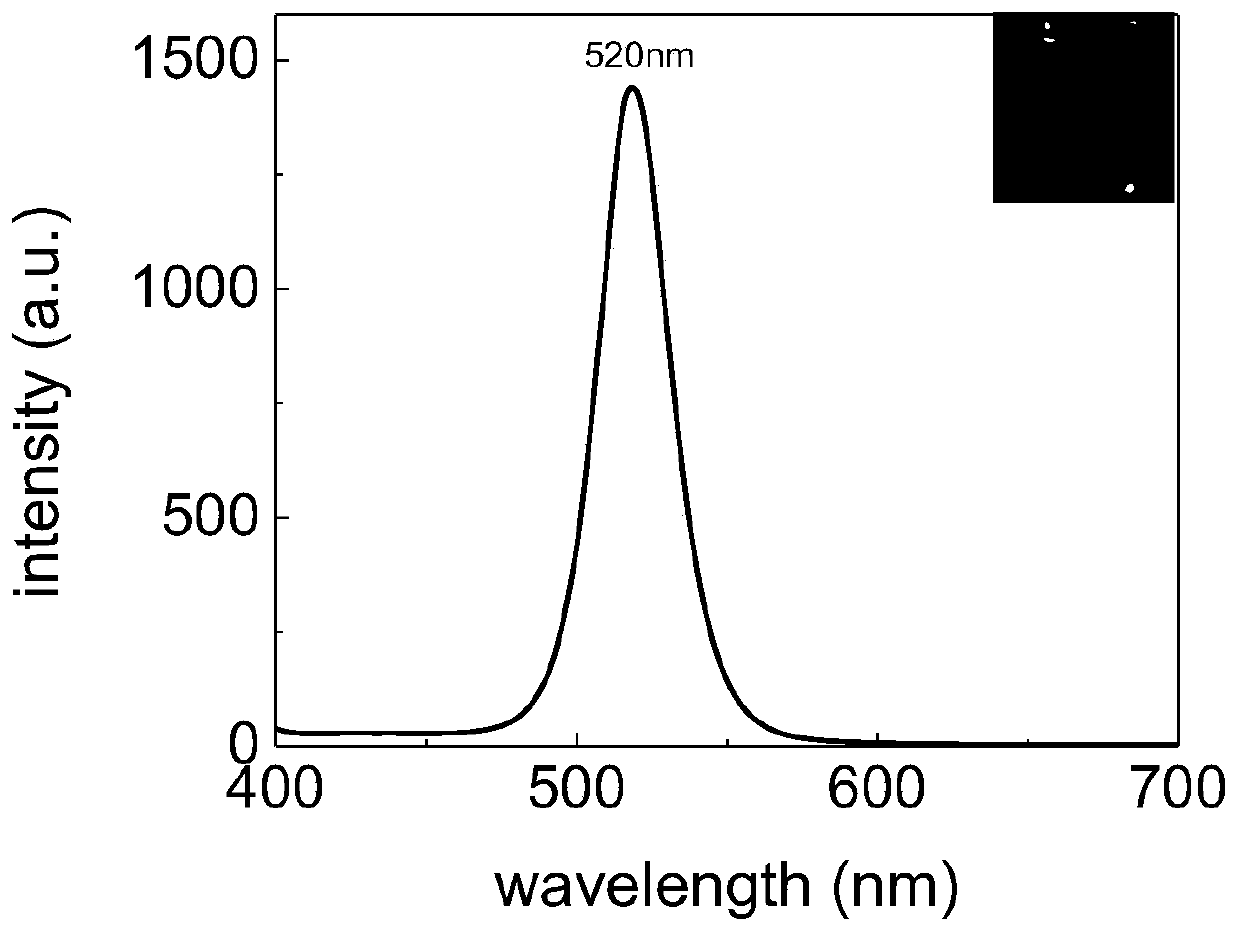
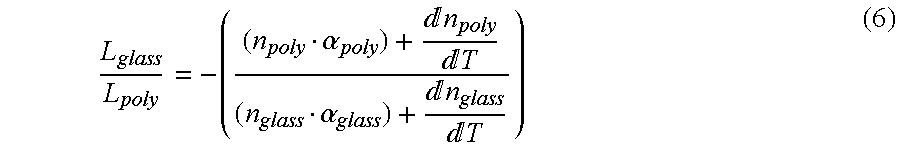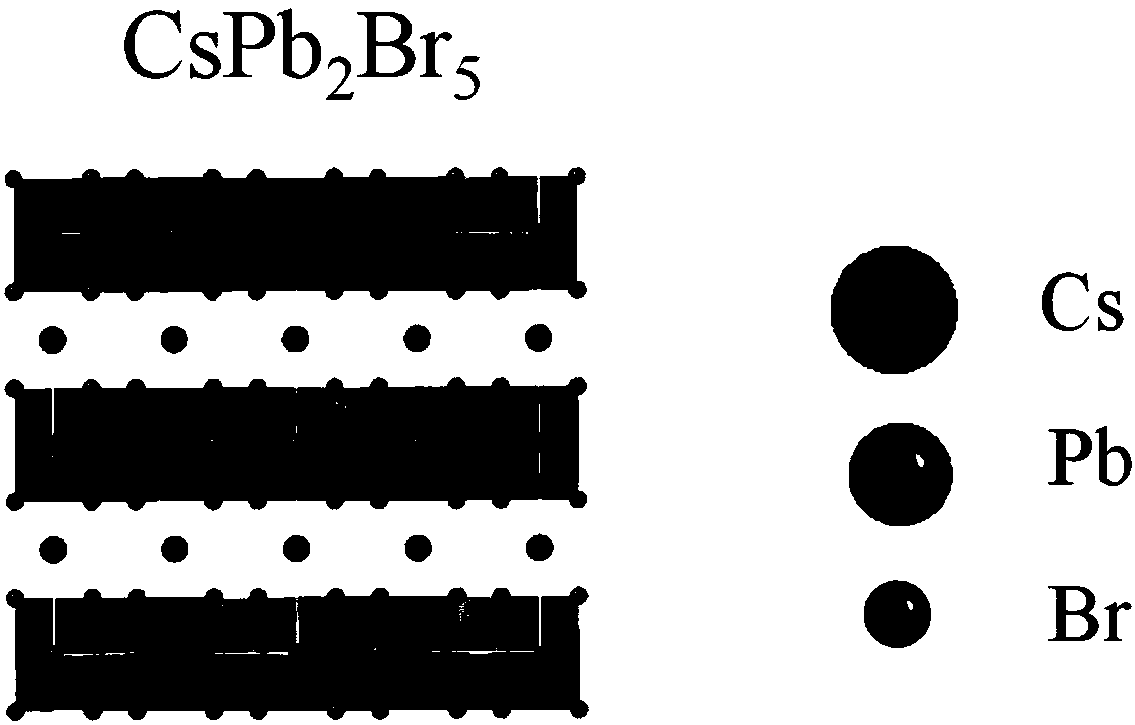Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
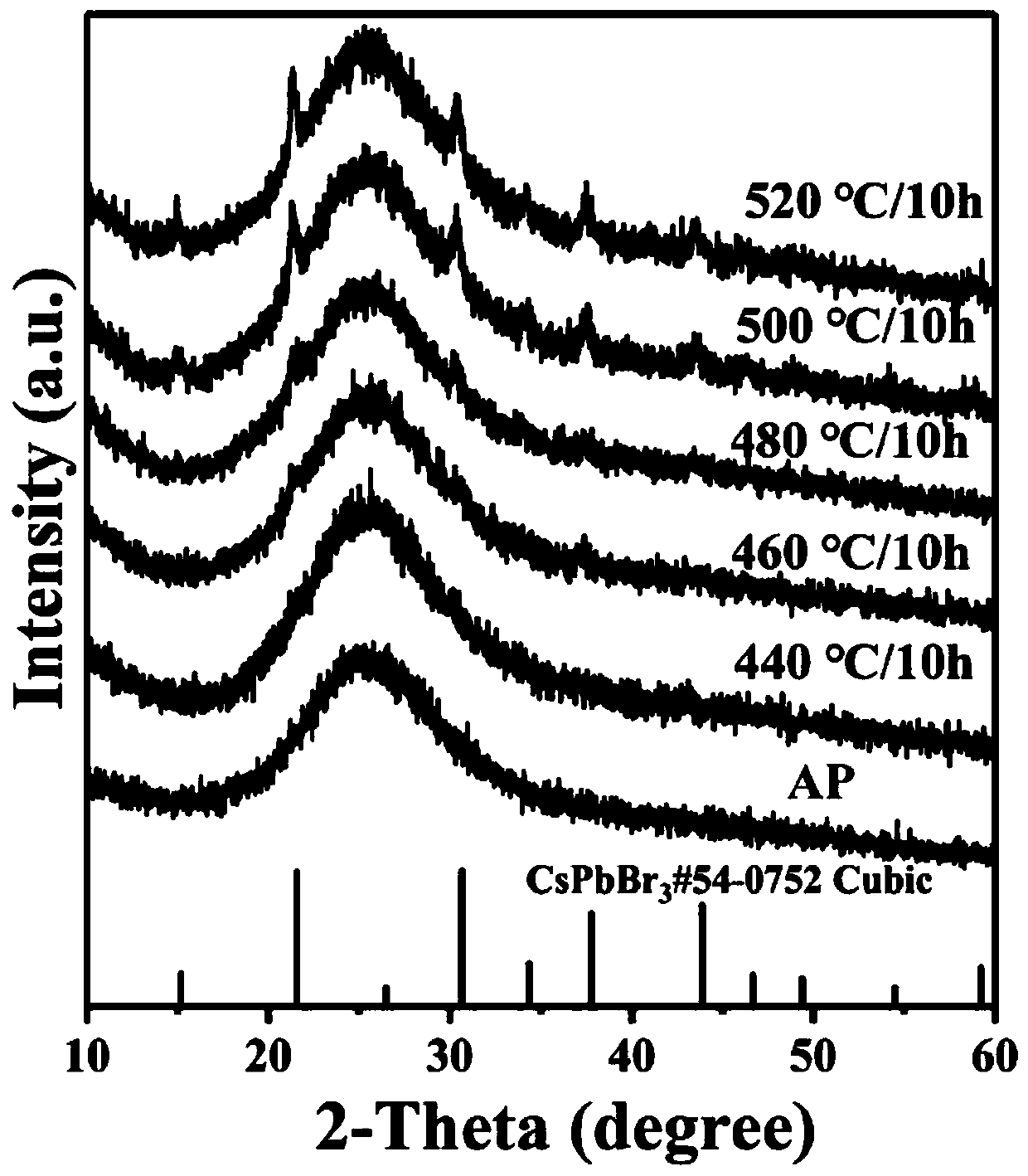
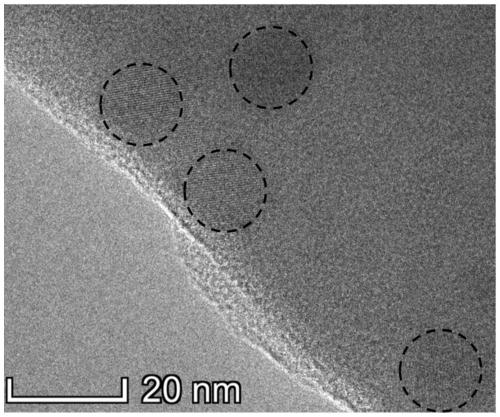
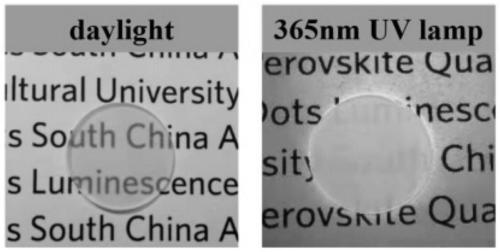
106 results about "Cesium bromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Caesium bromide or cesium bromide is an ionic compound of caesium and bromine with the chemical formula CsBr. It is a white or transparent solid with a melting point a 636 °C that readily dissolves in water. Its bulk crystals have the cubic CsCl structure, ...
Lamp unit and infrared night-vision system
InactiveUS20040021420A1Simple designReduce power consumptionClosed circuit television systemsSolid cathode detailsNight visionThallium
A lamp unit for emitting near-infrared light by electric discharge, has: a hollow discharge tube; a cesium halide enclosed in the hollow of the discharge tube; a near-infrared penetration filter covering around the discharge tube. The cesium halide may include at least one of cesium iodide and cesium bromide. Also, an indium halide and thallium halide may further be enclosed in the hollow discharge tube.
