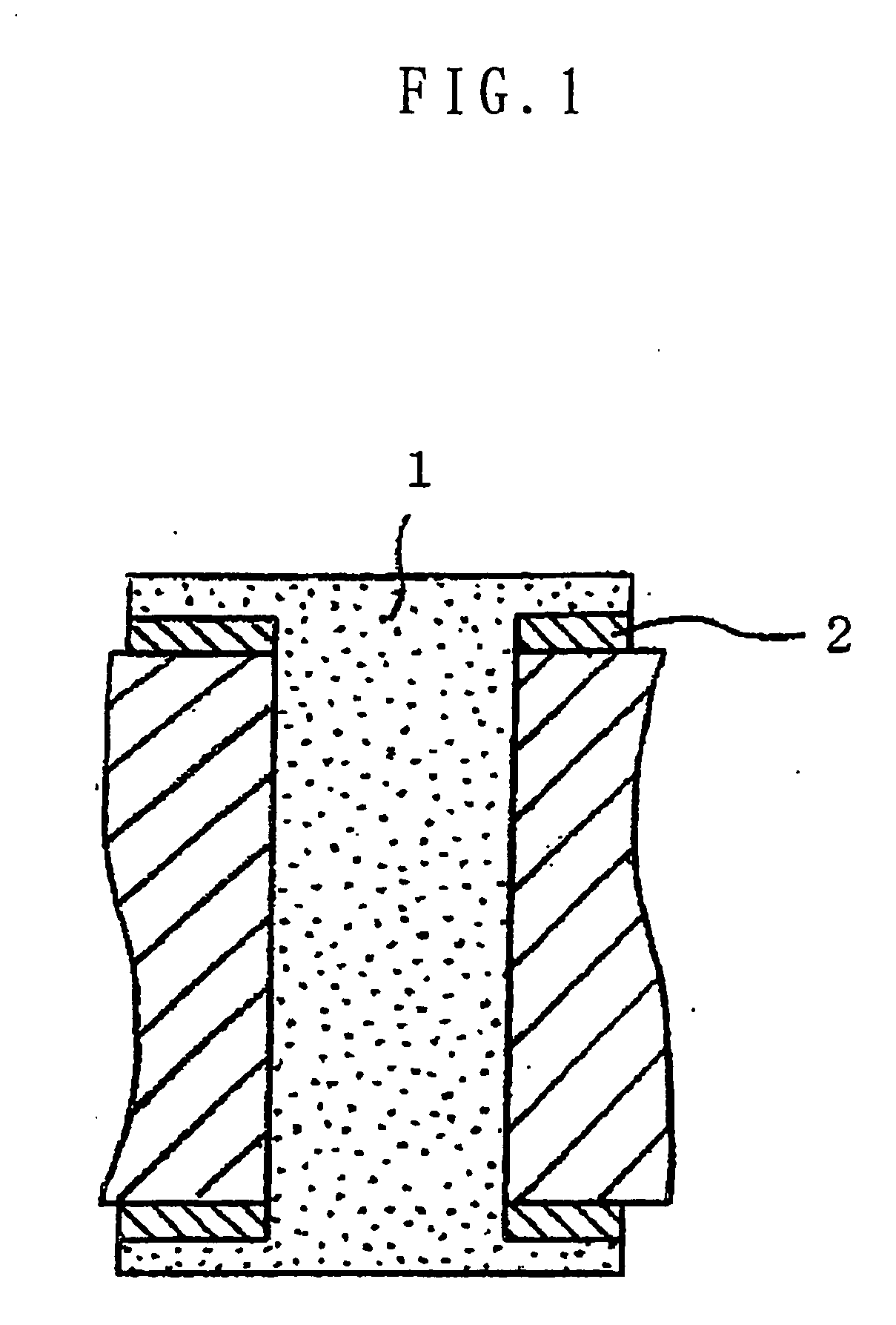
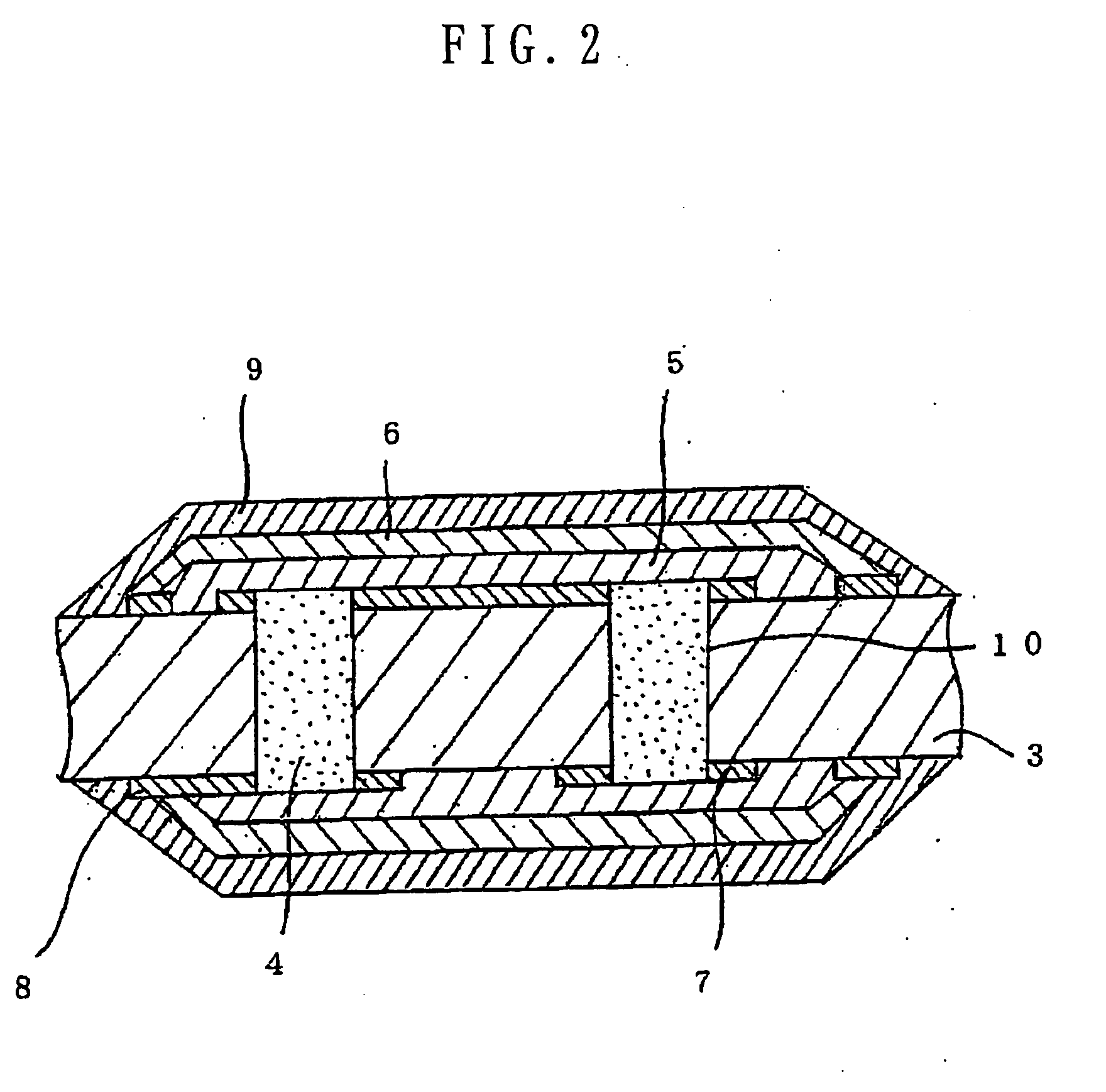
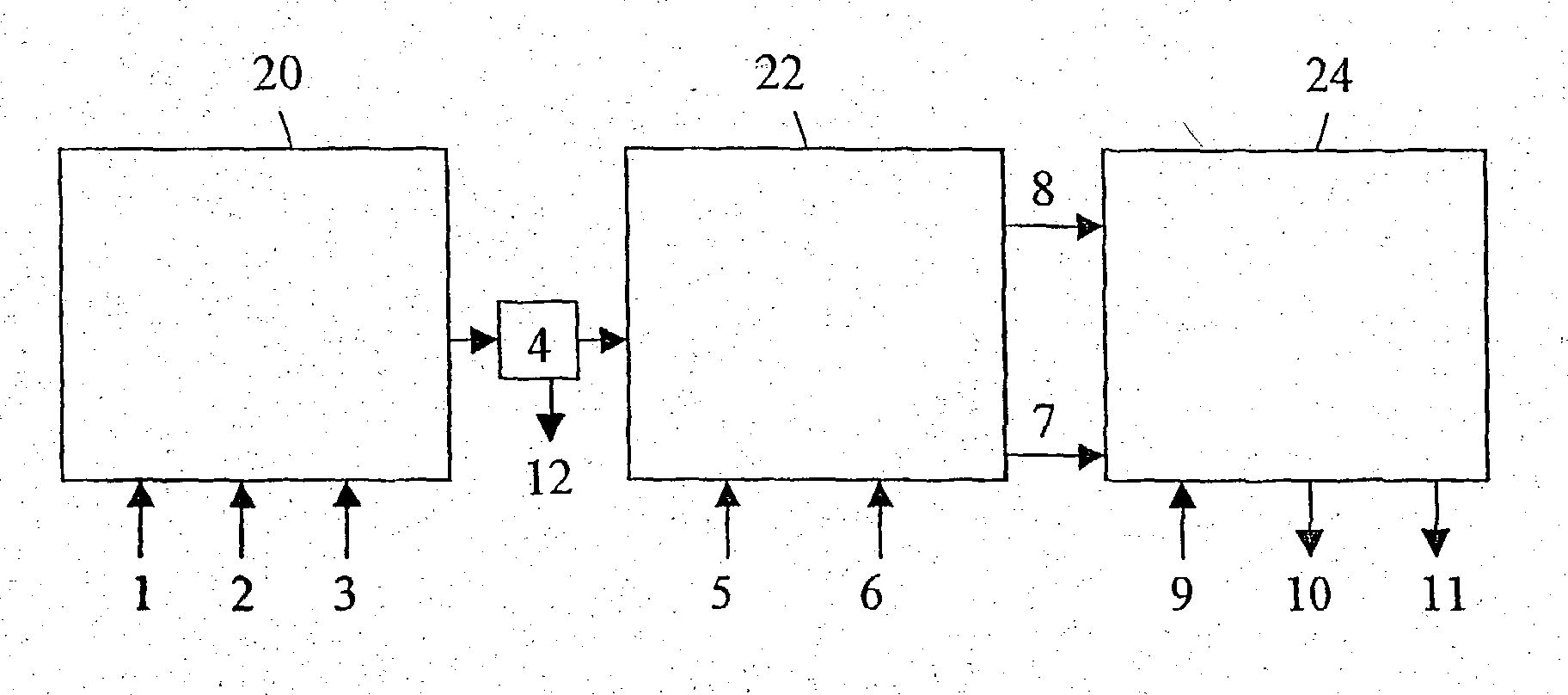
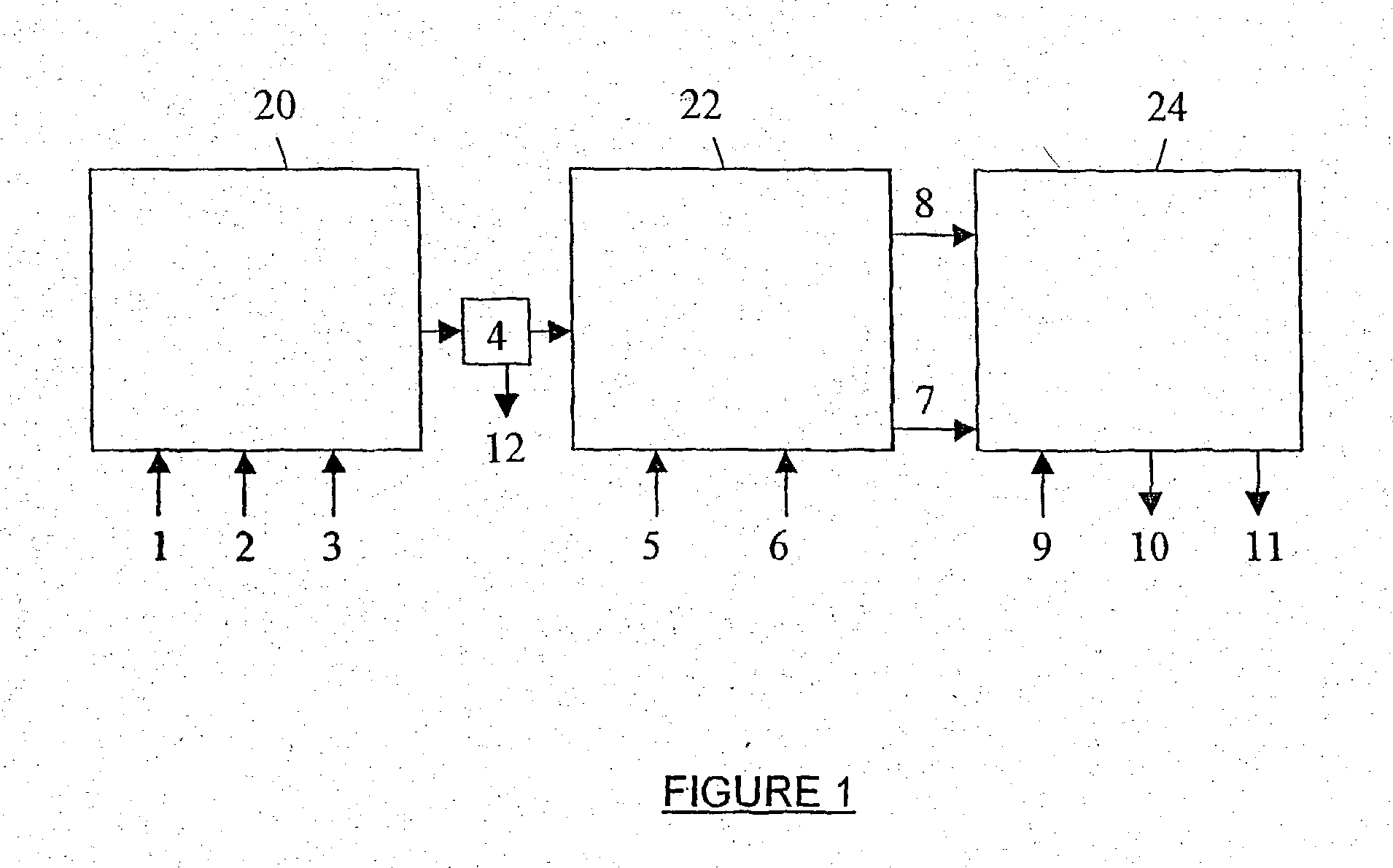
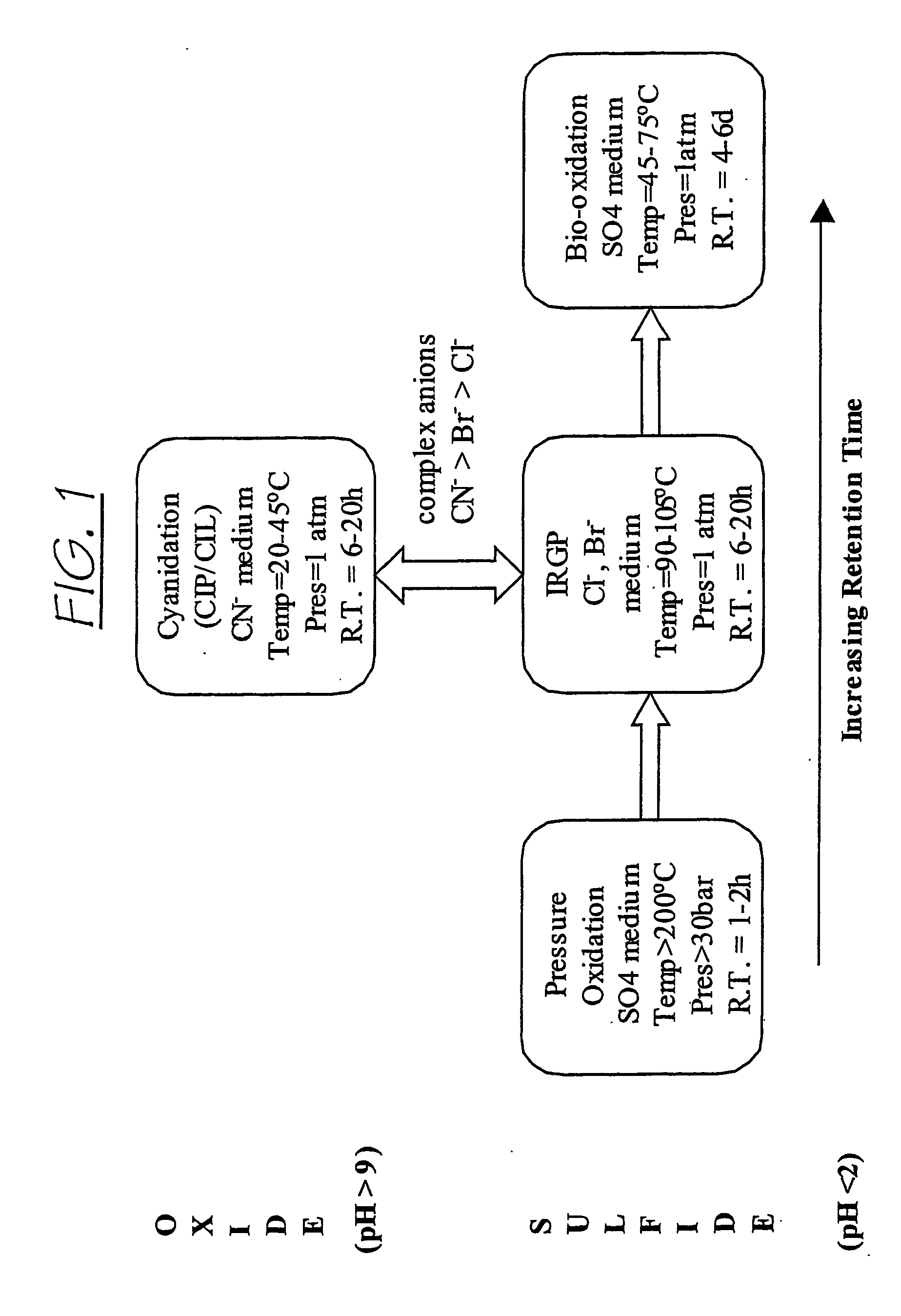
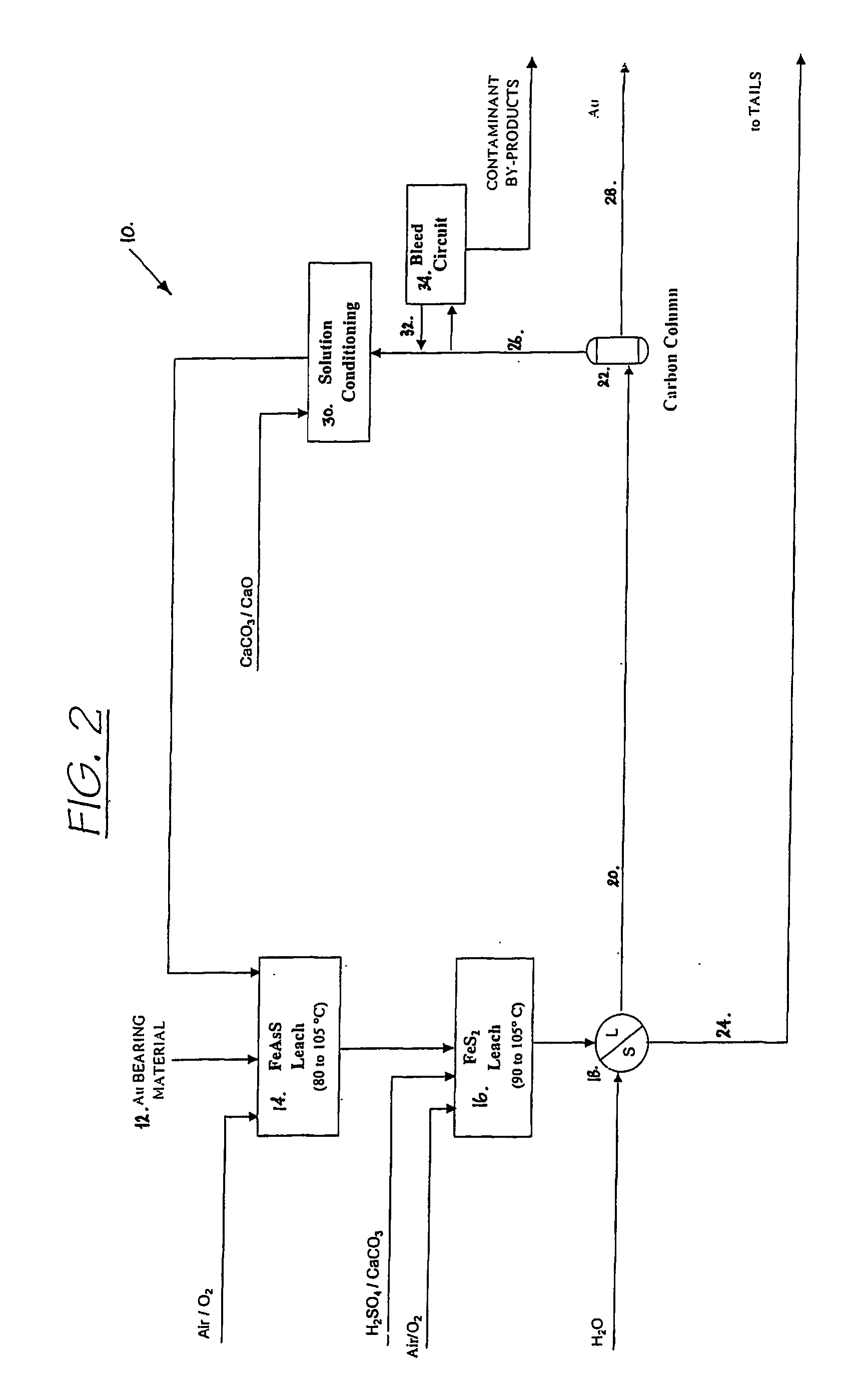
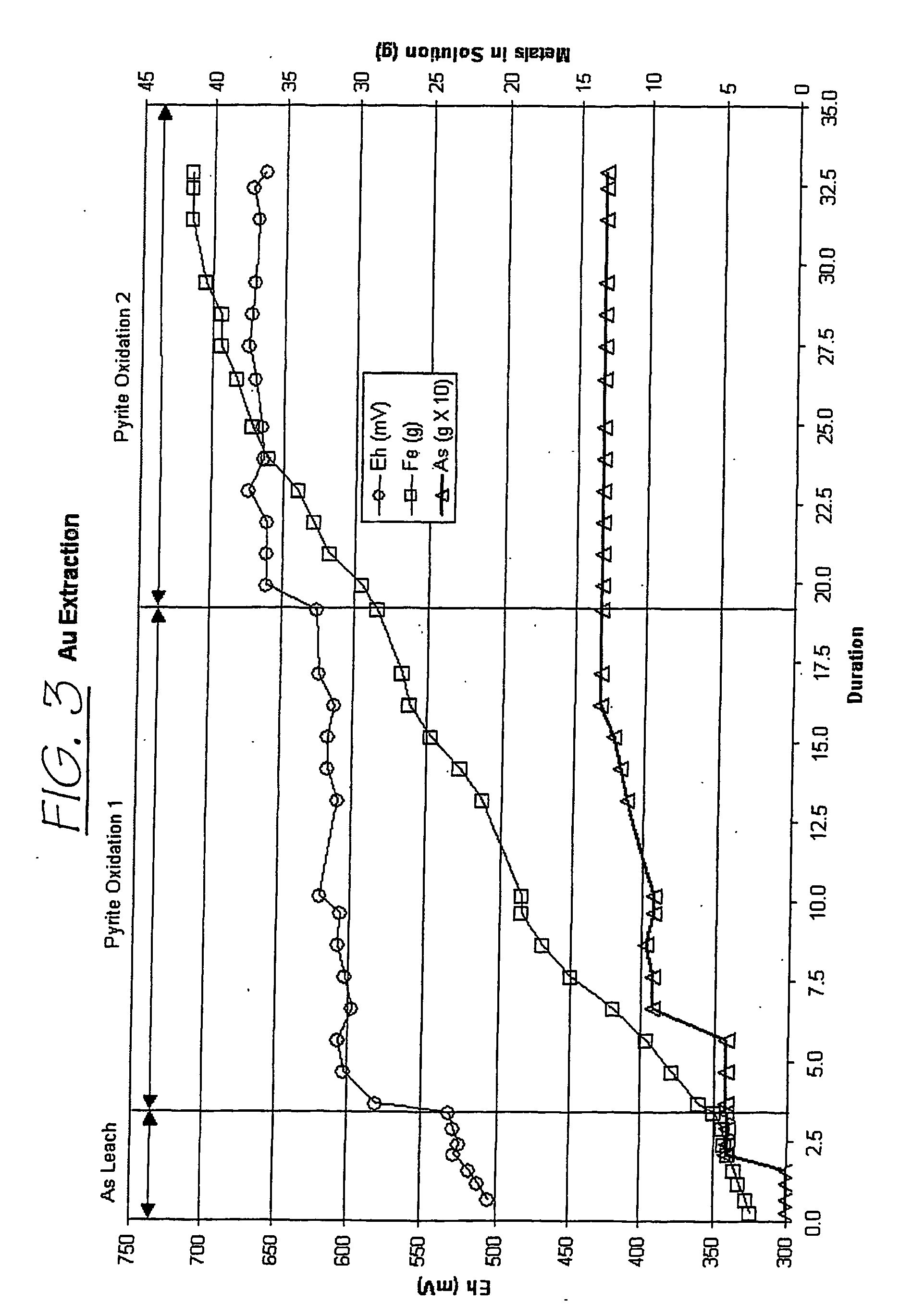
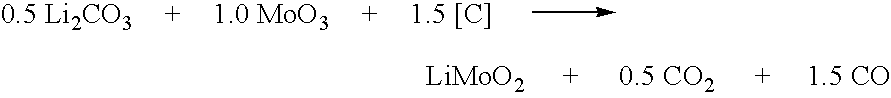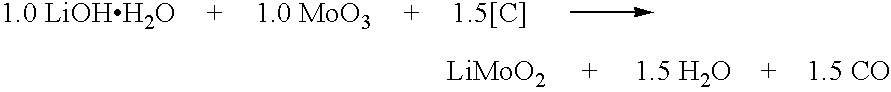Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
642results about "Copper compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Catalysts, activating agents, support media, and related methodologies useful for making catalyst systems especially when the catalyst is deposited onto the support media using physical vapor deposition
InactiveUS20050095189A1Improve performanceEasy to useMaterial nanotechnologyInternal combustion piston enginesGas phaseAdditive ingredient
Use of physical vapor deposition methodologies to deposit nanoscale gold on activating support media makes the use of catalytically active gold dramatically easier and opens the door to significant improvements associated with developing, making, and using gold-based, catalytic systems. The present invention, therefore, relates to novel features, ingredients, and formulations of gold-based, heterogeneous catalyst systems generally comprising nanoscale gold deposited onto a nanoporous support.
