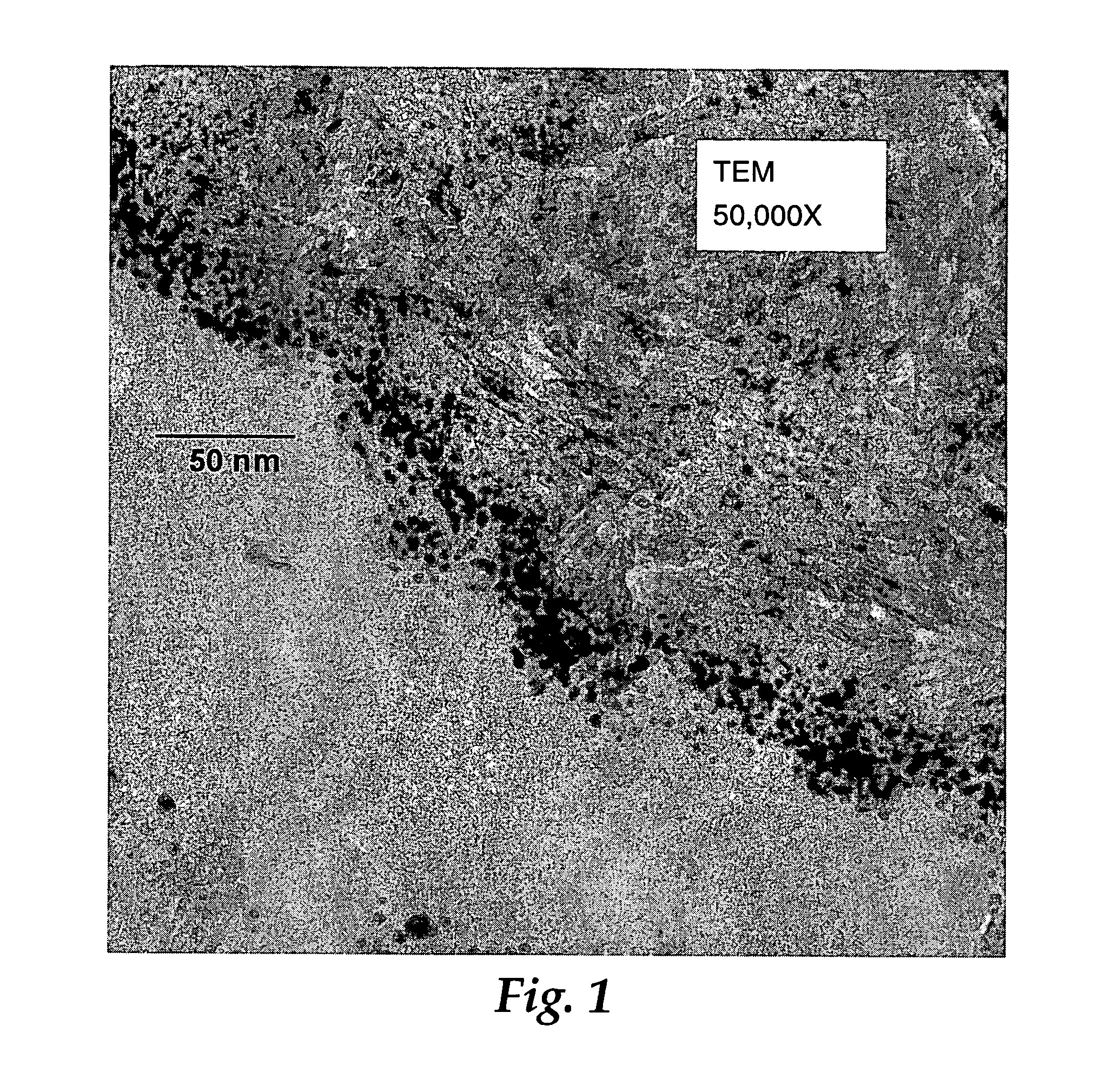
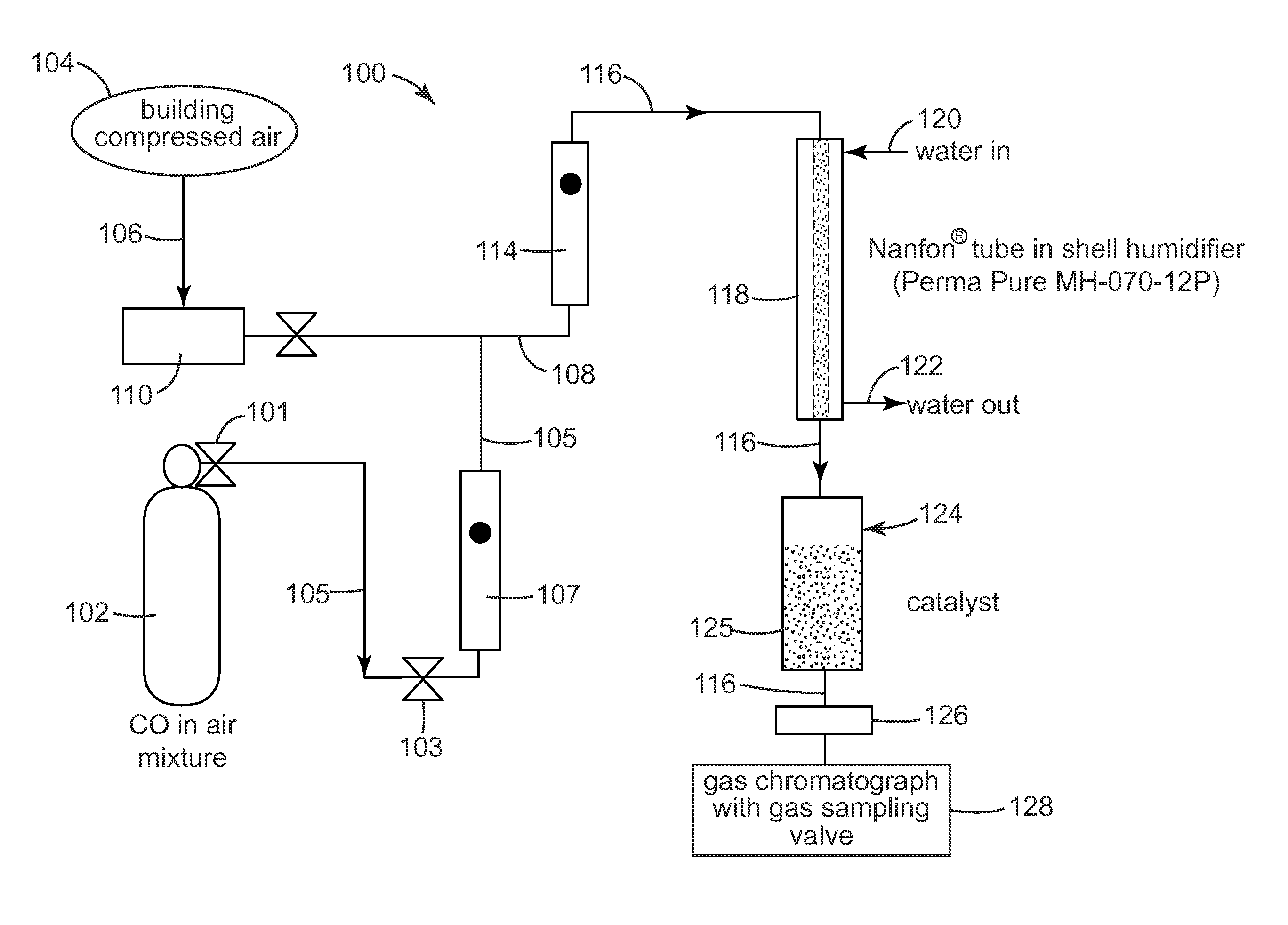
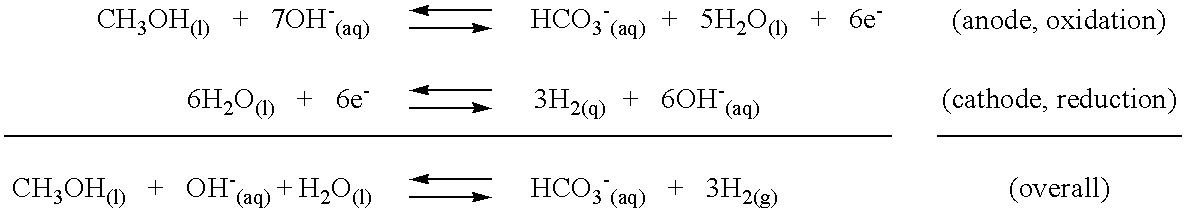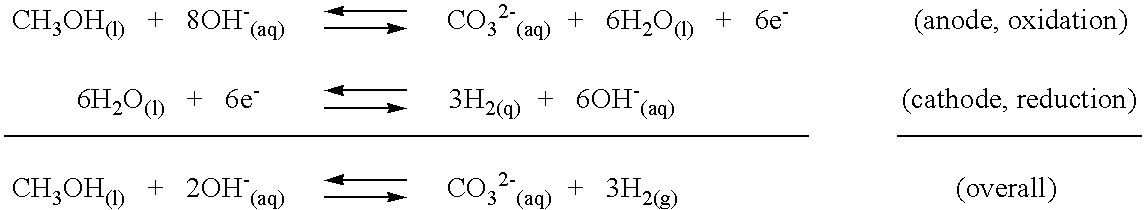Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67results about "Varying alkali metal carbonate water content" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
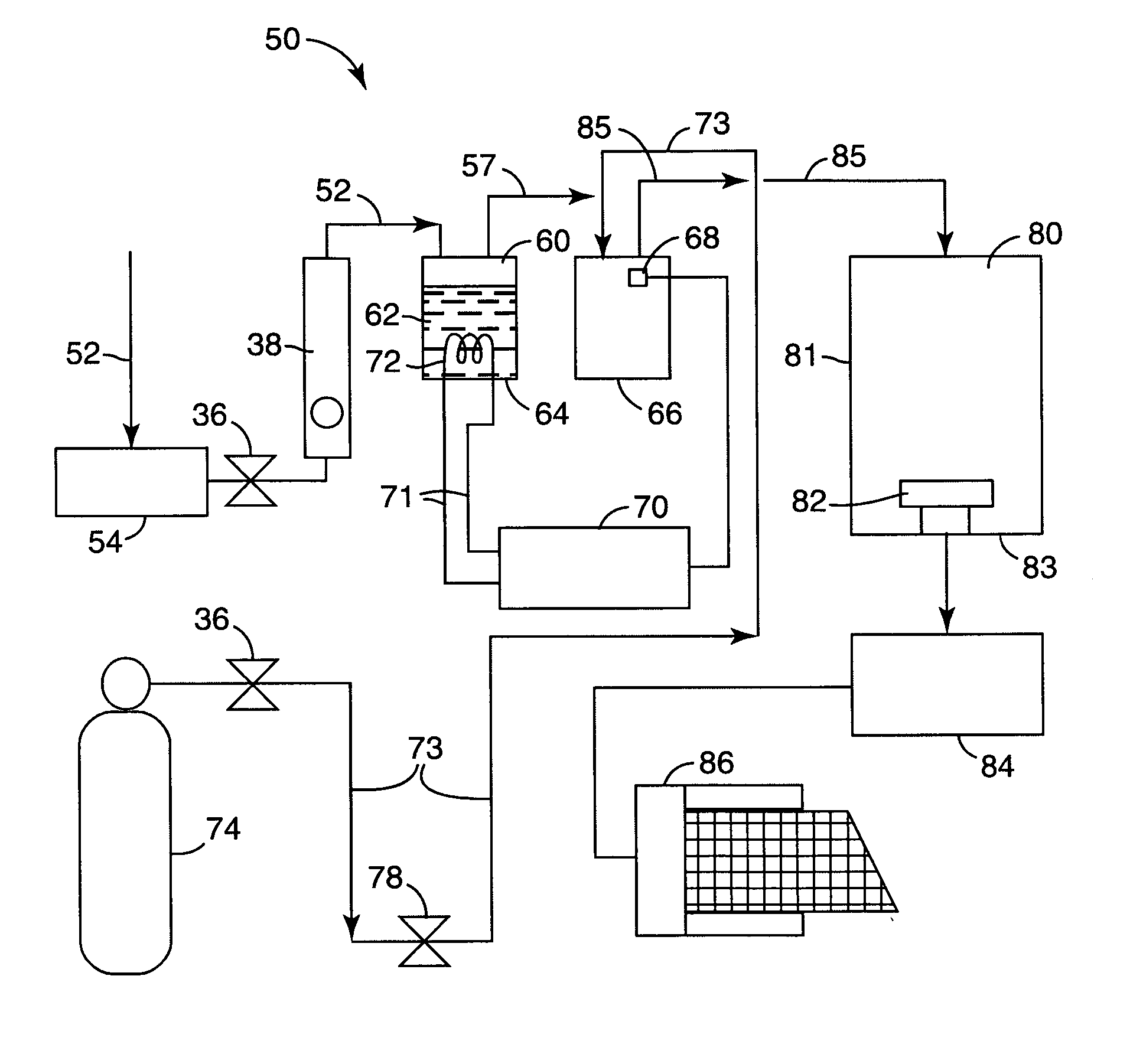
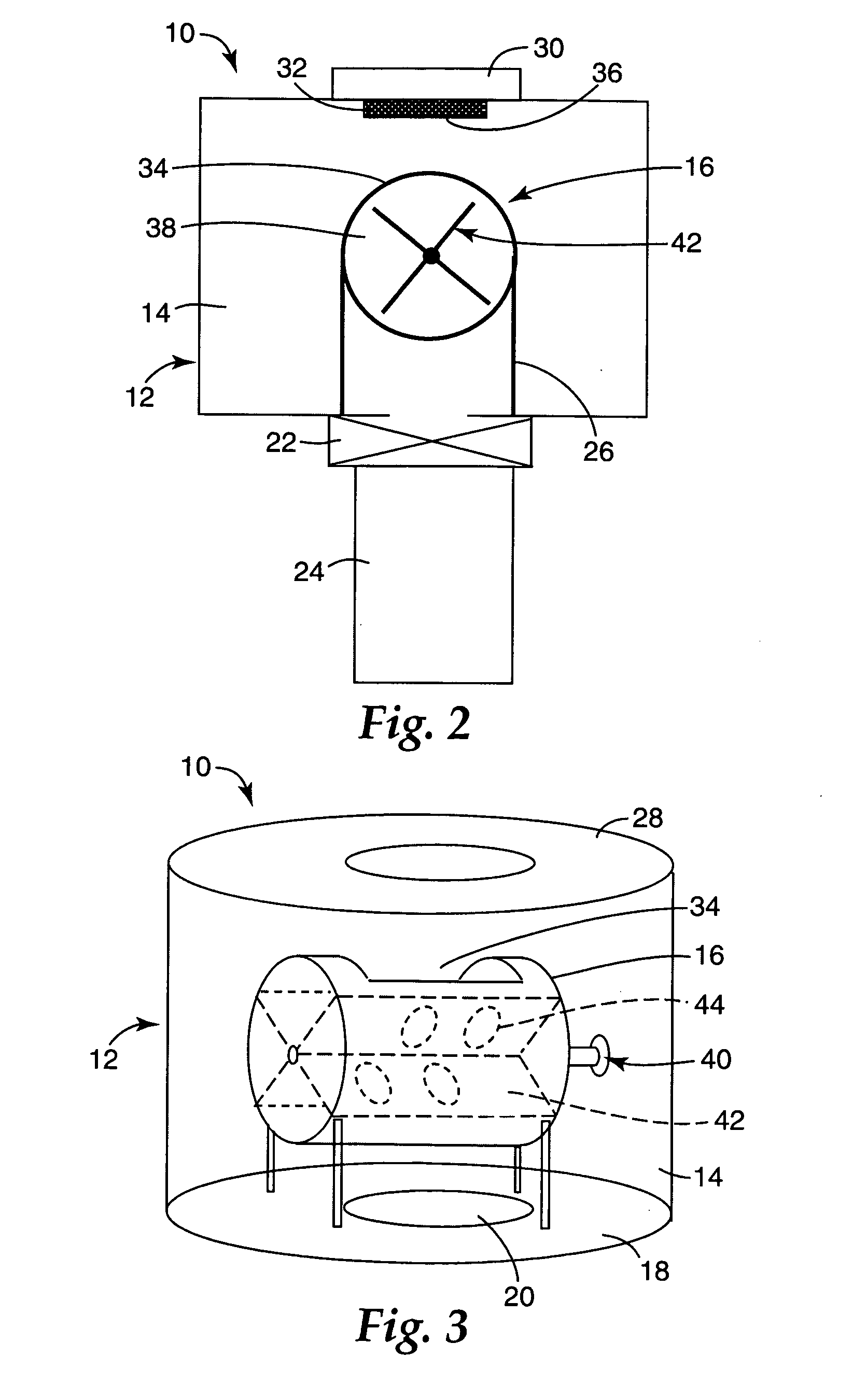
Catalysts, activating agents, support media, and related methodologies useful for making catalyst systems especially when the catalyst is deposited onto the support media using physical vapor deposition
InactiveUS20050095189A1Improve performanceEasy to useMaterial nanotechnologyInternal combustion piston enginesGas phaseAdditive ingredient
Use of physical vapor deposition methodologies to deposit nanoscale gold on activating support media makes the use of catalytically active gold dramatically easier and opens the door to significant improvements associated with developing, making, and using gold-based, catalytic systems. The present invention, therefore, relates to novel features, ingredients, and formulations of gold-based, heterogeneous catalyst systems generally comprising nanoscale gold deposited onto a nanoporous support.