Owner:KOITO MFG CO LTD
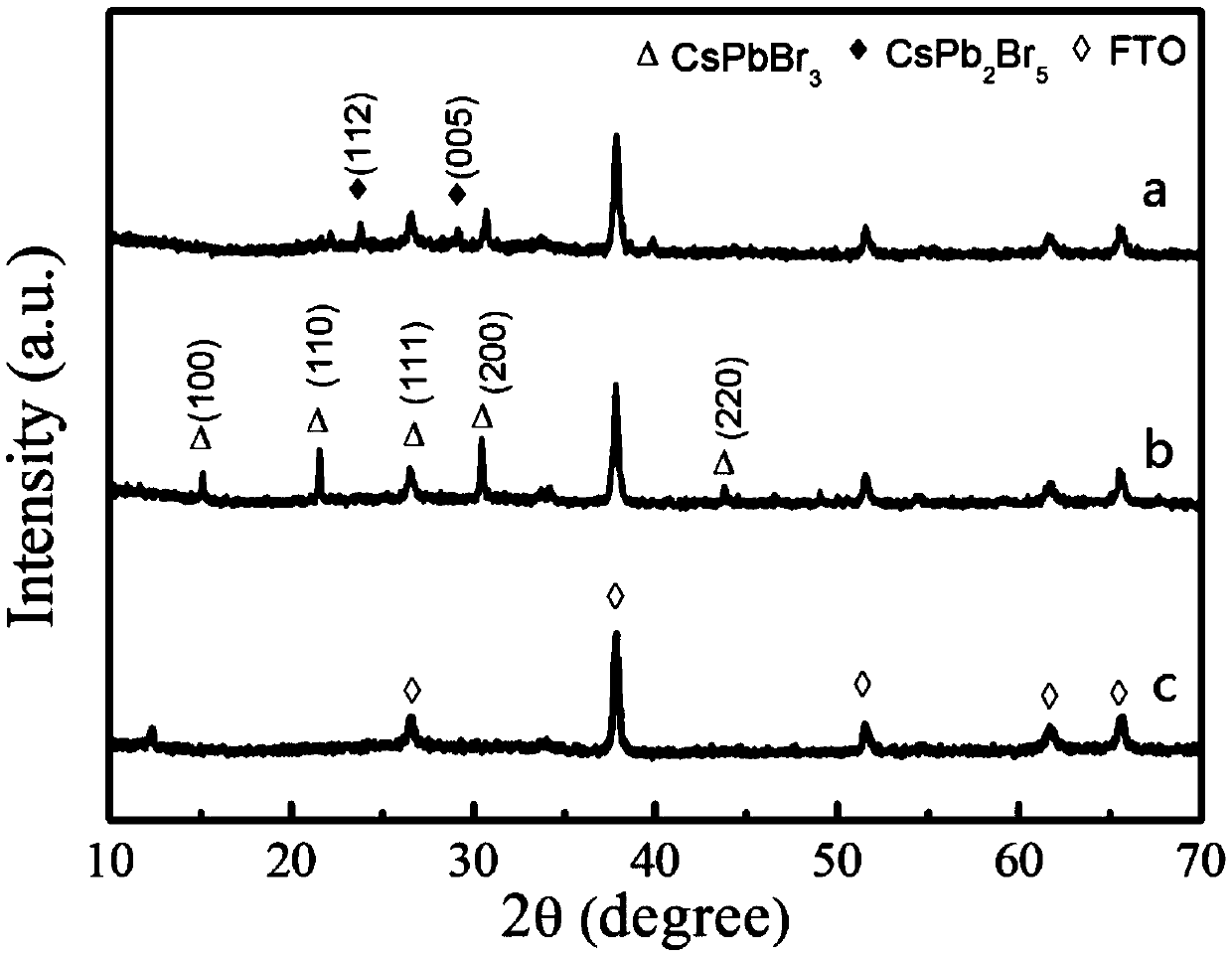
Temperature inversion solution growth method of all-inorganic perovskite single crystals
ActiveCN107829139AImprove crystal qualityPolycrystalline material growthFrom normal temperature solutionsOrganic solventSingle crystal
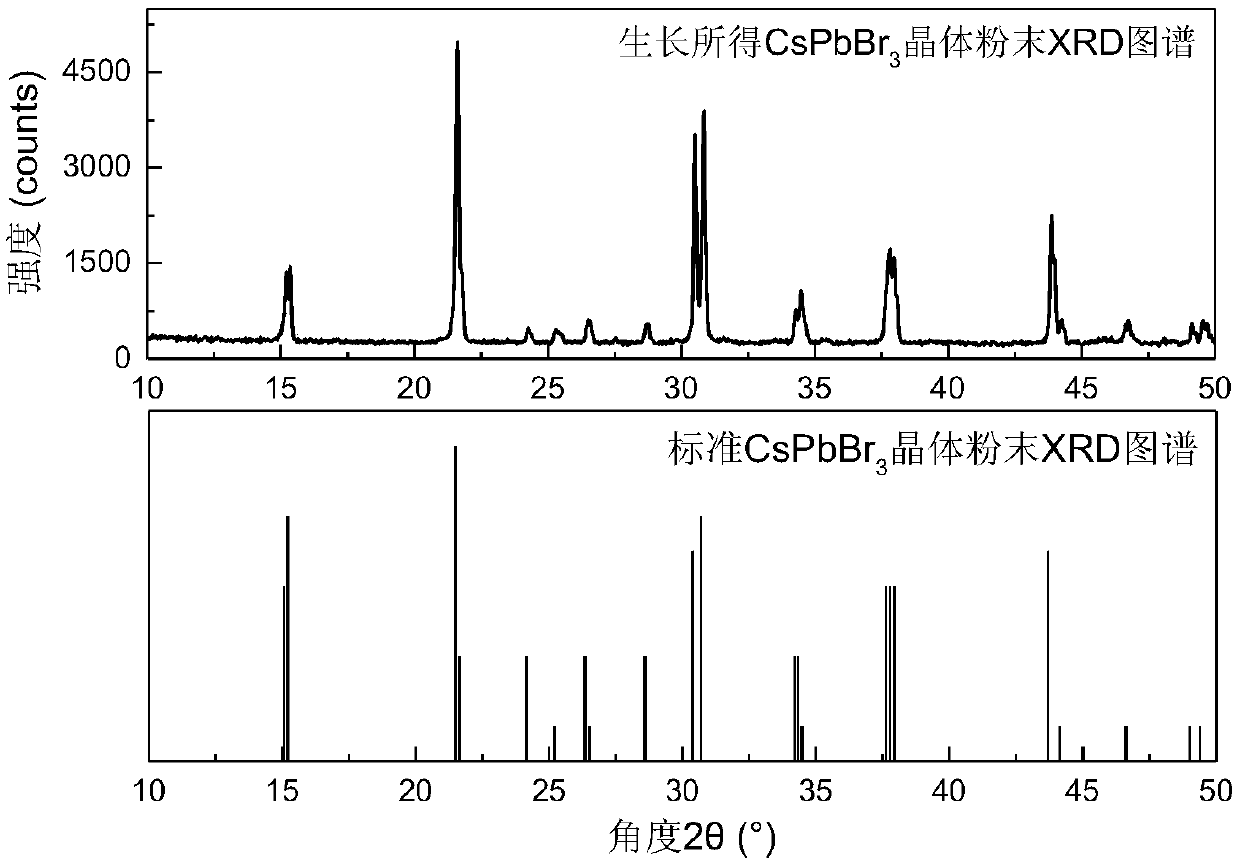
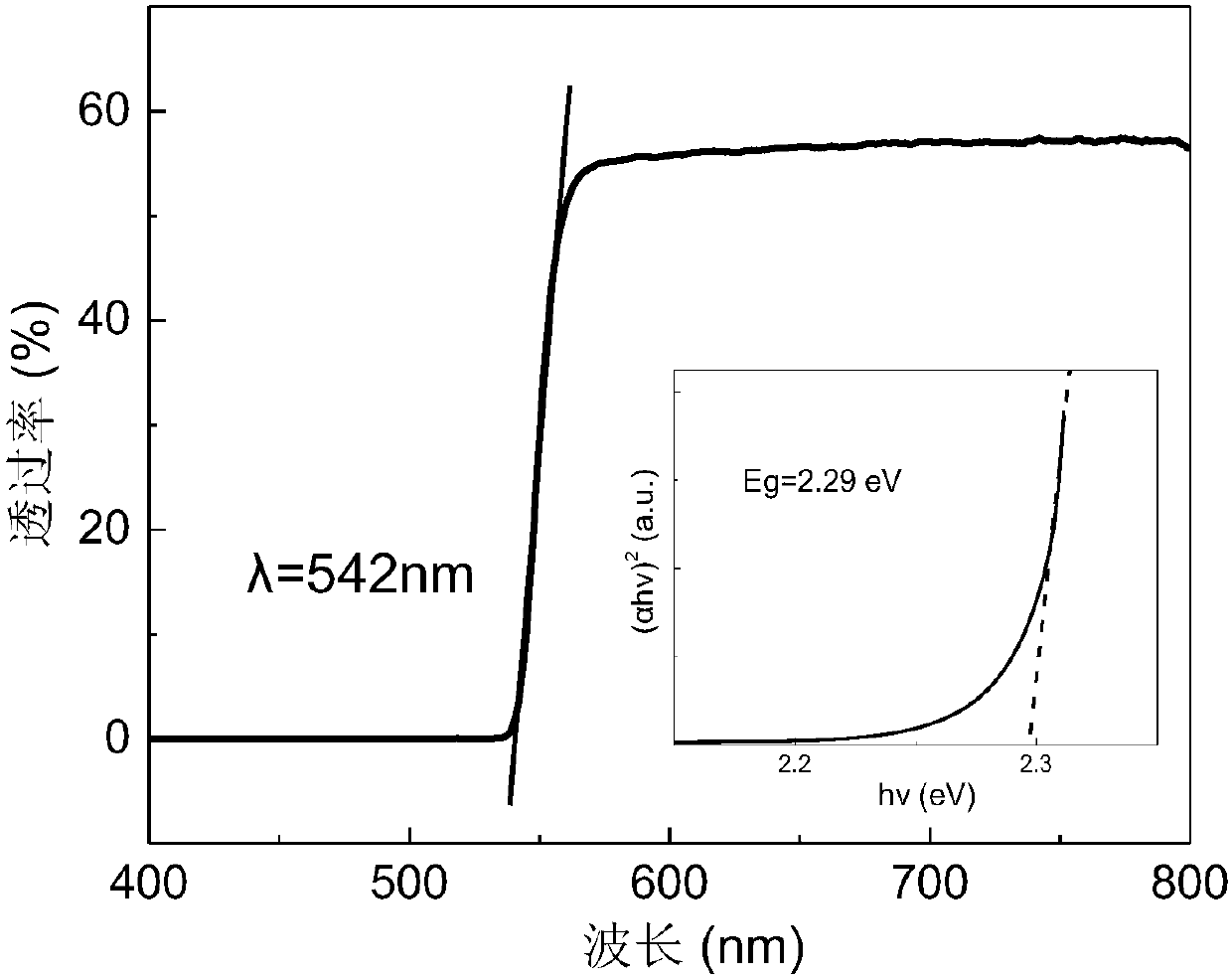
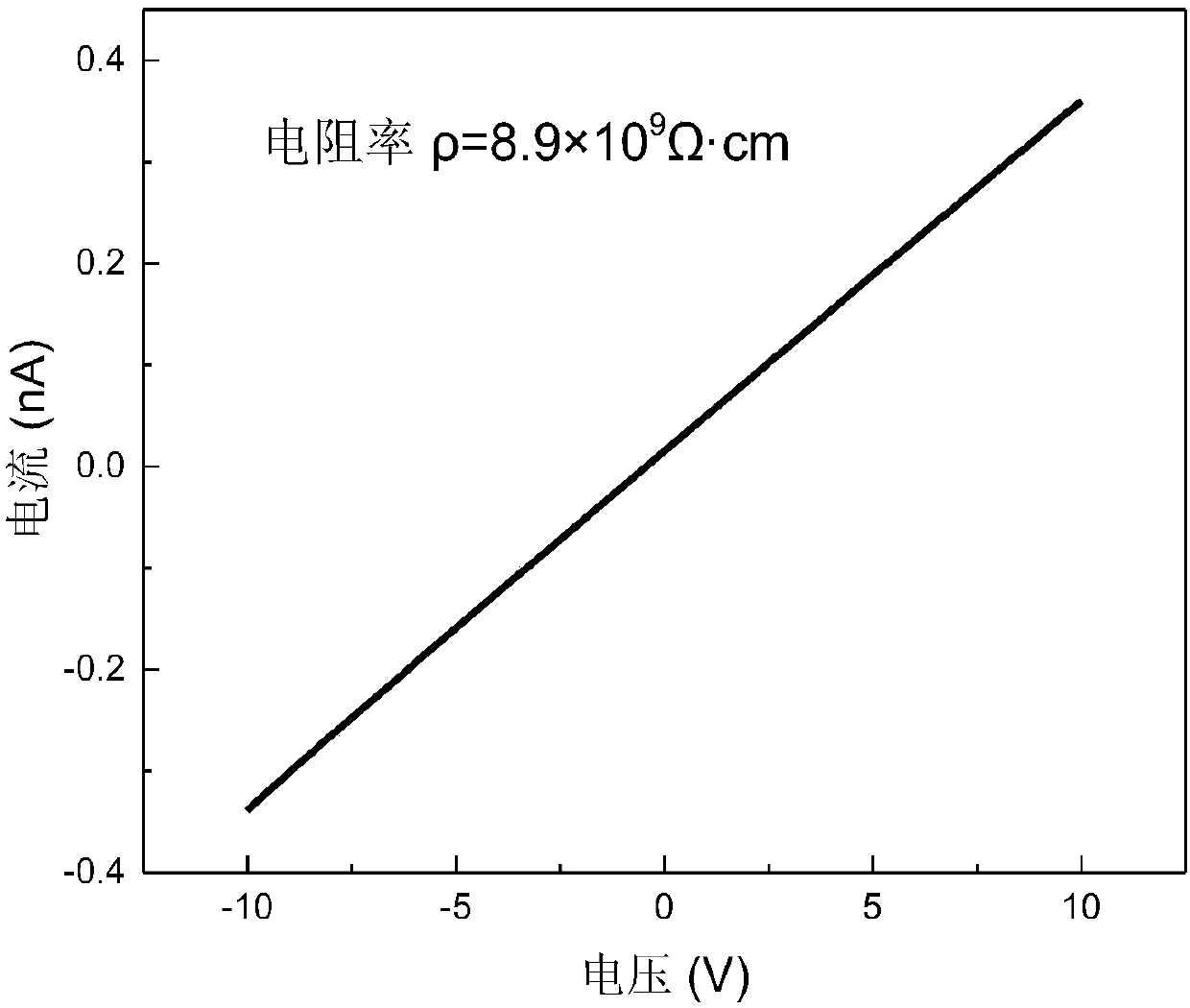
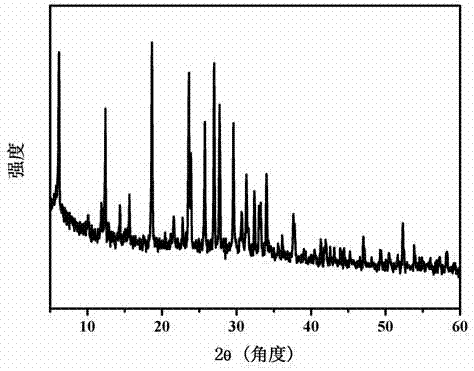
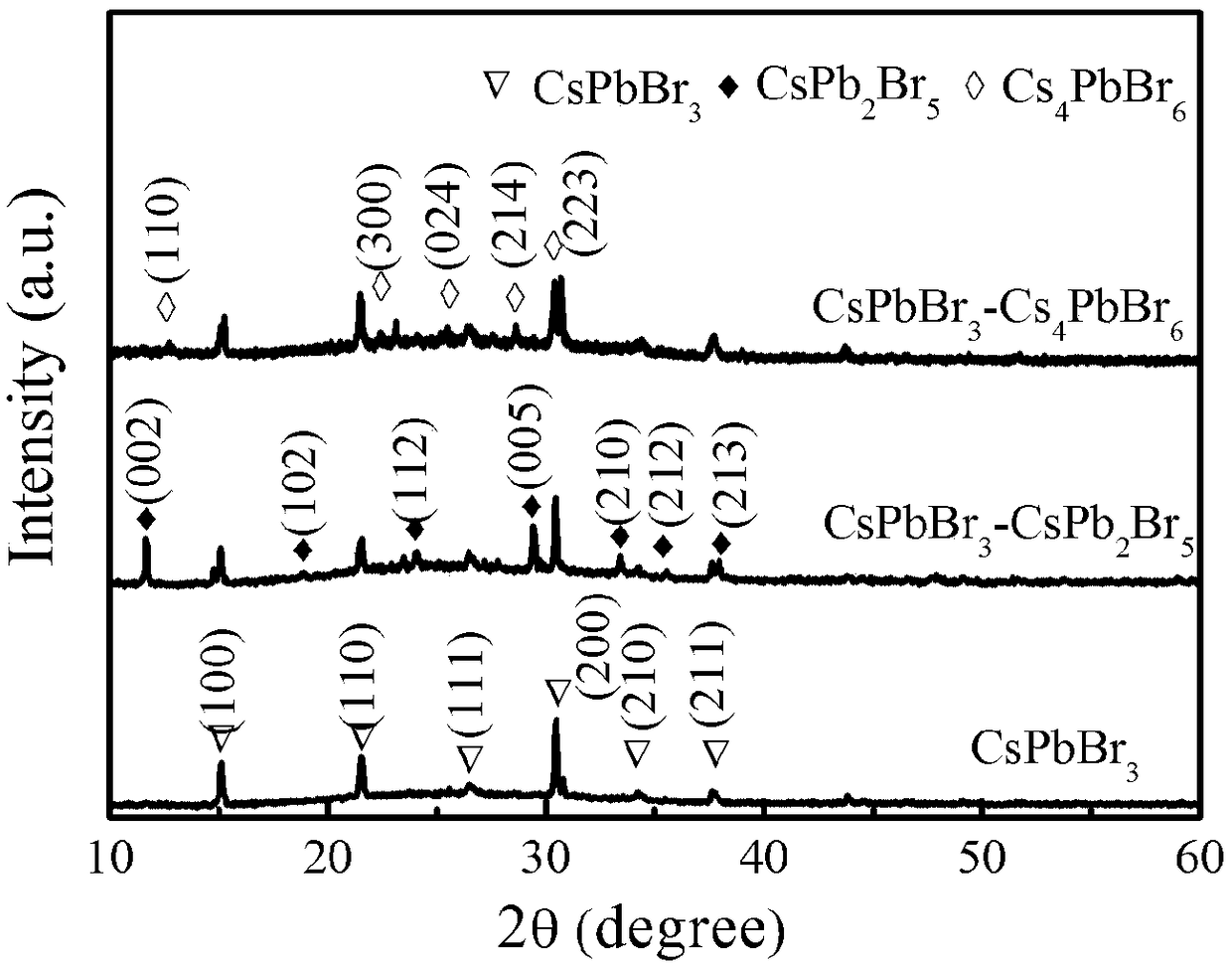
The invention discloses a temperature inversion solution growth method of all-inorganic perovskite single crystals, and aims to solve the technical problem of low practicability of the existing growthmethod of the all-inorganic perovskite single crystals. According to the technical scheme, the temperature inversion solution growth method of the all-inorganic perovskite single crystals comprises the following steps: firstly mixing cesium bromide and lead bromide which are used as raw materials, dissolving and stirring the materials in an organic solvent which is dimethyl sulfoxide, filtering,then adding a mixed solution with cyclohexanol and dimethyl formamide to obtain a precursor solution; secondly keeping the temperature of the precursor solution for a certain time under a certain temperature, and filtering the solution after separating crystals out; and finally putting filtrate in a water tank, heating and increasing the temperature to grow large-sized and high-crystallization-quality CsPbBr3 crystals. According to the method, an XRD spectrum of CsPbBr3 powder prepared by the method is consistent with an XRD spectrum of a standard structure card, thus proving that the CsPbBr3powder is a pure phase; CsPbBr3 single crystals which are 4-7mm in length, 3-5mm in width and 2-3mm in height are obtained; the method is high in practicability.
Owner:NANJING GONGCHENG RES INST OF ENERGY CONSERVATION & NEW MATERIALS TECH CO LTD
Perovskite LED device based on surface ligand control and preparation method thereof
InactiveCN110867532ALong luminous lifeImprove luminous efficiencySolid-state devicesSemiconductor/solid-state device manufacturingActive agentSurface-active agents
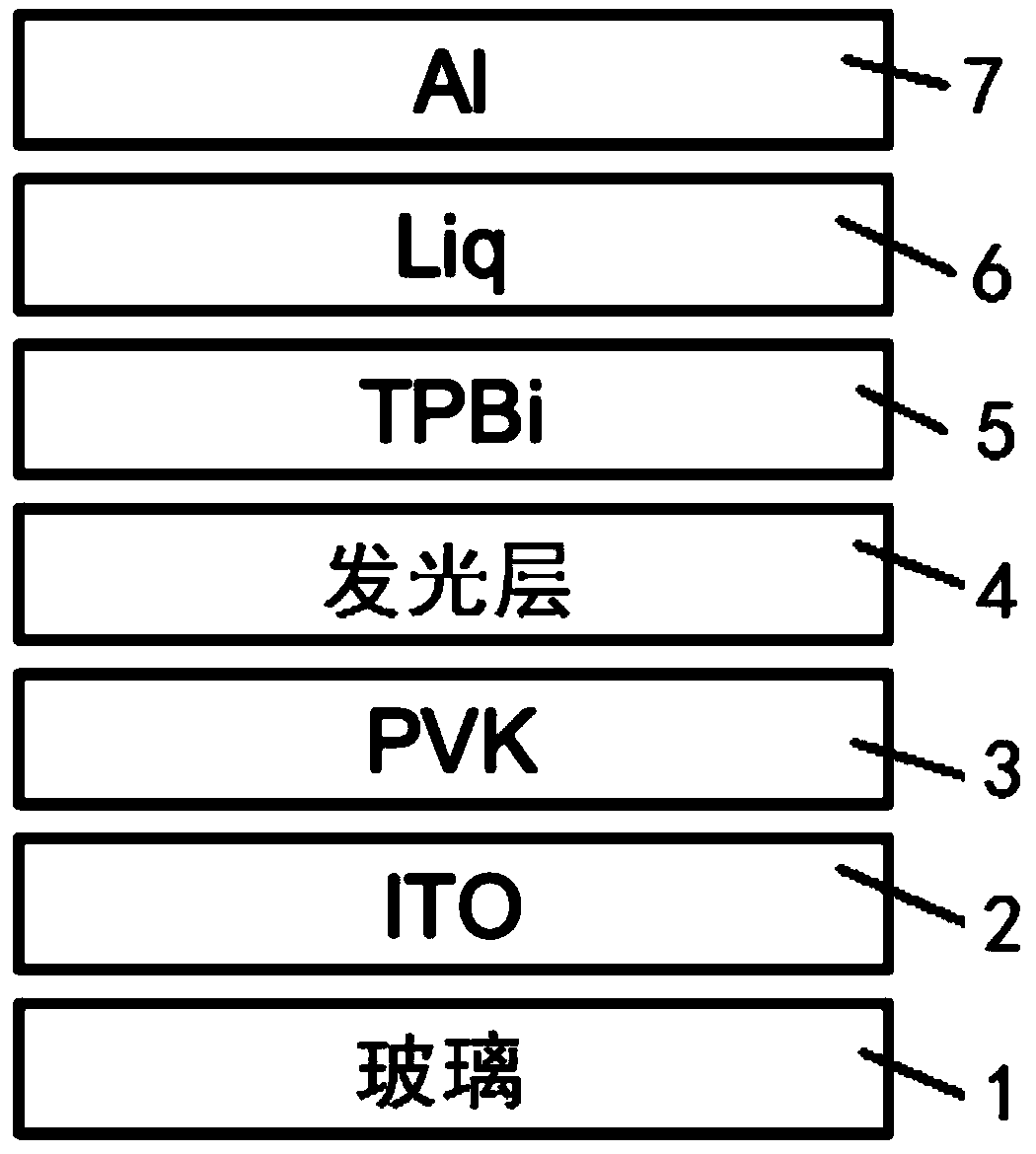