Owner:3M INNOVATIVE PROPERTIES CO
Monodisperse noble metal nanocrystals
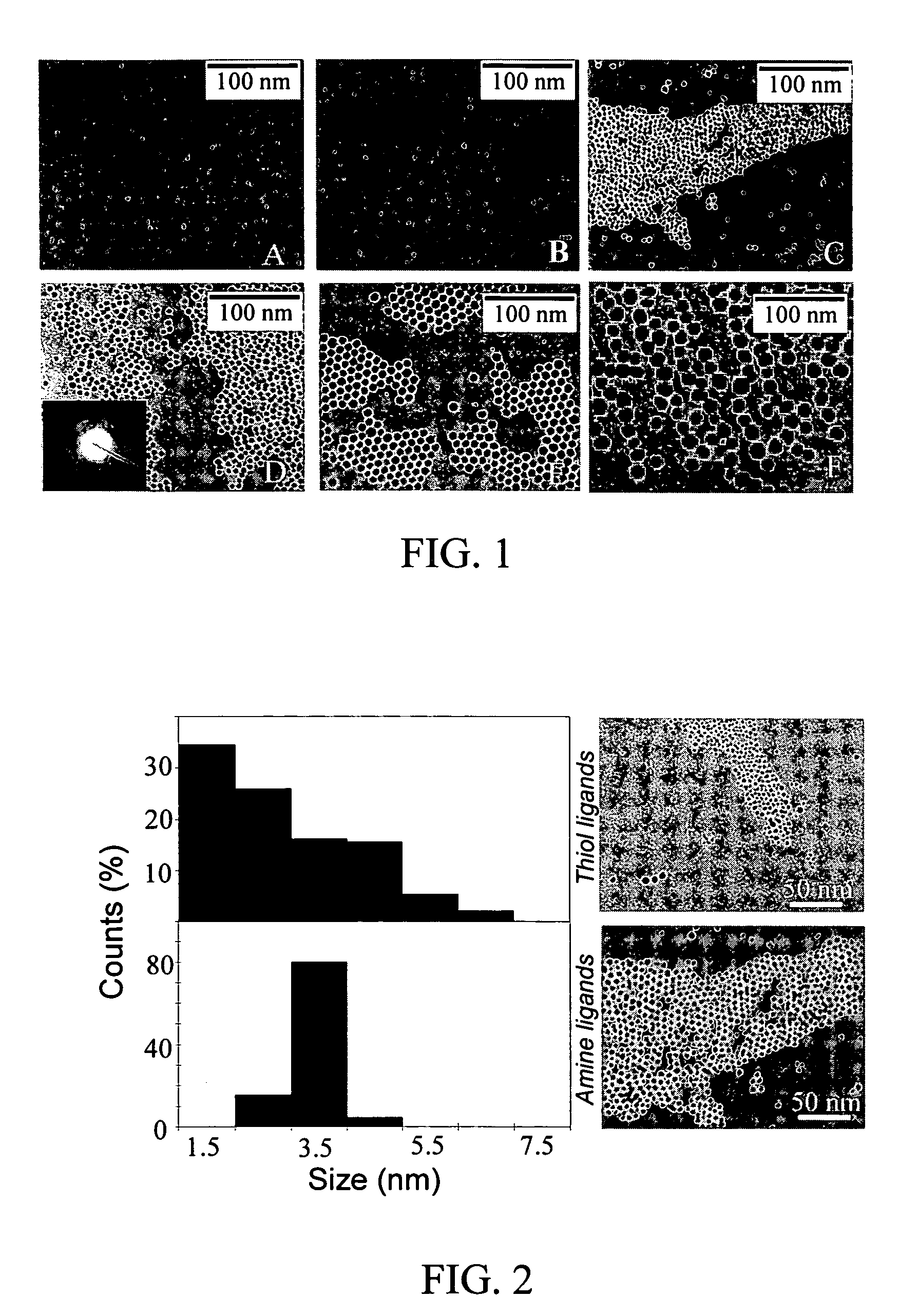
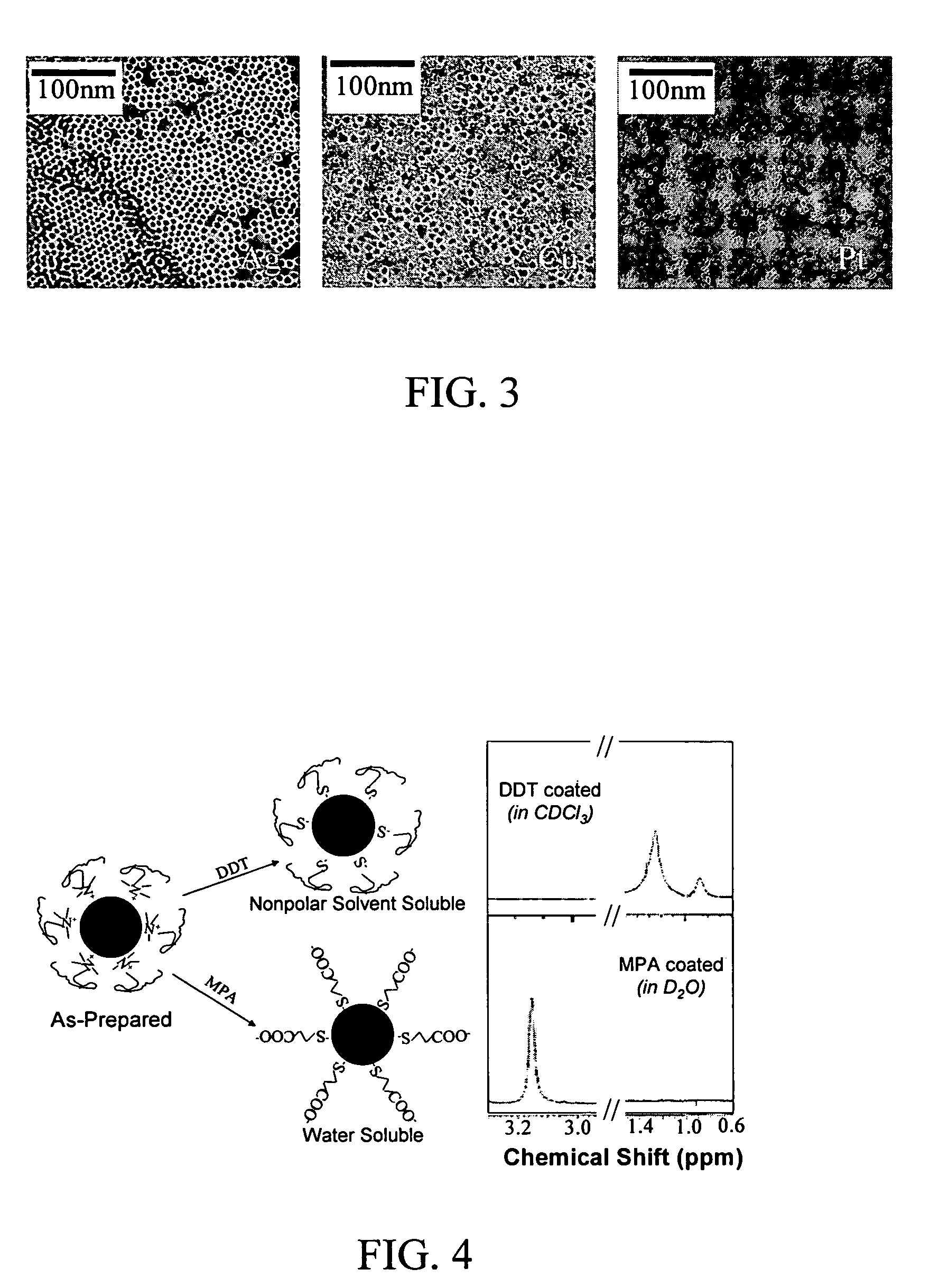
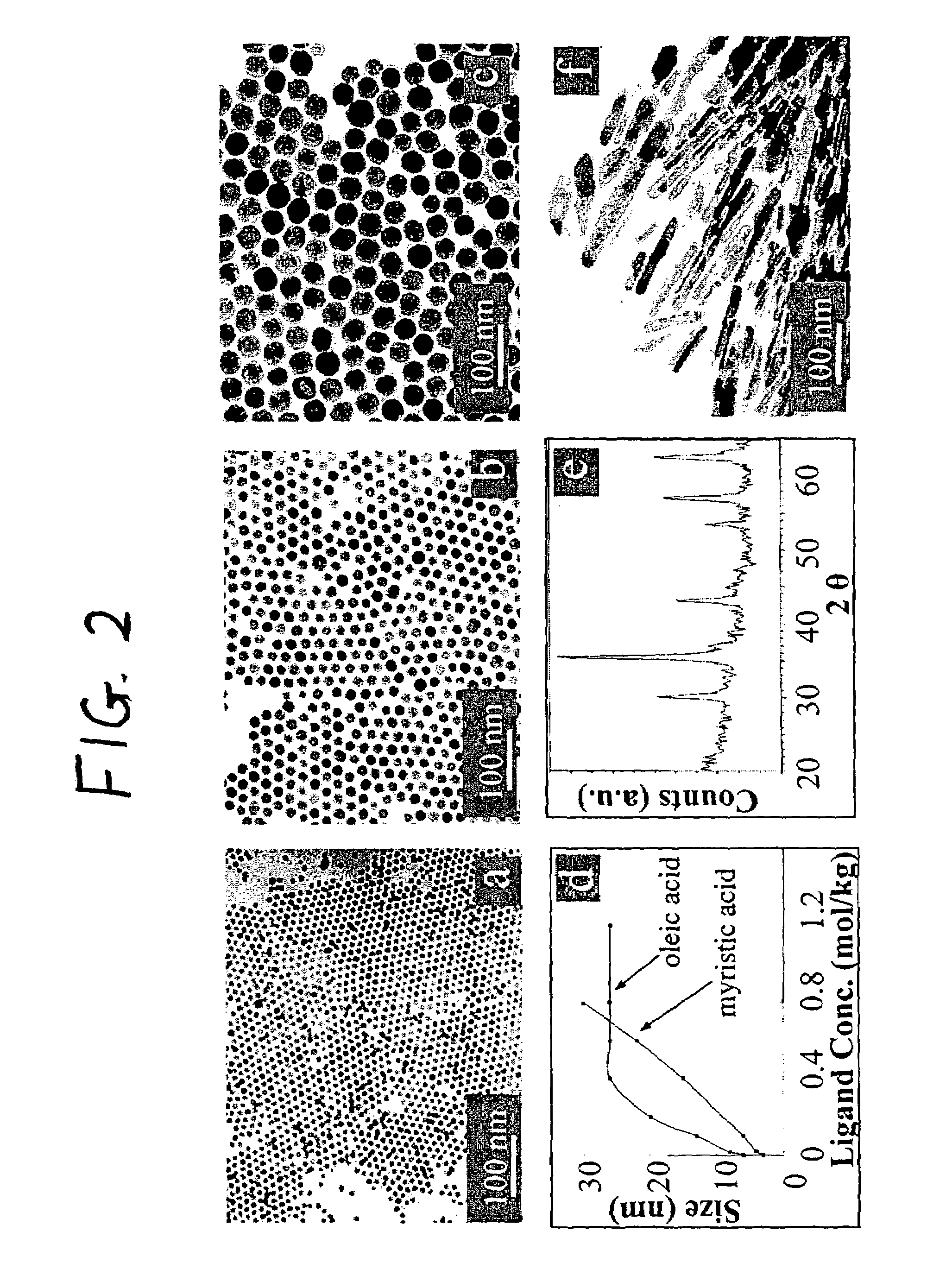
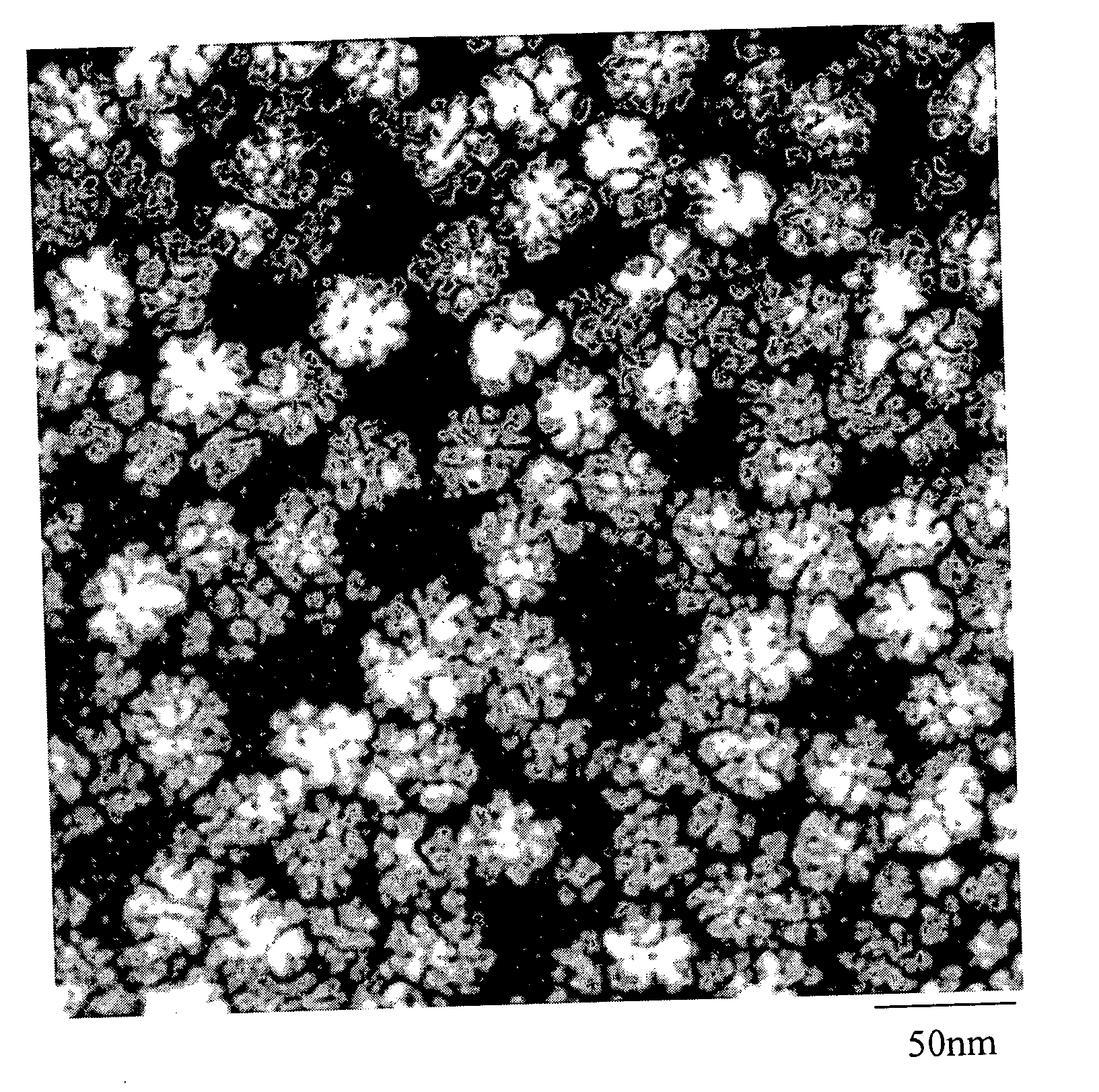
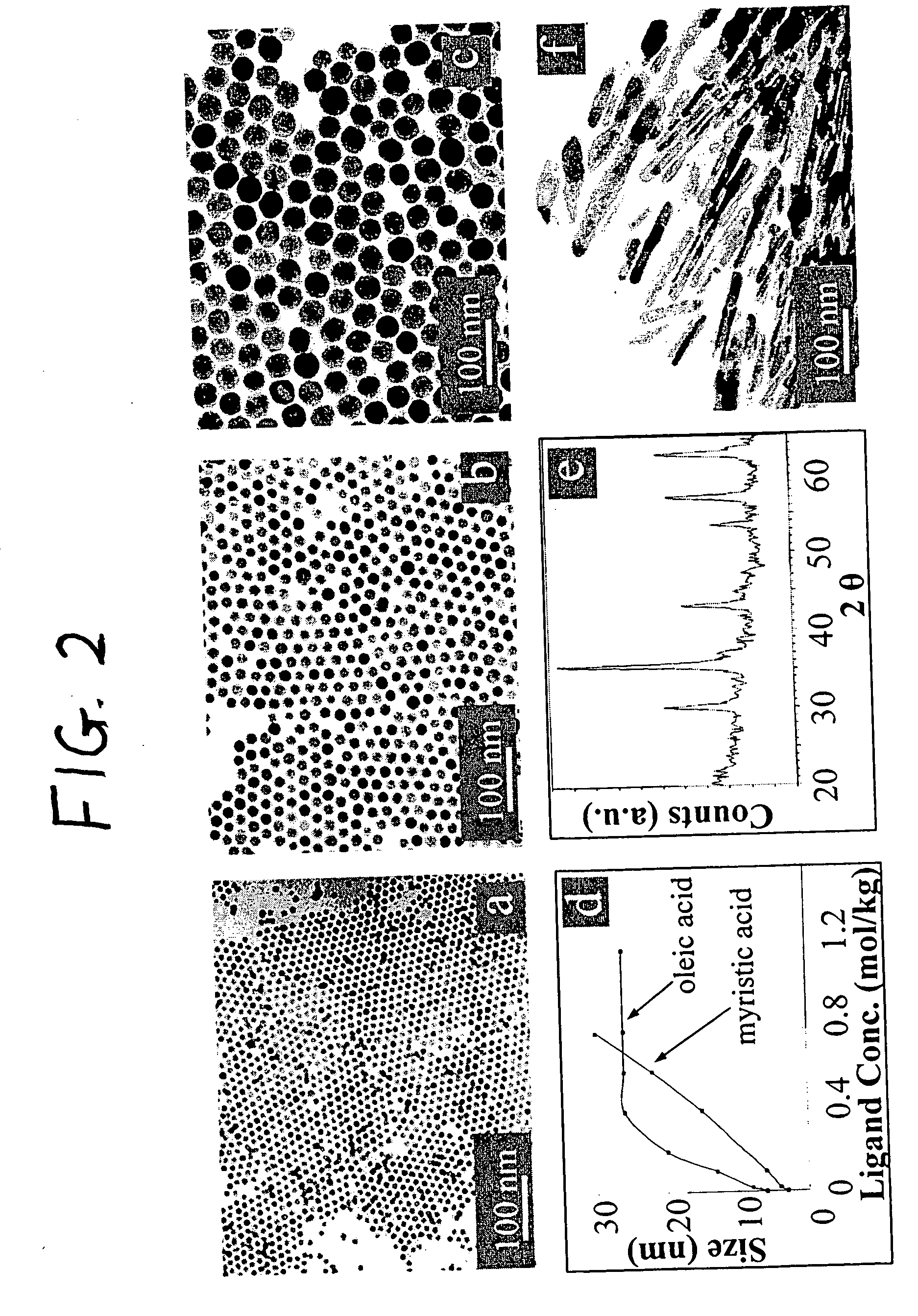
Nanoparticle compositions of noble metals, and methods of making them, are described. The nanoparticle compositions are made by reacting a salt or complex of a noble metal, such as Au, Ag, Cu or Pt, with a weak ligand, and a reducing agent, in a single liquid phase. The noble metal is typically provided as a halide or carboxylate. The ligand is preferably a fatty acid or aliphatic amine. The reducing agent is preferably a borohydride reagent, hydrazine, or a mixture thereof. Nanocrystals in the size range of 1 nm to 20 nm are produced, and can be made in substantially monodisperse form.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Catalysts, activating agents, support media, and related methodologies useful for making catalyst systems especially when the catalyst is deposited onto the support media using physical vapor deposition
InactiveUS7727931B2High catalytic activityTendency increaseMaterial nanotechnologyInternal combustion piston enginesGas phasePhysical chemistry
Use of physical vapor deposition methodologies to deposit nanoscale gold on activating support media makes the use of catalytically active gold dramatically easier and opens the door to significant improvements associated with developing, making, and using gold-based, catalytic systems. The present invention, therefore, relates to novel features, ingredients, and formulations of gold-based, heterogeneous catalyst systems generally comprising nanoscale gold deposited onto a nanoporous support.