Owner:3M INNOVATIVE PROPERTIES CO
Catalysts, activating agents, support media, and related methodologies useful for making catalyst systems especially when the catalyst is deposited onto the support media using physical vapor deposition
InactiveUS7727931B2High catalytic activityTendency increaseMaterial nanotechnologyInternal combustion piston enginesGas phasePhysical chemistry
Use of physical vapor deposition methodologies to deposit nanoscale gold on activating support media makes the use of catalytically active gold dramatically easier and opens the door to significant improvements associated with developing, making, and using gold-based, catalytic systems. The present invention, therefore, relates to novel features, ingredients, and formulations of gold-based, heterogeneous catalyst systems generally comprising nanoscale gold deposited onto a nanoporous support.
Owner:3M INNOVATIVE PROPERTIES CO
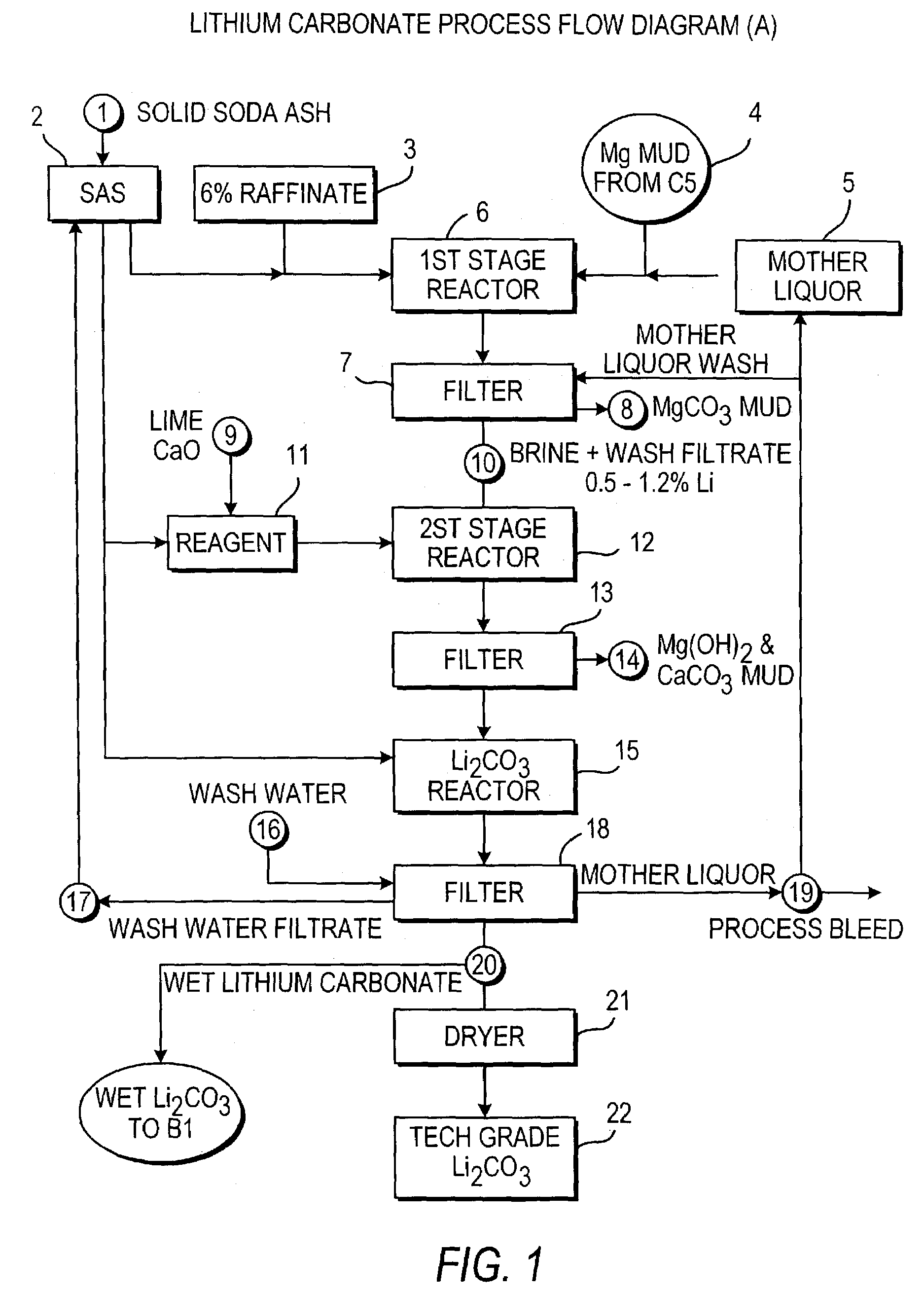
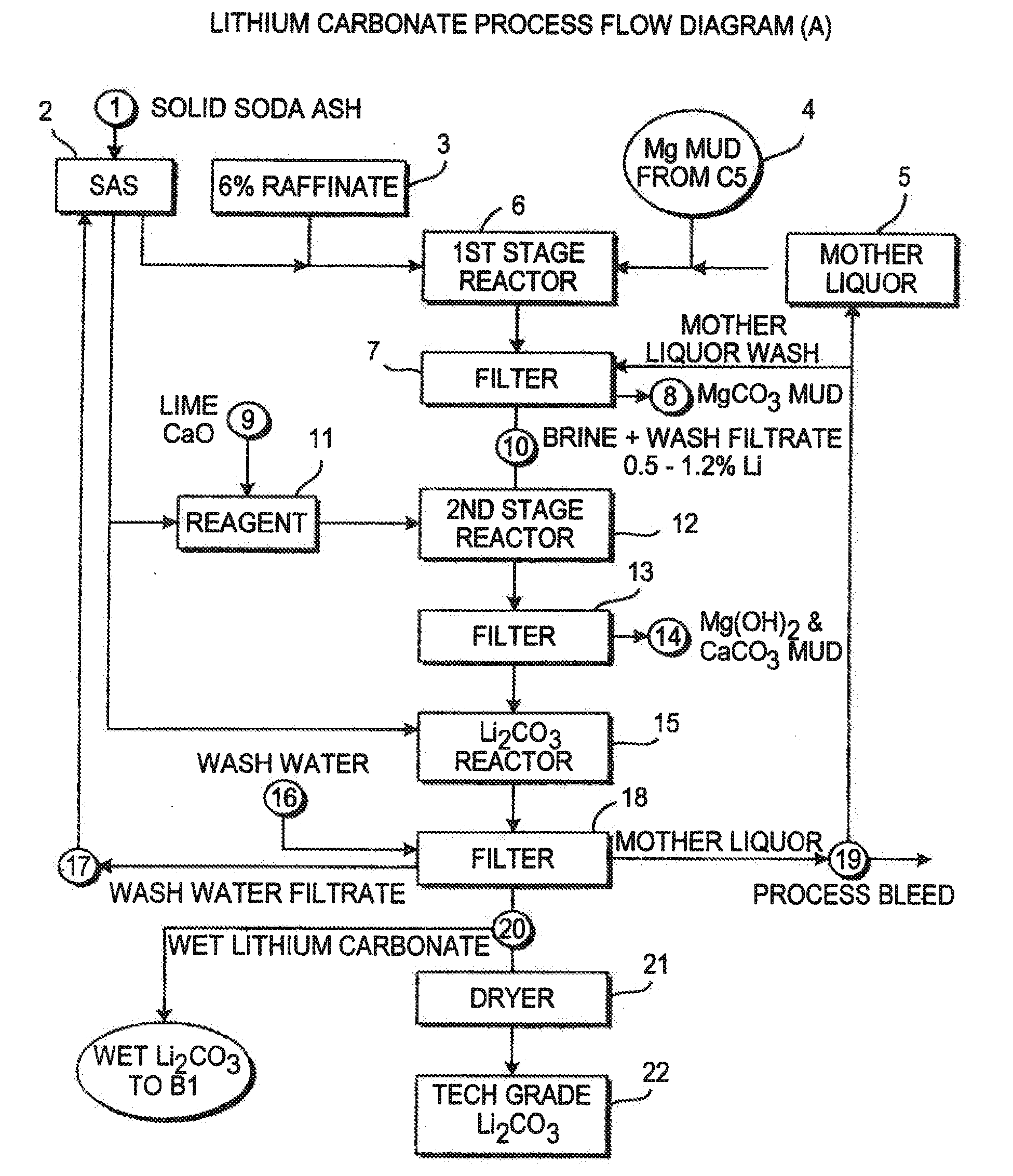
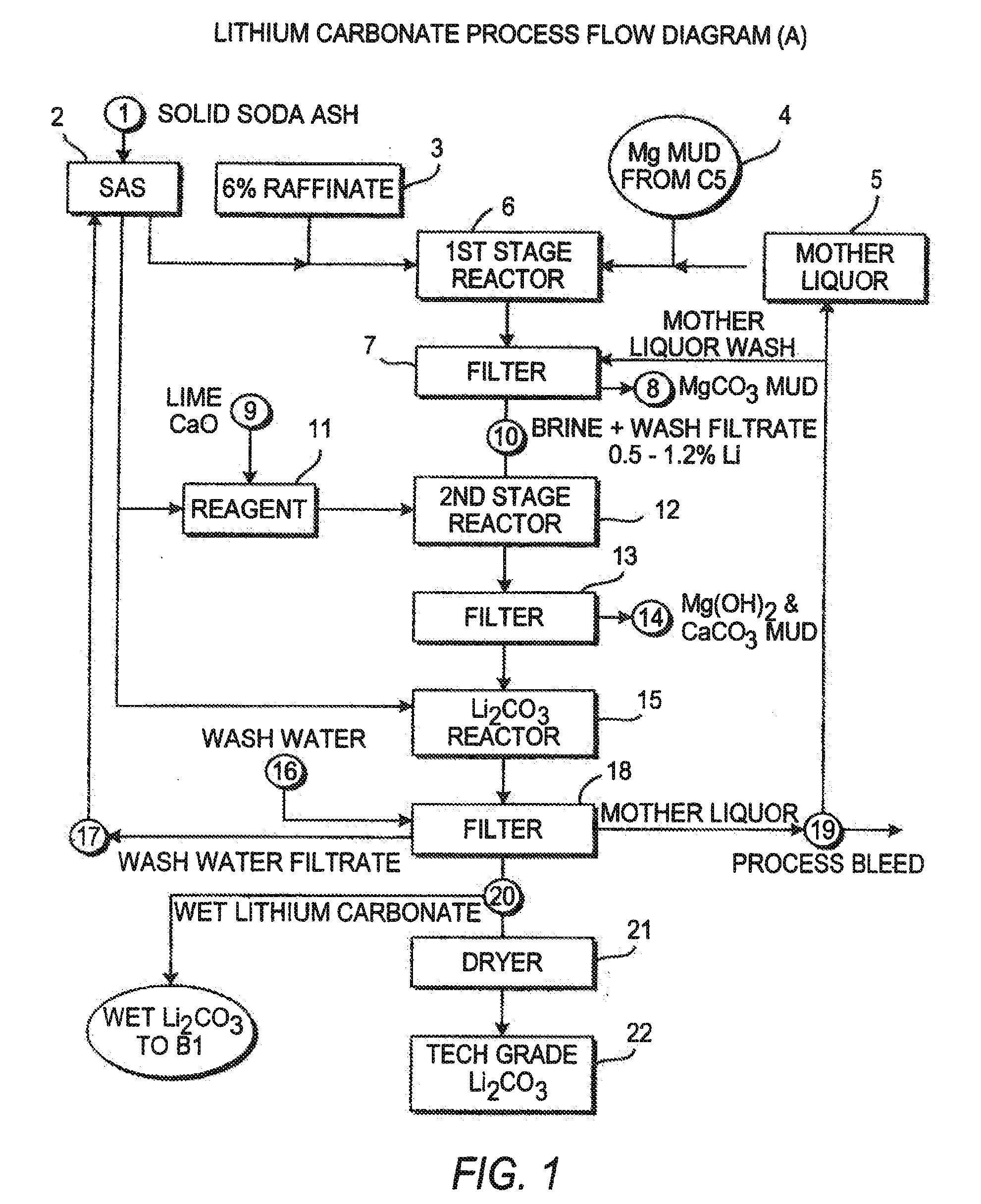