The invention relates to a method for preparing a perovskite LED device based on surface ligand control, including the following steps: applying an organic solution of a hole injection layer materialto the surface of a conductive substrate, and forming a hole injection layer after annealing; dissolving cesium bromide, lead bromide and phenethylamine bromide in an organic solvent under the effectof a 3-(decyl dimethyl ammonium) propane-1-sulfonic acid inner salt surfactant to obtain a perovskite precursor solution, applying the perovskite precursor solution to the surface of the hole injection layer and obtaining a perovskite film after annealing; treating the surface of the perovskite film with an alkylamine organic solution to form a light-emitting layer; and successively preparing an electron transport layer, an electron injection layer and a metal cathode electrode on the surface of the light-emitting layer. The method of the invention is simple and convenient, has a wide range ofmaterials and good repeatability, and can achieve the device performance. Through surface ligand exchange, the flatness and uniformity of the perovskite film are improved, the formation of defects iseffectively suppressed, and the overall performance of the device is significantly improved.
Owner:SUZHOU UNIV
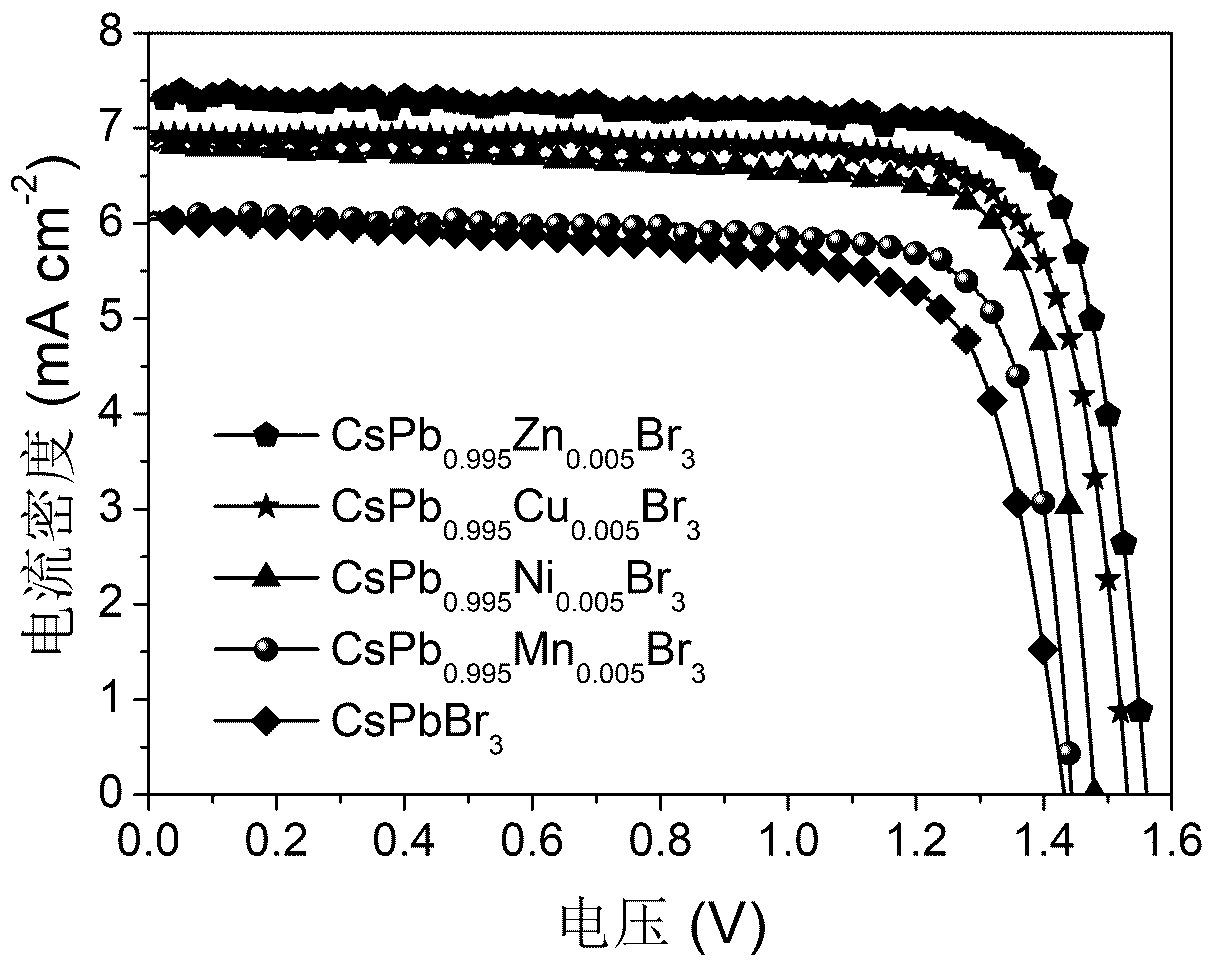
Preparation and application of all-inorganic perovskite cell based on transition metal ion doping
ActiveCN110047951AIncrease binding energyInhibition formationFinal product manufacturePhotovoltaic energy generationHole transport layerCrystallinity
The present invention provides an all-inorganic perovskite solar cell based on transition metal ions doped CsPbBr<3> and a preparation method and an application thereof. Specifically, the invention isthat conductive glass is first spin-coated with an electron transfer layer and subsequently spin-coated with a lead bromide solution mixed with transition metal ions, then is spin-coated with a cesium bromide solution repeatedly; the non-porous perovskite film with a high crystallinity and a large grain size is prepared; and finally the all-inorganic perovskite solar cell based on transition metal ions doped CsPbBr<3> is assembled by blade coating of carbon back electrode. The invention reduces the defect density in the perovskite film by doping transition metal ions, simultaneously the energy band structure is adjusted; the energy loss of charge migration is reduced; and the separation, extraction and transfer of photogenerated charges and charge recombination are reduced, thereby the photoelectric conversion efficiency and long-term operation stability of the cell are improved. The invention has the advantages such as simple and feasible preparation method, large optimization spaceof material combination, no noble metal back electrode or hole transporting layer and low cost.