Owner:3M INNOVATIVE PROPERTIES CO
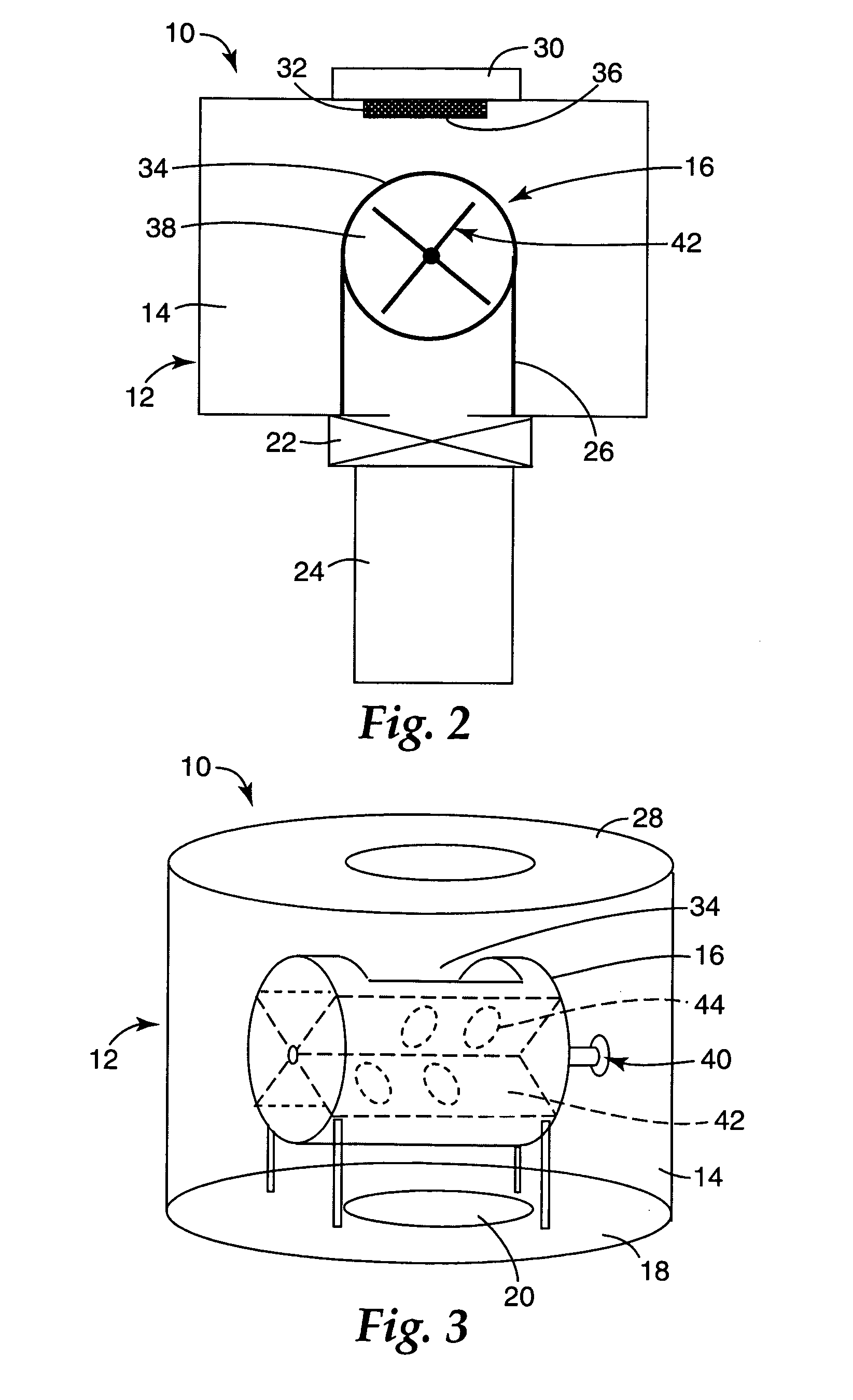
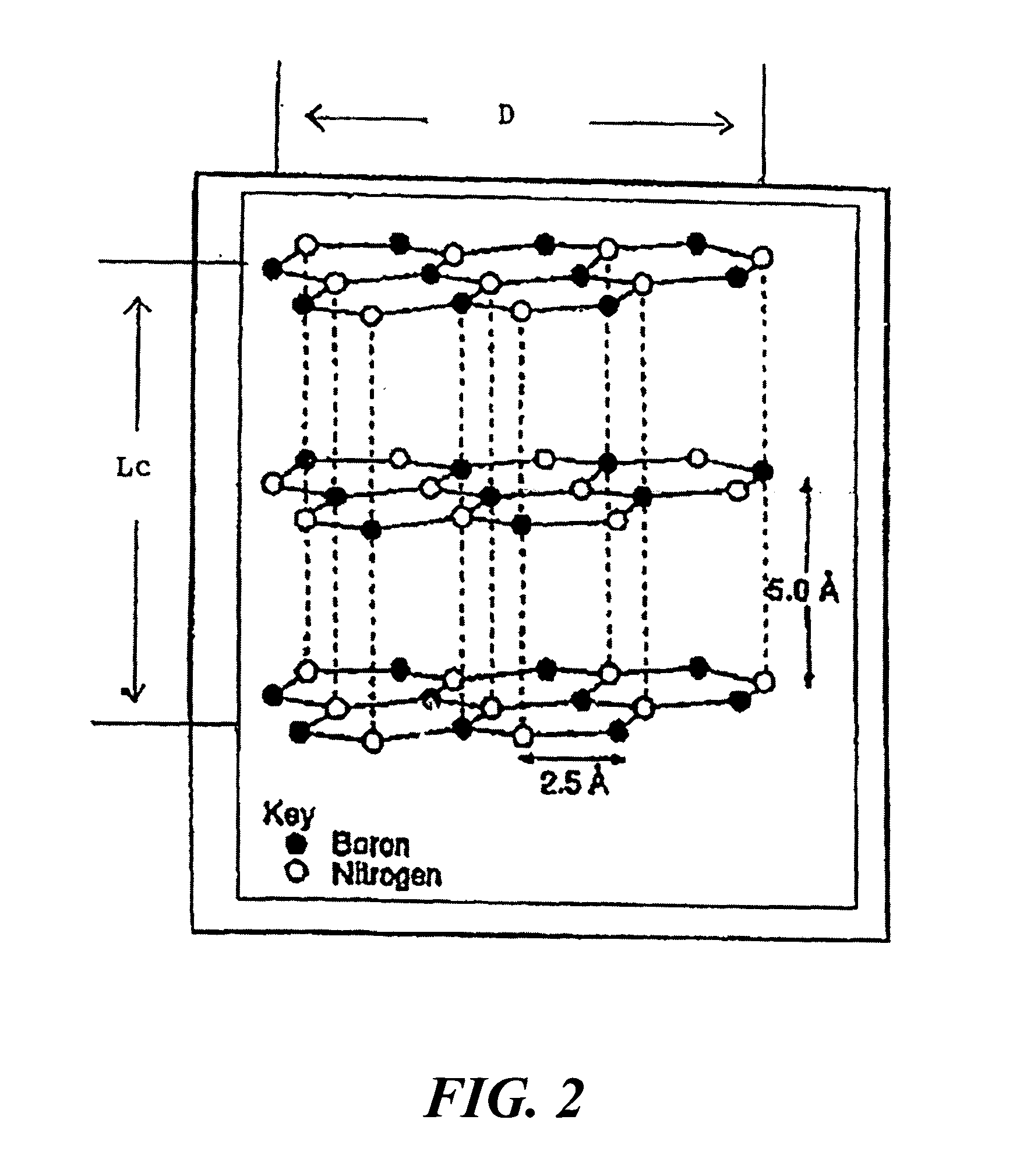
Boron nitride agglomerated powder
Novel boron nitride agglomerated powders are provided having controlled density and fracture strength features. In addition methods for producing same are provided. One method calls for providing a feedstock powder including boron nitride agglomerates, and heat treating the feedstock powder to form a heat treated boron nitride agglomerated powder. In one embodiment the feedstock powder has a controlled crystal size. In another, the feedstock powder is derived from a bulk source.
Owner:SAINT GOBAIN CERAMICS & PLASTICS INC
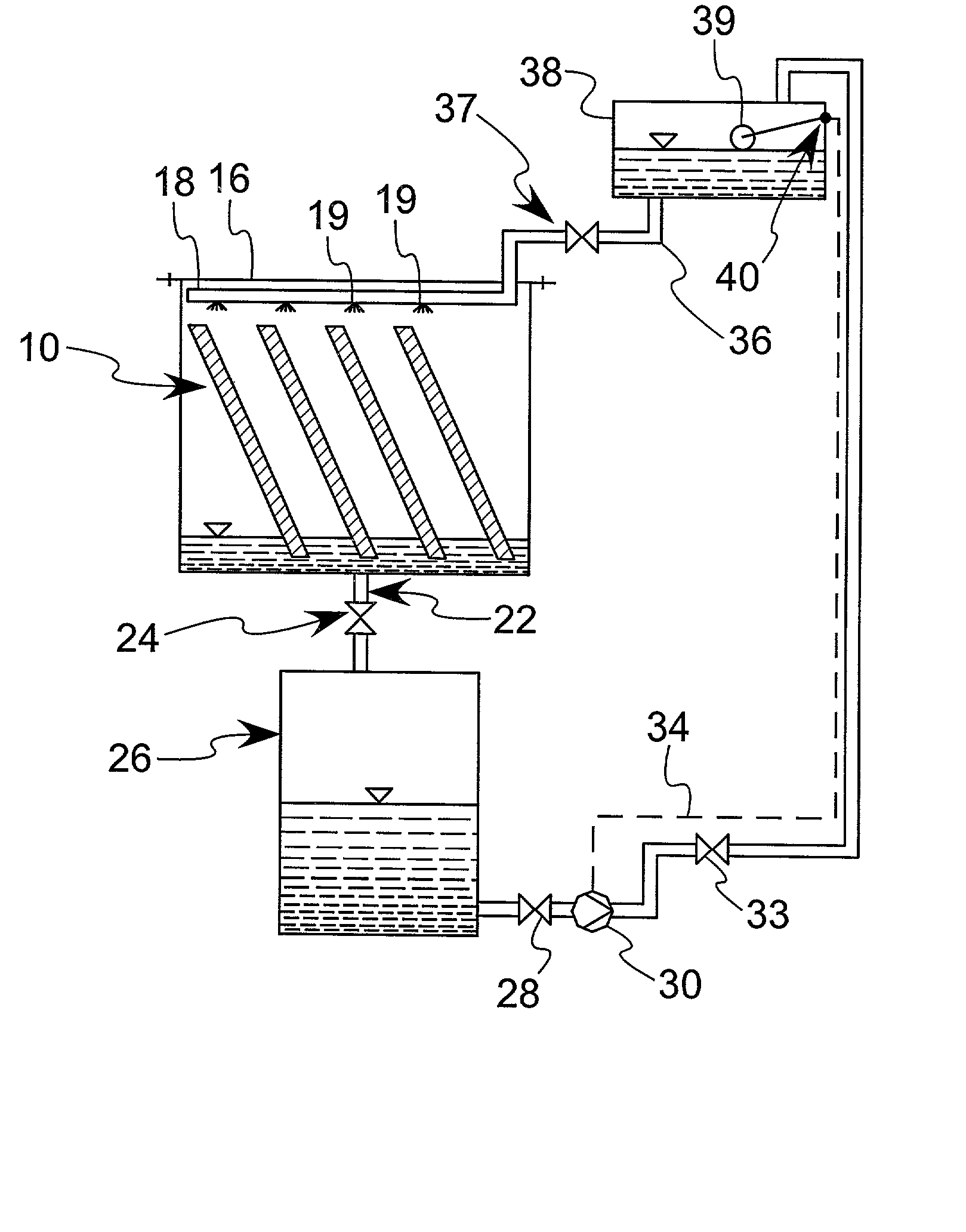
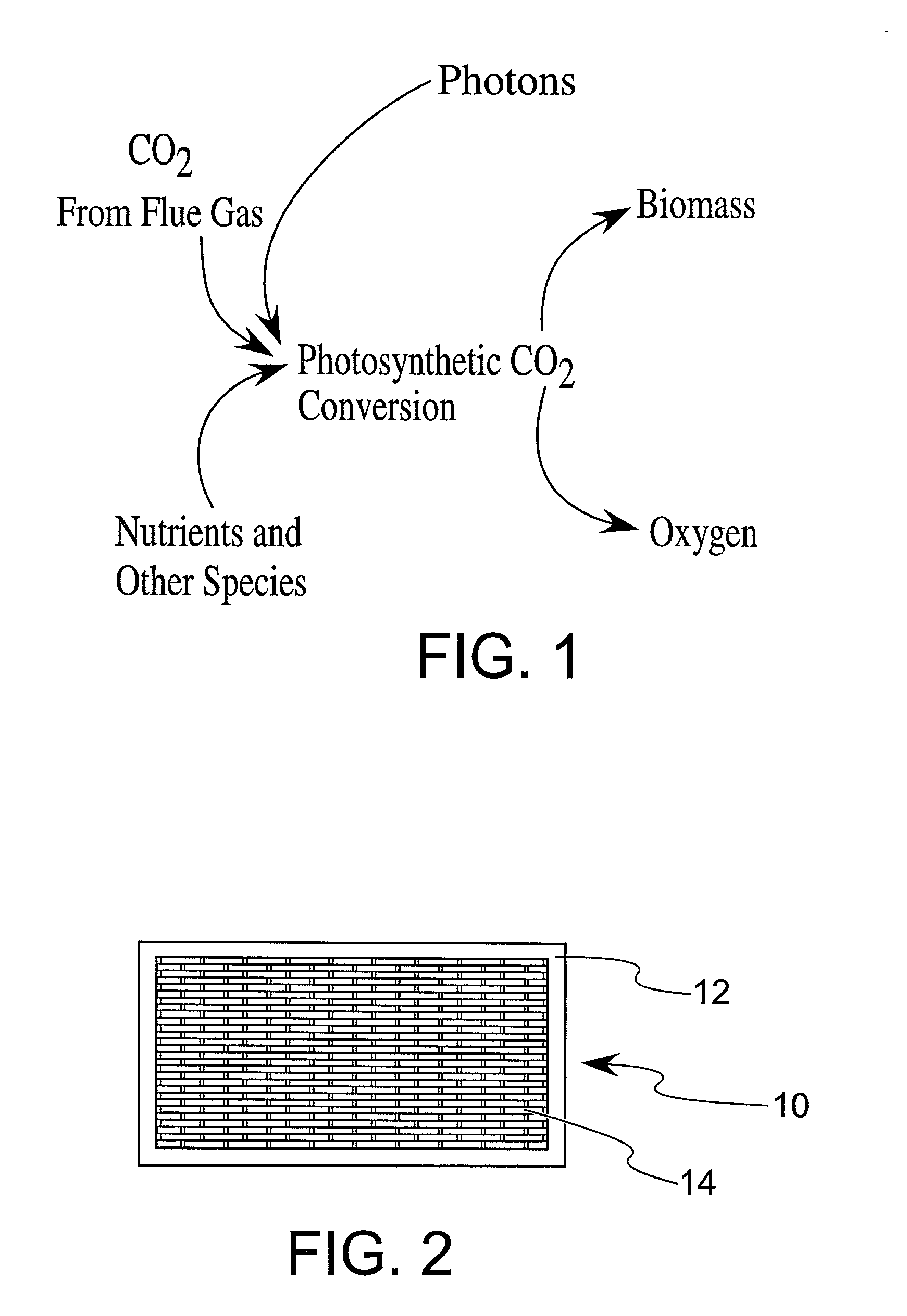
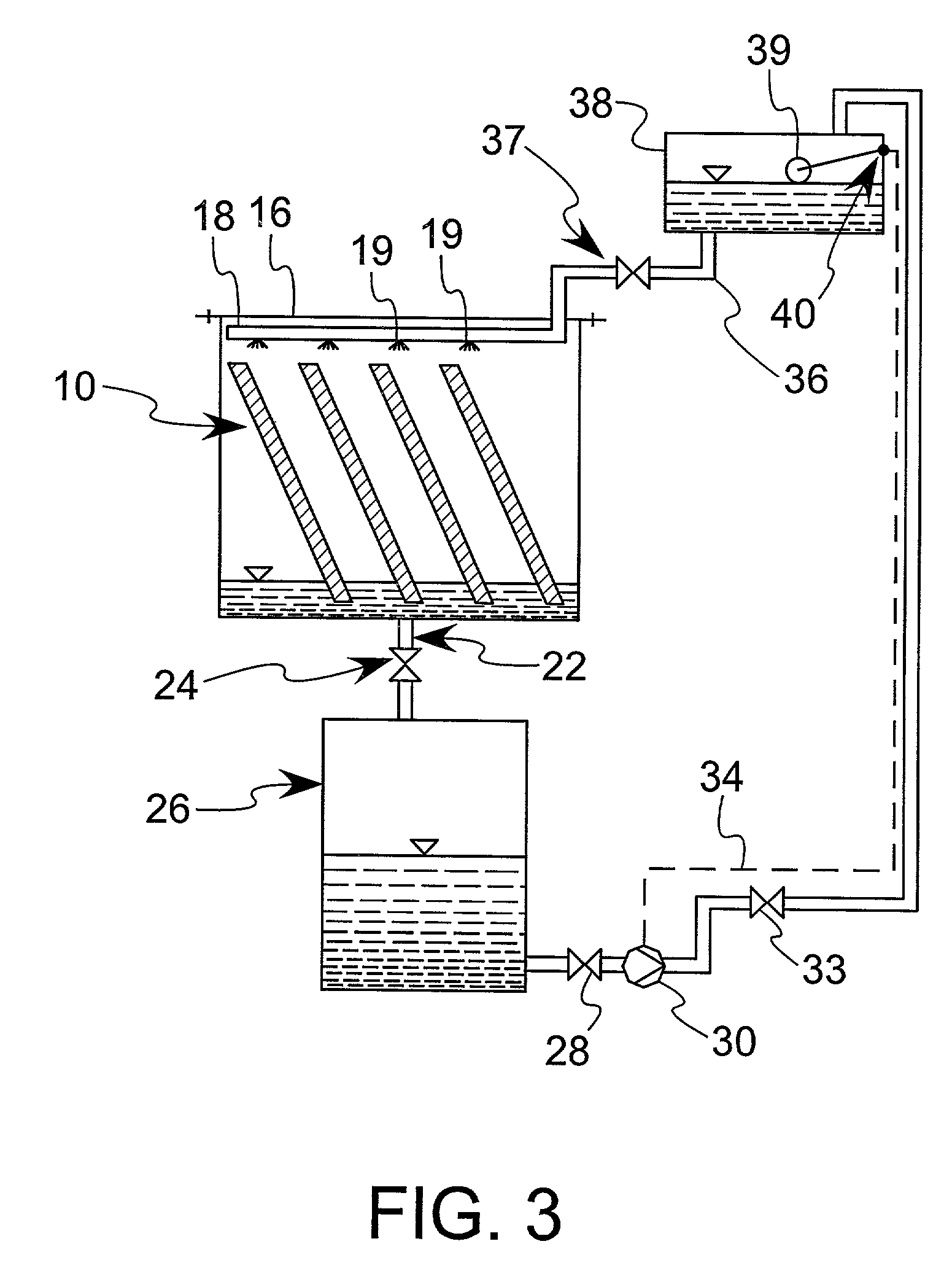
Enhanced practical photosynthetic CO2 mitigation
InactiveUS20020072109A1Increase the amount of waterBioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismCyanobacteria
This process is unique in photosynthetic carbon sequestration. An on-site biological sequestration system directly decreases the concentration of carbon-containing compounds in the emissions of fossil generation units. In this process, photosynthetic microbes are attached to a growth surface arranged in a containment chamber that is lit by solar photons. A harvesting system ensures maximum organism growth and rate of CO2 uptake. Soluble carbon and nitrogen concentrations delivered to the cyanobacteria are enhanced, further increasing growth rate and carbon utilization.
Owner:OHIO UNIV
Conductive paste
InactiveUS20060145125A1Pigmenting treatmentGroup 1/11 element organic compoundsConductive pasteAdhesive
The present invention provides an electroconductive paste that can contain a high proportion of an electroconductive powder, has excellent electroconductivity reliability and migration resistance, has a highly competitive price due to a reduced amount of silver plating, and is suitable for use in solder electrode formation, an electroconductive adhesive, etc. The electroconductive paste of the present invention comprises a binder and an electroconductive powder containing 80 to 97 wt % of a substantially spherical silver-coated copper powder in which the surface of a copper powder is coated with silver and the surface thereof is further coated with 0.02 to 0.5 wt % relative to the copper powder of a fatty acid, and 3 to 20 wt % of a flat-shaped silver-coated copper powder in which the surface of a copper powder is coated with silver and the surface thereof is further coated with 0.02 to 1.2 wt % relative to the copper powder of a fatty acid.
Owner:HITACHI CHEM CO LTD
Precipitated aragonite and a process for producing it
InactiveUS20030213937A1Less expensiveEfficient and less-expensiveCalcium/strontium/barium carbonatesInorganic/elemental detergent compounding agentsParticulatesAragonite
Disclosed is a novel form of particulate precipitated aragonite, and a novel process for producing it.
Owner:3P TECH
Metal oxide particle and its uses
InactiveUS20070154561A1High transparencyPromote absorptionMaterial nanotechnologyBiocideAcyl groupUltraviolet absorption
An object of the present invention is to provide a metal oxide particle which exercises more excellent ultraviolet absorbency as a matter of course and combines therewith merits of, for example, either being shifted in ultraviolet absorption edge toward the longer wavelength side and being excellent also in the absorption efficiency of a long-wavelength range of ultraviolet rays, or having good transparency and, for example, even in cases where added into or coated onto substrates, not damaging the transparency or hue of the substrates. As a means of achieving this object, a metal oxide particle according to the present invention is a metal oxide particle such that a hetero-element is contained in a particle comprising an oxide of a specific metal element (M), wherein the metal oxide particle is: 1) a metal oxide particle in the form of a fine particle wherein the hetero-element is at least one specific metal element (M′); 2) a metal oxide particle wherein the hetero-element includes at least two specific metal elements (M′); 3) a metal oxide particle wherein: the hetero-element is a more specified metal element (M′) and at least a part thereof is 2 in valence; or the metal element (M) is a more specified metal element and the metal oxide particle is in a specific range in crystal grain diameter in the vertical direction to each of the (002) plane and the (100) plane; or 4) a metal oxide particle wherein: the hetero-element is at least one specific nonmetal element and an acyl group is contained in the particle; or the hetero-element includes at least two specific nonmetal elements; or the hetero-element is at least one specific nonmetal element and a component derived from a metal element (M′) other than the metal element (M) is contained in the particle.