Production of lithium compounds directly from lithium containing brines
InactiveUS7157065B2Reduce in quantityPromote absorptionCalcium/strontium/barium carbonatesChemical/physical/physico-chemical processesSlurryLithium compound
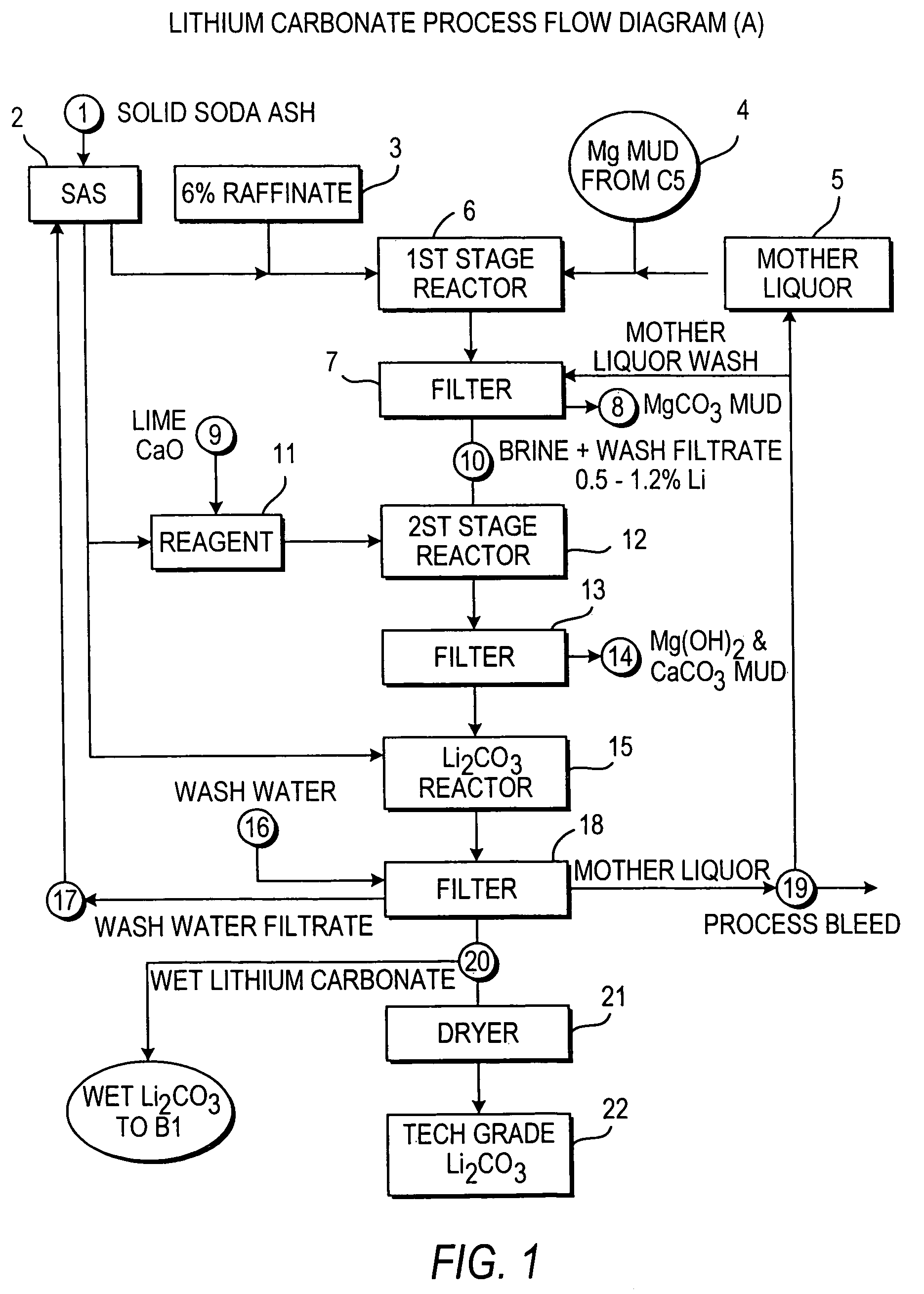
A continuous process for directly preparing high purity lithium carbonate from lithium containing brines by preparing a brine containing about 6.0 wt % lithium and further containing other ions naturally occurring in brines; adding mother liquor containing carbonate to precipitate magnesium; adding a solution of CaO and sodium carbonate to remove calcium and any residual magnesium; precipitating lithium carbonate from the purified brine by adding soda ash solution; filtering to obtain solid lithium carbonate; preparing an aqueous slurry of the lithium carbonate and introducing carbon dioxide gas at a temperature from at least minus 10 to +40° C.; passing the lithium bicarbonate solution through a filter to clarify the solution; introducing said filtered lithium bicarbonate solution into a reactor and adjusting the temperature of the solution to from 60–100° C. to precipitate ultra-pure lithium carbonate.