Owner:OCEAN UNIV OF CHINA
CsPbBr3 (cesium-lead-bromide) perovskite quantum dot fluorescent glass for display of wide color gamut and preparation method and application thereof
ActiveCN110294597AImprove internal quantum efficiencyImprove thermal stabilitySemiconductor devicesFluorescenceGermanium dioxide
The invention belongs to the technical field of display of fully-organic perovskite quantum dots, and discloses a CsPbBr3 (cesium-lead-bromide) perovskite quantum dot fluorescent glass for display ofwide color gamut and a preparation method and application thereof. The CsPbBr3 perovskite quantum dot fluorescent glass comprises the following components in percentage by mole: 0 to 45% of SiO2 (silicon dioxide), 0 to 45% of GeO2 (germanium dioxide), 30 to 40% of B2O3 (boron oxide), 2 to 8% of Al2O3 (aluminum oxide), 3 to 7% of MCO3 (metal carbonate), 1 to 5% of ZnO (zinc oxide), 5 to 15% of CsBr(cesium bromide) or Cs2CO3 (cesium carbonate), 2 to 10% of PbBr2 (lead bromide) or PbO (lead oxide), and 3 to 15% of NaBr (sodium bromide) or KBr (potassium-bromide), wherein M is Ca (calcium) or Sr(strontium); the sum of the components in percentage by mole is 100%. The CsPbBr3 perovskite quantum dot fluorescent glass has the advantages that the technology is simple, and the operation is easy;after in-situ crystallization, the quantum efficiency is higher, the light emitting stability is obviously improved, and the broad application prospect is realized.
Owner:SOUTH CHINA AGRI UNIV
Phase-change-controllable totally-inorganic perovskite thin film preparation method and device application
ActiveCN109355638AImprove crystal qualityReduce the density of defect statesFinal product manufactureChemical vapor deposition coatingGas phaseChemical vapor deposition
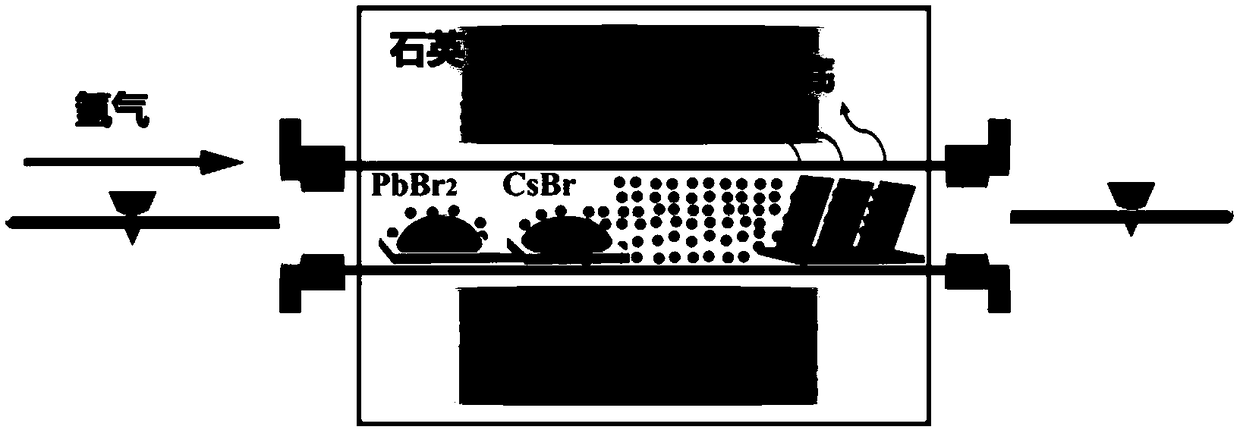
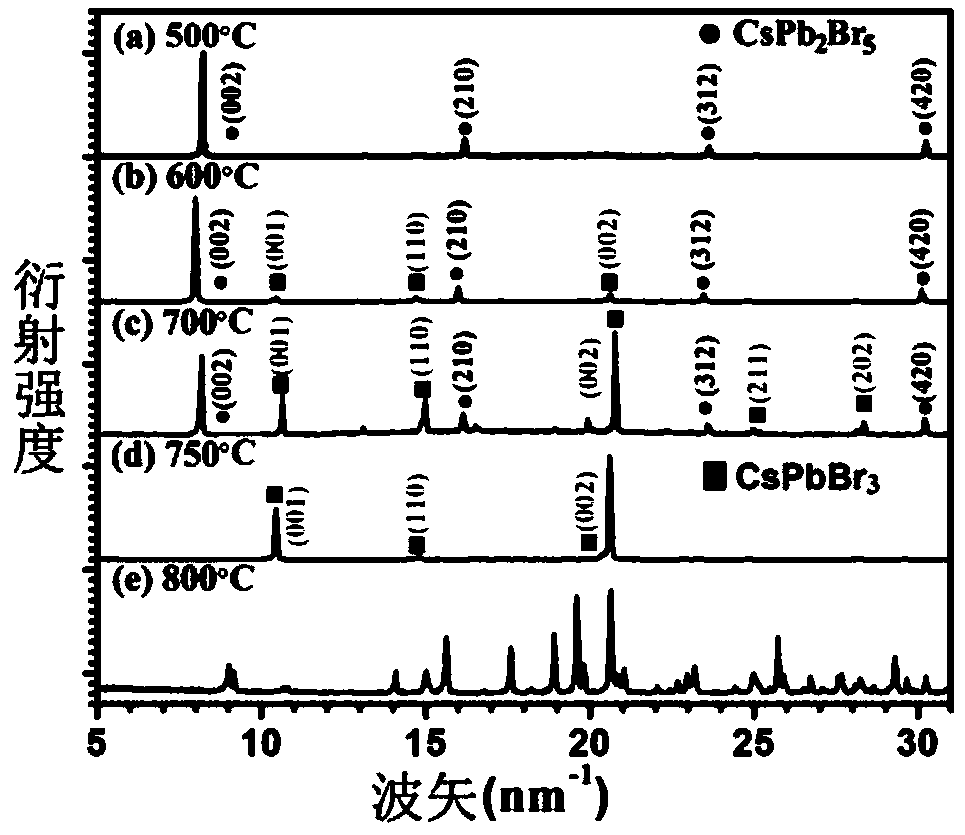
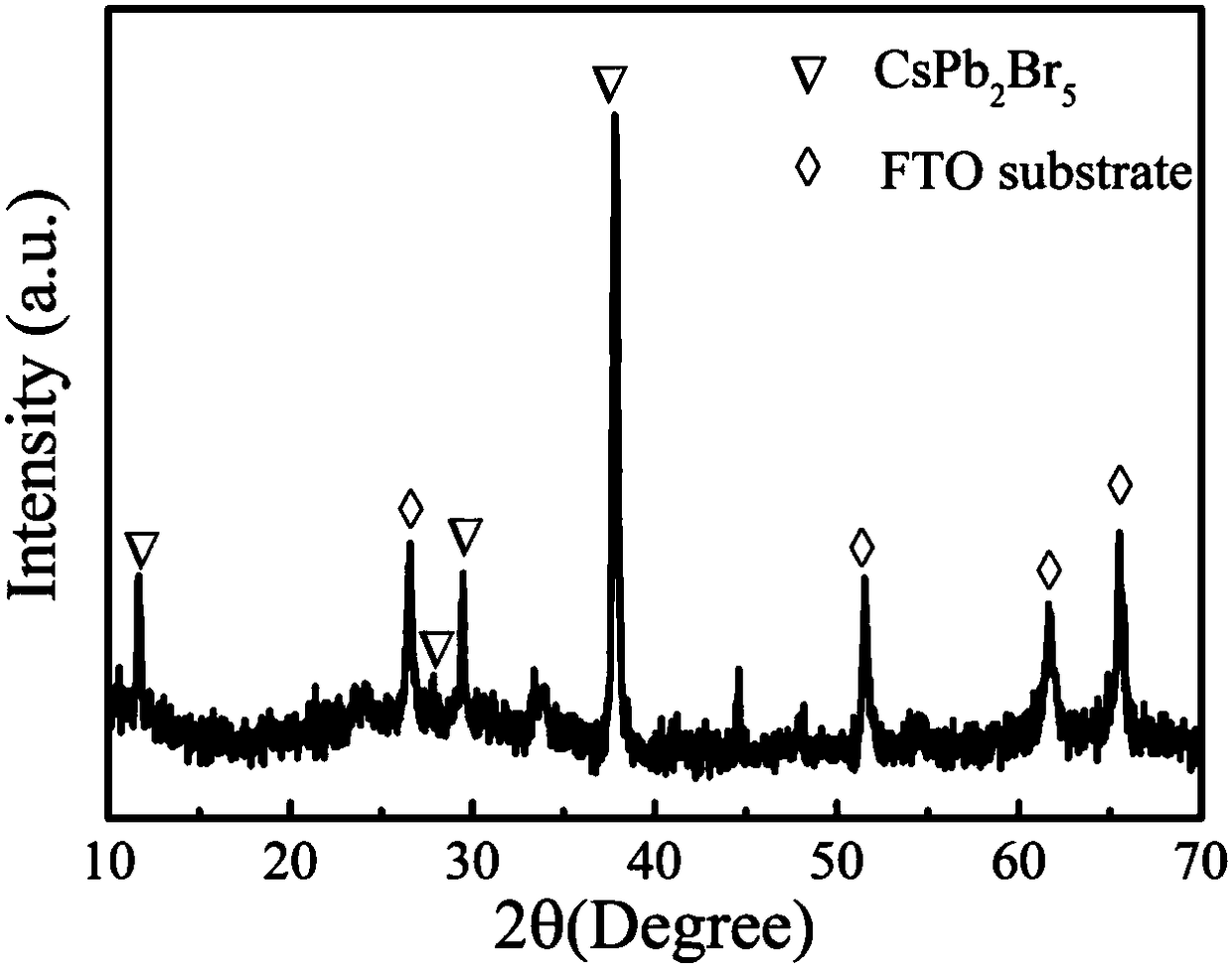
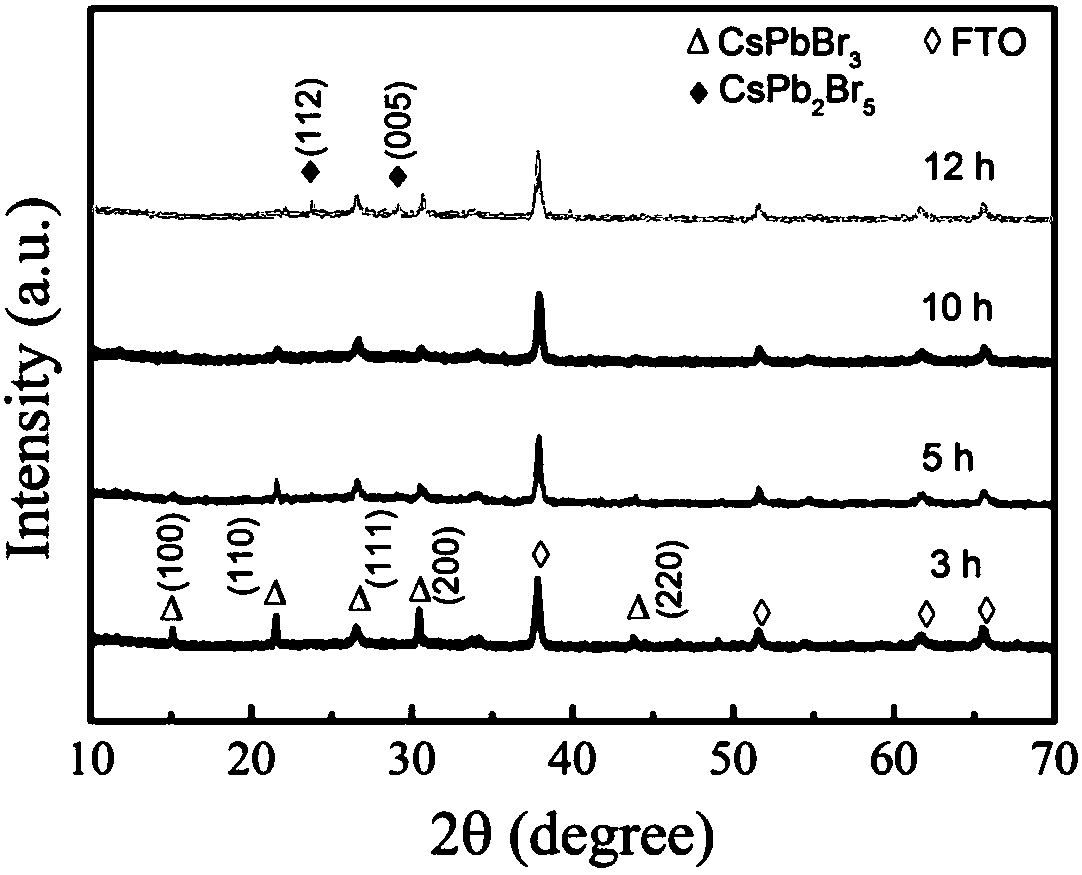
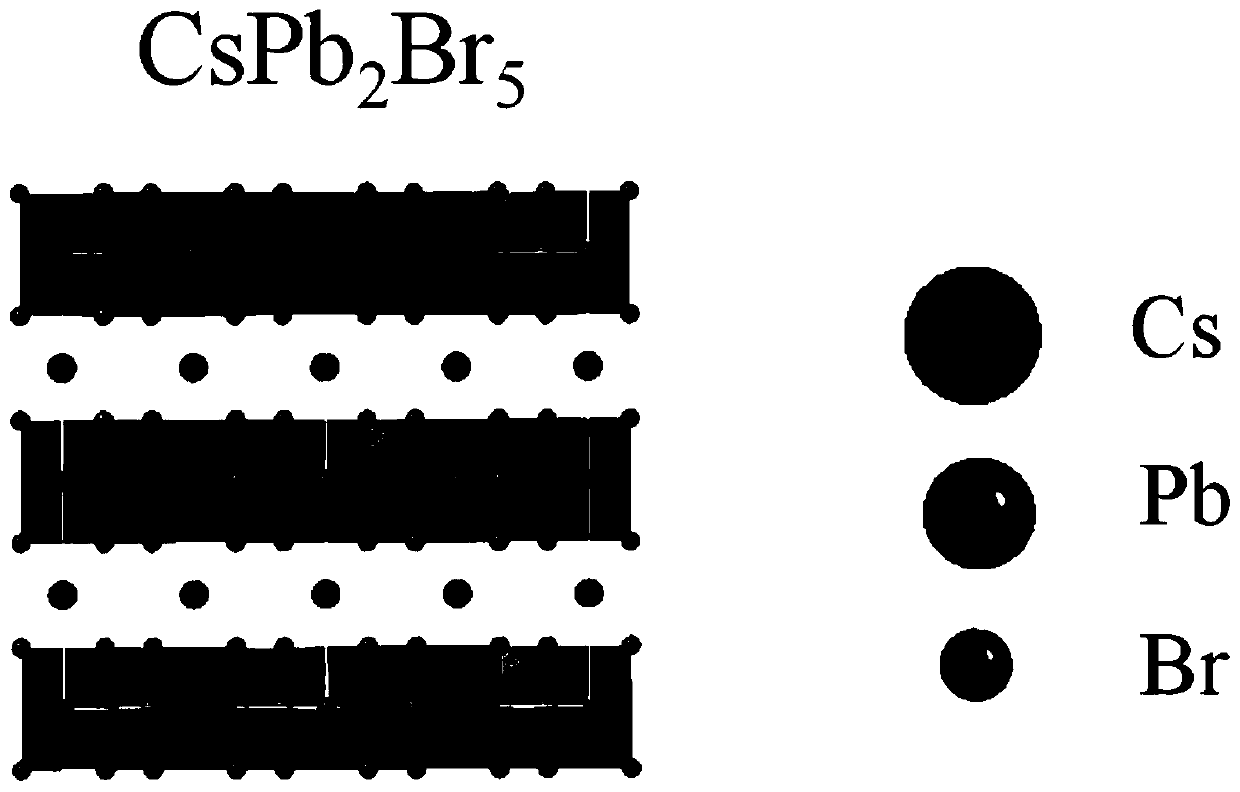
The invention belongs to the technical field of photoelectric functional materials and particularly relates to a phase-change-controllable totally-inorganic perovskite thin film preparation method anddevice application. The method comprises the steps that (1), precursor lead bromide and cesium bromide are placed into a vapor deposition device correspondingly, a substrate is placed in a depositionarea, and vacuum pumping is performed on the whole device; (2), an inert gas is introduced into the vapor deposition device; (3), the deposition temperature and deposition time are set, the deposition temperature is selected from 500-800 DEG C, and at the different deposition temperature, perovskite thin film has different composition and crystal forms. The phase-change-controllable totally-inorganic perovskite thin film preparation method and the device application adopt a chemical vapor deposition method, are simple in process condition, easy to control accurately and suitable for industrial production; meanwhile, the thin film has good uniformity and has good adhesiveness and spreadability with the substrate material, and the prepared optoelectronic thin film has broad application prospects; and by means of change of the deposition temperature, controllable growth of perovskite phase from CsPb2Br5 to CsPbBr3 is realized, and a photoelectric detector prepared on base of the perovskite thin film obtained by the phase-change-controllable totally-inorganic perovskite thin film preparation method shows good photoelectric response and switching characteristics.
Owner:CHANGZHOU INST OF TECH
Trifluoroacetate induction based small-grain CsPbX3 perovskite thin film preparation method
InactiveCN110054214ALow costEasy to industrializeSemiconductor/solid-state device manufacturingLead compoundsHalogenTrifluoroacetic acid
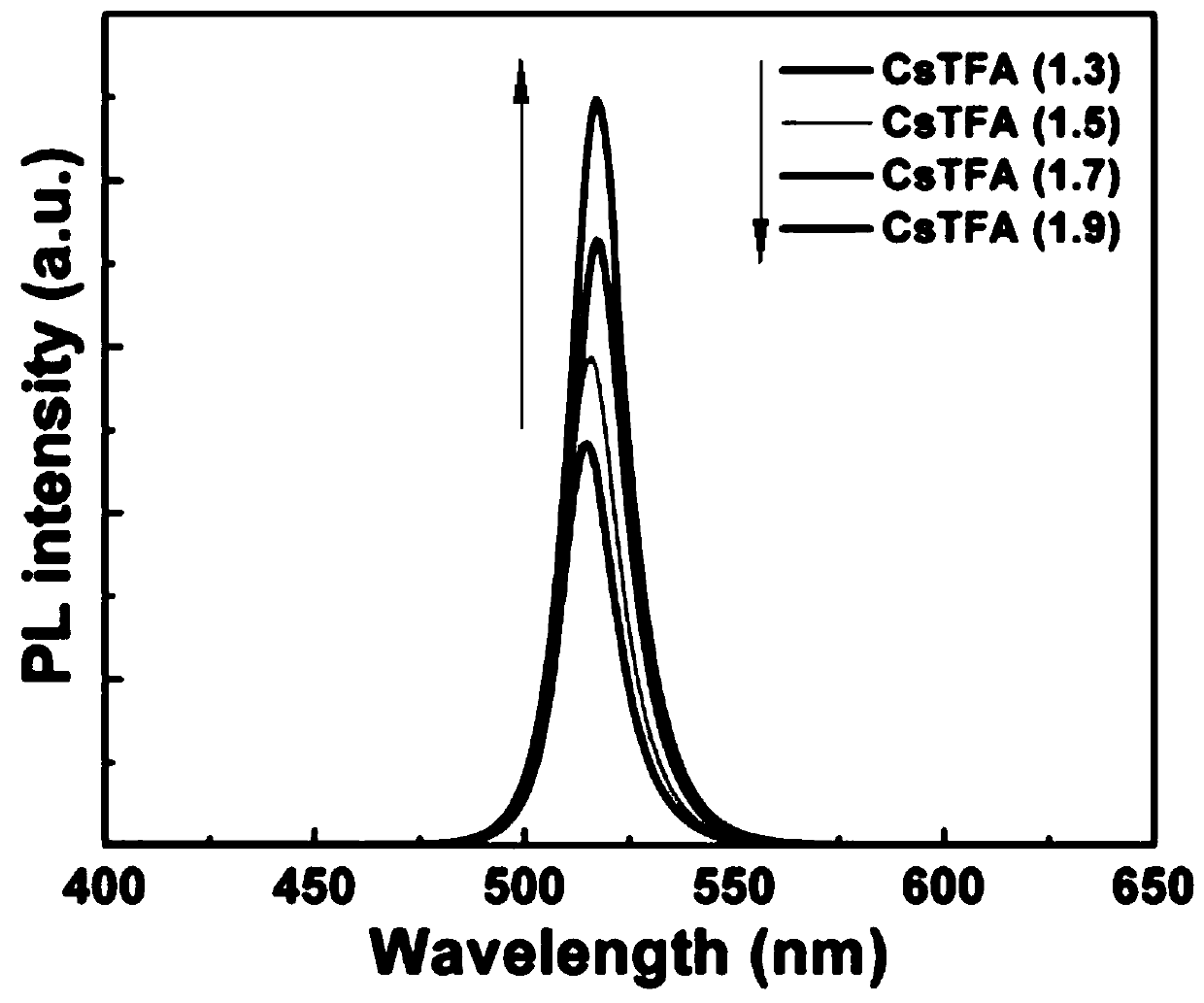
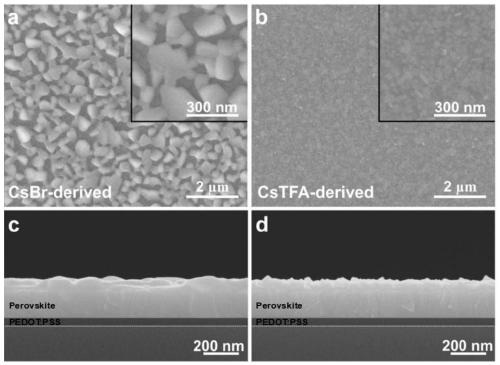