Owner:NIPPON SHOKUBAI CO LTD
Synthetic control of metal oxide nanocrystal sizes and shapes
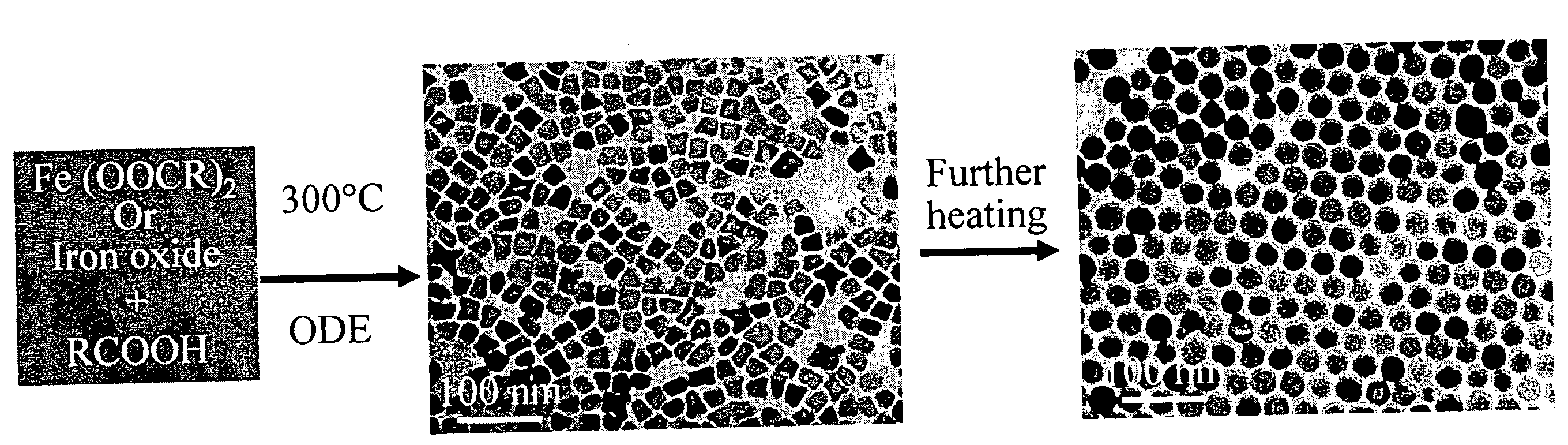
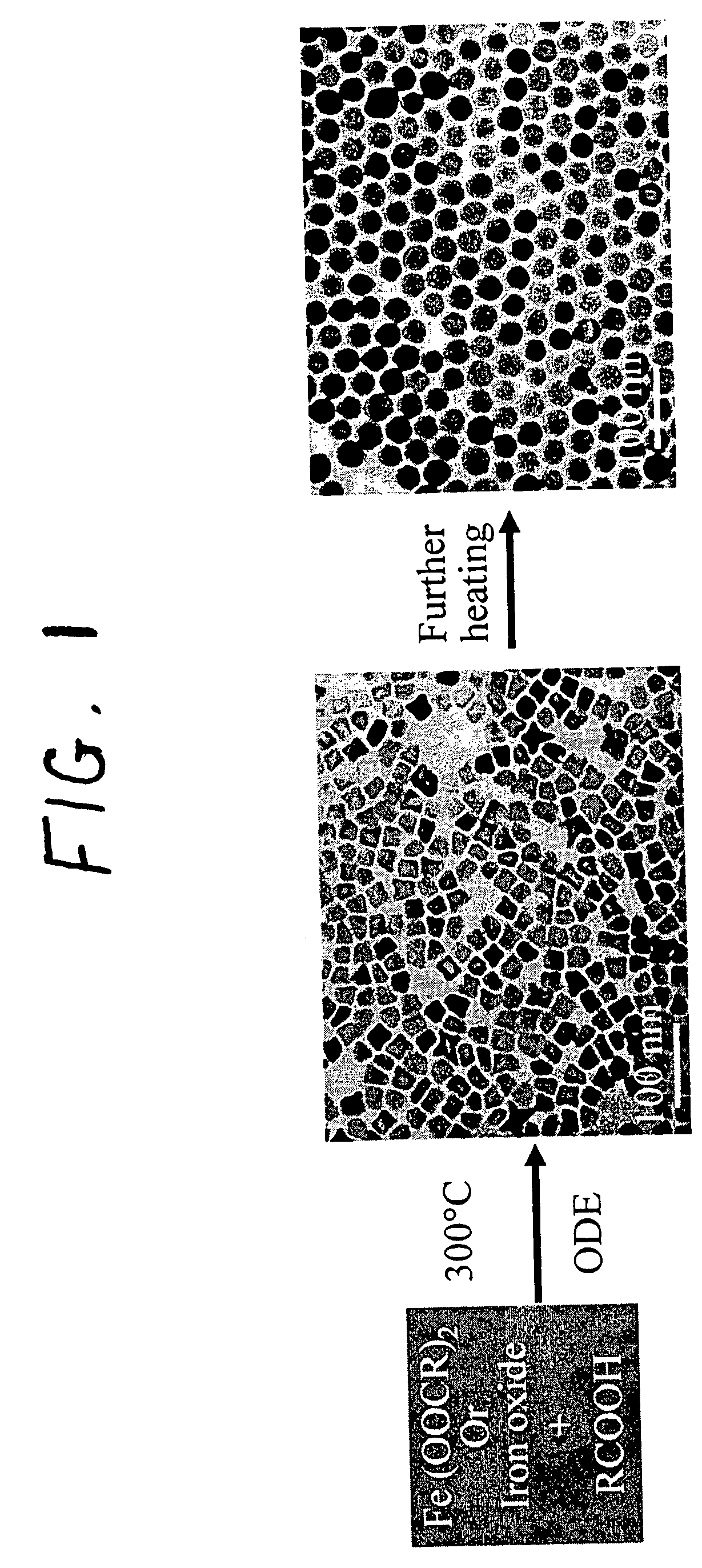
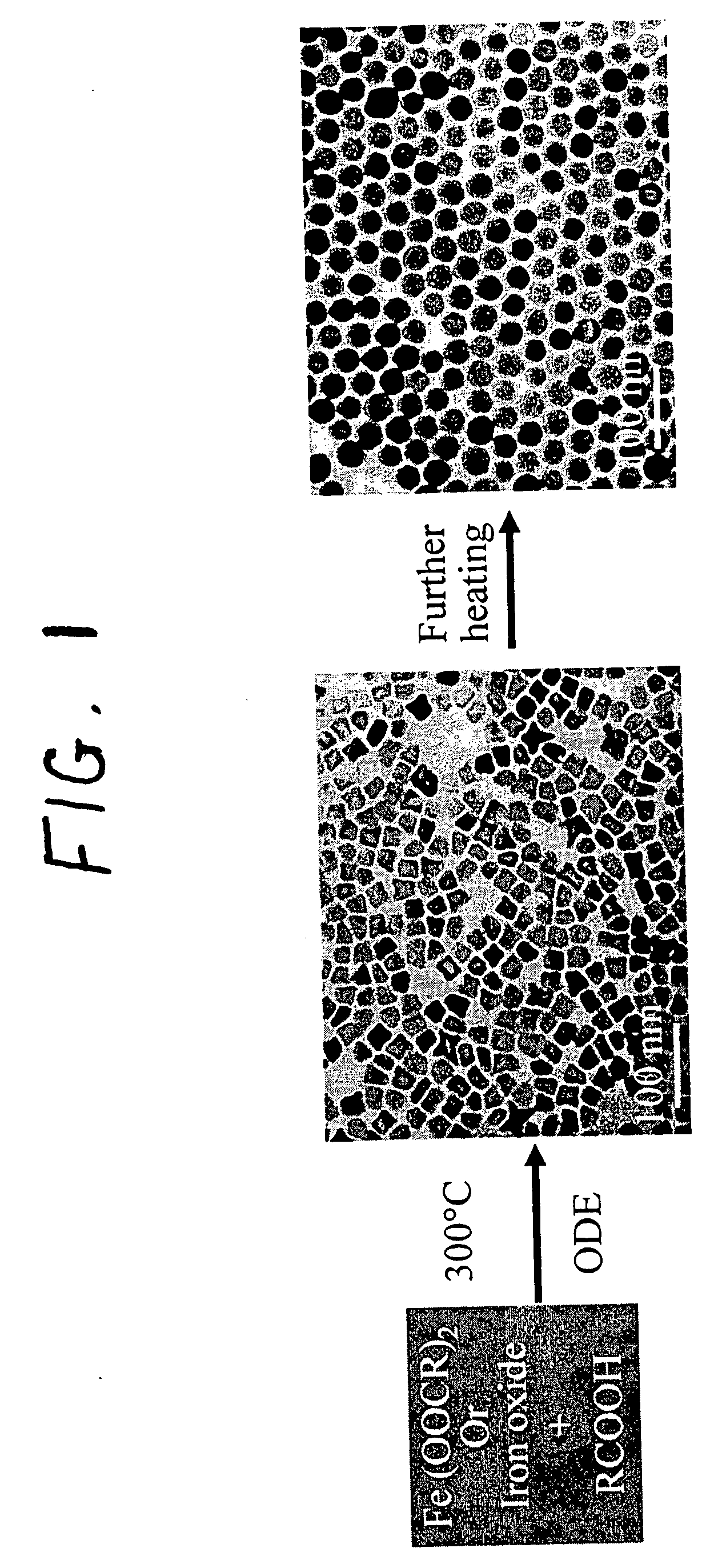
A general, reproducible, and simple synthetic method that employs readily available chemicals permits control of the size, shape, and size distribution of metal oxide nanocrystals. The synthesis entails reacting a metal fatty acid salt, the corresponding fatty acid, and a hydrocarbon solvent, with the reaction product being pyrolyzed to the metal oxide. Nearly monodisperse oxide nanocrystals of Fe3O4, Cr2O3, MnO, Co3O4, NiO, ZnO, SnO2, and In2O3, in a large size range (3-50 nm), are described. Size and shape control of the nanocrystals is achieved by varying the reactivity and concentration of the precursors.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Methods for removing heavy metals from water using chemical precipitation and field separation methods
InactiveUS6896815B2Small sizeChemical cost reductionSolid sorbent liquid separationGold compoundsWater useSludge
A two-step chemical precipitation process involving hydroxide precipitation and sulfide precipitation combined with “field separation” technology such as magnetic separation, dissolved air flotation, vortex separation or expanded plastics flotation, effectively removes chelated and non-chelated heavy metal precipitates and other fine particles from water. In the first-step, the non-chelated heavy metals are precipitated as hydroxides and removed from the water by a conventional liquid / solids separator such as an inclined plate clarifier to remove a large percentage of the dissolved heavy metals. The cleaned water is then treated in a second precipitation step to remove the residual heavy metals to meet discharge limits. In the second precipitation step, any metal precipitant more effective than hydroxide for metal precipitation can be used. The invention improves metal removal, lowers cost because fewer chemicals are used, produces less sludge, and reduces the discharge of toxic metals and metal precipitants to the environment.
Owner:CORT STEVEN L
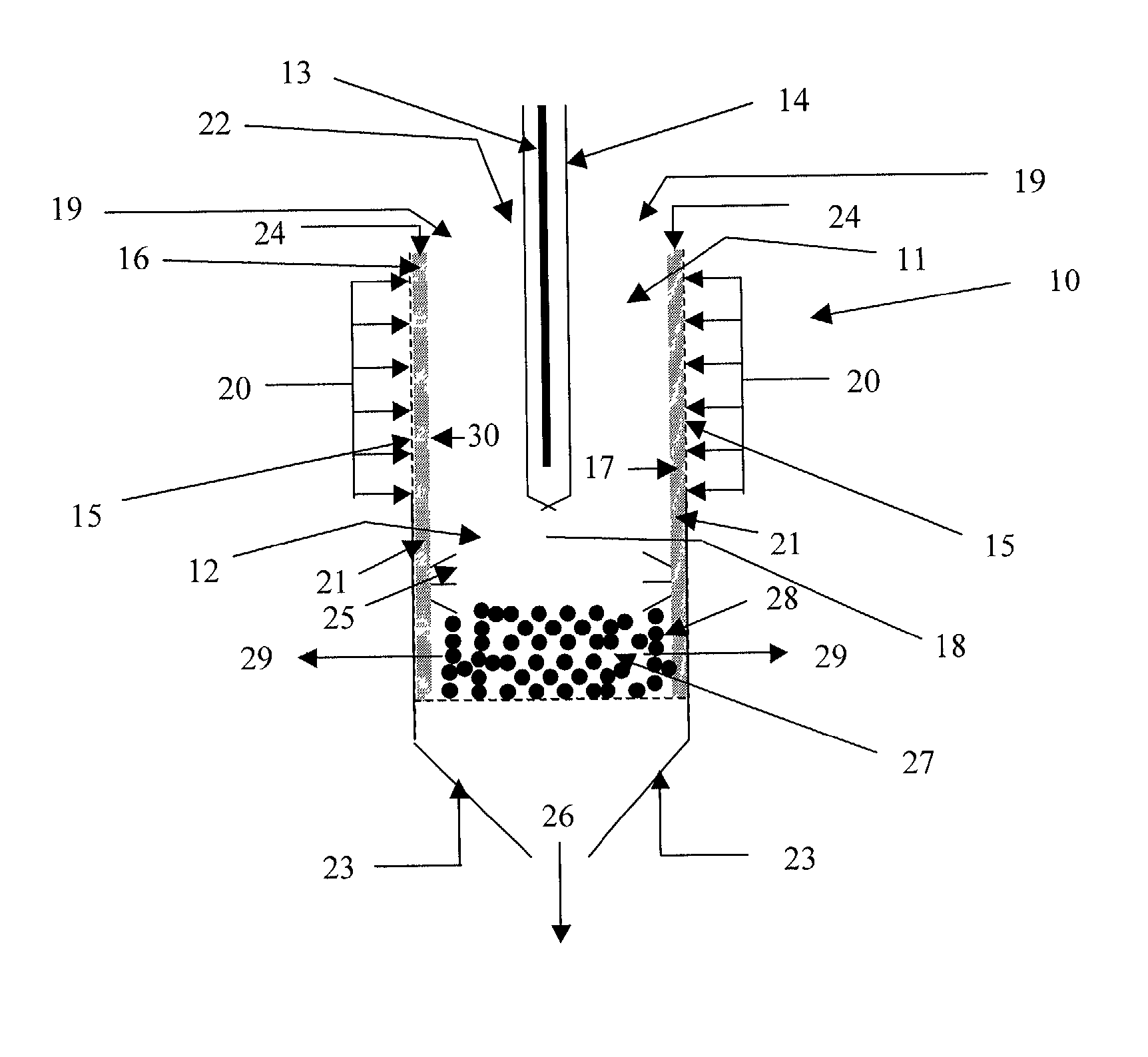
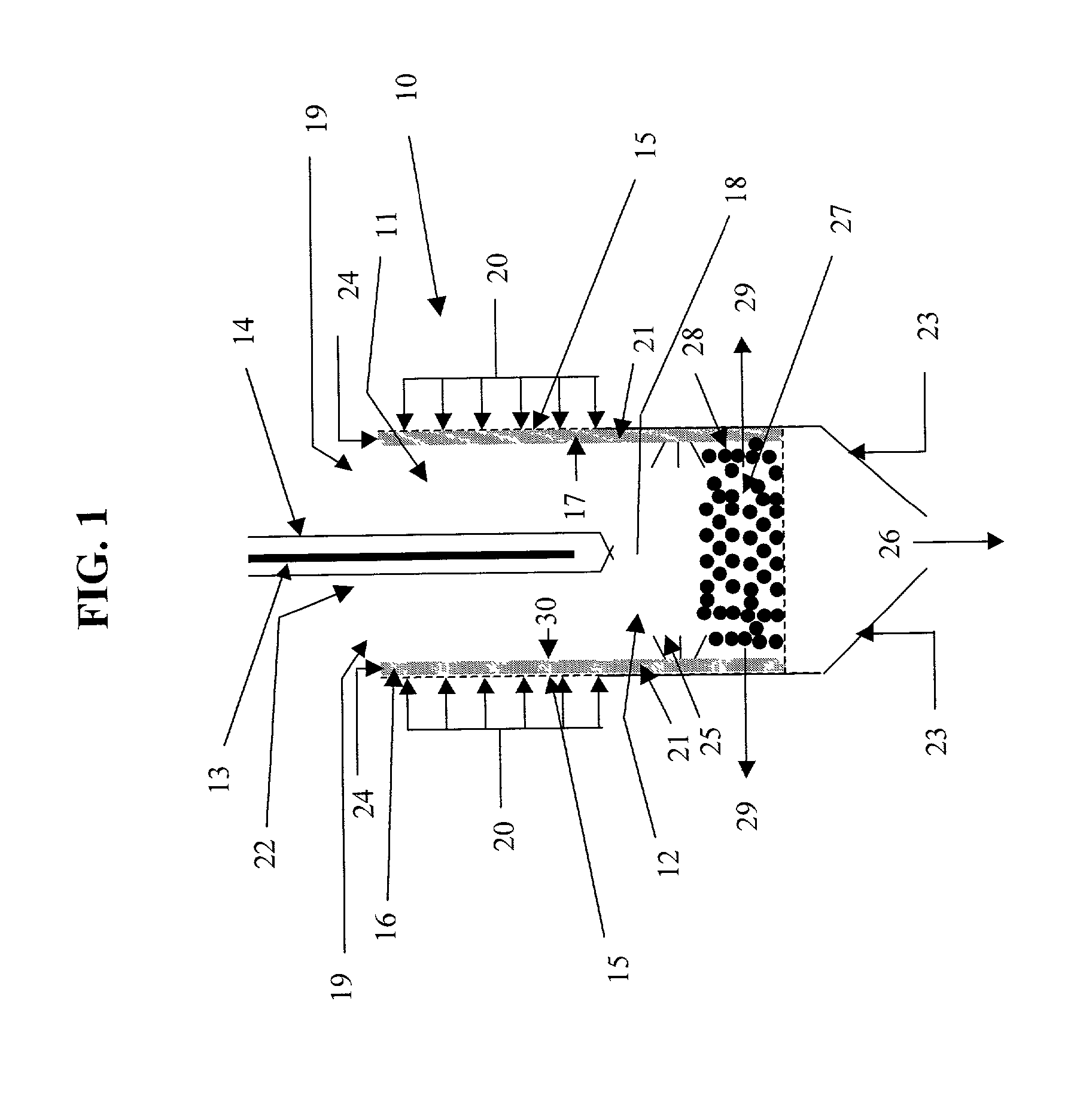
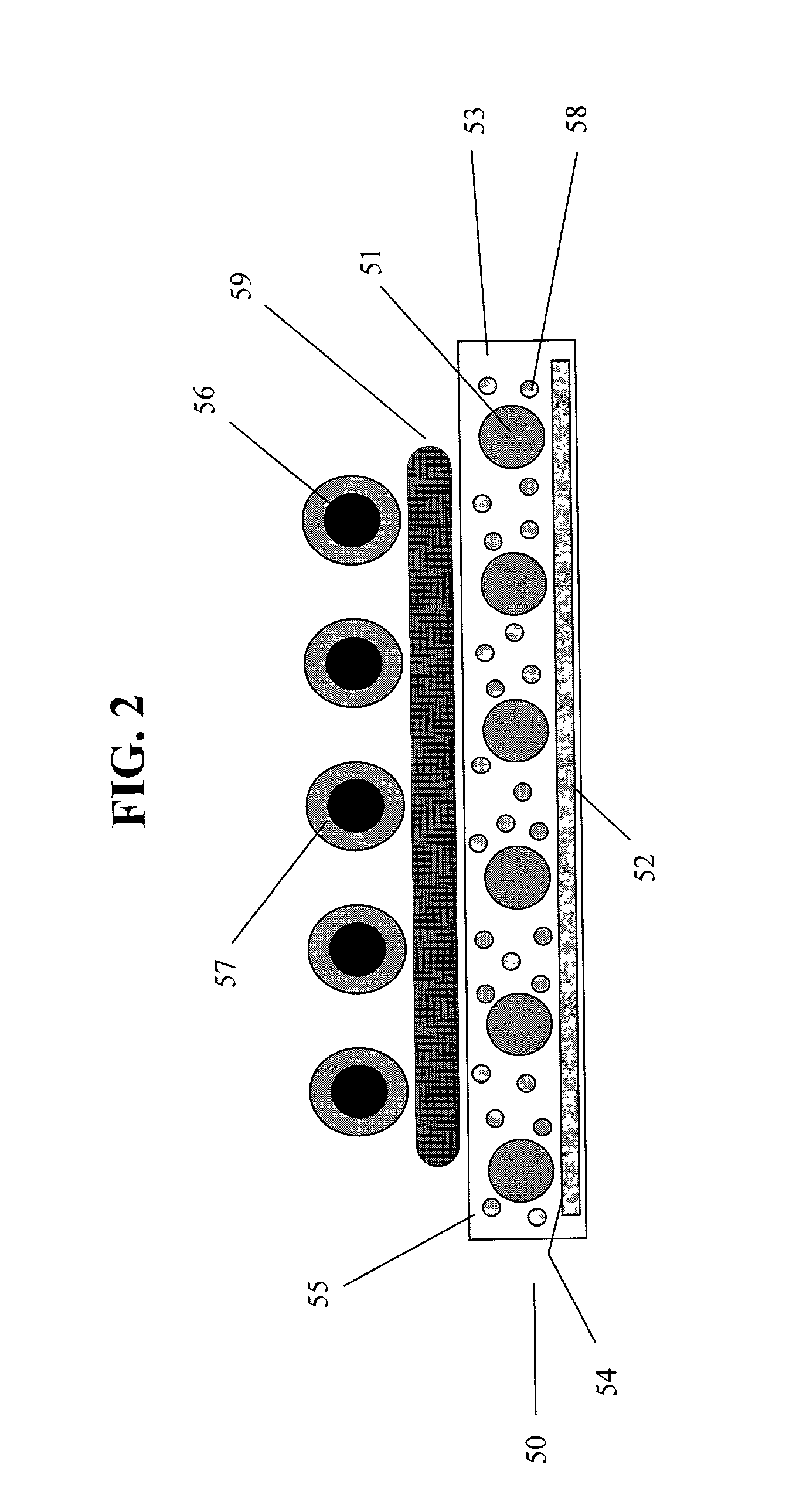
Shrouded-plasma process and apparatus for the production of metastable nanostructured materials
InactiveUS20070044513A1Improve compactnessImprove sintering performanceMaterial nanotechnologyNitrogen compoundsLiquid jetShort range order
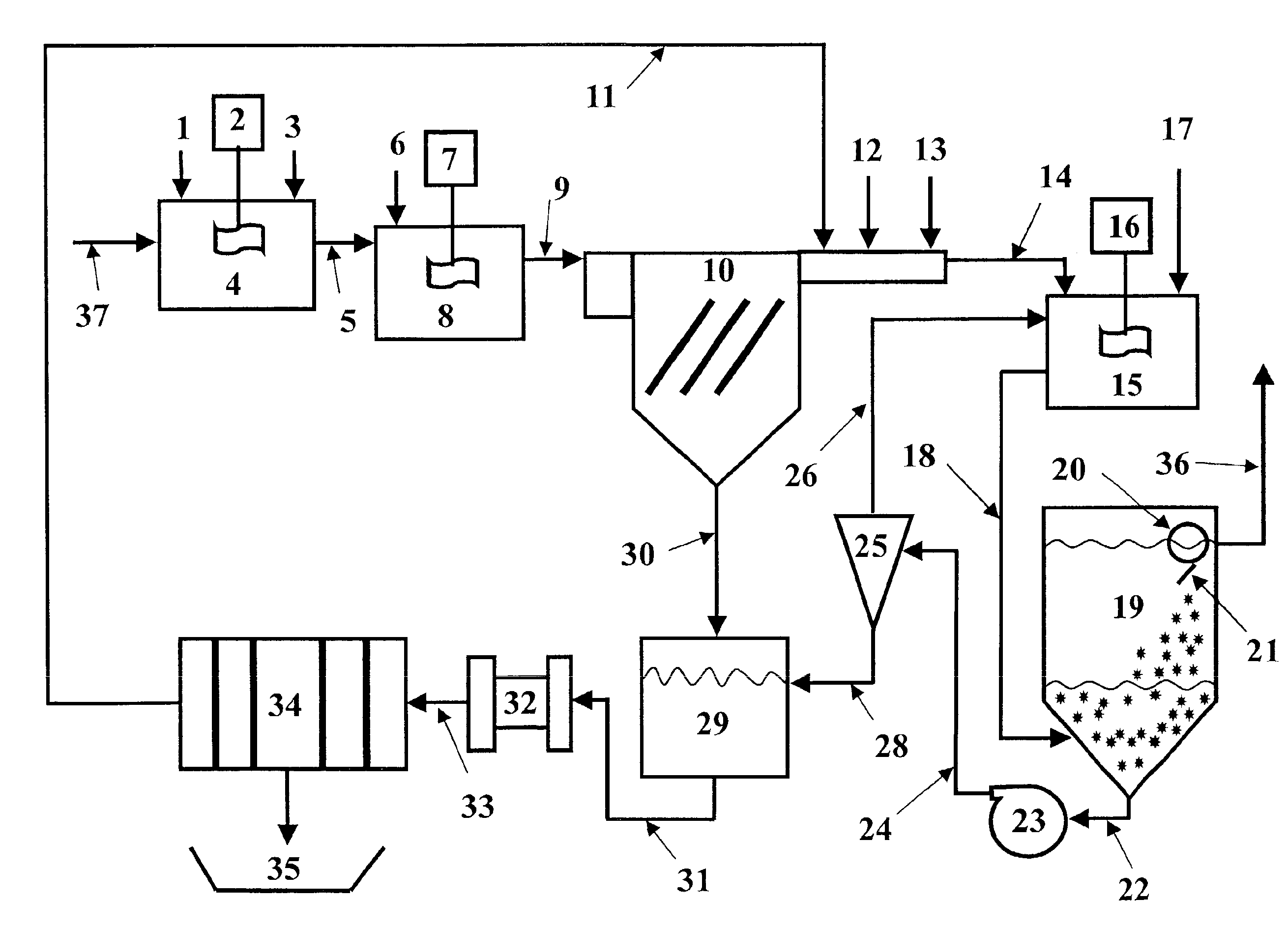
A method and apparatus for producing metastable nanostructured materials employing a ceramic shroud surrounding a plasma flame having a steady state reaction zone into which an aerosol or liquid jet of solution precursor or powder material is fed, causing the material to be pyrolyzed, melted, or vaporized, followed by quenching to form a metastable nanosized powder that has an amorphous (short-range ordered), or metastable microsized powder that has a crystalline (long-range ordered) structure, respectively.