Owner:ROCKWOOD LITHIUM INC
Production of lithium compounds directly from lithium containing brines
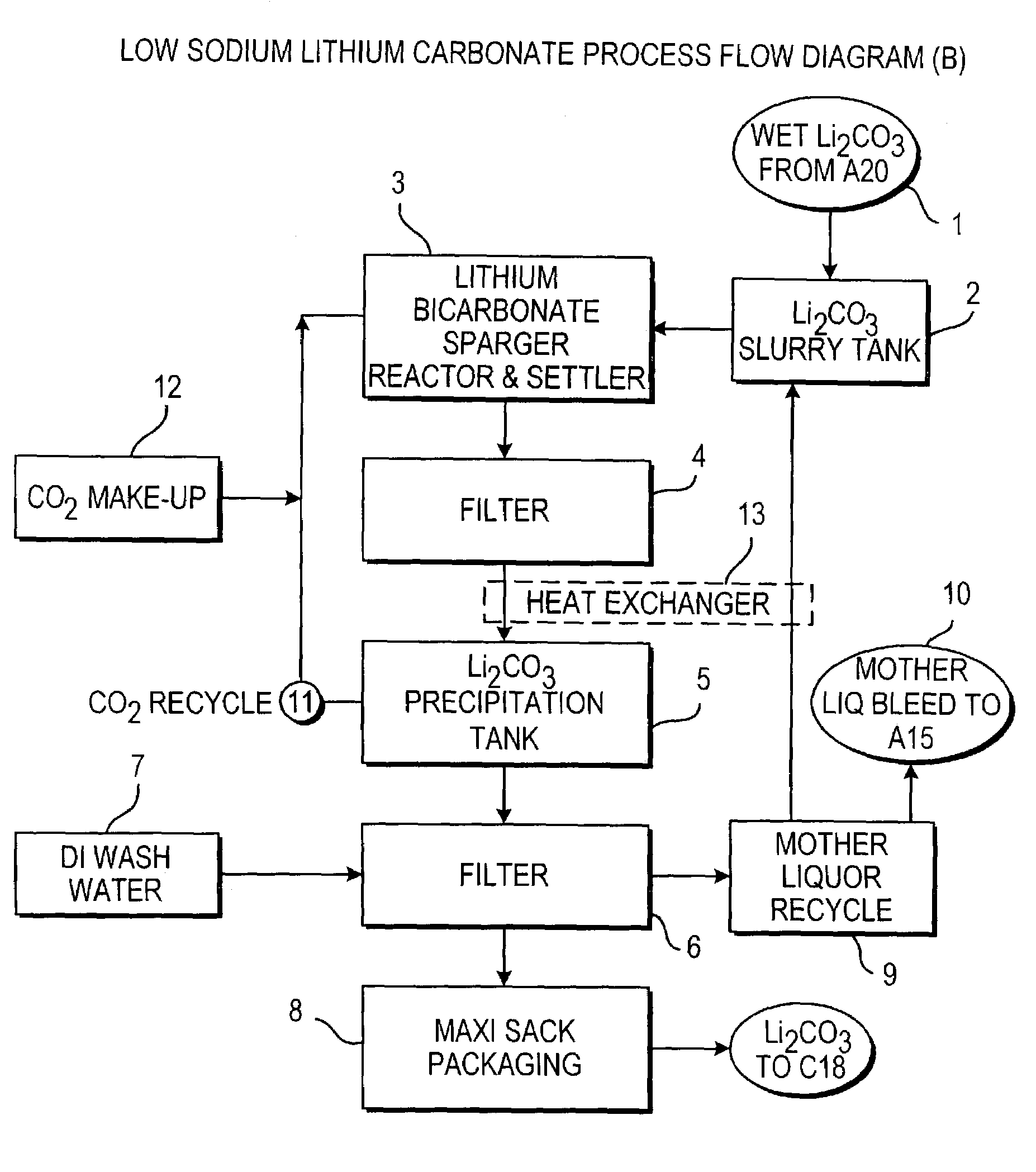
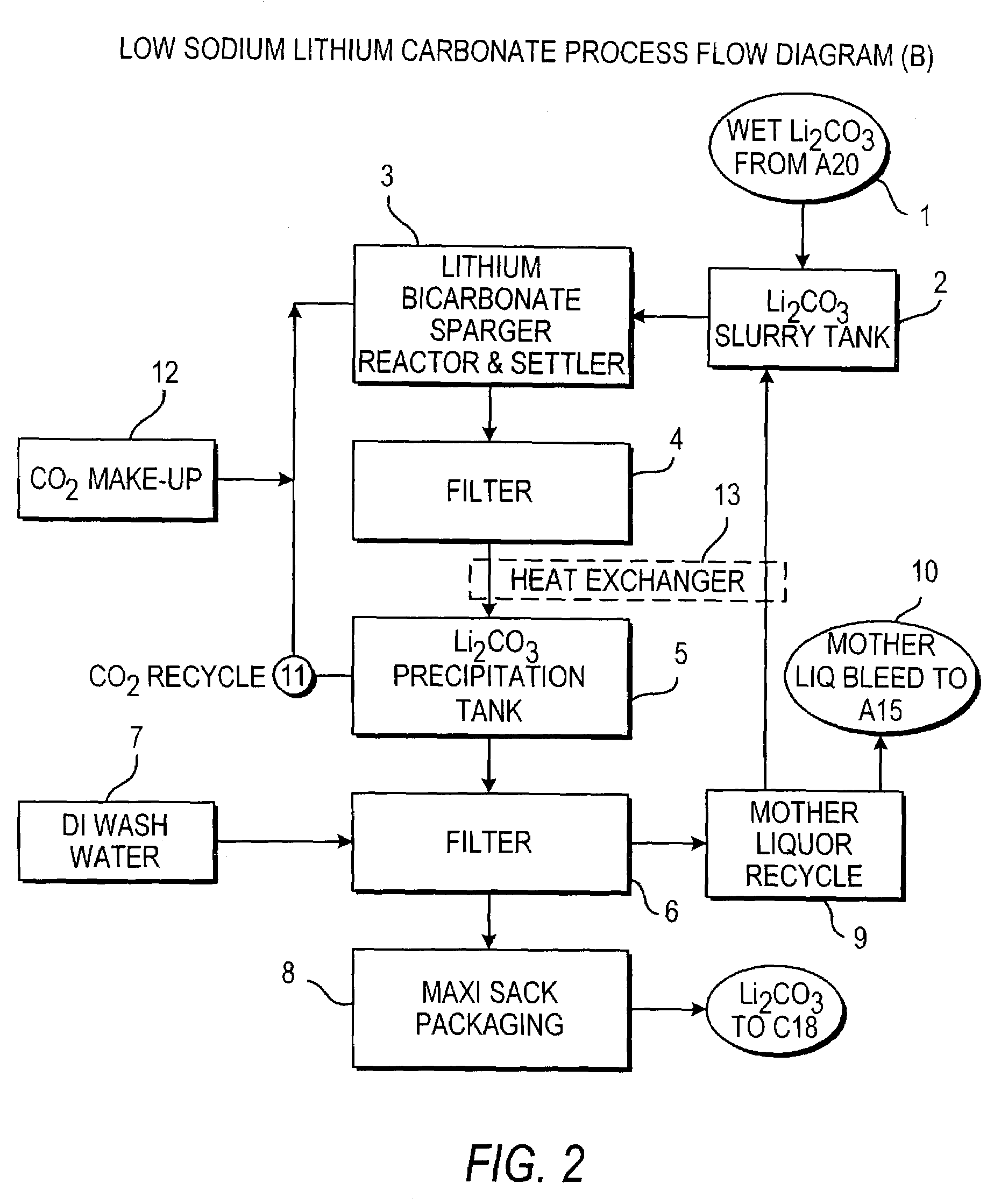
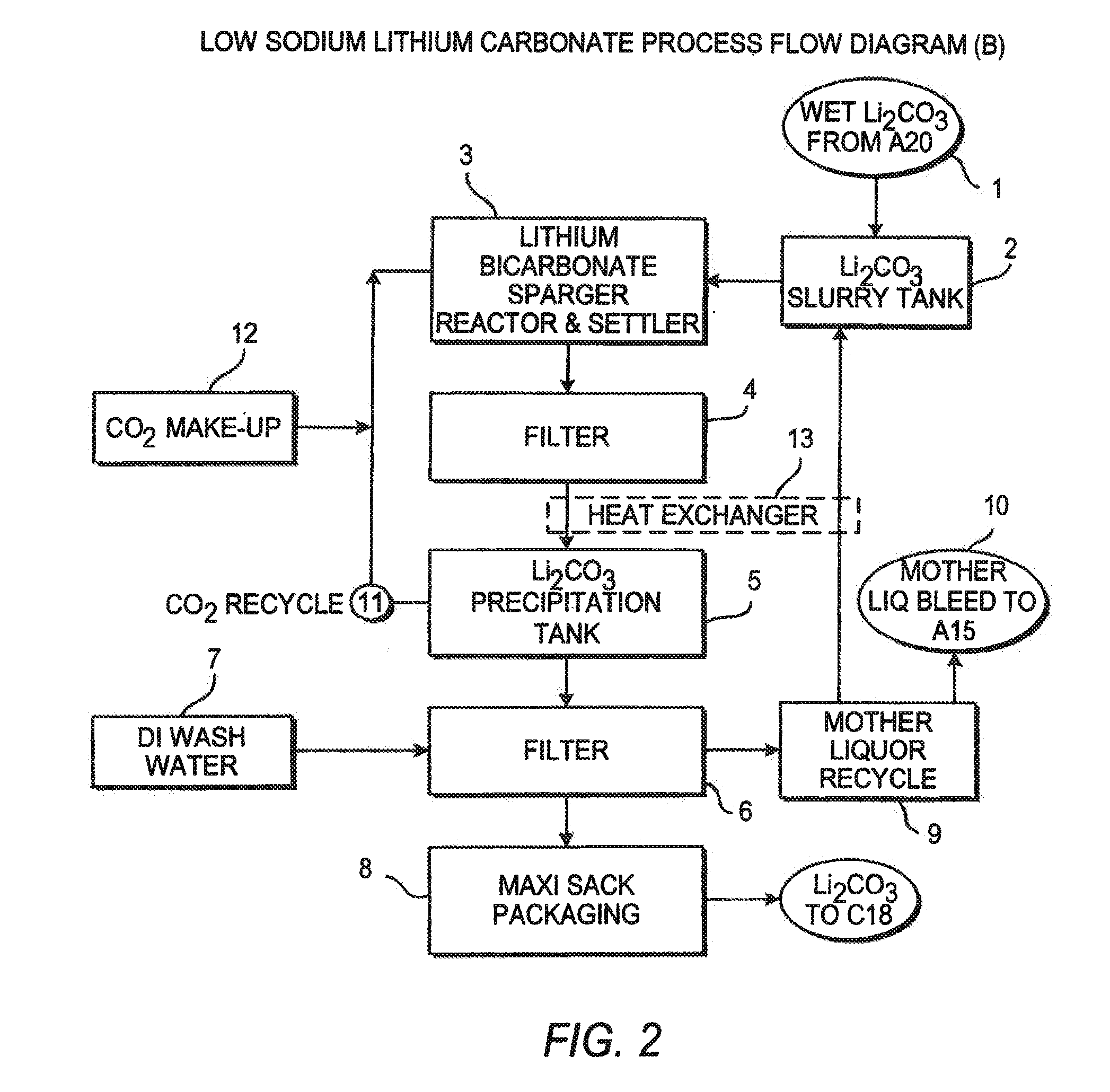
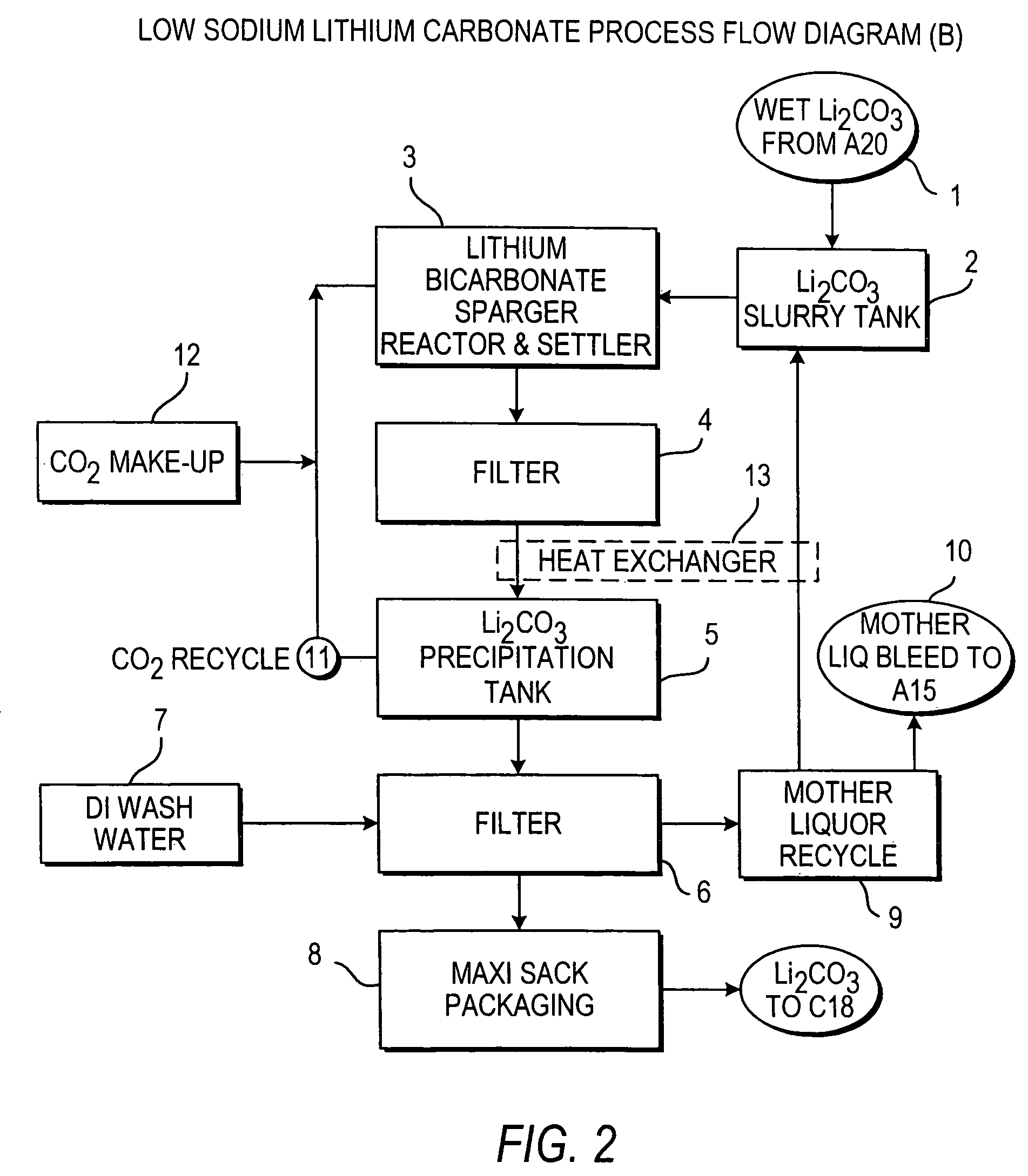
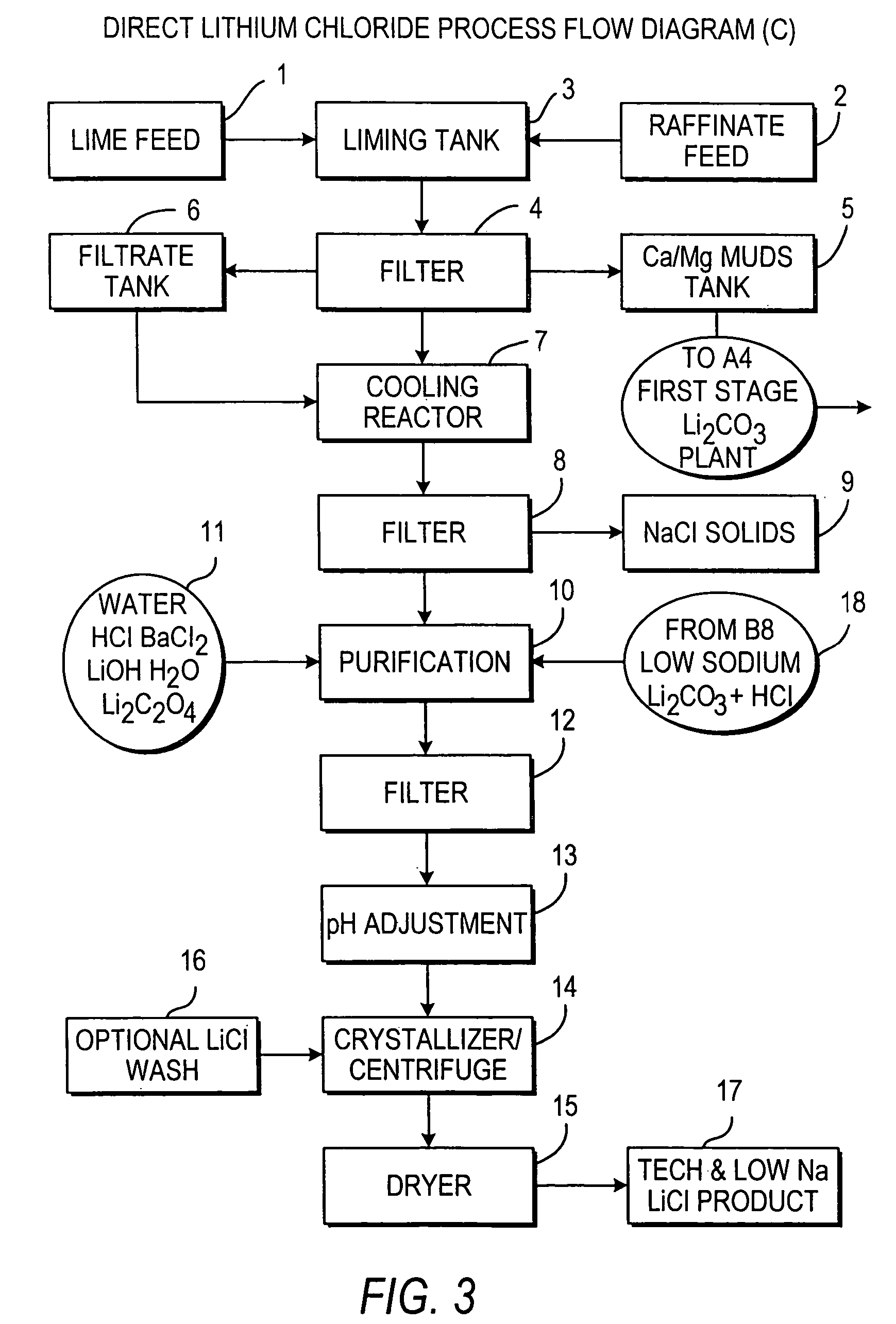
InactiveUS20110123427A1Reduce in quantityPromote absorptionVarying alkali metal carbonate water contentRubidium/caesium/francium compoundsLithium chlorideLithium carbonate
Methods and apparatus for the production of low sodium lithium carbonate and lithium chloride from a brine concentrated to about 6.0 wt % lithium are disclosed. Methods and apparatus for direct recovery of technical grade lithium chloride from the concentrated brine are also disclosed.
Owner:ROCKWOOD LITHIUM INC
Carbonate recycling in a hydrogen producing reaction
InactiveUS6994839B2Calcium/strontium/barium carbonatesElectrolysis componentsCarbonateMetal hydroxide
A process for producing hydrogen gas from a reaction of an organic substance and a base with a recycling of a carbonate or bicarbonate by-product and a regeneration of the base. In one embodiment, reaction of an organic substance and a base produces hydrogen gas and a metal carbonate. The instant invention provides recycling of the metal carbonate by-product. In a preferred embodiment, the metal carbonate by-product is soluble and recycling includes a three step process. In a first step, the soluble metal carbonate is reacted with a metal hydroxide to form a weakly soluble or insoluble metal carbonate that precipitates in a metathesis reaction. The