The invention discloses a trifluoroacetate induction based small-grain CsPbX3 perovskite thin film preparation method. The method includes steps: firstly, preparing CsPbX3 perovskite precursor solution; secondly, setting the CsPbX3 perovskite precursor solution on a substrate, and performing curing and annealing to form a film. Under irradiation of an ultraviolet lamp, a CsPbX3 perovskite thin film varies in color according to different halogens. According to the method, cesium trifluoroacetate (CsTFA) is adopted as a new cesium source to take the place of cesium bromide (CsBr) which is a traditional cesium source for preparing the CsPbX3 perovskite thin film, and cost of the prepared perovskite thin film is evidently reduced. Compared with a CsPbX3 thin film prepared according to a traditional method, the CsPbX3 thin film prepared according to the method has advantages that stability is evidently improved, the perovskite grain size is evidently reduced, and the preparation process isenvironmentally friendly, green and healthy.
Owner:SHANGHAI UNIV
Manganese doped CsPbBr3 perovskite quantum dot and molecular sieve composite luminescent material as well as preparation method and application thereof
ActiveCN107384387AGood environmental stabilityReduce the dosage ratioMaterial nanotechnologyNanoopticsGreen-lightOleylamine
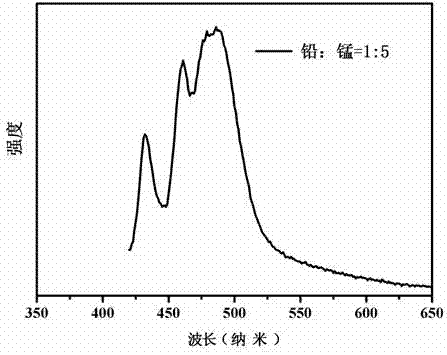
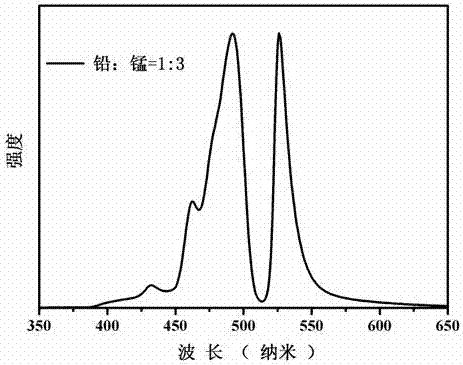
The invention discloses a manganese doped CsPbBr3 perovskite quantum dot and molecular sieve composite luminescent material as well as a preparation method and an application thereof, and belongs to the technical field of luminescent materials. The method comprises the following steps: (1) stirring, centrifugation, washing and drying are carried for molecular sieve and a cesium bromide solution in order to obtain Cs+ exchanged molecular sieve; (2) octadecene, oleic acid and oleylamine are used for lead bromide and manganous bromide in order to obtain a bromide solution; (3) under the condition of inert gas and stirring, in octadecene, the bromide solution and the Cs+ exchanged molecular sieve are reacted, and cooling, washing and drying are carried out in order obtain the manganese doped CsPbBr3 perovskite quantum dot and molecular sieve composite luminescent material. The composite luminescent material has multi-peak emission in blue light and green light zones, and luminescent peak positions and each luminescent peak intensity can be modulated according to proportion between manganese ions and lead ions; the composite luminescent material has good environmental stability, and can be applied to the field of white light LED.
Owner:SOUTH CHINA UNIV OF TECH
Thermal battery electrolyte with low melting point and high electric conductivity and preparation method thereof
InactiveCN102437345ALow melting pointWide working lower limit temperatureDeferred-action cellsInorganic saltsLithium bromide
The invention discloses a thermal battery electrolyte with low melting point and high electric conductivity and a preparation method thereof. The thermal battery electrolyte is composed of the following component in percentage by weight: 25%-35% of analytically pure lithium bromide (LiBr), 10%-22% of analytically purepatassium bromide (KBr), 45%-53% of analytically pure cesium bromide (CsBr) and 8%-13% of analytically pure lithium iodide (LiI). The thermal battery electrolyte with low melting point and high electric conductivity is prepared by sintering a eutectic salt composed by the four inorganic salts (LiBr, KBr, CsBr and LiI) according to a certain proportion under protection of an inert gas; the eutectic melting point is lower than 200 DEG C; and the electric conductivity is greater than 1.8S / cm at 500 DEG C. The thermal battery electrolyte with low melting point and high electric conductivity can obviously prolong working time of thermal cells, as well as reduce the surface temperature of thermal cells at work.