Owner:RUTGERS THE STATE UNIV
Method for producing mixed metal oxides and metal oxide compounds
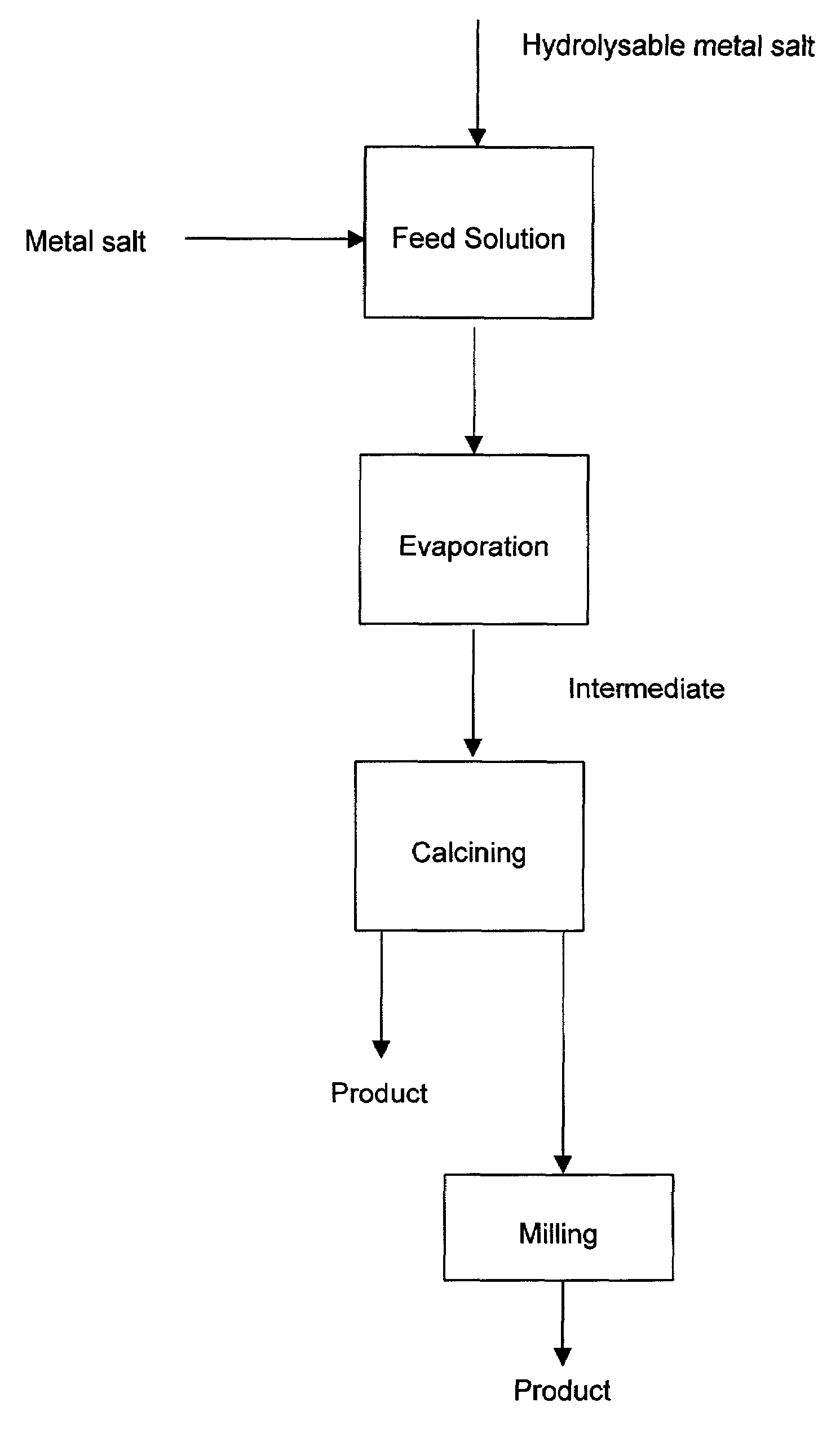
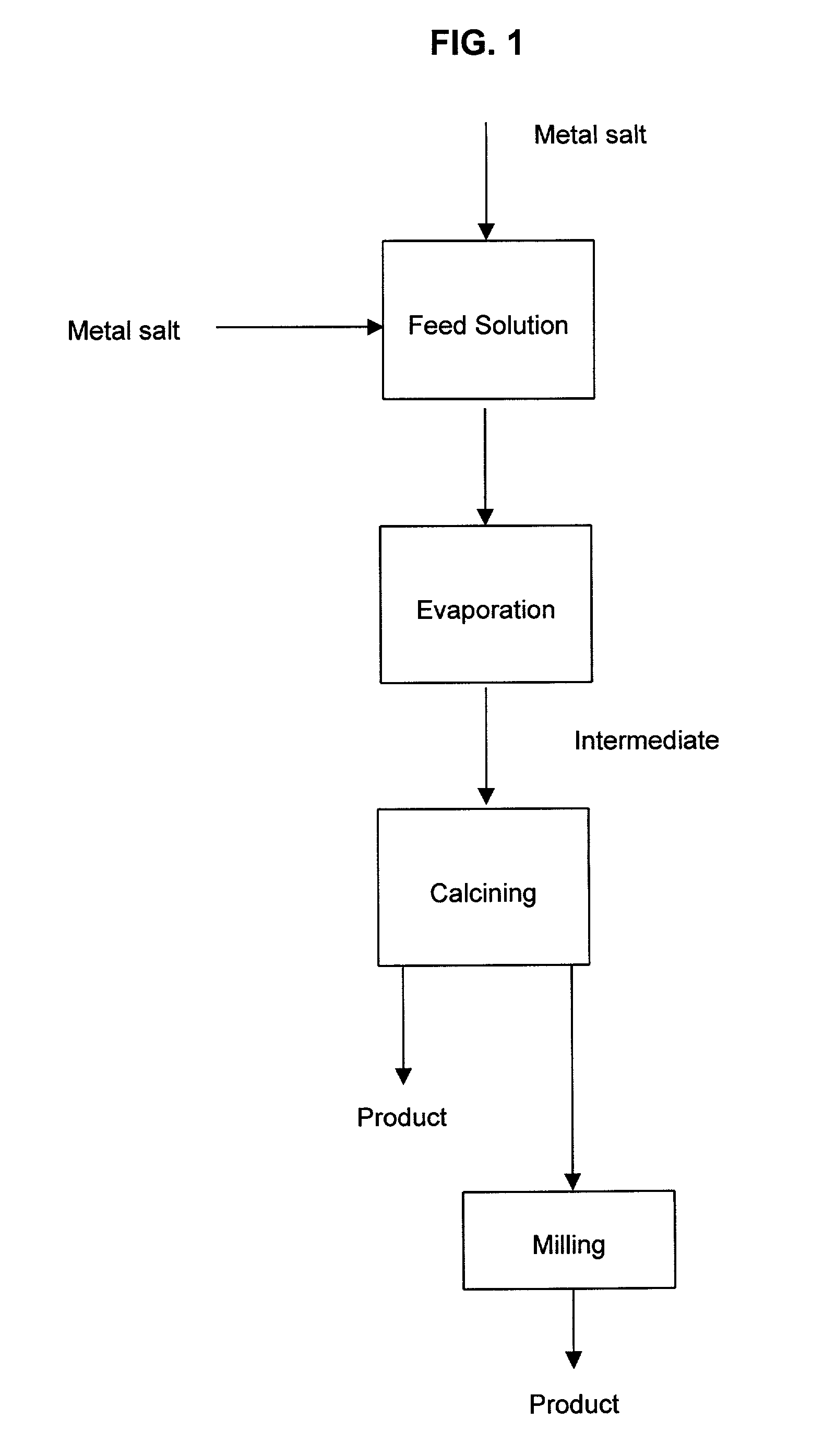
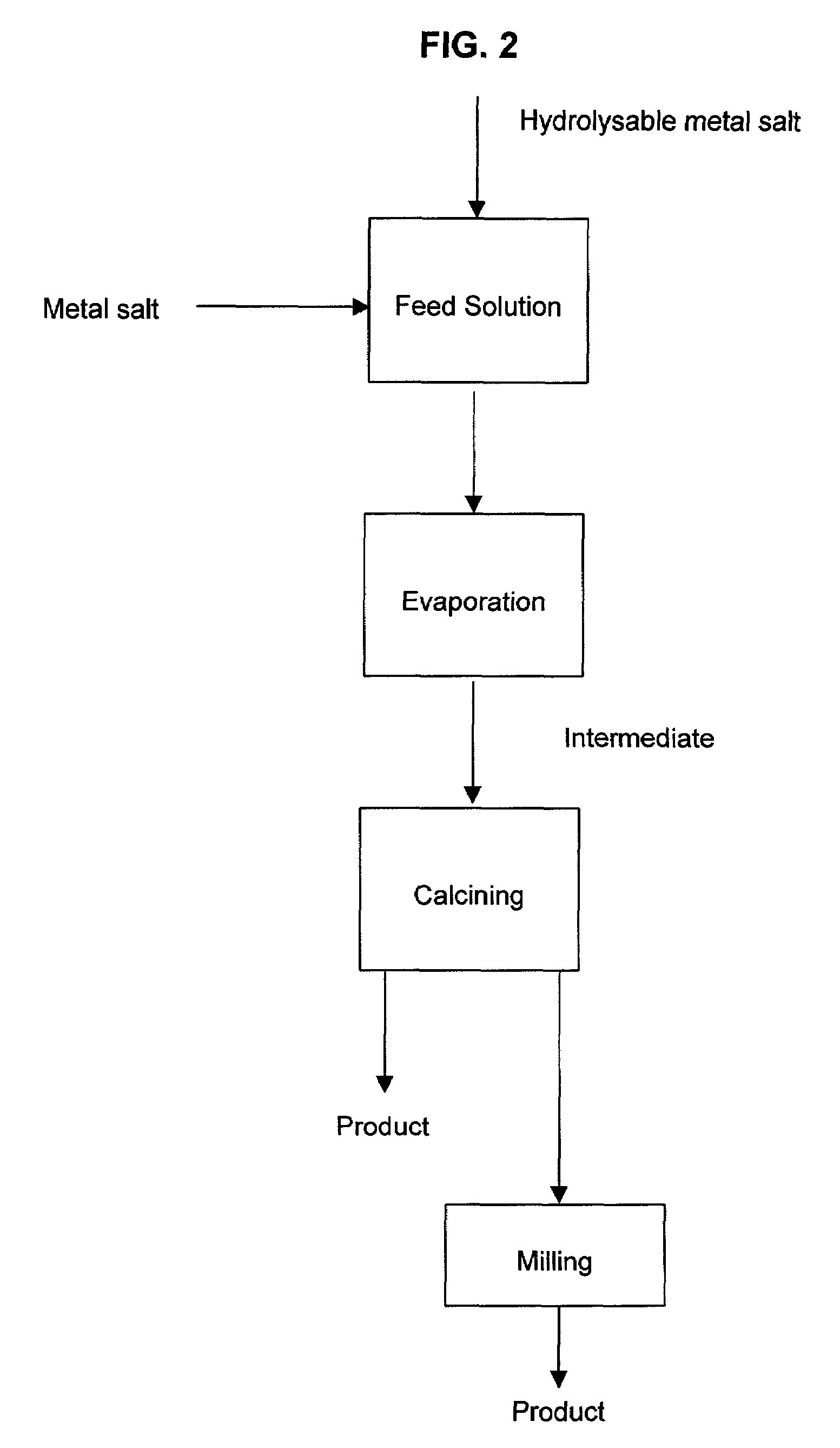
A process to produce mixed metal oxides and metal oxide compounds. The process includes evaporating a feed solution that contains at least two metal salts to form an intermediate. The evaporation is conducted at a temperature above the boiling point of the feed solution but below the temperature where there is significant crystal growth or below the calcination temperature of the intermediate. The intermediate is calcined, optionally in the presence of an oxidizing agent, to form the desired oxides. The calcined material can be milled and dispersed to yield individual particles of controllable size and narrow size distribution.
Owner:ALTAIR NANOMATERIALS INC
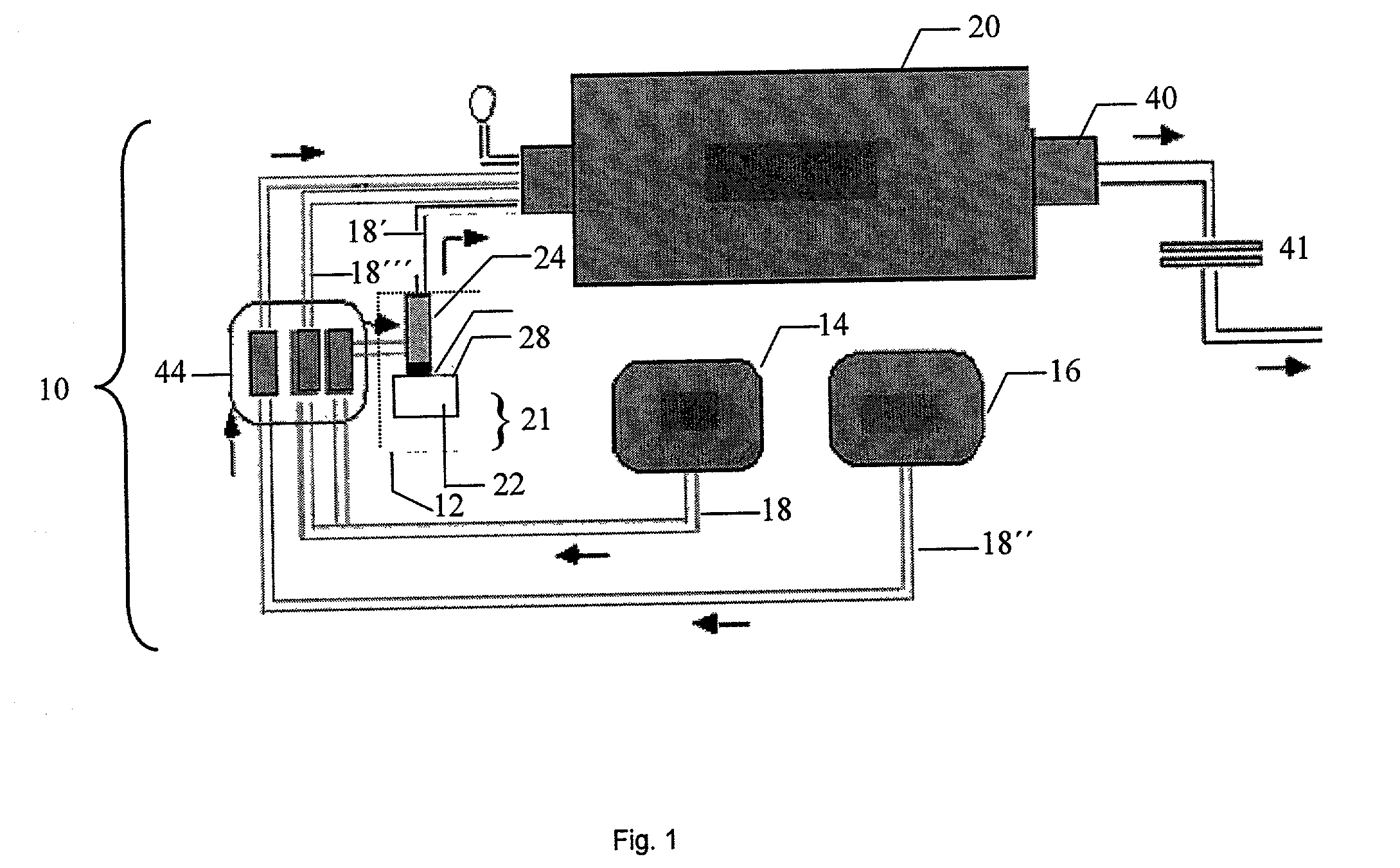
Apparatus and method for preparing cerium oxide nanoparticles
InactiveUS20050031517A1Quick responseReaction time is thus limitedMaterial nanotechnologyMixing methodsCerium nitrateNanoparticle
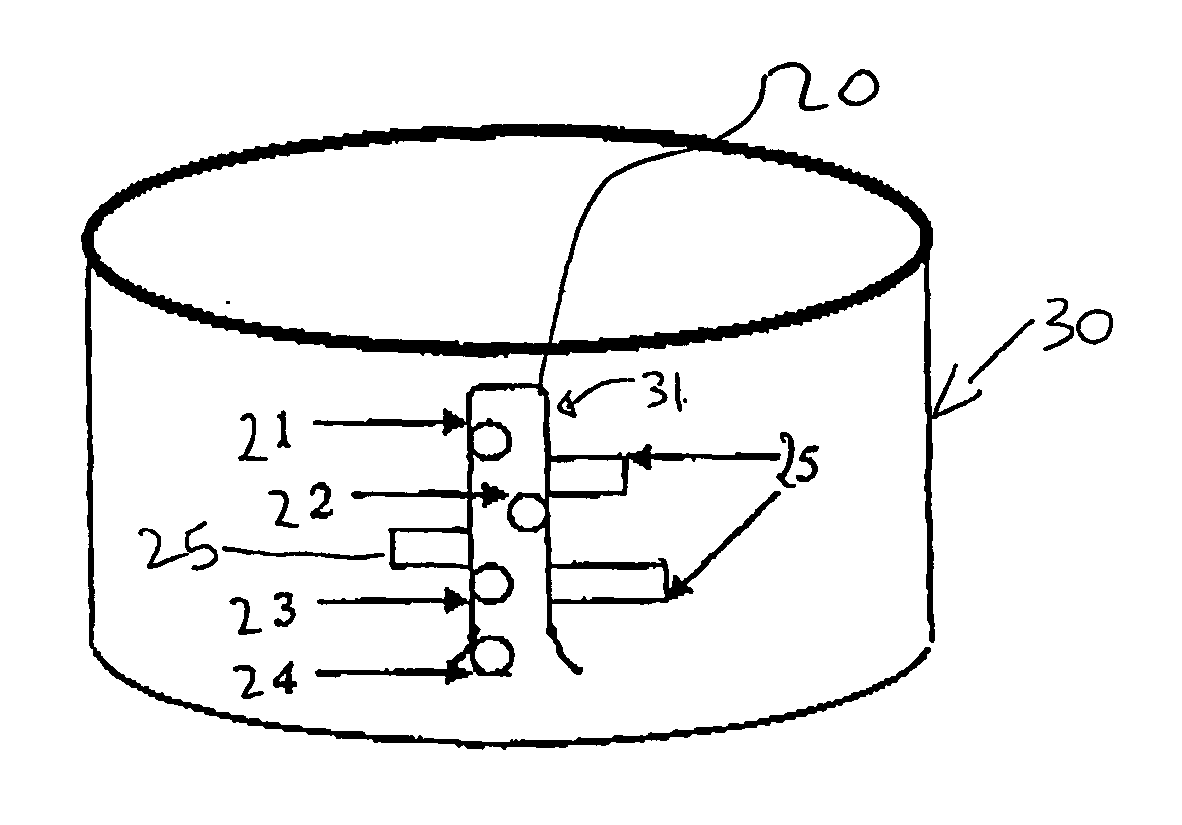
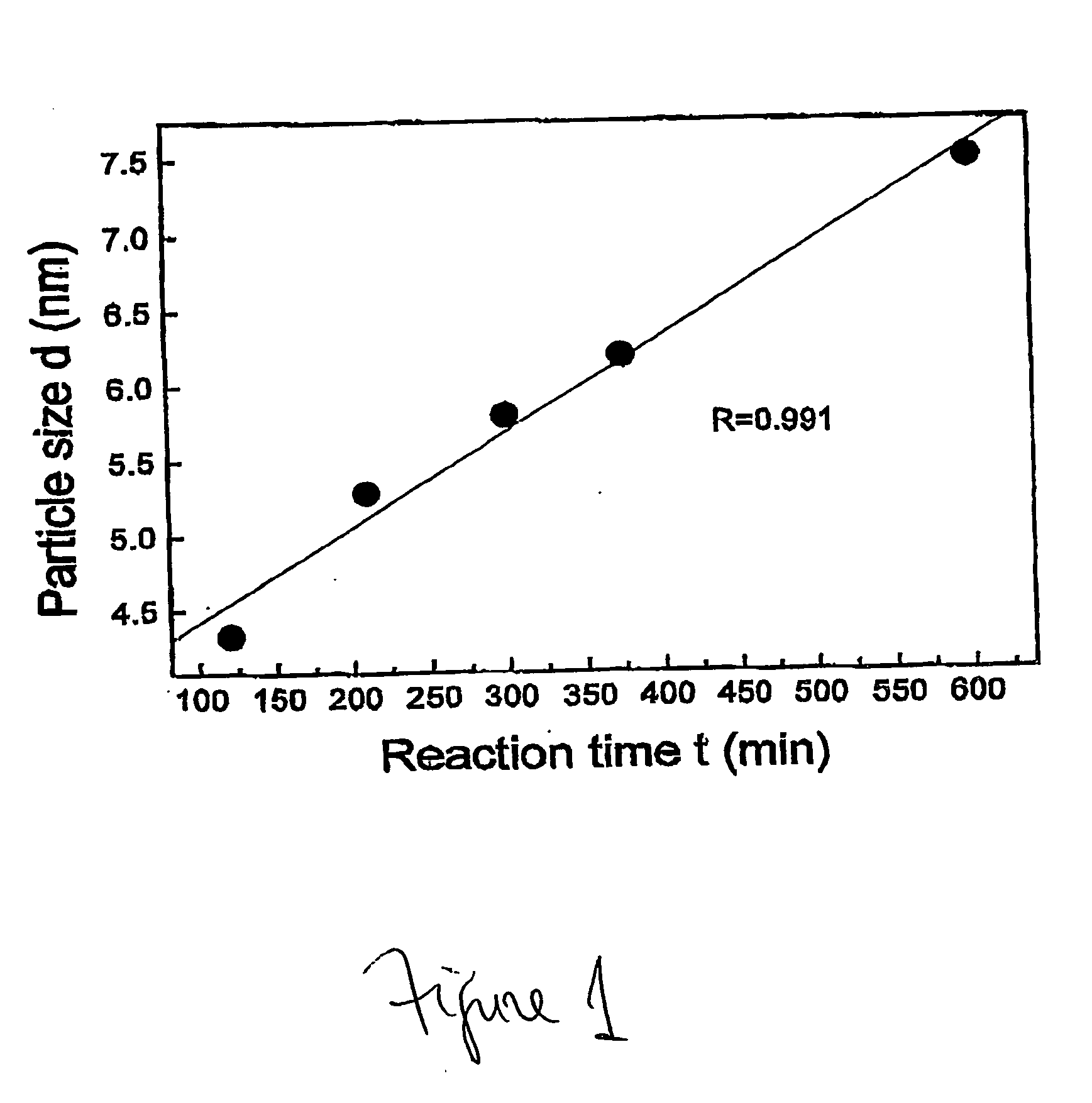
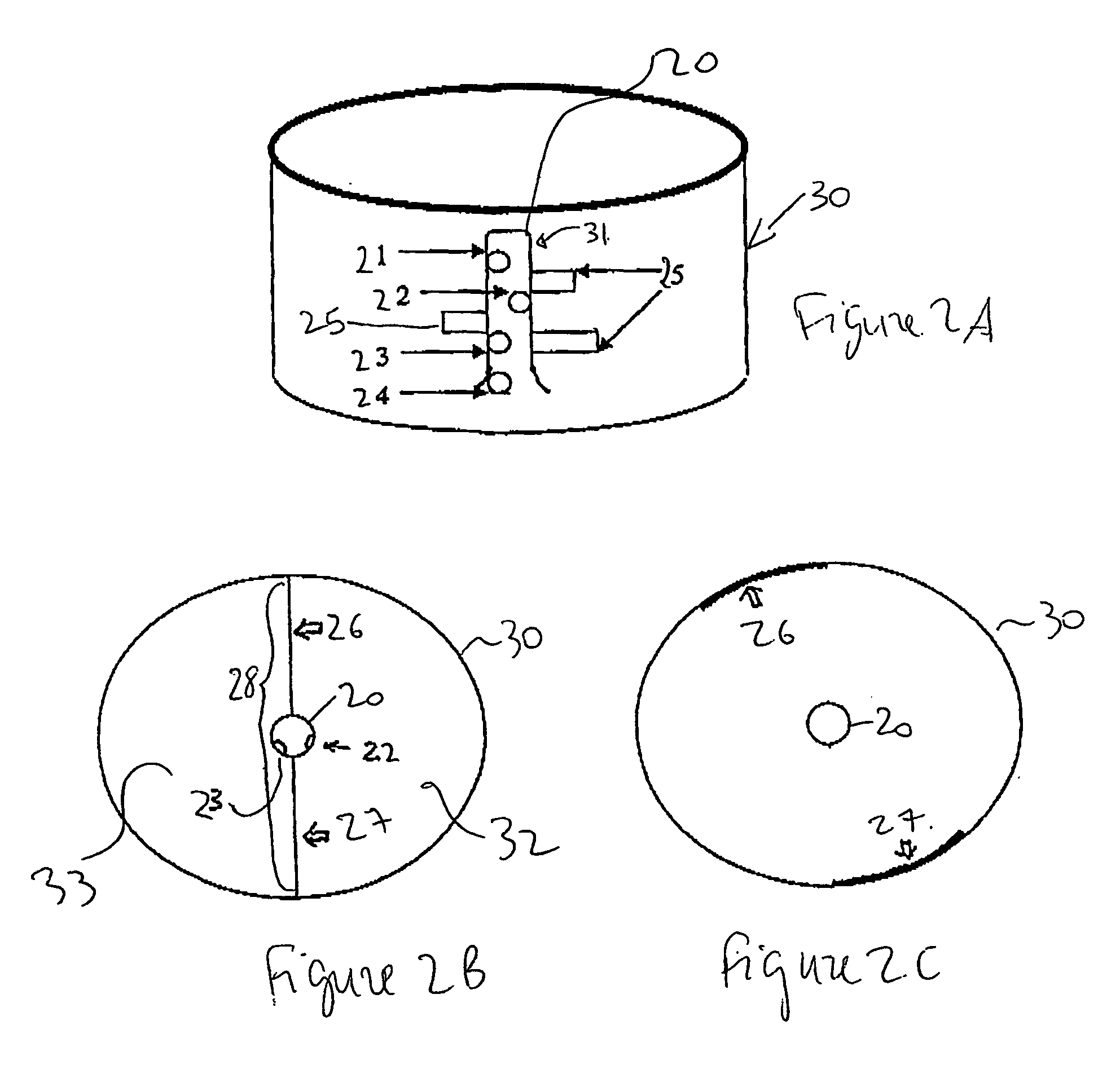
This invention provides a method for preparing cerium oxide nanoparticles with a narrow size distribution. The cerium oxide nanoparticles obtained by the method of the invention are nearly all crystalline. The method comprises providing a first aqueous solution comprising cerium nitrate and providing a second aqueous solution comprising hexamethylenetetramine. The first and second aqueous solutions are mixed to form a mixture, and the mixture is maintained at a temperature no higher than about 320° K to form nanoparticles. The nanoparticles that are formed are then separated from the mixture. A further aspect of the present invention is an apparatus for preparing cerium oxide nanoparticles. The apparatus comprises a mixing vessel having a first compartment for holding a first aqueous solution comprising cerium nitrate and a second compartment for holding a second aqueous solution comprising hexamethylenetetramine. The mixing vessel has a retractable partition separating the first and second compartments. When the retractable partition is retracted, rapid mixing of the first aqueous solution with the second aqueous solution takes place to form a mixture, and the mixture is maintained at a temperature no higher than about 320° K to form nanoparticles therein.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Synthetic control of metal oxide nanocrystal sizes and shapes
A general, reproducible, and simple synthetic method that employs readily available chemicals permits control of the size, shape, and size distribution of metal oxide nanocrystals. The synthesis entails reacting a metal fatty acid salt, the corresponding fatty acid, and a hydrocarbon solvent, with the reaction product being pyrolyzed to the metal oxide. Nearly monodisperse oxide nanocrystals of Fe3O4, Cr2O3, MnO, Co3O4, NiO, ZnO, SnO2, and In2O3, in a large size range (3-50 nm), are described. Size and shape control of the nanocrystals is achieved by varying the reactivity and concentration of the precursors.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Shrouded-Plasma Process and Apparatus for the Production of Metastable Nanostructured Materials
A method and apparatus for producing metastable nanostructured materials employing a ceramic shroud surrounding a plasma flame having a steady state reaction zone into which an aerosol or liquid jet of solution precursor or powder material is fed, causing the material to be pyrolyzed, melted, or vaporized, followed by quenching to form a metastable nanosized powder that has an amorphous (short-range ordered), or metastable microsized powder that has a crystalline (long-range ordered) structure, respectively.