metal hydroxide reactant of the hydrogen producing reaction is also formed in the metathesis reaction and remains in solution. Precipitation of the carbonate thus permits ready isolation of the carbonate by-product, while leaving behind an aqueous metal hydroxide phase that can be returned to and further utilized in the hydrogen producing reaction. The metal carbonate precipitate of the metathesis reaction is thermally decomposed to form a metal oxide solid in a second step. In a third step, the metal oxide is reacted with water to reform the metal hydroxide reactant of the metathesis reaction. The hydrogen producing reaction and recycling process are sustainable in that the metal hydroxide reactant of each reactant is regenerated in the recycling process. In an alternative embodiment, the hydrogen producing reaction produces a metal carbonate precipitate directly and recycling occurs through thermal decomposition of the metal carbonate to form a metal oxide followed by reaction of the metal oxide with water to reform the metal hydroxide employed in the hydrogen producing reaction. In yet another embodiment, a bicarbonate by-product is formed by a hydrogen producing reaction of an organic substance and a base and bicarbonate recovery occurs by heating the bicarbonate to form a carbonate and recycling according to the instant carbonate recycling process.
Owner:TACTICAL FUEL CELLS
Heterogeneous, composite, carbonaceous catalyst system and methods that use catalytically active gold
ActiveUS20070207079A1Low densityInexpensive materialsMaterial nanotechnologyGas treatmentActivated carbonHost material