Owner:GUIZHOU MEILING POWER SUPPLY CO LTD
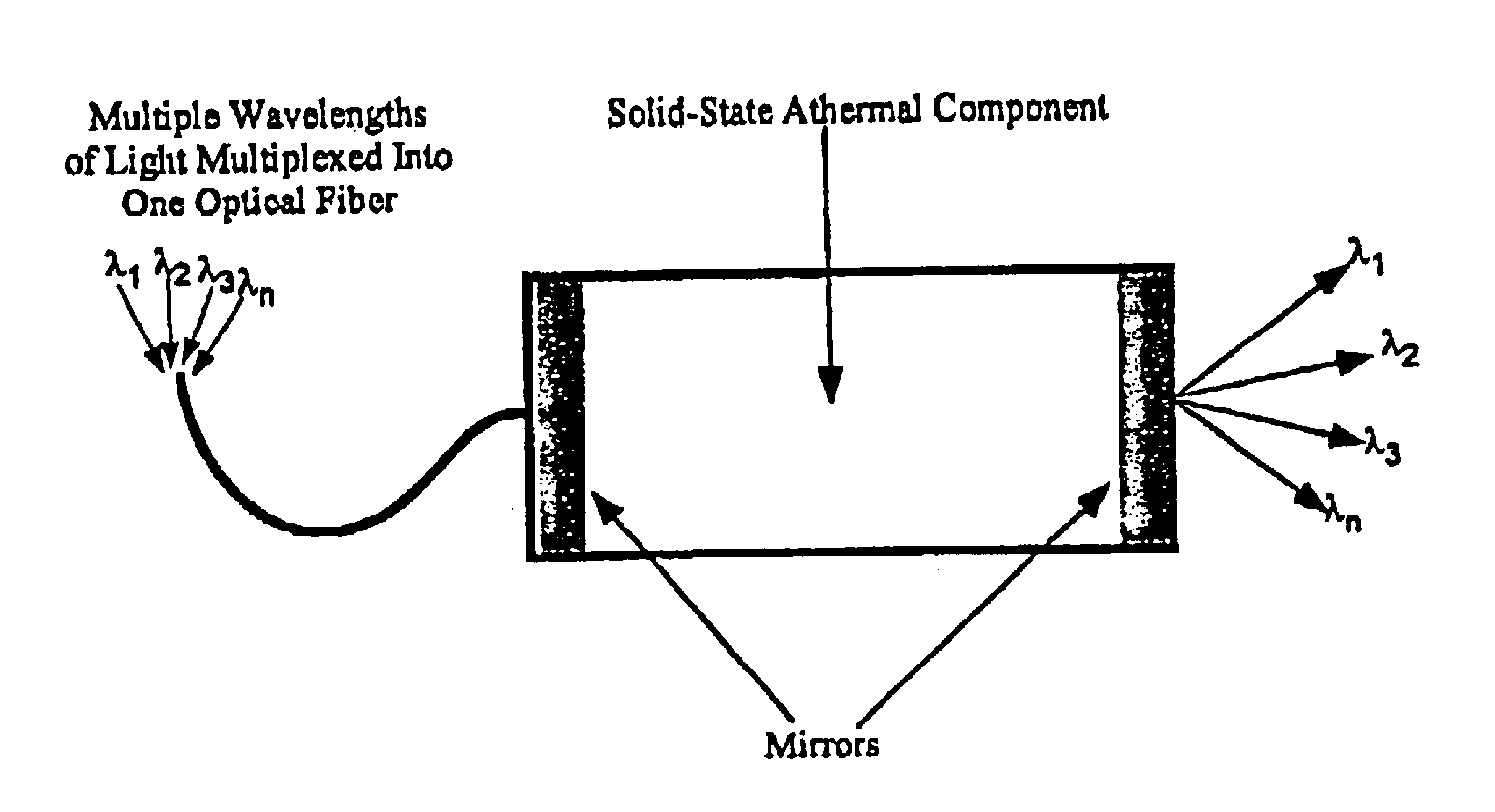
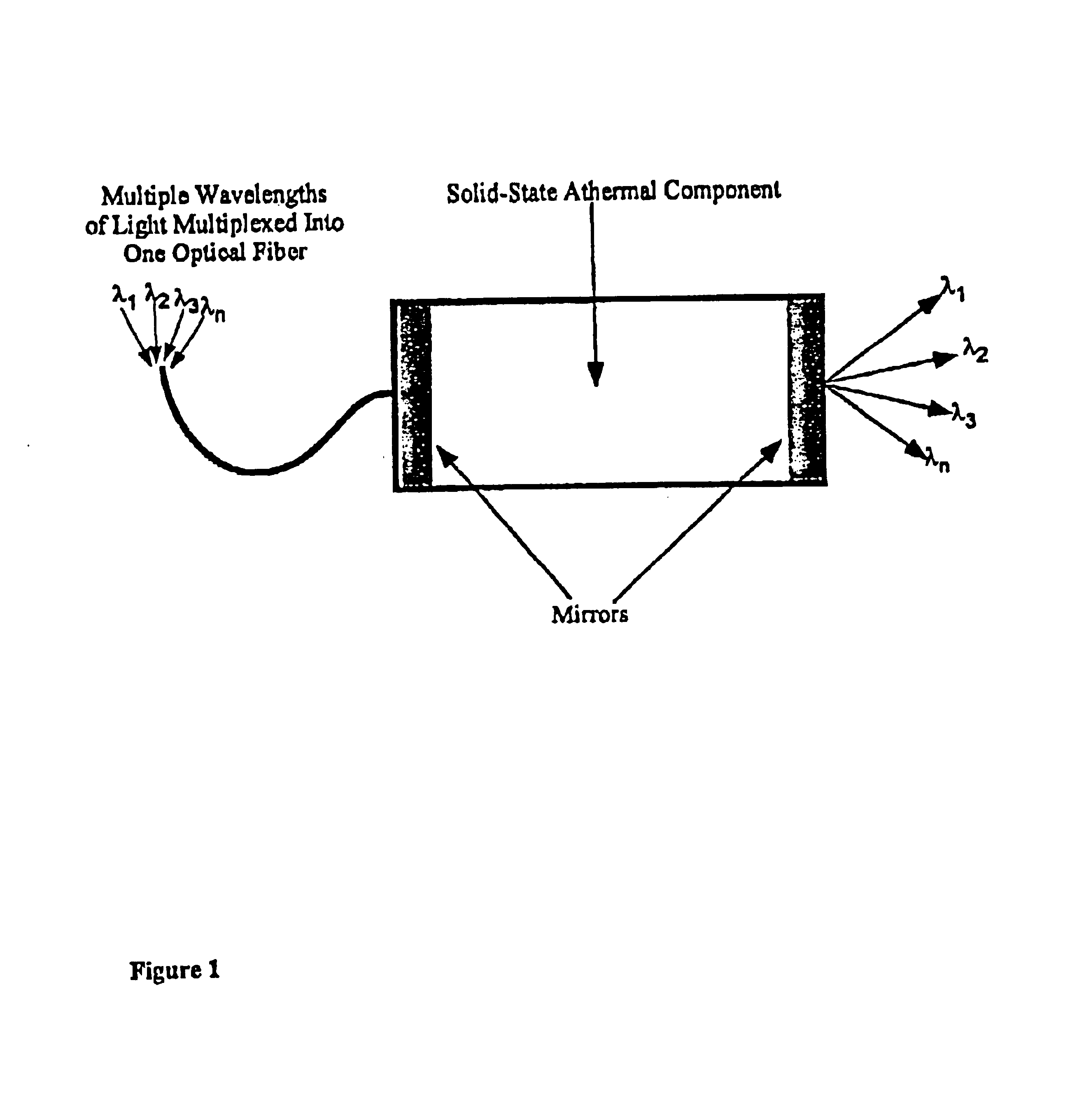
Athermal optical components
InactiveUS6865318B1High refractive indexImprove insertion lossCladded optical fibreCoupling light guidesCrystalline materialsSilver chloride
Athermal optical components comprise cubic crystalline materials including silver chloride and cesium bromide, or comprise composites of at least two layers of different compositions wherein the total optical pathlength, nL, across said layers is essentially independent of temperature.
Owner:SCHOTT AG
Method for preparing layered structure inorganic perovskite negative electrode material by using gas phase method
ActiveCN108682791ALow priceHigh thermodynamic stabilitySecondary cellsVapour deposition manufacturingGas phaseCopper foil
The invention discloses a method for preparing a layered structure inorganic perovskite negative electrode material by using a gas phase method, and relates to the technical field of preparation of lithium ion battery negative electrode materials. The method comprises: sequentially or simultaneously depositing lead bromide and cesium bromide on a copper foil substrate under a vacuum condition through a gas phase method, and carrying out heating annealing in air to obtain the layered structure inorganic perovskite negative electrode material. According to the present invention, according to theprinciples of physical vapor deposition and chemical vapor deposition, inorganic lead bromide and cesium bromide are used as raw materials and are deposited on the surface of the copper foil substrate through heating evaporation, the evaporation rate, the deposition thickness and the thickness ratio are regulated, and the subsequent annealing treatment is performed to prepare the dense and uniform pure inorganic perovskite material. With the applications of the layered structure inorganic perovskite negative electrode material in lithium ion battery negative electrode materials, good thermodynamic stability and excellent physical and chemical properties can be achieved, and the process is simple.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
Preparation method of two-dimensional inorganic perovskite anode material for lithium battery
InactiveCN108511706AImprove stabilityExcellent physical and chemical propertiesCell electrodesSecondary cellsCarbon coatingN dimethylformamide
The invention discloses a preparation method of a two-dimensional inorganic perovskite anode material for a lithium battery. The preparation method comprises the following steps of adding lead bromideand cesium bromide into N,N-dimethylformamide, heating and stirring, and introducing protective gas in the stirring process, so as to obtain a mixture; drying the mixture under the vacuum condition,so as to obtain a precursor material; adding toluene into the precursor material, centrifuging and separating, and drying the obtained centrifuged solid material under the vacuum condition, so as to obtain the two-dimensional inorganic perovskite anode material for the lithium battery. The preparation method of the two-dimensional inorganic perovskite anode material for the lithium battery has theadvantages that the simple one-step synthesis technology is adopted; by performing simple dissolving and extracting process, the perovskite material with high purity and high crystallizing degree canbe obtained; the problems of complicated preparation technology of graphitizing and carbon coating at high temperature and higher cost in the conventional anode material of the lithium battery are solved.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
Method for preparing two-dimensional inorganic perovskite negative electrode material by solid-liquid phase reaction
InactiveCN108455660AImprove electrochemical performanceSolve complexityNegative electrodesSecondary cellsCarbon coatingN dimethylformamide
The invention discloses a method for preparing a two-dimensional inorganic perovskite negative electrode material by solid-liquid phase reaction. The method comprises the following steps of adding lead bromide and cesium bromide into N,N-dimethylformamide, heating and stirring, and drying under the vacuum condition, so as to obtain precursor powder; mixing the precursor powder and the lead bromide, performing ball milling, grinding, and drying under the vacuum condition, so as to obtain the two-dimensional inorganic perovskite negative electrode material. The method for preparing the two-dimensional inorganic perovskite negative electrode material by the solid-liquid phase reaction has the advantages that by adopting the simple solution synthesizing and ball milling technology, the precursor material is in contact with an interface in the high-energy impacting process, and then reacts, so as to obtain the inorganic perovskite material with high crystallizing degree; the problems of complicated preparation technology and higher cost due to graphitizing and carbon coating required by the existing negative electrode material of the lithium ion battery at high temperature are solved; the large-batch synthesizing preparation purpose is realized, the production process of the negative electrode material is greatly shortened, and the material cost is greatly reduced.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
Method for preparing pure inorganic perovskite film under assistance of polyethylene glycol
ActiveCN107324665AEvenly distributedImprove compactnessCoatingsLuminescent compositionsQuantum efficiencyPolyethylene glycol
The invention discloses a method for preparing a pure inorganic perovskite film under assistance of polyethylene glycol. The method comprises steps as follows: a polyethylene glycol solution is mixed with a solution containing lead bromide and cesium bromide and stirred at room temperature, a mixed liquid is obtained, then the mixed liquid is subjected to spin coating and thermal treatment sequentially, and the pure inorganic perovskite film is obtained; alternatively, lead bromide and cesium bromide are directly dissolved in the polyethylene glycol solution serving as a solvent and stirred at room temperature, and a mixed liquid is obtained; the mixed liquid is subjected to spin coating and thermal treatment sequentially, and the pure inorganic perovskite film is obtained. The preparation process is simple, the product can be obtained only through one-step spin coating and thermal treatment, industrial production is facilitated, the prepared pure inorganic perovskite film has a compact and flat surface, has the quantum efficiency nearly 3 times that of a film without polyethylene glycol and can be widely applied to solar cells, laser and LEDs.
Owner:SUZHOU UNIV
Method for synthesizing CsPbBr3 nanocrystal through centrifugal stirring
InactiveCN107792877AHigh fluorescence efficiencySimple processLead halidesNanotechnologyFluorescenceSolvent
The invention discloses a method for synthesizing CsPbBr3 nanocrystal through centrifugal stirring. The method comprises the following steps: dissolving cesium bromide and lead bromide into dimethyl sulfoxide, then transferring to a centrifuge for centrifugation, standing after completion of the centrifugation, removing a supernatant, washing the dimethyl sulfoxide, filtering, and performing vacuum drying to obtain CsPbBr3 nanocrystal powder. The CsPbBr3 nanocrystal synthesized by the method provided by the invention has high fluorescence efficiency, can be unquenched for a long time at high temperature, has a wide application prospect; meanwhile, by the method provided by the invention, a technology is simple, any surface modifier and any toxic solvent are added, environment friendlinessis achieved, raw materials are cheap, operation is convenient, the efficiency is high, and industrial production is facilitated.