Owner:KEAR BERNARD H +2
Method for smelting noble metal
InactiveUS6126720AShorten the timeReduce impurityPhotography auxillary processesSolvent extractionDecompositionMaterials science
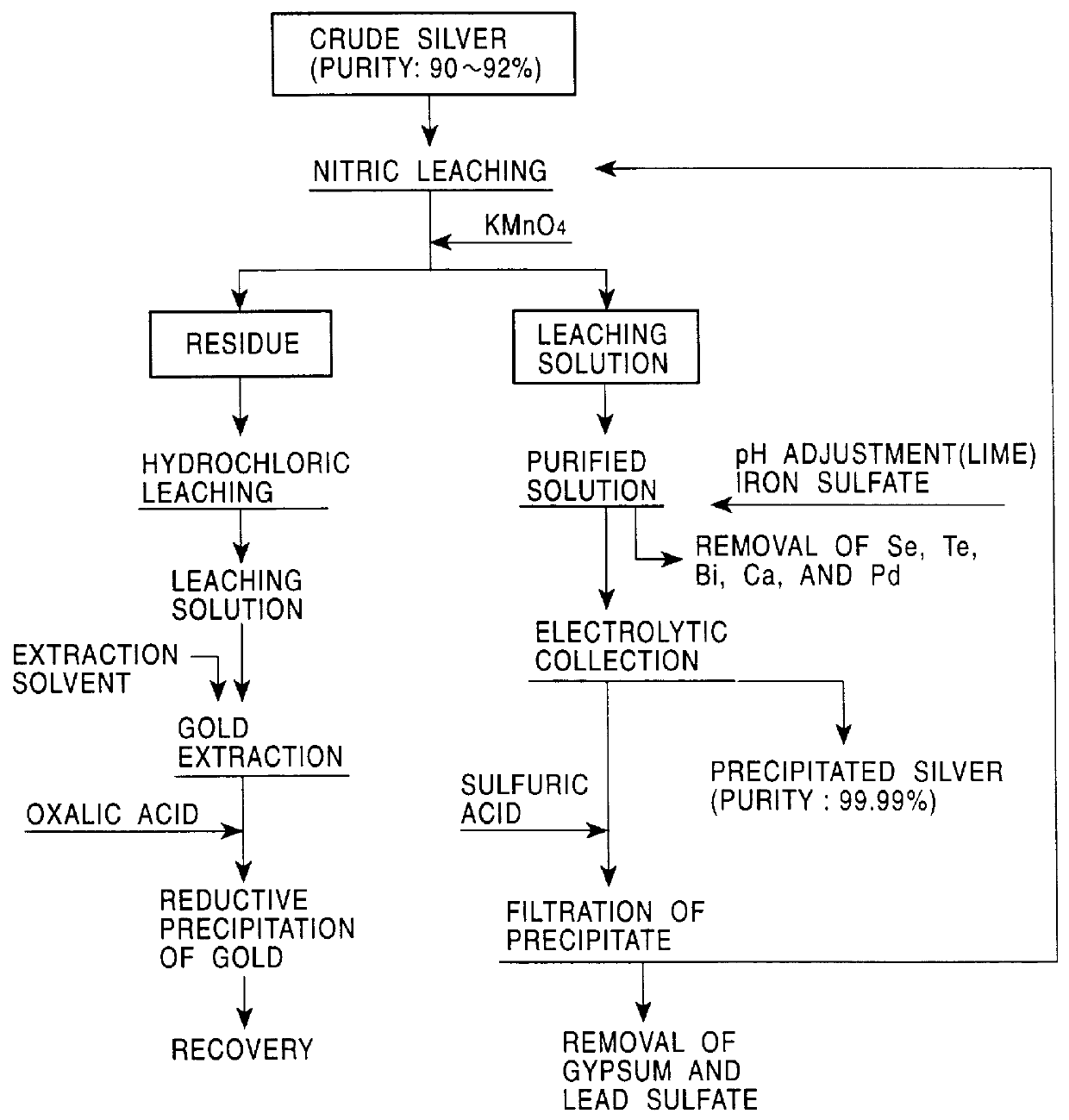
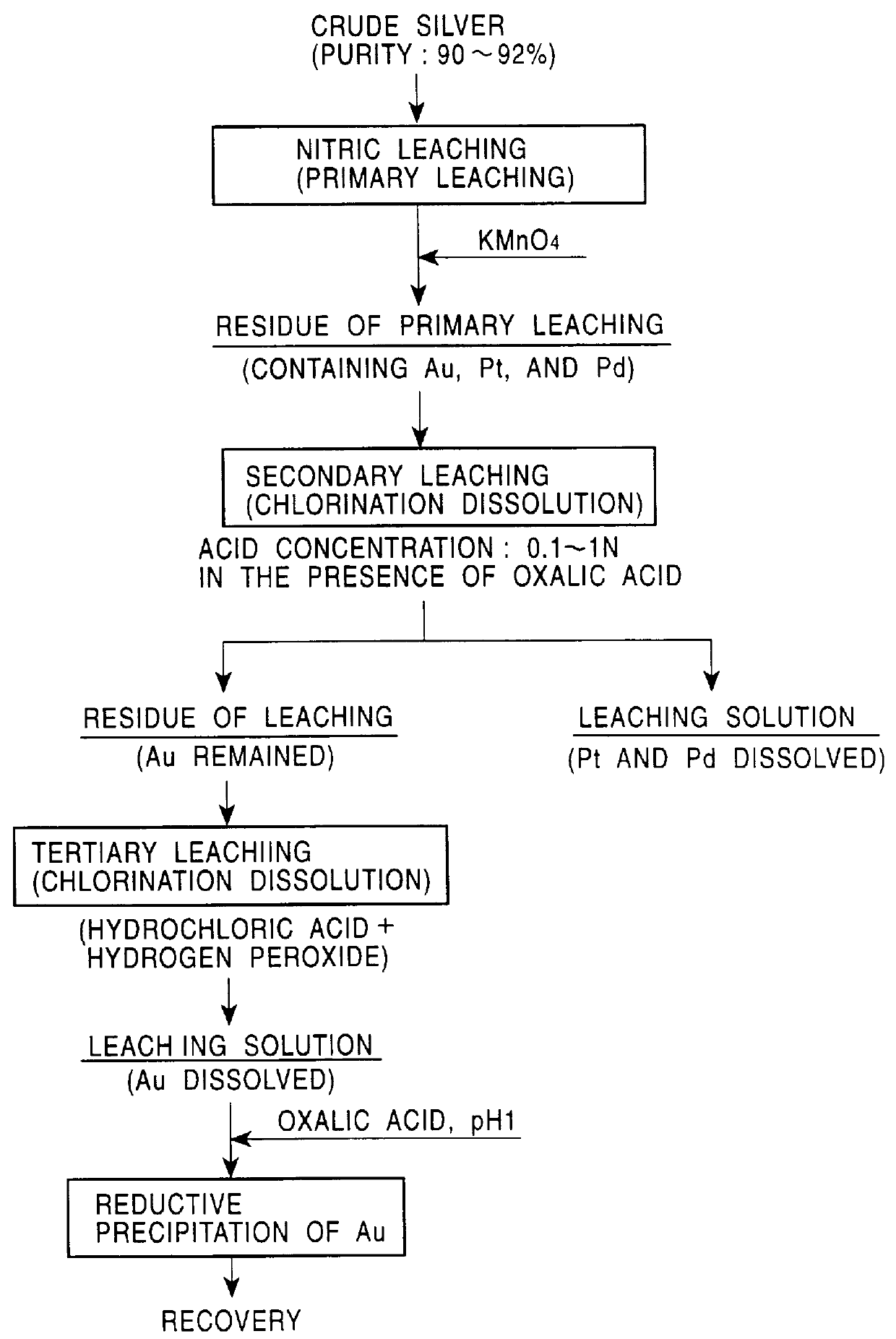
PCT No. PCT / JP98 / 02479 Sec. 371 Date Jan. 15, 1999 Sec. 102(e) Date Jan. 15, 1999 PCT Filed Jun. 4, 1998 PCT Pub. No. WO98 / 58089 PCT Pub. Date Dec. 23, 1998A method for refining noble metals has a silver treating process including a nitric acid leaching step of silver, a purification step of the leaching solution, an electrolytic decomposition step of silver, and a recycling step after the electrolytic decomposition, wherein in the purification step, lime is added in order to precipitate the metallic impurities, such as selenium, tellurium, bismuth, and copper, by neutralization of the leaching solution, and in the recycling step, sulfuric acid is added to the solution after electrolytic decomposition to regenerate nitric acid for recycling use by precipitation of calcium in the solution as gypsum. Preferably, the refining method has a gold recovery process, as well as the silver treating process, wherein the residue of the nitric leaching of the crude silver is dissolved by chlorination and gold is recovered from the leaching solution by solvent extraction or reductive precipitation. High purity gold and silver can be readily obtained, and the refining time for gold is significantly shorter than that in conventional methods.
Owner:MITSUBISHI MATERIALS CORP
Synthesis of Metal Compounds Under Carbothermal Conditions
InactiveUS20050255026A1Economical and convenient processLower capability requirementsPhosphatesLithium compoundsOxidation stateGraphite
Active materials of the invention contain at least one alkali metal and at least one other metal capable of being oxidized to a higher oxidation state. Preferred other metals are accordingly selected from the group consisting of transition metals (defined as Groups 4-11 of the periodic table), as well as certain other non-transition metals such as tin, bismuth, and lead. The active materials may be synthesized in single step reactions or in multi-step reactions. In at least one of the steps of the synthesis reaction, reducing carbon is used as a starting material. In one aspect, the reducing carbon is provided by elemental carbon, preferably in particulate form such as graphites, amorphous carbon, carbon blacks and the like. In another aspect, reducing carbon may also be provided by an organic precursor material, or by a mixture of elemental carbon and organic precursor material.
Owner:LITHIUM WERKS TECH BV +1
Recovering metals from sulfidic materials
InactiveUS20070014709A1Facilitate precious metal dissolution precious metalFacilitate precious metal subsequent precious metal recoveryPhotography auxillary processesSolvent extractionOxidation stateSulfide
A process for recovering a precious metal from a sulfidic material comprises the steps of preparing an acidic aqueous halide solution having an oxidation potential sufficient to oxidise the sulfidic material and render the precious metal soluble in the solution, adding the material to the acidic aqueous halide solution so that the sulfidic material is oxidised and the precious metal is solubilised and separating the precious metal from the oxidised sulfidic material. In addition, a process for removing a contaminant from a contaminated sulfidic material comprises the steps of mixing the material in an aqueous solution wherein a multi-valent species of a relatively high oxidation state oxidises the contaminant to render it soluble in the solution, produces a contaminant refined material, and is reduced to a relatively lower oxidation state; and removing the contaminant from the solution whilst regenerating the multi-valent species to its relatively high oxidation state.
Owner:INTEC INT PROJECTS
Apparatus and method for preparing cerium oxide nanoparticles
InactiveUS7141227B2Quick responseMaterial nanotechnologyTransportation and packagingCerium nitrateHexamethylenetetramine
This invention provides a method for preparing cerium oxide nanoparticles with a narrow size distribution. The cerium oxide nanoparticles obtained by the method of the invention are nearly all crystalline. The method comprises providing a first aqueous solution comprising cerium nitrate and providing a second aqueous solution comprising hexamethylenetetramine. The first and second aqueous solutions are mixed to form a mixture, and the mixture is maintained at a temperature no higher than about 320° K to form nanoparticles. The nanoparticles that are formed are then separated from the mixture. A further aspect of the present invention is an apparatus for preparing cerium oxide nanoparticles. The apparatus comprises a mixing vessel having a first compartment for holding a first aqueous solution comprising cerium nitrate and a second compartment for holding a second aqueous solution comprising hexamethylenetetramine. The mixing vessel has a retractable partition separating the first and second compartments.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Method for recovery of copper, indium, gallium, and selenium
InactiveUS20100329970A1Simple processShorten operation timePhotography auxillary processesGallium/indium/thallium compoundsProduction lineIndium
A method for the recovery of copper, indium, gallium, and selenium is provided. The method includes steps of using a mixed solution containing a hydrochloric acid and hydrogen peroxide to dissolve the copper, indium, gallium, and selenium. After using the hydrazine to separate the selenium out, the copper is reduced by indium metal. Later, a combination of a supported liquid membrane (SLM) and a strip dispersion solution separates the gallium from the indium. The acid performed in all the steps of the method is hydrochloric acid. Therefore, the copper, indium, gallium, and selenium can be separated one by one in a single production line without changing the solution during the operation process, thereby simplifying the process, shortening the operation time and lowering the manufacture cost.
Owner:SOLAR APPLIED MATERIALS TECHNOLOGY CORPORATION
Synthesis of metal compounds under carbothermal conditions
InactiveUS7060206B2Economical and convenient processLower capability requirementsCell electrodesSulfur compoundsOxidation stateGraphite
Active materials of the invention contain at least one alkali metal and at least one other metal capable of being oxidized to a higher oxidation state. Preferred other metals are accordingly selected from the group consisting of transition metals (defined as Groups 4–11 of the periodic table), as well as certain other non-transition metals such as tin, bismuth, and lead. The active materials may be synthesized in single step reactions or in multi-step reactions. In at least one of the steps of the synthesis reaction, reducing carbon is used as a starting material. In one aspect, the reducing carbon is provided by elemental carbon, preferably in particulate form such as graphites, amorphous carbon, carbon blacks and the like. In another aspect, reducing carbon may also be provided by an organic precursor material, or by a mixture of elemental carbon and organic precursor material.
Owner:LITHIUM WERKS TECH BV
Solution for forming rare-earth superconductive film and production method thereof
InactiveUS8865628B2Save resourcesSave energyConductive materialSuperconductor detailsCarbon numberRare-earth element
Provided is a coating solution where, upon producing a rare-earth superconductive composite metal oxide film by means of a coating-pyrolysis method, cracks are not generated in the heat treatment process for eliminating organic components, even when the thickness of the rare-earth superconductive film produced in a single coating is 500 nm or more, and without having to repeat the coating and annealing process. A solution for producing a rare-earth superconductive film which is made into a homogeneous solution by dissolving, in a solvent formed by adding a polyhydric alcohol to a univalent linear alcohol having a carbon number of 1 to 8 and / or water, a metal complex coordinated, relative to metal ions of a metallic species containing rare-earth elements, barium and copper, with pyridine and / or at least one type of tertiary amine, at least one type of carboxylic acid having a carbon number of 1 to 8, and, as needed, an acetylacetonato group.
Owner:NAT INST OF ADVANCED IND SCI & TECH
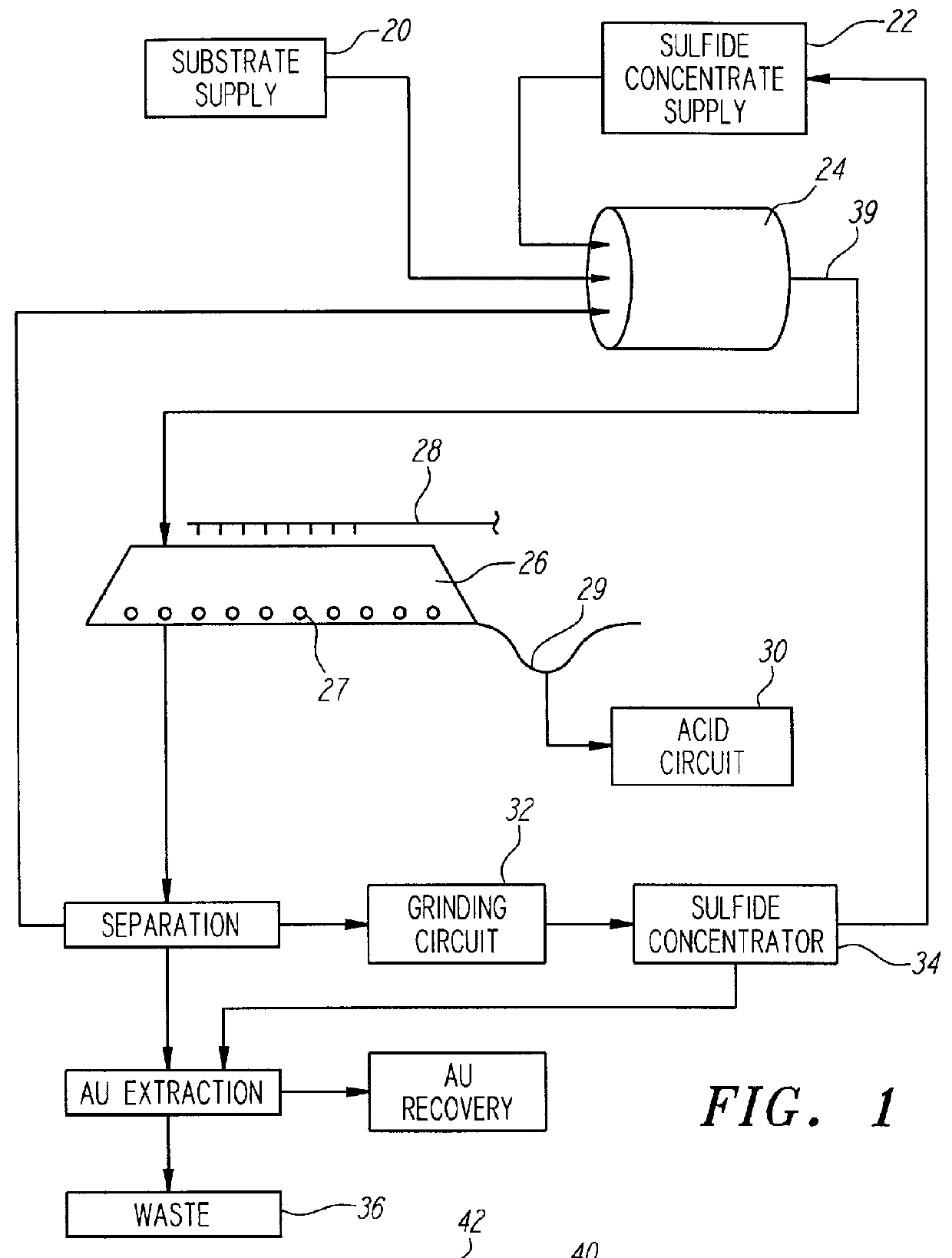
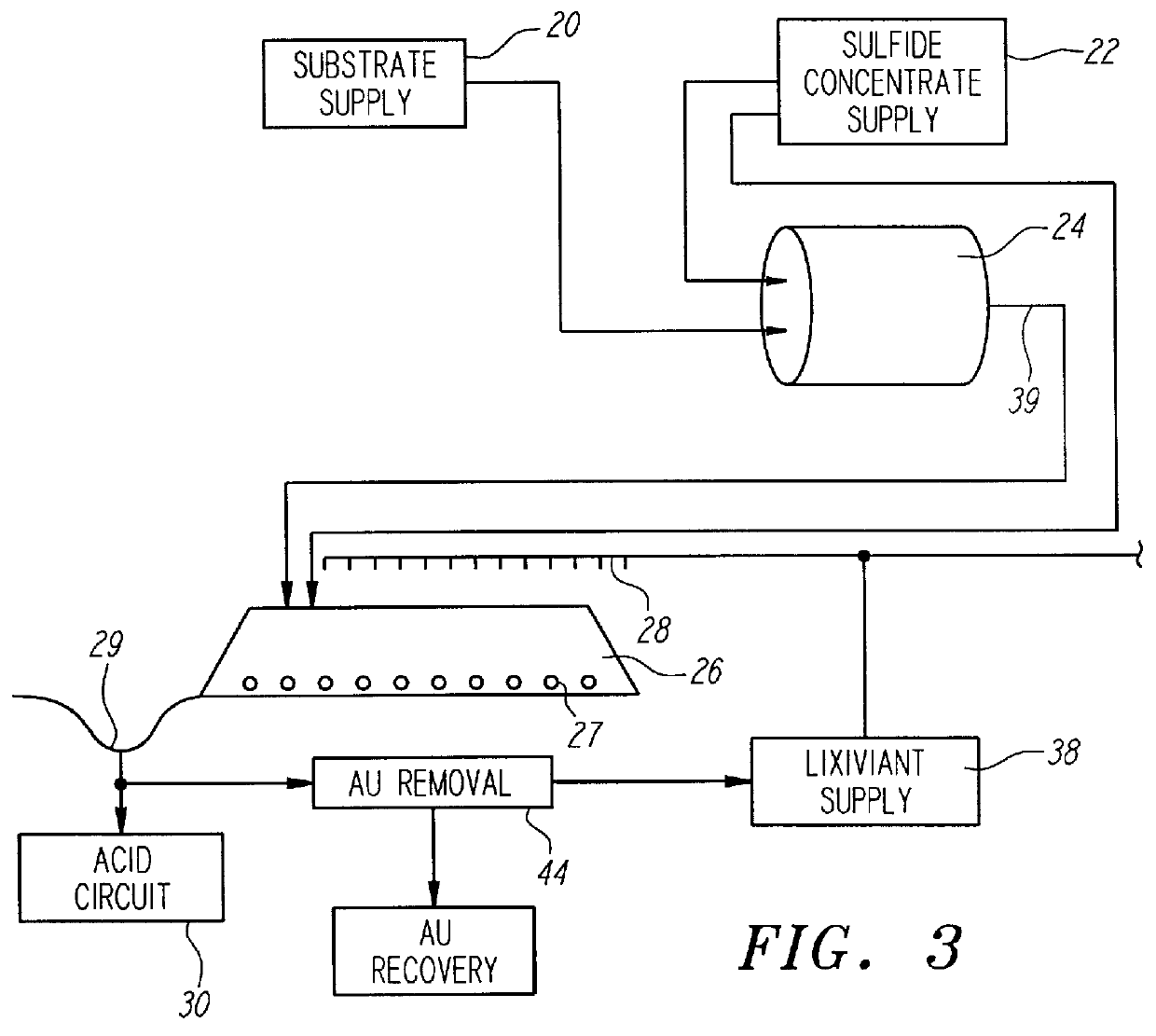
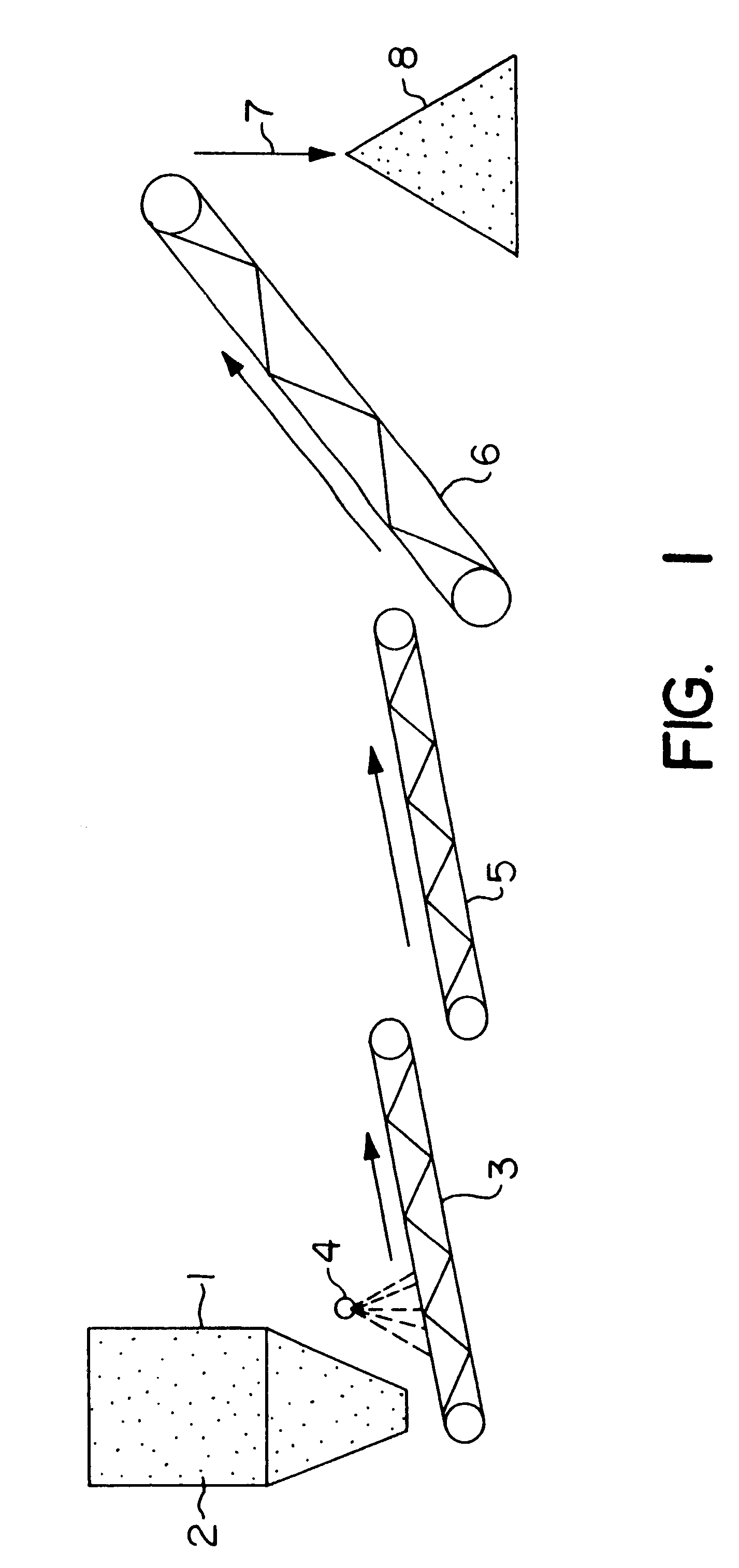
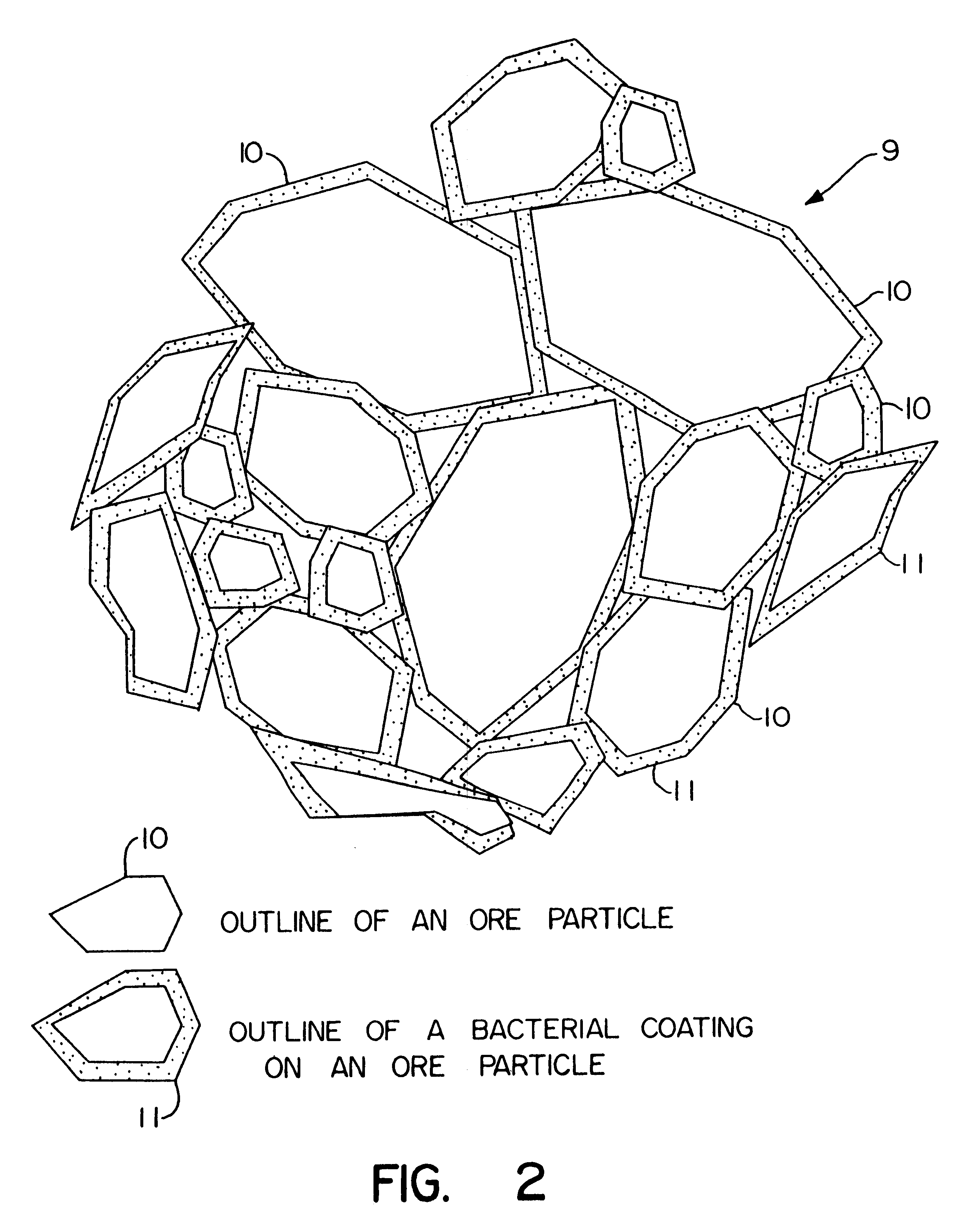
Nonstirred bioreactor for processing refractory sulfide concentrates and method for operating same
InactiveUS6083730AReduced pHIncrease equipment costSolvent extractionContaminated soil reclamationSulfide mineralsMetallic sulfide
A method of biooxidizing sulfide minerals in a nonstirred bioreactor is provided. According to the disclosed method, a concentrate of sulfide minerals is coated onto a substrate, such as coarse ore particles, lava rock, gravel or rock containing mineral carbonate as a source of CO2 for the biooxidizing bacteria. After the sulfide minerals are coated onto the substrate, a heap is formed with the coated substrates or the coated substrates are placed within a tank. The sulfide minerals are then biooxidized to liberate the metal value of interest. Depending on the particular ore deposit being mined, the sulfide mineral concentrates used in the process may comprise sulfide concentrates from precious metal bearing refractory sulfide ores or they may comprise sulfide concentrates from metal sulfide type ores, such as chalcopyrite, millerite or sphalorite. The distinction being that in the former, the metal of interest is a precious metal occluded within the sulfide minerals, whereas in the latter, the metal to be recovered is copper, nickel or zinc and is present as a metal sulfide in the sulfide concentrate.