Heterogeneous catalyst systems, methods of making these systems, and methods of using these systems, wherein catalytically active gold is deposited onto composite support media. The composite support media is formed by providing nanoporous material on at least a portion of the surfaces of carbonaceous host material. In representative embodiments, relatively fine, nanoporous guest particles are coated or otherwise provided on surfaces of relatively coarser activated carbon particles. Catalytically active gold may be deposited onto one or both of the guest or host materials either before or after the guest and host materials are combined to from the composite host material. PVD is the preferred catalyst system of depositing gold.
Owner:3M INNOVATIVE PROPERTIES CO
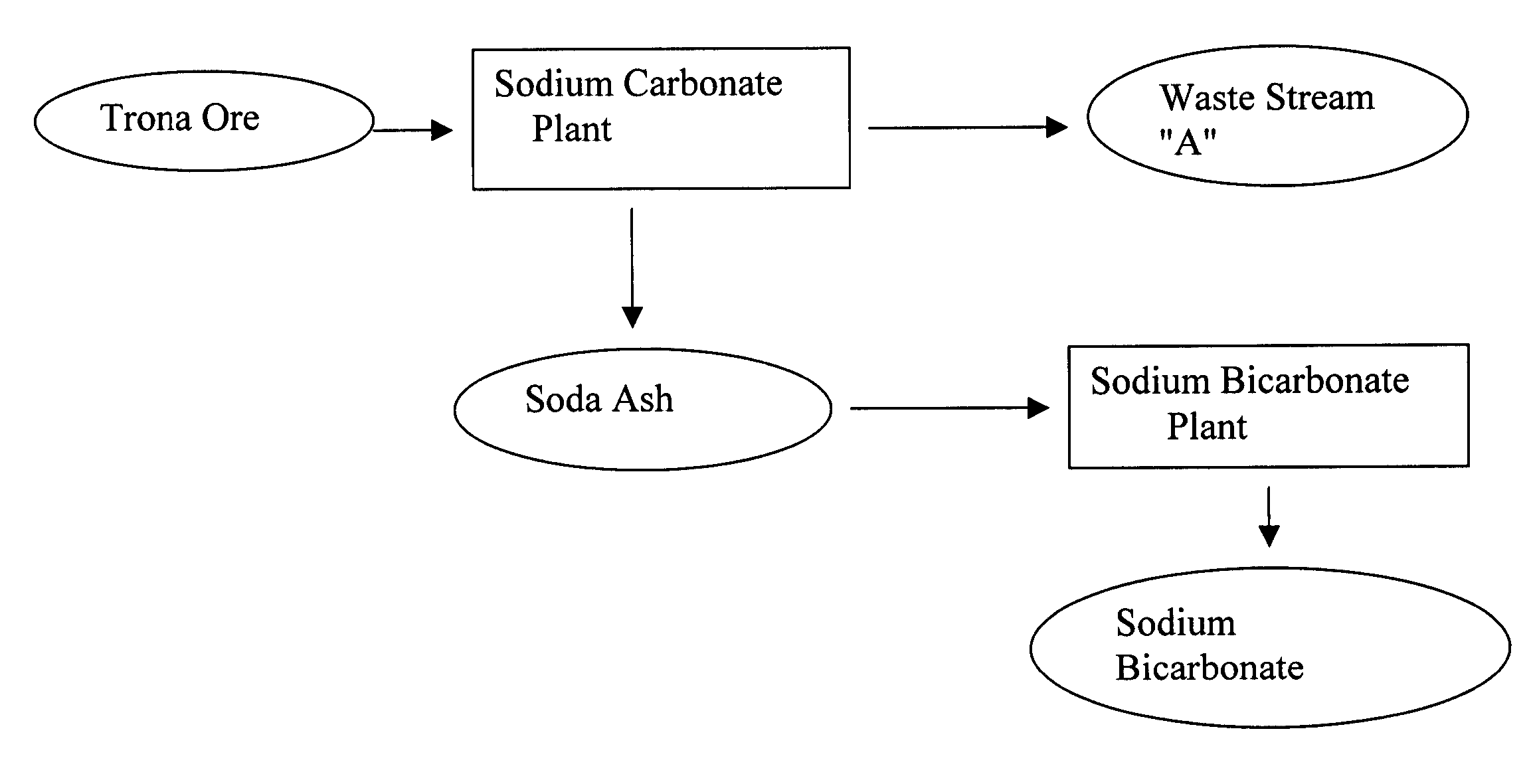
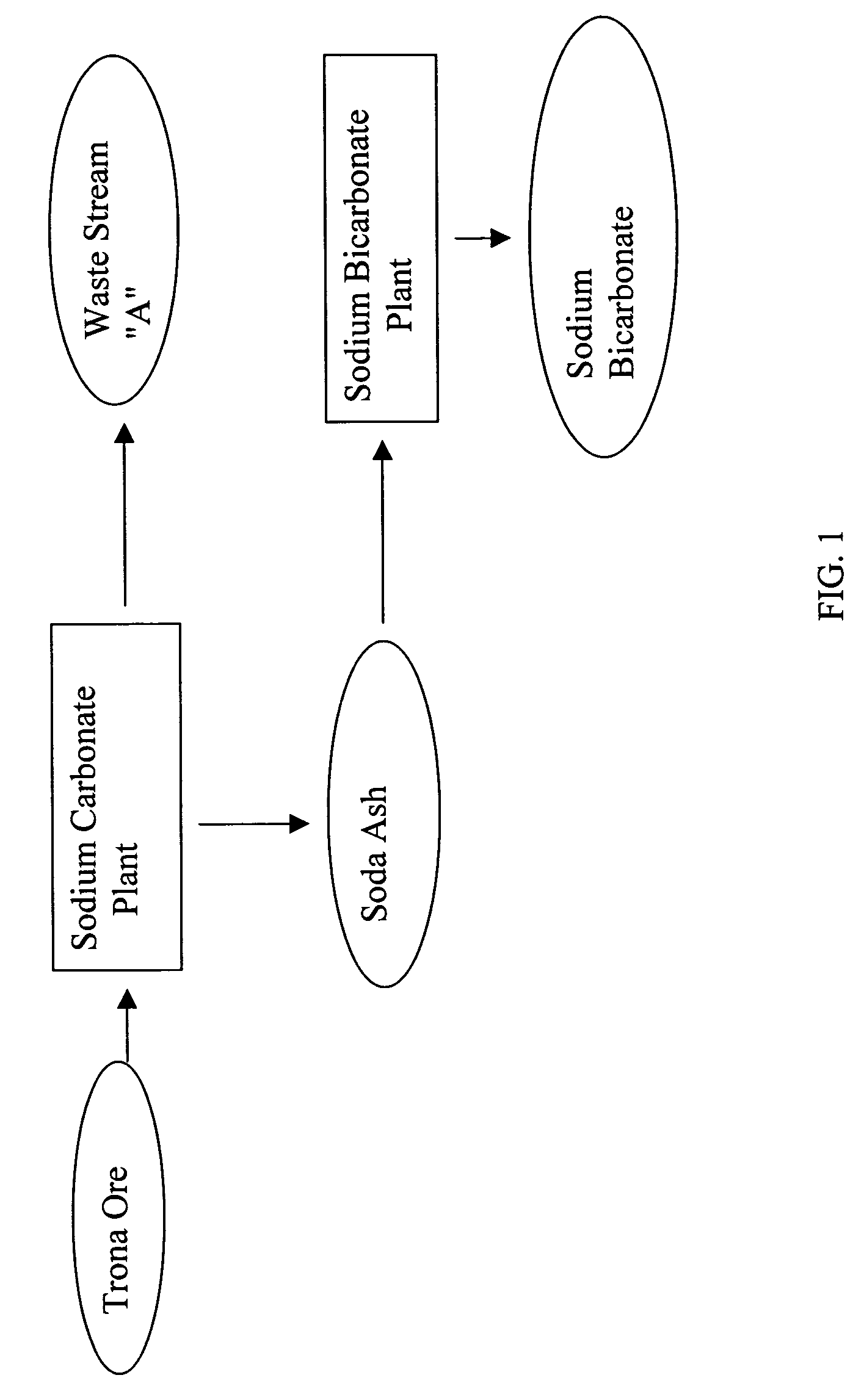
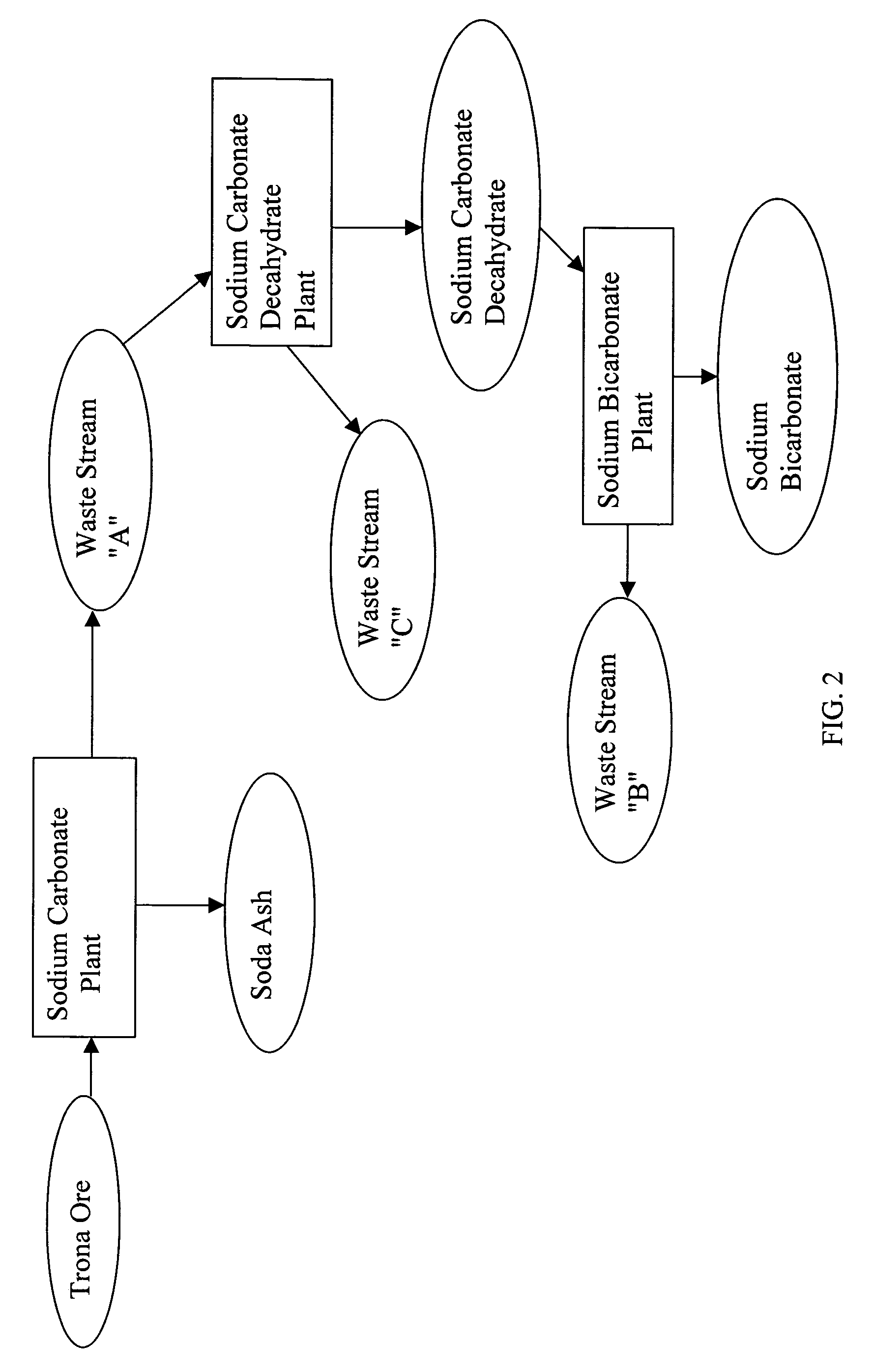
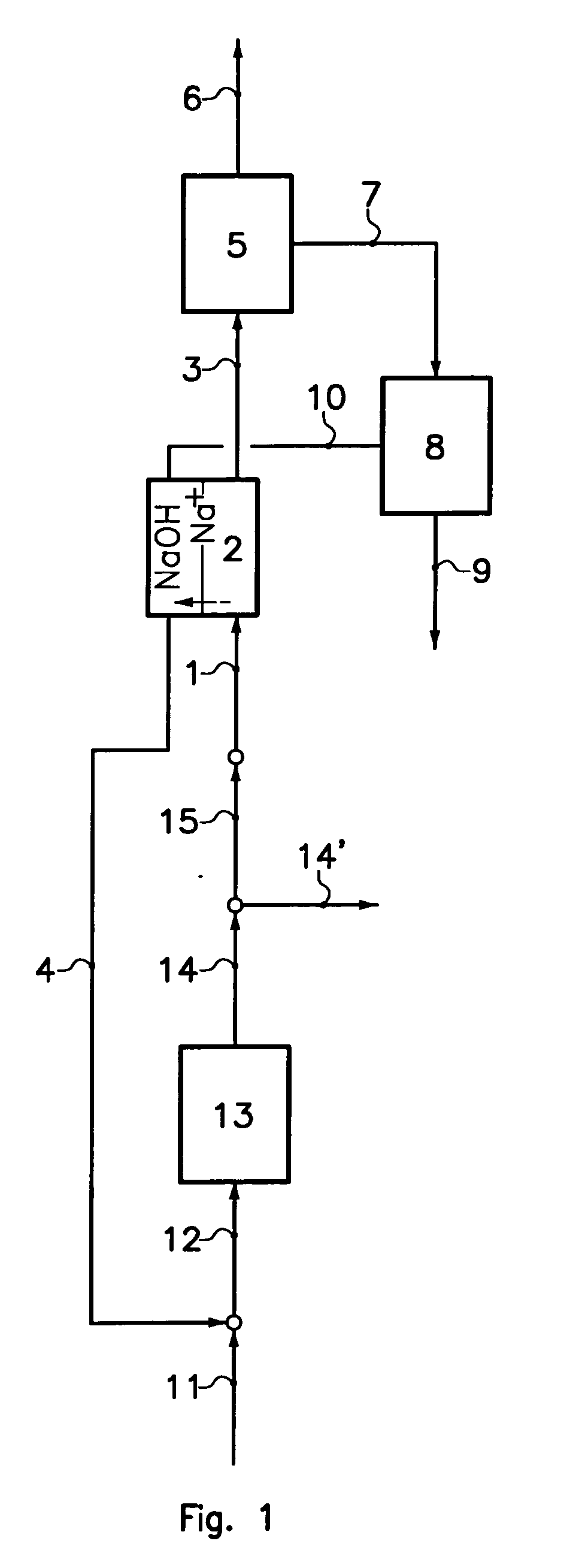
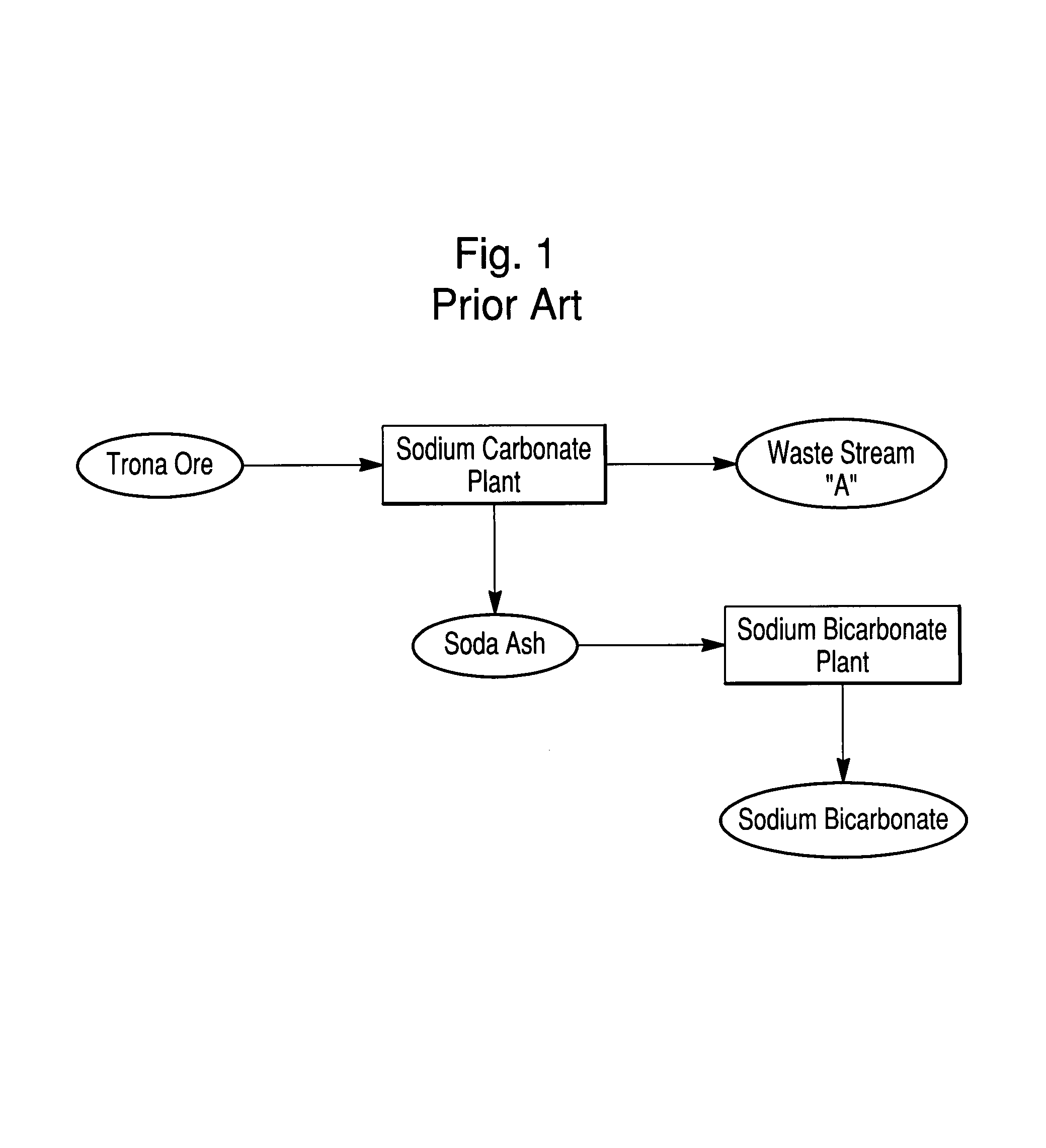
Sodium bicarbonate production method
InactiveUS20040057892A1Improvement in recovered sodium valuePromote recoveryBicarbonate preparationVarying alkali metal carbonate water contentWastewaterEngineering
A method of producing sodium bicarbonate having a high degree of purity and obtaining a net reduction in effluent waste water, as compared to prior processes, when starting from trona ore is disclosed. The process entails utilizing the waste-water effluent stream from the conversion of trona ore to sodium carbonate as the feed for the conversion of sodium carbonate to sodium bicarbonate.