Owner:SOUTH CHINA UNIV OF TECH
Composite perovskite material with CsPbBr3 doped in CsPb2Br5, preparation method thereof, and application thereof
ActiveCN110950375AHigh fluorescence intensityStable structureLuminescent compositionsLead compoundsAlcohol ethylMetal-organic framework
The invention discloses a composite perovskite material with CsPbBr3 doped in CsPb2Br5 and a preparation method thereof. The doped perovskite material is formed by doping a small amount of CsPbBr3 ina CsPb2Br5 material. The design of the preparation method provided by the invention is skillful, firstly, a metal organic framework Pb-MOF taking lead as a framework is synthesized by utilizing a trimesic acid ligand and lead ions, then a cesium bromide aqueous solution and the Pb-MOF react under an ultrasonic condition in an alcoholic solution, and after the Pb-MOF is dissociated in the reaction,a composite material with a small amount of CsPbBr3 doped in CsPb2Br5 is obtained. The synthesis process has few steps, the process is easy to implement, heating and inert gas protection are not needed, and energies are saved. Used synthetic solvents are only pure water and ethanol and are environment-friendly, and little pollution is caused to the environment.
Owner:SHANDONG ANALYSIS & TEST CENT
Recrystallization-based all-bromo perovskite blue light quantum dot and preparation method thereof
ActiveCN112125332ASmall sizeReduce solubilityNanoopticsLuminescent compositionsQuantum yieldOleylamine
The invention belongs to the technical field of nano materials and inorganic perovskite light-emitting materials, and particularly relates to an all-bromo perovskite blue light quantum dot based on arecrystallization theory and a preparation method of the all-bromo perovskite blue light quantum dot. The preparation method comprises the following steps: preparing a precursor solution by a one-potmethod, taking cesium bromide and lead bromide as reactants, taking oleic acid and oleylamine as ligands, and taking N, Ndimethylformamide (DMF) as a solvent; adding a proper amount of methylbenzene into the precursor solution to form a mixed solution, so that the solution system is in a supersaturated state; at the moment, a small amount of the mixed solution is taken out, methylbenzene with thevolume being 1050 times that of the mixed solution is injected again, the CsPbBr3 blue light quantum dots which are extremely small in size and approximately uniform in size are obtained under the recrystallization effect, the fluorescence quantum yield reaches up to 87.20%, and the obtained emission peak wavelength ranges from 460-480 nm. The method skillfully uses the principle, is simple to operate, quick and efficient, and provides a new thought and a new method for the synthesis of the high-performance blue light material.
Owner:NAT UNIV OF DEFENSE TECH
All-inorganic perovskite solar cell based on inorganic perovskite nanocrystalline interface layer and preparation method and application thereof
ActiveCN110311012AOptimizing Energy Level DifferencesReduce the density of defect statesFinal product manufacturePhotovoltaic energy generationSolventCarbon coated
The invention provides an all-inorganic perovskite solar cell based on an inorganic perovskite nanocrystalline interface layer and a preparation method and an application thereof. The method is characterized in that the lead bromide is spin-coated on a surface of a conductive glass-supported titanium dioxide electron transport layer by the multi-step spin coating technique, a high-purity CsPbBr3 light absorbing layer is prepared by spin coating of cesium bromide in multiple steps, n-hexane solution with the inorganic perovskite nanocrystals is then spin-coated onto the CsPbBr3 layer for interfacial modification, and lastly, a carbon coated back cell is assembled into the all-inorganic perovskite solar cell. The method is advantaged in that high hole conductivity and energy level adjustability of the inorganic perovskite nanocrystal are fully utilized, electron-hole separation is promoted, energy loss is reduced, perovskite is treated with the n-hexane solvent, the perovskite grain sizeis increased, defect density and charge recombination are reduced, energy conversion efficiency of the cell is effectively improved, the preparation process is simple, cost is controllable, the material optimization space is large, and the commercial production prospect is further wide.
Owner:OCEAN UNIV OF CHINA
Red perovskite light emitting diode based on mixed halogen and preparation method thereof
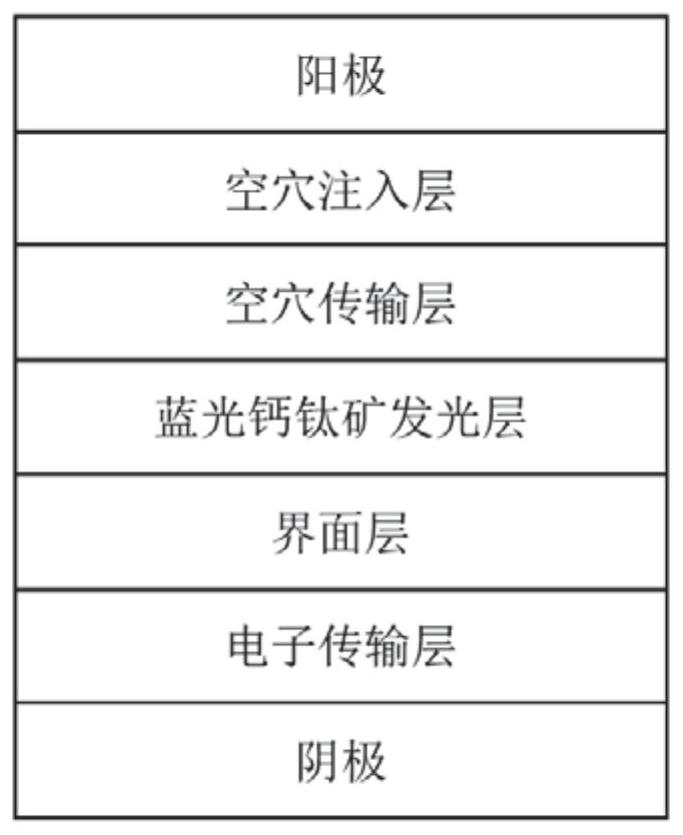
ActiveCN111916571ASuppress generationReduce movementSolid-state devicesSemiconductor/solid-state device manufacturingCesium iodideLight-emitting diode
The invention discloses a red perovskite light-emitting diode based on mixed halogen and a preparation method thereof. The light-emitting diode sequentially comprises a transparent substrate, an anode, a hole injection layer, a hole transport layer 1, a hole transport layer 2, a perovskite light-emitting layer, an electron transport layer, an electron injection layer and a cathode, wherein the perovskite light-emitting layer is prepared by spin-coating a perovskite precursor solution; dropwise adding an anti-solvent in the spin coating process, wherein the perovskite precursor solution is prepared by dissolving cesium iodide CsI, cesium bromide CsBr, lead bromide PbBr2, lead iodide PbI2, organic ammonium bromide salt LBr and organic ammonium iodide salt LI in a polar solvent. By combininglarge-size organic cations with mixed halogens, the generation of a non-luminous active perovskite phase is inhibited, the movement of halogen ions is reduced, and the spectral stability is realized while the brightness and efficiency of the red perovskite LED are improved.
Owner:SOUTH CHINA UNIV OF TECH
Method for efficient synthesis of Cs2AgBr3 non-lead all-inorganic perovskite
InactiveCN110862103AShort timeEasy to operateLuminescent compositionsSilve compoundsFluorescenceSilver bromide
Belonging to the technical field of preparation of semiconductor nanomaterials, the invention discloses a method for efficient synthesis of Cs2AgBr3 non-lead all-inorganic perovskite. The method includes: firstly mixing cesium bromide and silver bromide according to a molar ratio of 2:1, then adding tri-n-octyl phosphorus, performing grinding, gradually hardening the mixture from fluffy faint yellow powder, and attaching the mixture to the wall of a container, gradually turning the faint yellow powder to white powder along with the grinding, performing monitoring by a 302nm ultraviolet lamp inthe grinding process, and stopping the action when the brightness of the product does not increase any more; and subjecting the obtained product to heat treatment in a vacuum oven at 80-300DEG C for3h, and then conducting freezing treatment at a temperature ranging from -10DEG C to -50DEG C for 1h-3h, thus obtaining the Cs2AgBr3 non-lead all-inorganic perovskite with improved fluorescence yield.The method provided by the invention has the advantages of short time, easy operation and suitability for large-scale production.
Owner:JILIN UNIV
Method for synthesizing copper-based perovskite powder by low-temperature solvent method
InactiveCN112280555ALow costShort preparation cycleCopper compoundsLuminescent compositionsCupric bromideCopper chloride
The invention relates to a method for synthesizing copper-based perovskite powder by a low-temperature solvent method. The method comprises the following steps: adding CsX and CuX into a polar organicmixed solution, and stirring for 4-6 hours at 40-70 DEG C, wherein the polar organic mixed solution is a mixed solution of N, N-dimethylformamide (DMF)and dimethyl sulfoxide (DMSO), the CsX is cesiumchloride, cesium bromide or cesium iodide, and the CuX is copper chloride, copper bromide or copper iodide. According to the synthesis process, constant-temperature growth on a hot plate is not needed, synthesis is directly conducted on a magnetic stirrer, the synthesis process has the advantages of being short in manufacturing period, low in cost, simple in growth step, environmentally friendlyand the like, and Cs3Cu2X5 powder with high crystallization quality is separated out within 5 hours.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
A preparation method of lithium ion using inorganic perovskite derivative phase as negative electrode material
ActiveCN109065834AEasy to getLow priceElectrode thermal treatmentSecondary cellsCooking & bakingCopper foil
The invention discloses a preparation method of an inorganic perovskite derivative phase used as a negative electrode material for lithium ions, which relates to the technical fields of preparation and research of the negative electrode material of lithium ion batteries and the like. The precursor of lead bromide and cesium bromide was evaporated by vacuum thermal deposition process and depositedon the copper foil of cathode current collector. The cathode material was homogeneous and dense by subsequent heating and baking. The invention utilizes the principle of chemical solid state reactionand thermal evaporation vapor deposition, Using inorganic lead bromide and cesium bromide as raw materials, the pure inorganic perovskite materials were prepared by vacuum heating and evaporation on current collector copper foil substrate. The evaporation rate of precursor material was adjusted and the thickness and thickness ratio of the precursor material were adjusted during the reaction. Applying it to the cathode material of lithium ion battery, it exhibits good thermodynamic stability and excellent physicochemical properties, and the process is simple.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
Pulsed CO2 laser including an optical damage resistant electro-optical switching arrangement
InactiveUS7113529B2Mitigating optical damageActive medium materialCoupling light guidesRefractive indexEngineering
An electro-optical switch includes an optical assembly arranged to transmit laser-radiation. The optical assembly comprises an active optical crystal. On one of two opposite surfaces of the crystal is an optical window formed from a material having a refractive index lower than the material of the crystal. Heat deposited, as a result of transmission of the laser-radiation, at the surface of the crystal in contact with the widow is transferred to the window, thereby reducing the potential for optical damage to the crystal surface. The window is sufficiently thick that it has an odd integer multiple of quarter-wavelengths optical thickness at a wavelength about equal to a wavelength of the laser-radiation, thereby behaving as an antireflection device for the crystal at that wavelength. In one embodiment of the optical assembly, the active optical crystal is a cadmium telluride crystal and the window is formed from cesium bromide.