Owner:GEOSYNFUELS LLC (US)
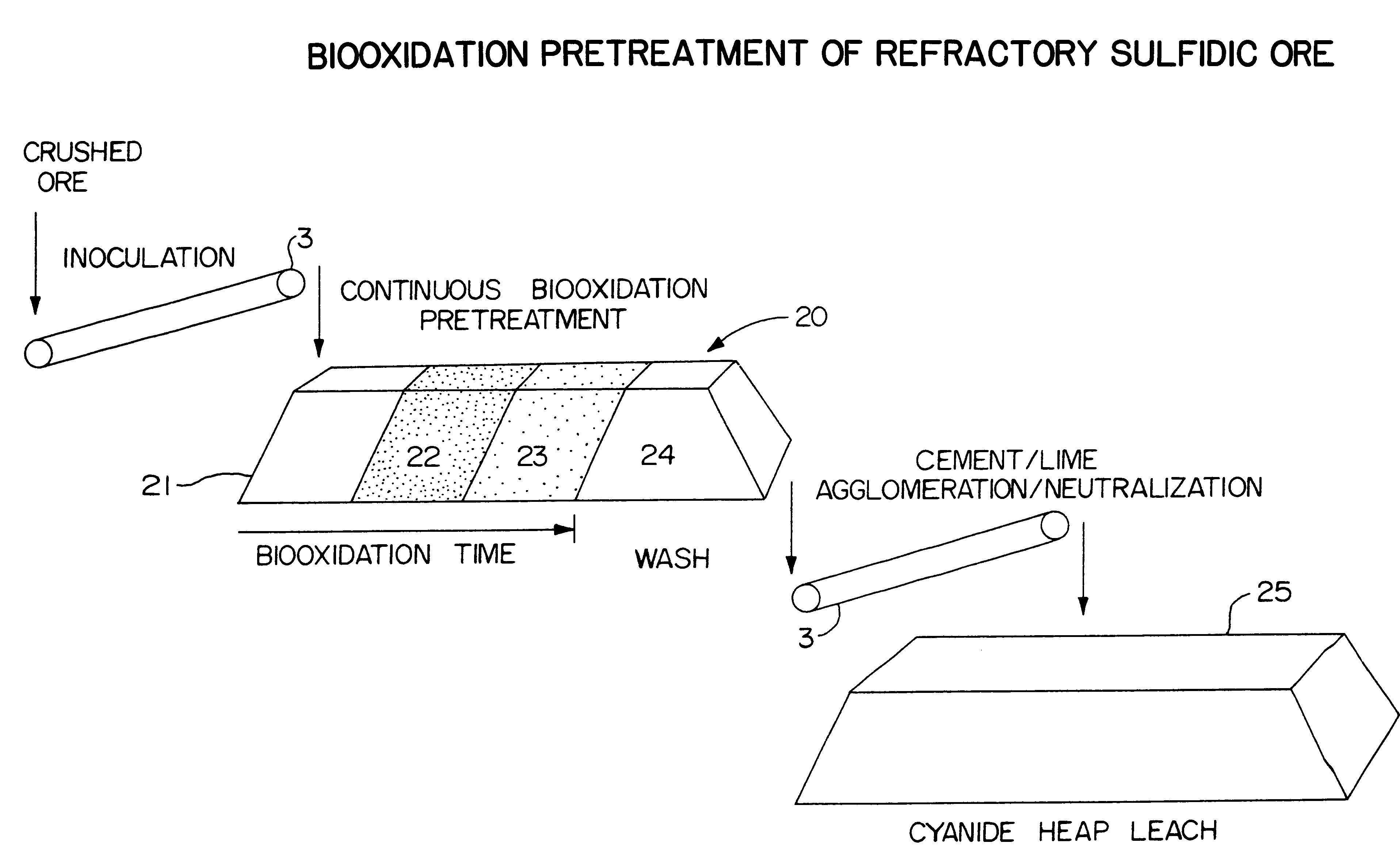
Biooxidation process for recovery of metal values from sulfur-containing ore materials
InactiveUS6383458B1Increase ratingsIncrease attractivenessTransuranic element compoundsSolvent extractionParticulatesPartial oxidation
A process for the recovery of one or more metal values from a metal ore material comprising those of one or more values and a matrix material having a sulfur content wherein the sulfur is present in an oxidation-reduction state of zero or less comprisinga. forming particulates from particles of said ore and an inoculate comprising bacteria capable of at least partially oxidizing the sulfur content;b. forming a heap of said particulates;c. biooxidizing the sulfur content andd. recovering those one or more metal values.
Owner:NEWMONT USA LTD
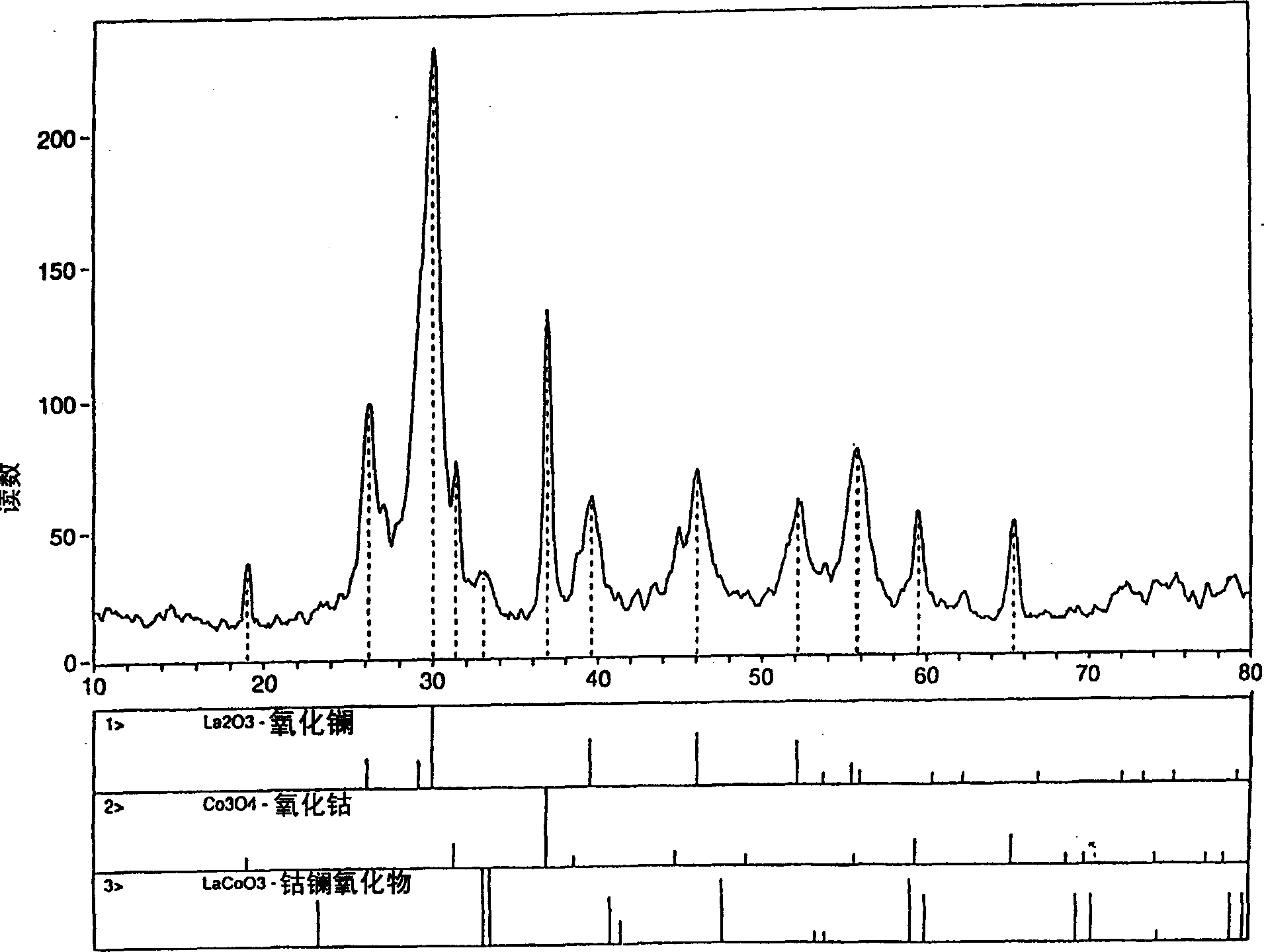
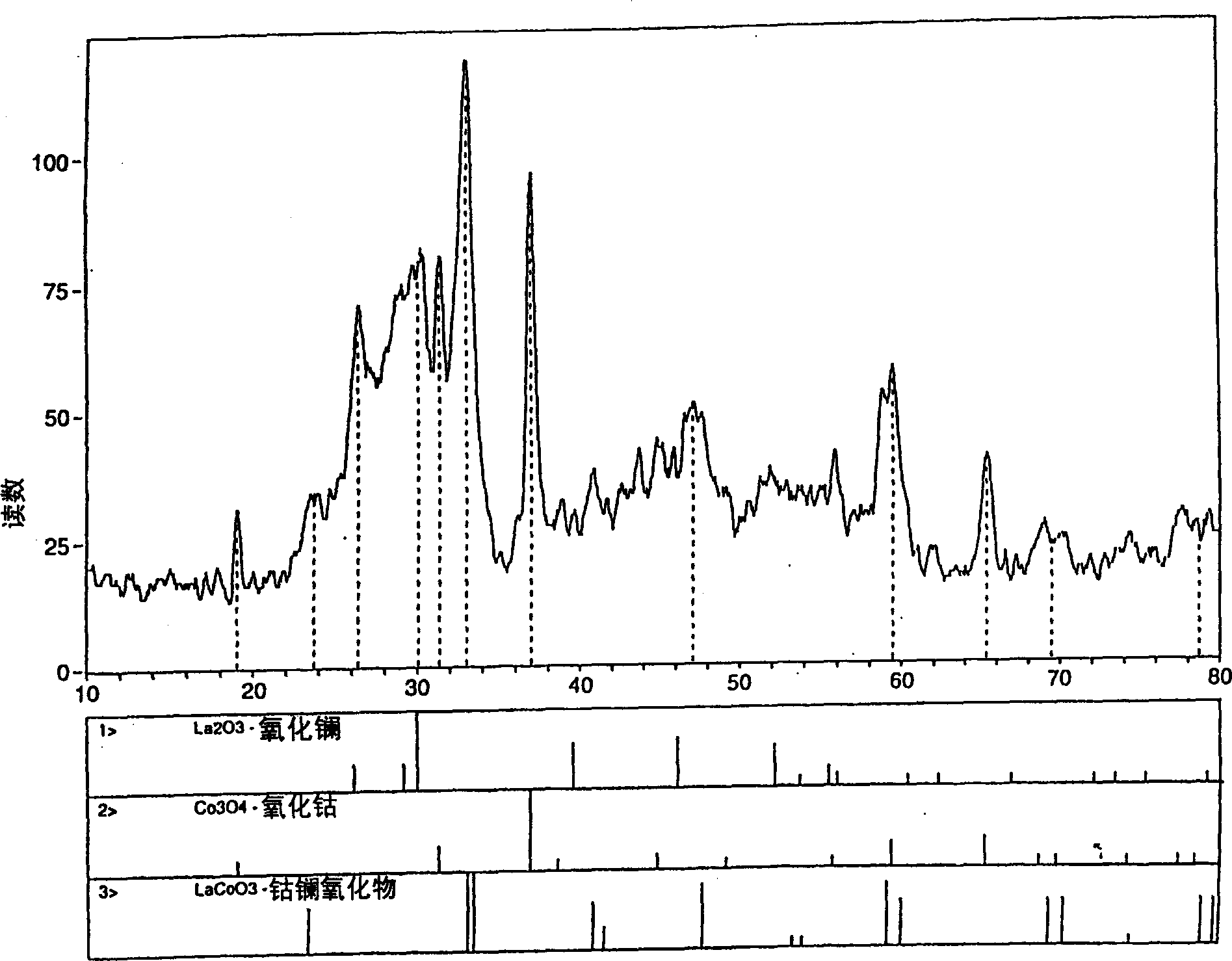
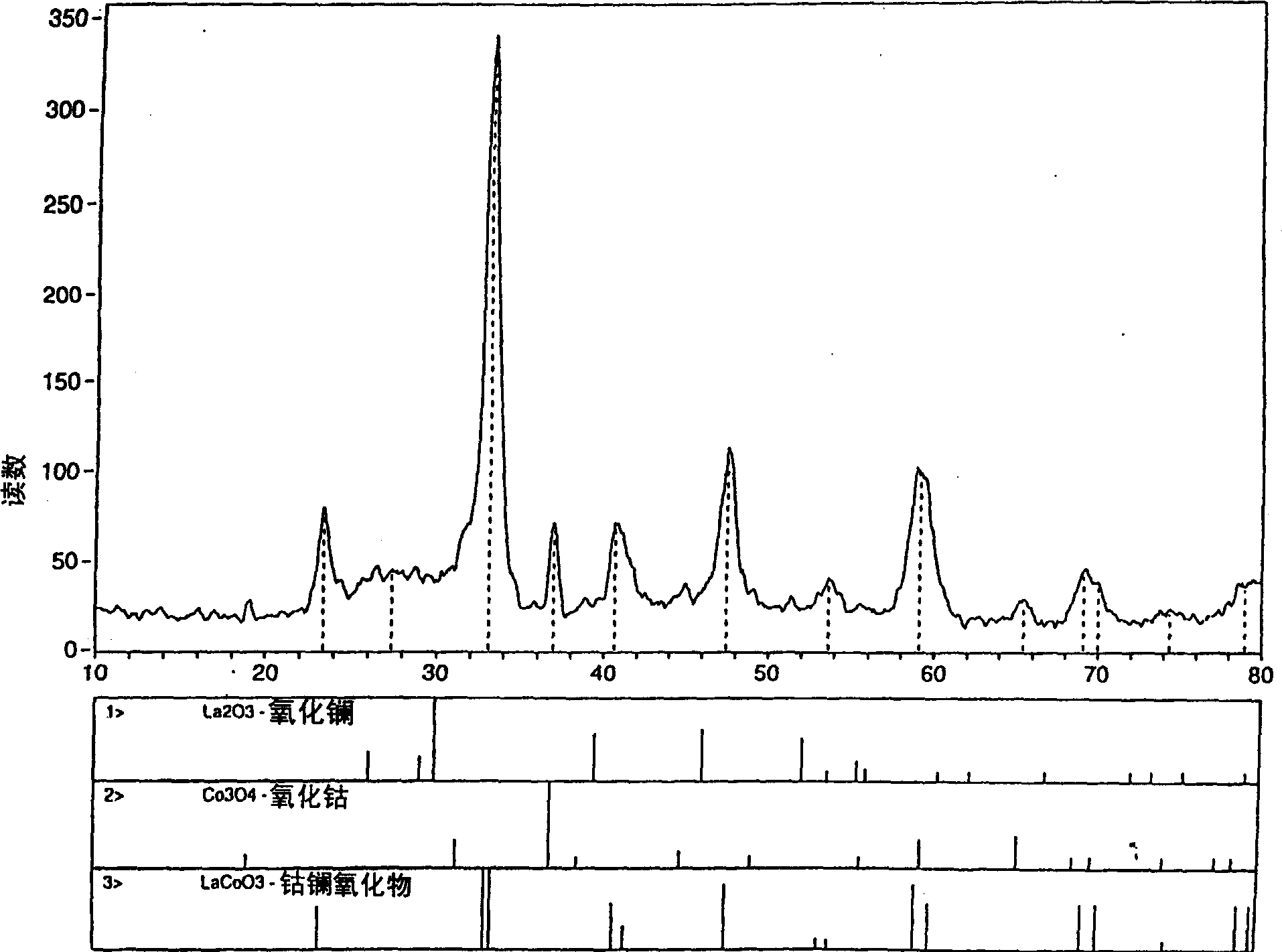
Process for synthesizing metal oxides and metal oxide having perovskite or perovskite-like crystal structure
InactiveCN1315920ALarge specific surface areaSimple methodNanotechOxide/hydroxide preparationHigh densityLattice defects
Perovskite-type structure compounds having the general empirical formula ABO3 are prepared by a process comprising subjecting a mixture of starting powders formulated to contain the components represented by A and B in the formula to a high energy milling sufficient to induce chemical reaction of the components and thereby synthesize a mechanically-alloyed powder comprising the perovskite in the form of nanostructural particles. The process according to the present invention is simple, efficient, not expensive and does not require any heating step for producing a perovskite that may easily show a very high specific surface area. Another advantage is that the perovskite obtained according to the present invention also has a high density of lattice defects thereby showing a higher catalytic activity, a characteristic which is highly desirable in their eventual application as catalysts and electronic conductors.
Owner:UNIV LAVAL
Processes and apparatuses for treating halogen-containing gases
InactiveUS20030012718A1Decreasing and eliminating amountReduce corrosionGas treatmentSolvent extractionHalogenLiquid water
There are disclosed various processes, apparatuses and systems for treating a halogen-containing gas such as F2 that involve generating a plasma in order to reduce chemically the halogen-containing gas into products that are more environmentally manageable. According to a particular embodiment, a reducing agent is mixed with the halogen-containing gas to produce a feed gas mixture and a non-thermal plasma is generated in the feed gas mixture in the presence of liquid water.