Owner:CHURCH & DWIGHT CO INC
Heterogeneous, composite, carbonaceous catalyst system and methods that use catalytically active gold
ActiveUS8058202B2High catalytic activityTendency increaseMaterial nanotechnologyGas treatmentActivated carbonHost material
Heterogeneous catalyst systems, methods of making these systems, and methods of using these systems, wherein catalytically active gold is deposited onto composite support media. The composite support media is formed by providing nanoporous material on at least a portion of the surfaces of carbonaceous host material. In representative embodiments, relatively fine, nanoporous guest particles are coated or otherwise provided on surfaces of relatively coarser activated carbon particles. Catalytically active gold may be deposited onto one or both of the guest or host materials either before or after the guest and host materials are combined to from the composite host material. PVD is the preferred catalyst system of depositing gold.
Owner:3M INNOVATIVE PROPERTIES CO
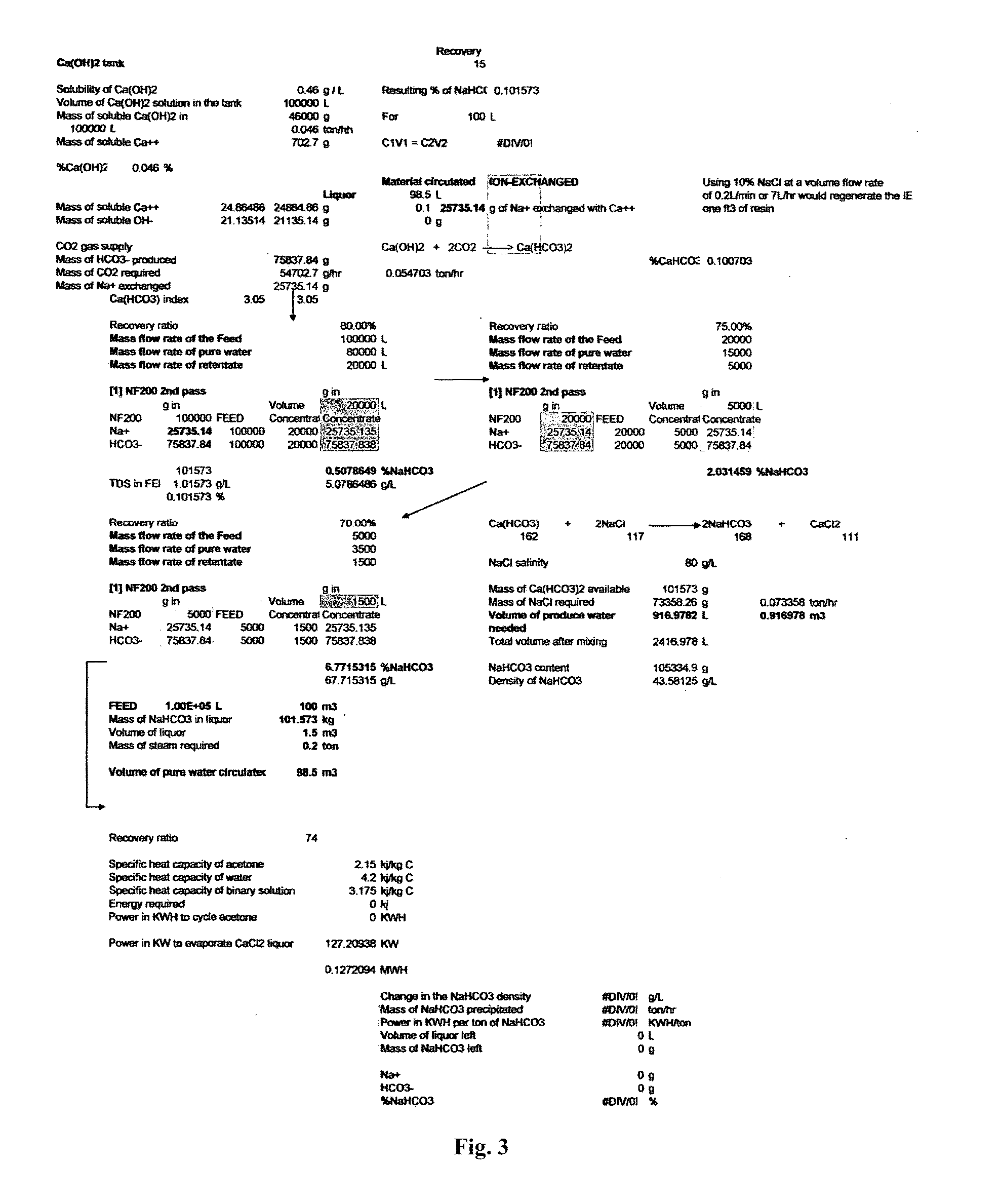
Combined solid waste, carbon dioxide quicklime sparging, brine water, and reverse osmosis/ion exchange processes for the production of soda chemicals
InactiveUS20110217227A1Calcium/strontium/barium carbonatesSemi-permeable membranesWaste processingChemical equation
The proposed invention uses a classical chemical equation where carbon dioxide CO2 is reacted with quick lime Ca(OH)2 to produce soda carb NaHCO3 and concentrating it to 6% using advanced membrane and resin technology. The invention requires three chemicals CO2, Ca(OH)2, and sodium chloride NaCl to produce NaHCO3. The output of many industrial processes lacks waste heat and in many instances CO2 and the present invention combines a solid waste processing unit to the above processes which allows the production of solid products or high % liquors. Availability of waste heat sources can lead to high efficiency in NaHCO3, Na2CO3, and NaOH production. The process is not chloro-alkali electrochemical or Solvay column ammonia processing technique. Advanced membrane uses technologies of reverse osmosis and nanofiltration systems while resin technology uses ion exchange systems. Therefore, we conveniently call it the solid waste-quicklime membrane SWQM process.
Owner:ENGSL FZE +1
Process for producing sodium salts
InactiveUS6207123B1Sesquicarbonate preparationVarying alkali metal carbonate water contentSodium bicarbonateAqueous solution
A process for producing sodium salts, which comprises adding solid sodium carbonate to a first aqueous solution containing sodium carbonate and sodium hydrogencarbonate, to prepare a second aqueous solution, precipitating, separating and recovering from the second aqueous solution sodium sesquicarbonate crystals containing at least 50 mol % of the sodium hydrogencarbonate component contained in the second aqueous solution, and further recovering sodium carbonate from a mother liquor remaining after separating the sodium sesquicarbonate crystals from the second aqueous solution.
Owner:ASAHI GLASS CO LTD
Process for producing sodium salts
InactiveUS6576209B2Sesquicarbonate preparationVarying alkali metal carbonate water contentSodium bicarbonateAqueous solution
A process for producing sodium salts, which comprises adding solid sodium carbonate to a first aqueous solution containing sodium carbonate and sodium hydrogencarbonate, to prepare a second aqueous solution, precipitating, separating and recovering from the second aqueous solution sodium sesquicarbonate crystals containing at least 50 mol % of the sodium hydrogencarbonate component contained in the second aqueous solution, and further recovering sodium carbonate from a mother liquor remaining after separating the sodium sesquicarbonate crystals from the second aqueous solution.