Owner:COHERENT INC
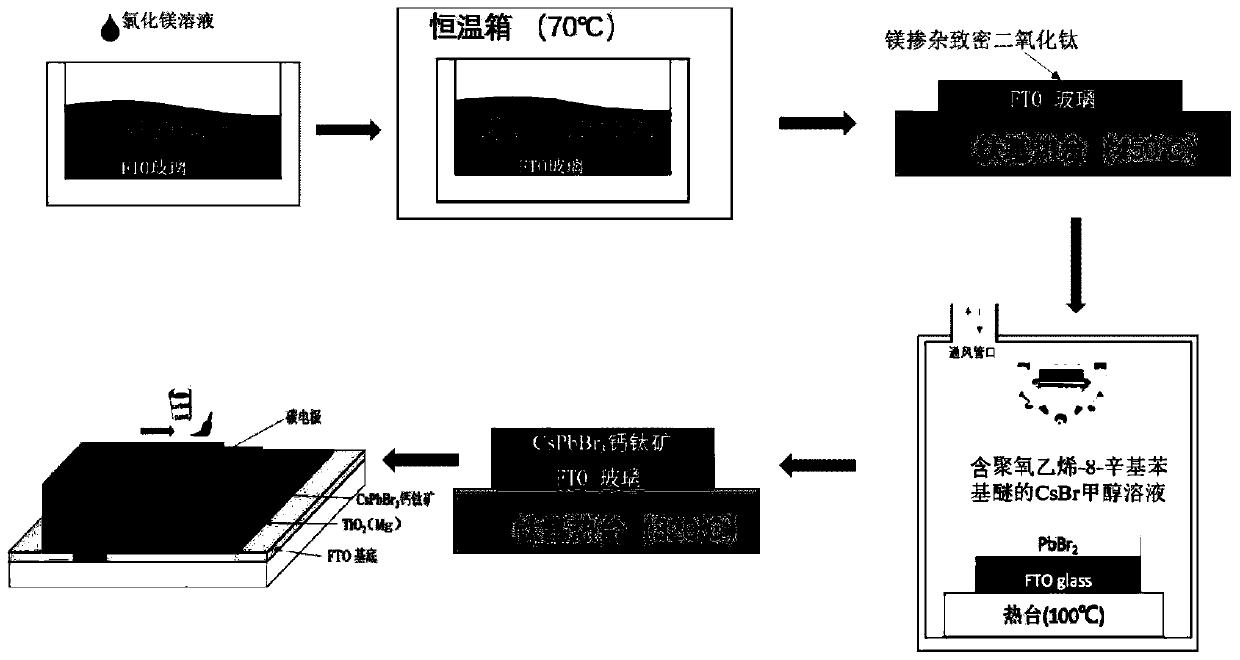
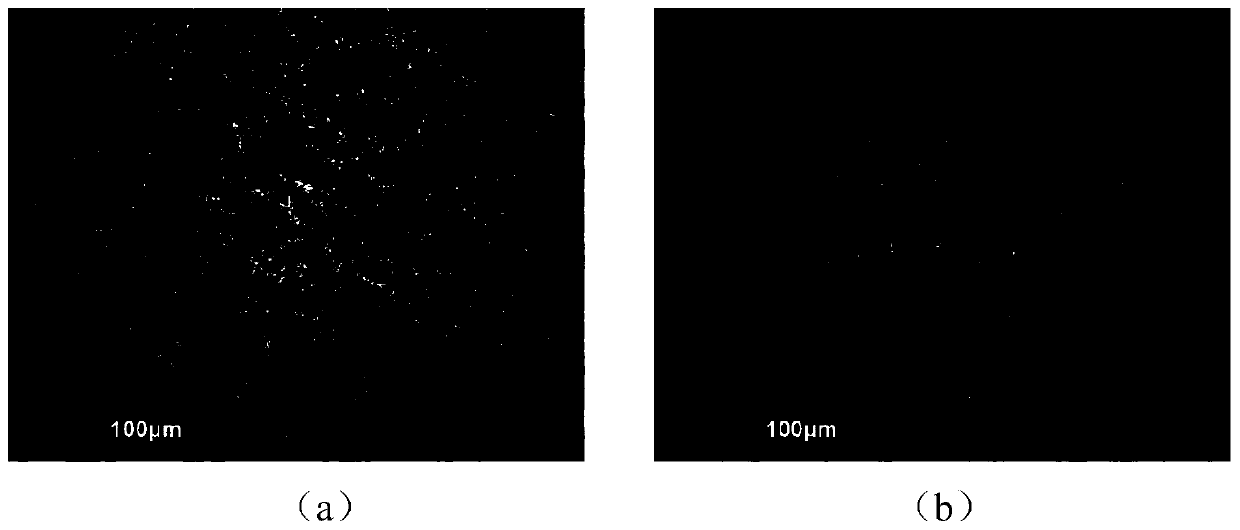
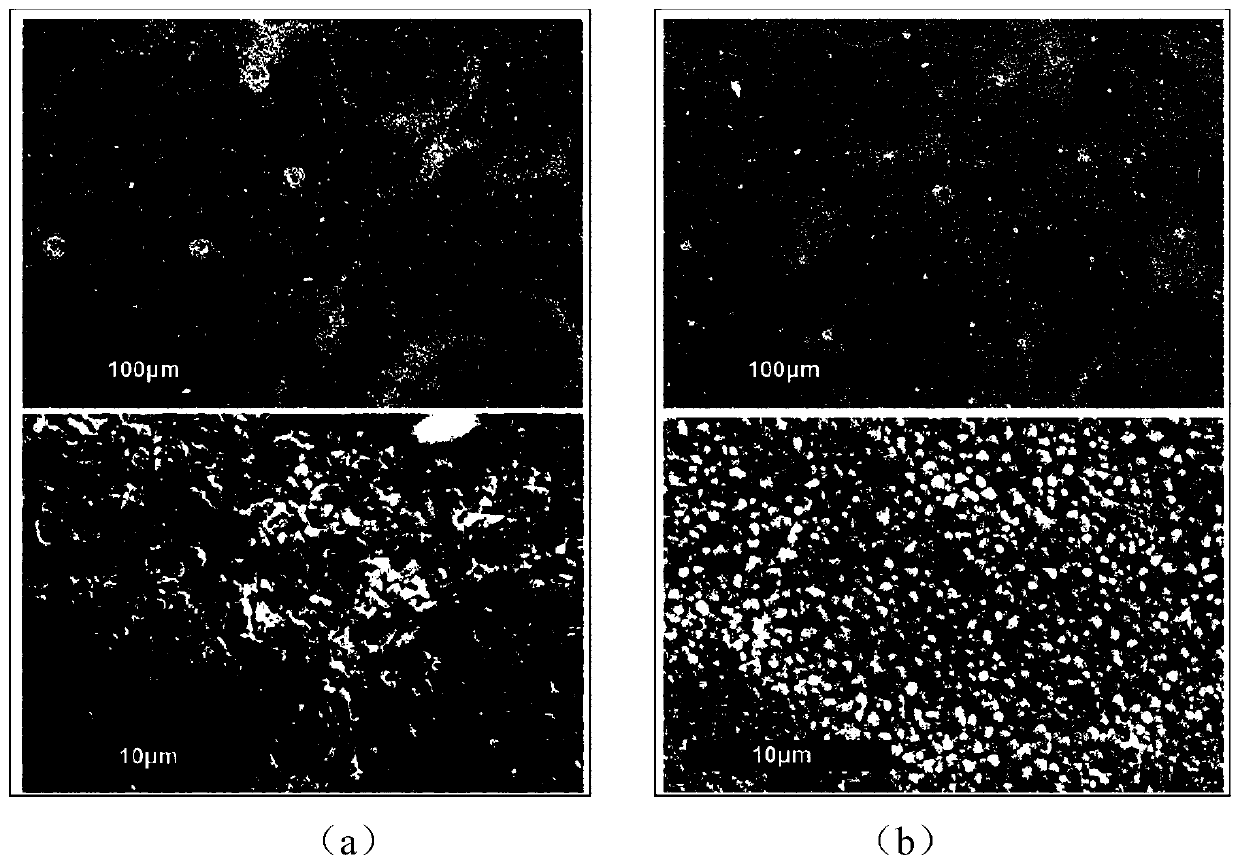
Preparation method and application of CsPbBr3 inorganic perovskite film
ActiveCN110190151AOptimizing the interface energy level structureLower resistanceFinal product manufacturePhotovoltaic energy generationTwo stepFermi level
The invention provides a preparation method and an application of a CsPbBr3 inorganic perovskite film. The preparation method of the CsPbBr3 inorganic perovskite film comprises the steps of firstly, preparing a magnesium doped titanium dioxide compact layer by adopting a mode of chemical bath deposition, and then depositing lead bromide and cesium bromide on the magnesium doped titanium dioxide compact layer according to a two-step deposition process by adopting ultrasonic spraying. The prepared CsPbBr3 inorganic perovskite film is enabled to be generated in a large area, and the application range of the CsPbBr3 inorganic perovskite film is improved. According to the invention, the magnesium element is taken as an effective doping agent and introduced into the titanium dioxide compact layer in a simple way, thereby enabling the Fermi level of titanium dioxide to move up, thus optimizing the interface energy level structure between a CsPbBr3 perovskite layer and a titanium dioxide electron transport layer, increasing the free carrier concentration of the film, enabling the resistance of TiO2 to be reduced, and reducing the series resistance of the whole device. The photoelectric conversion efficiency of all-inorganic perovskite solar cells doped with magnesium can reach up to 5.75%.
Owner:WUHAN UNIV OF TECH
Chemical sensitivity screening test
ActiveUS20160169872A1Analysis using chemical indicatorsMaterial analysis by observing effect on chemical indicatorBoron trioxideErythrosine
The present document describes a screening composition comprising a marker compound, chosen from at least one of iodine, and fluorescein; eosin Y, erythrosine, ponceau S, calcein, a catalyst, chosen from at least one boron trioxide (B2O3), potassium (K), Gallium (III) oxide (Ga2O3), Nickel (II) oxide (NiO), Vanadium (V) oxide (V2O5), magnesium oxide (MgO), a bismuth oxide chosen from bismuth subcarbonate [Bi2O2(CO3)], bismuth chloride oxide (BiClO), and bismuth oxide (Bi2O3), cesium bromide (CsBr), lanthanum (III) oxide (La2O3), molybdenum (VI) oxide (MoO3), neodymium oxide (Nd2O3), Nickel (II) carbonate anhydrous (NiCO3); and a pigment, chosen from at least one of scandium (III) oxide (Sc2O3), Lead (IV) oxide (PbO2), Sulfur (S) powder, and Tungsten (VI) oxide (WO3), chromium (III) oxide (Cr2O3), copper (II) oxide (CuO), copper (I) oxide (Cu2O), iron (III) oxide (Fe2O3), lead (II) oxide (PbO). The document also describes method of using the same.
Owner:BRYSON PATENTS
Room-temperature synthetic method of ultra pure inorganic halogen perovskite blue light nanosheet
InactiveCN108359457AImprove luminosityMake up for the shortcoming of low luminous efficiency and instabilityLuminescent compositionsSolventOleic Acid Triglyceride
The invention provides a room-temperature synthetic method of an ultra pure inorganic halogen perovskite blue light nanosheet. The method comprises the following steps: 1) preparing mixed liquor of oleylamine, oleic acid and toluene; 2) adding a precursor of lead bromide in the mixed liquor after the reaction in the step 1) is completed, uniformly stirring the materials, and adding a precursor ofcesium bromide; taking the precursor of lead bromide as a solvent of dimethyl sulfoxide; taking a hydrobromic acid solution with 40% weight ratio of the precursor of cesium bromide as a solvent; 3) injecting a butanol solution in the mixed liquor after the reaction in the step 2) is completed, fully reacting the materials, and performing centrifugation collection to obtain a supernatant; and 4) performing microwave heating on the supernatant and performing re-centrifugation, and depositing and dispersing the centrifuged precipitate at a ground floor in a toluene solution. The method has the advantages of simple operation and high efficiency, the luminescence property of the ultra pure inorganic halogen perovskite blue light nanosheet is excellent, and the shortage of the low luminescence efficiency and non stabilization of an inorganic halogen perovskite blue light nano-material in the prior art can be effectively made up.
Owner:NANJING UNIV OF SCI & TECH
Preparation method of inorganic perovskite-coated MXene two-dimensional layered anode material
ActiveCN109786718AImprove structural stabilityImprove stabilityCell electrodesSecondary cellsShielding gasVacuum drying
The invention discloses a preparation method of an inorganic perovskite-coated MXene two-dimensional layered anode material and relates to the technical field of preparation of lithium ion battery anode materials. The preparation method comprises the following steps: adding lead bromide and cesium bromide into N,N-dimethyl formamide, introducing protective gas, heating and stirring, so that an inorganic perovskite material precursor is obtained; adding Ti3AlC2 into HF aqueous solution, stirring and reacting, centrifuging, washing, and performing vacuum drying, so that Ti3C2 powder is obtained;adding the Ti3C2 powder into the inorganic perovskite material precursor, heating, and stirring, so that precursor solution is obtained; adding methylbenzene into the precursor solution, centrifuging, and performing vacuum drying, so that the inorganic perovskite-coated MXene two-dimensional layered anode material is obtained. The preparation method disclosed by the invention enhances structuralstability of a MXene two-dimensional layered anode material Ti3C2 by coating the MXene two-dimensional layered anode material Ti3C2 with the inorganic perovskite material, so that the anode material has good stability and excellent physical and chemical properties; meanwhile, compared with the conventional high-cost technology that a lithium battery anode material needs to be graphitized and coated with carbon at high temperature, the preparation method disclosed by the invention is simple in technology and low in cost.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
Blue-light perovskite thin film, preparation thereof, and inverted quasi-two-dimensional blue-light perovskite light-emitting diode
ActiveCN113130802AGood lookingEfficient transferSolid-state devicesSemiconductor/solid-state device manufacturingOrganosolvHole transport layer
The invention belongs to the technical field of light-emitting diodes, and discloses a blue-light perovskite thin film, preparation thereof and an inverted quasi-two-dimensional blue-light perovskite light-emitting diode. The method comprises the following steps of: 1) preparing lead bromide, large-group organic halide and cesium bromide into a perovskite precursor solution by adopting an organic solvent; and 2) spin-coating the precursor solution, dropwise adding an anti-solvent in the spin-coating process, and removing the solvent to obtain the blue-light perovskite thin film. The inverted quasi-two-dimensional blue-light perovskite light-emitting diode comprises a cathode, an electron transport layer, an interface layer, a light-emitting layer, a hole transport layer, a hole injection layer and an anode distributed from bottom to top sequentially, wherein the light-emitting layer is the blue-light perovskite thin film. According to the method, the exciton binding energy of the blue-light perovskite can be increased, the morphology of the thin film is improved, meanwhile, formation of a low-dimensional phase (n = 1) is inhibited, and the luminous efficiency of the quasi-two-dimensional blue-light perovskite is improved. The blue light perovskite thin film is used for a light emitting diode, and therefor, blue light emission is achieved, and the light emitting efficiency is improved.
Owner:SOUTH CHINA UNIV OF TECH
Ultra-small perovskite quantum dots and preparation method thereof
ActiveCN110684529AControllable growthGood chemistryFinal product manufactureNanoopticsIce waterMesoporous silica
The invention relates to a preparation method of ultra-small perovskite quantum dots, which comprises the following steps: weighing solid powders of lead bromide and cesium bromide respectively, dissolving them in ethanol solution, and performing heat preservation treatment to obtain reactant precursor solution; dispersing mesoporous silica in chloroform solution containing oleylamine and oleic acid, stirring, and placing the obtained mesoporous silica suspension in an ice water bath for later use; adding the reactant precursor solution into the mesoporous silica suspension, reacting and crystallizing under stirring conditions, then separating and washing the solids in the mixed liquid to obtain the ultra-small perovskite quantum dots. Compared with the prior art, the prepared ultra-smallquantum dot has the advantages of stable, orderly and controllable growth, has excellent chemical and optical stability, and has good application prospect on LED devices.
Owner:SHANGHAI INST OF TECH
Method for solid-phase synthesis of green fluorescent perovskite
ActiveCN110776912AThe synthesis method is simpleSynthetic raw materials are readily availableLuminescent compositionsLead compoundsPhysical chemistryCesium bromide
The invention discloses a method for solid-phase synthesis of green fluorescent perovskite, and belongs to the technical field of perovskite fluorescent materials. The preparation method comprises: uniformly mixing lysine, cesium bromide and lead bromide to obtain mixed powder; putting the mixed powder into a centrifugal tube, carrying out light-shielding treatment on the centrifugal tube, puttingthe light-shielding centrifugal tube into a vortex mixer, carrying out an oscillating mixing reaction for 1-30 min, and carrying out light-shielding placement treatment for 1-30 min so as to obtain agreen fluorescent CsPbBr3 material; or grinding the mixed powder for 1-5 min or carrying out stirring mixing treatment for 5-30 min to obtain a green fluorescent CsPbBr3 material. According to the invention, high-stability and high-fluorescence-intensity CsPbBr3 is synthesized in one step by using cesium bromide, lysine and lead bromide as synthesis raw materials without a solution substrate, sothat the synthesis method is simple, the synthesis raw materials are easy to obtain, and the consumed time is short.
Owner:YUNNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com