Owner:BATTELLE MEMORIAL INST
Process to increase the bioleaching speed of ores or concentrates of sulfide metal species, by means of continuous inoculation with leaching solution that contains isolated microorganisms, with or without presence of native microorganisms
ActiveUS20080127779A1Decrease ore bioleaching timeImprove bioleaching conditionSolvent extractionGold compoundsTailings damPotassium
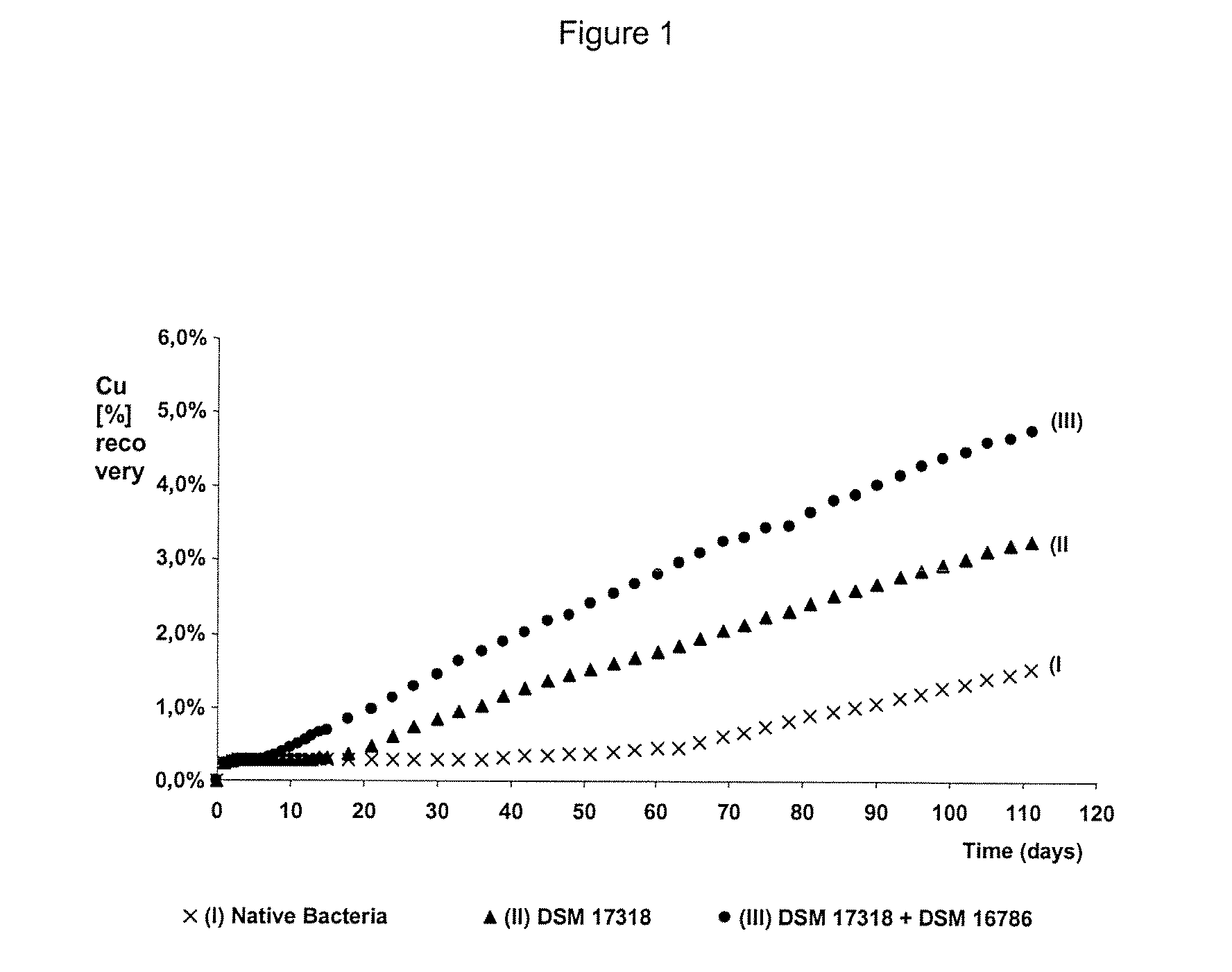
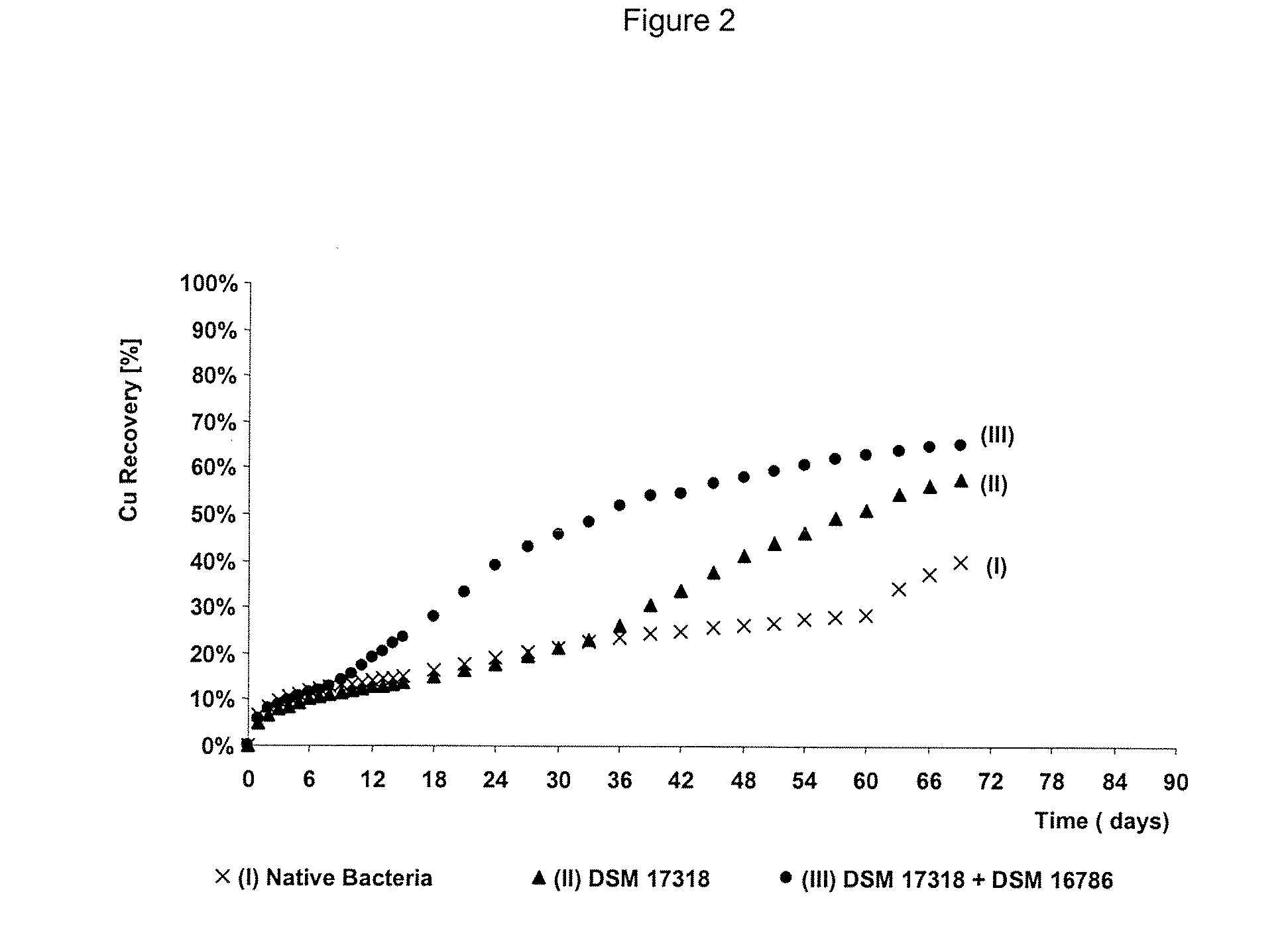
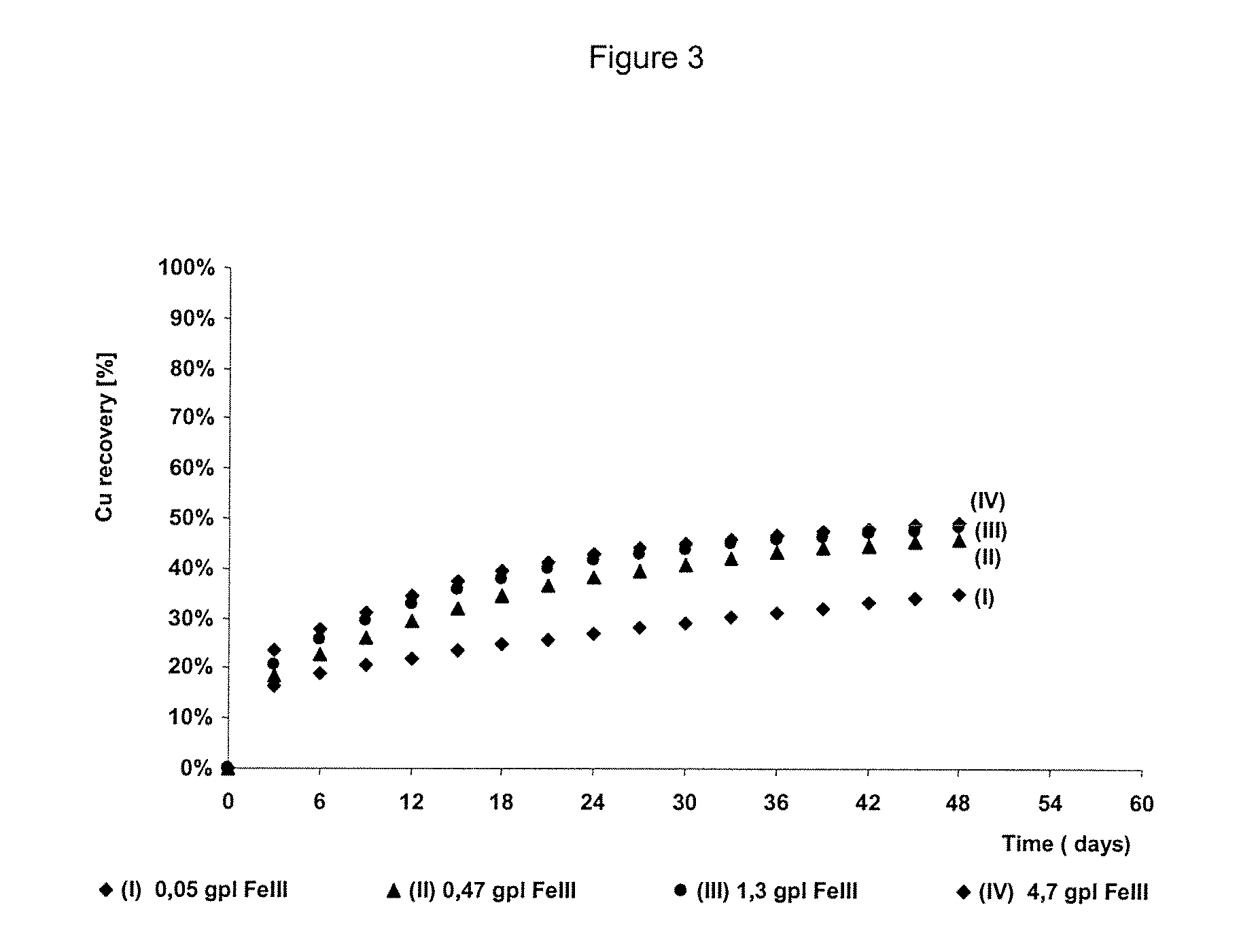
The invention publishes a process to increase the bioleaching speed of ores or concentrates of sulfide metal species in heaps, tailing dams, dumps, or other on-site operations. The process is characterized by the continuous inoculation of the ores or concentrates with isolated microorganisms of the Acidithiobacillus thiooxidans type, together with isolated microorganisms of the Acidithiobacillus ferrooxidans type, with or without native microorganisms, in such a way that the total concentration of microorganisms in the continuous inoculation flow is of around 1×107 cells / ml to 5,6×107 cells / ml. In particular, the invention publishes the continuous inoculation of Acidithiobacillus thiooxidans Licanantay DSM 17318 together with Acidithiobacillus ferrooxidans Wenelen DSM 16786 microorganisms, or with other native microorganisms at a concentration higher than 5×107 cells / ml. In addition to the inoculation of isolated bacteria, the invention includes the addition of oxidizing agents such as the ferric ion produced externally, together with nutrients in the shape of salts of ammonium, magnesium, iron, potassium, as well as air enriched continuously with carbon dioxide to promote bacterial action in the bioleaching process of ores or concentrates.
Owner:BIOSIGMA
Carboxylic acid stabilized silver nanoparticles and process for producing same
ActiveUS20100090179A1Group 1/11 organic compounds without C-metal linkagesConductive materialOrganic solventCarboxylic acid
Processes for producing carboxylic acid-stabilized silver nanoparticles are disclosed. The processes comprise (a) forming a suspension of silver salt particles in a carboxylic acid; (b) forming a solution of an organohydrazine and a first organic solvent; (c) heating the suspension; (d) adding the solution to the suspension to form a mixture; and (e) reacting the mixture to form carboxylic acid-stabilized silver nanoparticles.
Owner:XEROX CORP
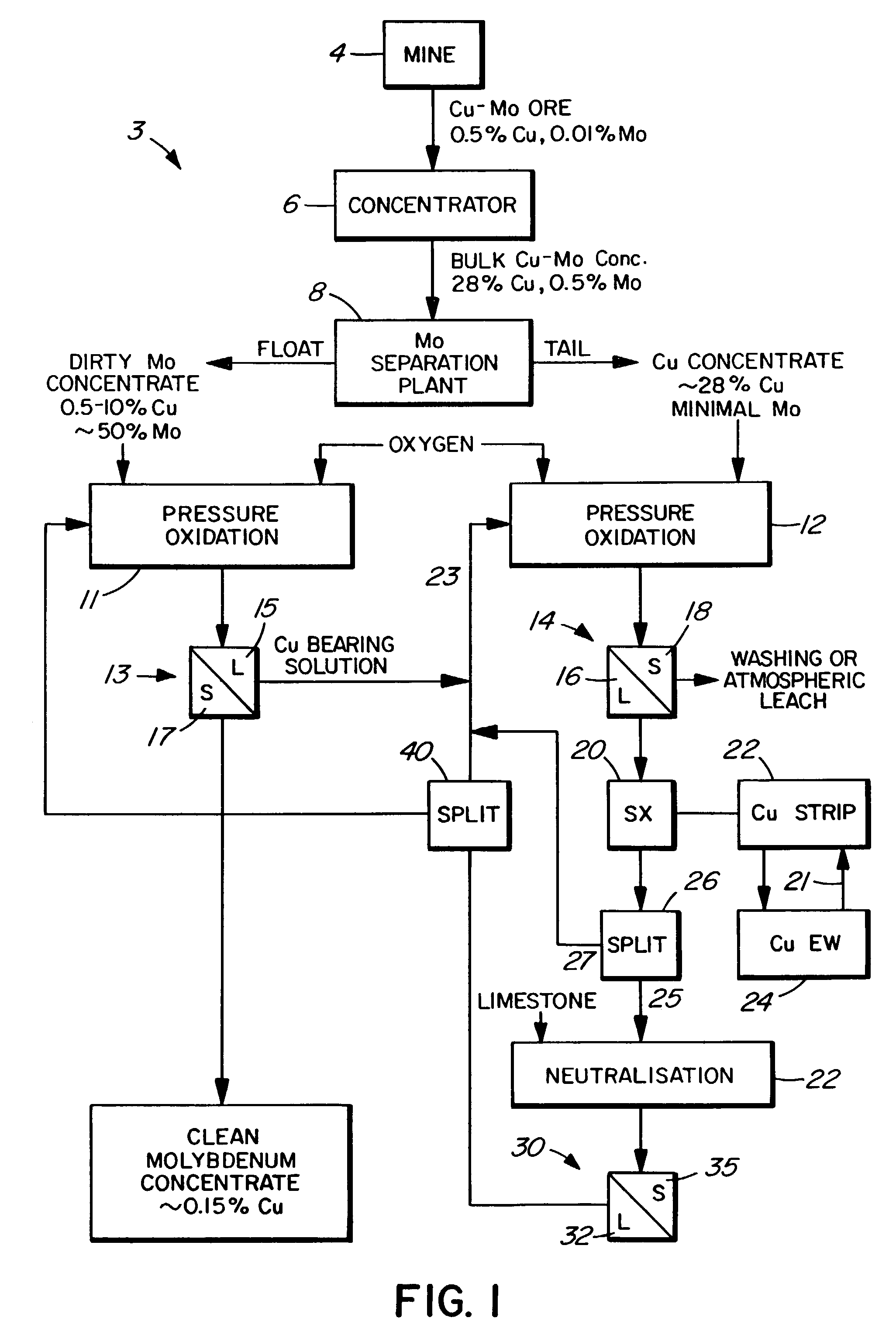
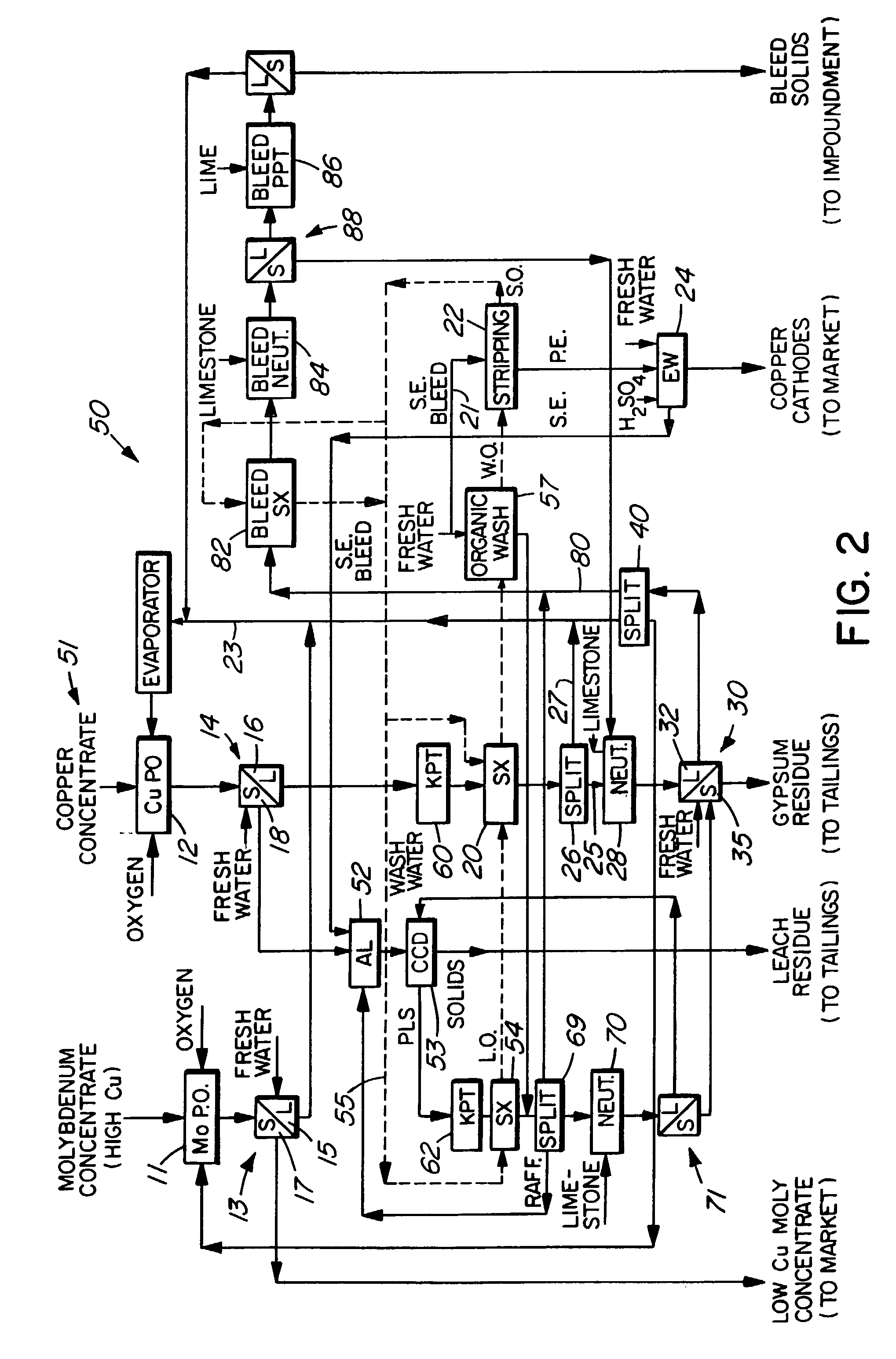
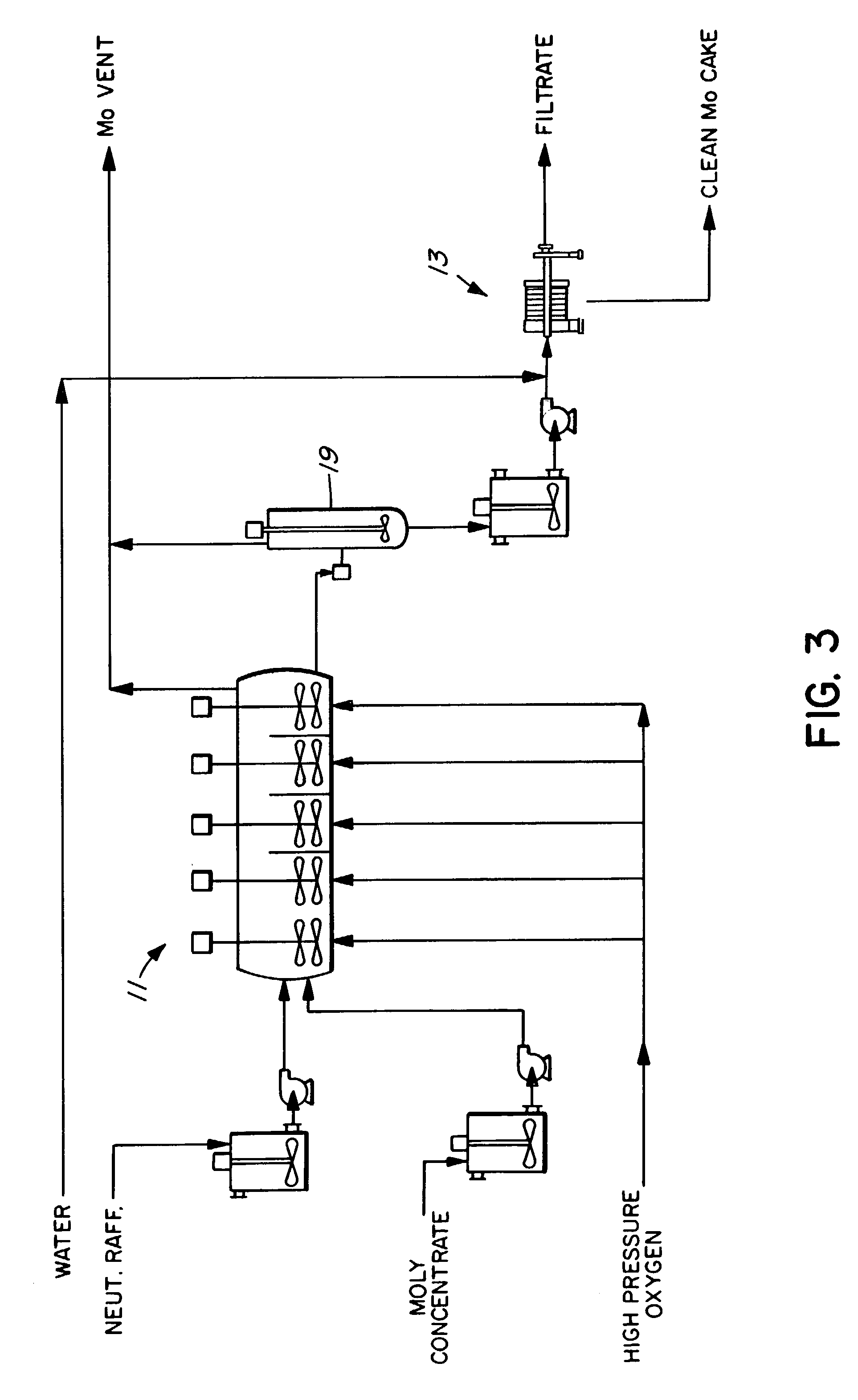
Process for the treatment of molybdenum concentrate
A method of treatment or purification of a molybdenum concentrate also containing copper, comprises the step of subjecting the molybdenum concentrate to pressure oxidation in the presence of oxygen and a feed solution containing copper (e.g. CuSO4) and halide (e.g. CuCl2) to produce a pressure oxidation solution containing copper and a solid residue containing molybdenum. The pressure oxidation solution may be combined with feed solution to a second pressure oxidation in which a copper concentrate is treated for the recovery of copper therefrom.
Owner:CESL LIMITED
Organoboron route and process for preparation of boron nitride
InactiveUS20020155052A1Simple preparation processEasy to purifyMaterial nanotechnologyPolycrystalline material growthCrystal structureWhiskers
An organoboron route and process for preparation of boron nitride utilizing aerosol assisted vapor phase synthesis (AAVS) wherein organoboron precursors are nitrided in one or two heating steps, and wherein a boron oxide nitride intermediary composition is formed after the first heating step and may be further nitrided to form resultant spheroidal boron nitride powders including spheroidal particles that are smooth, bladed, have protruding whiskers, and are of turbostratic or hexagonal crystalline structure.
Owner:NEW MEXICO UNIV OF
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com