Owner:ASAHI GLASS CO LTD
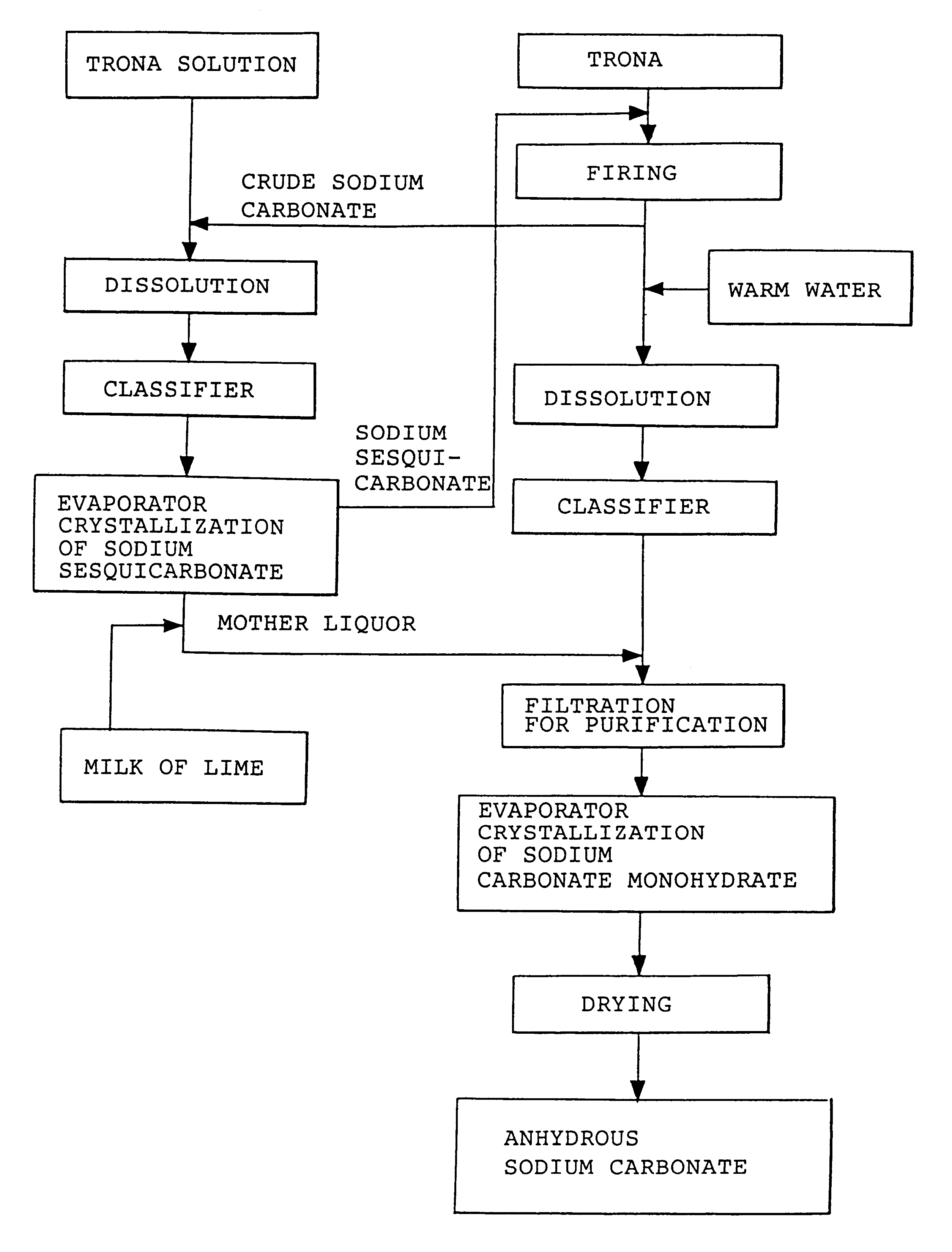
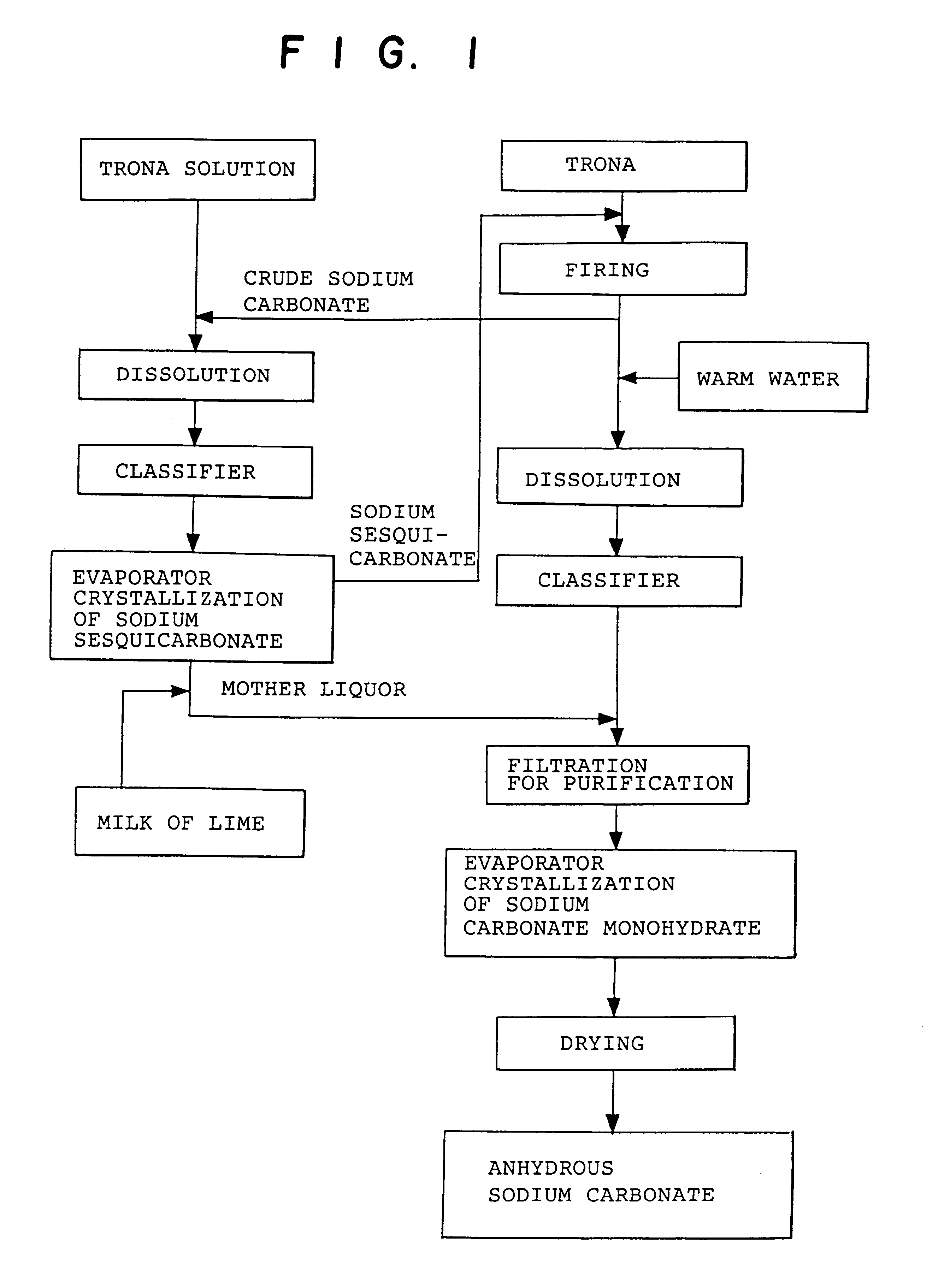
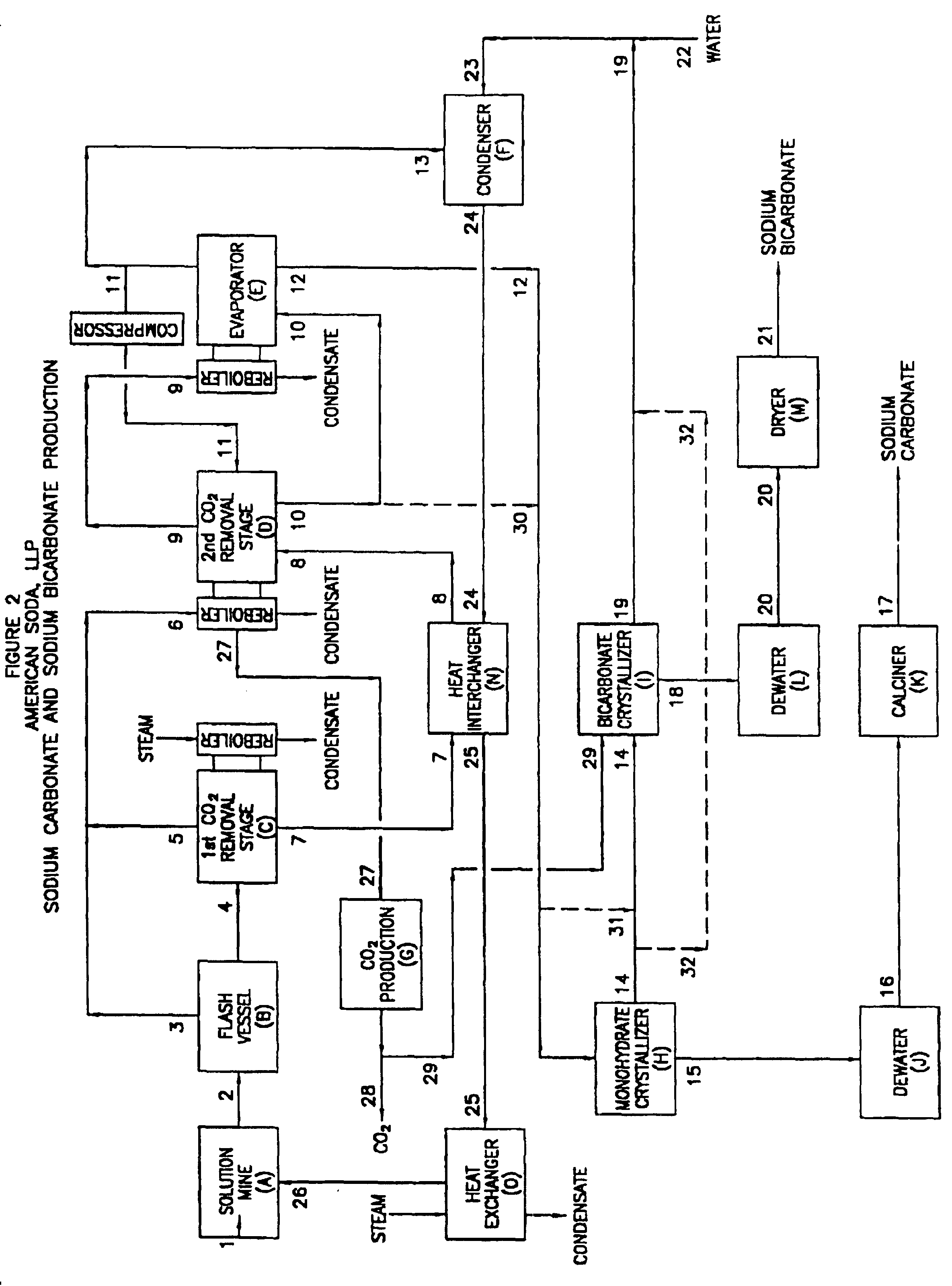
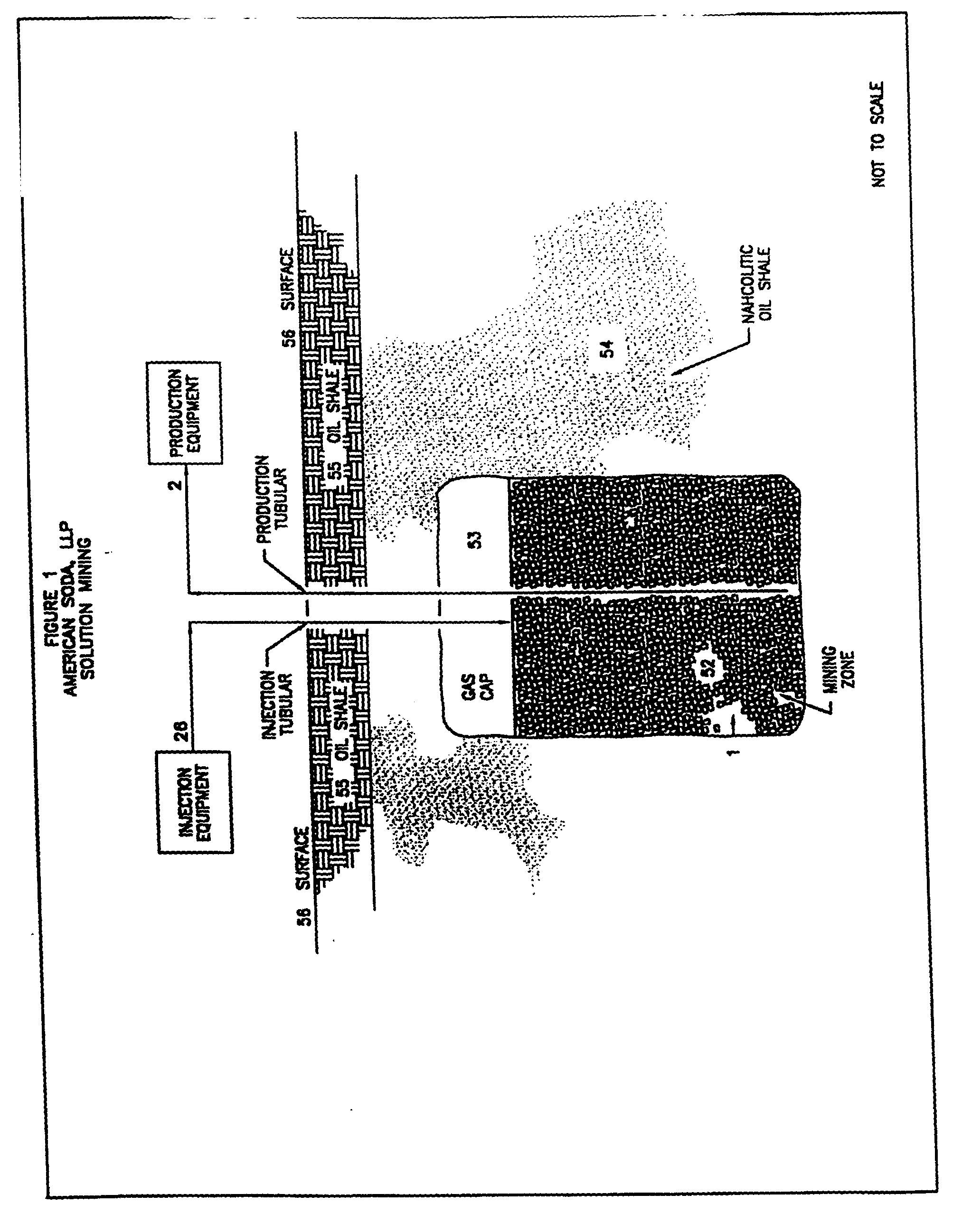
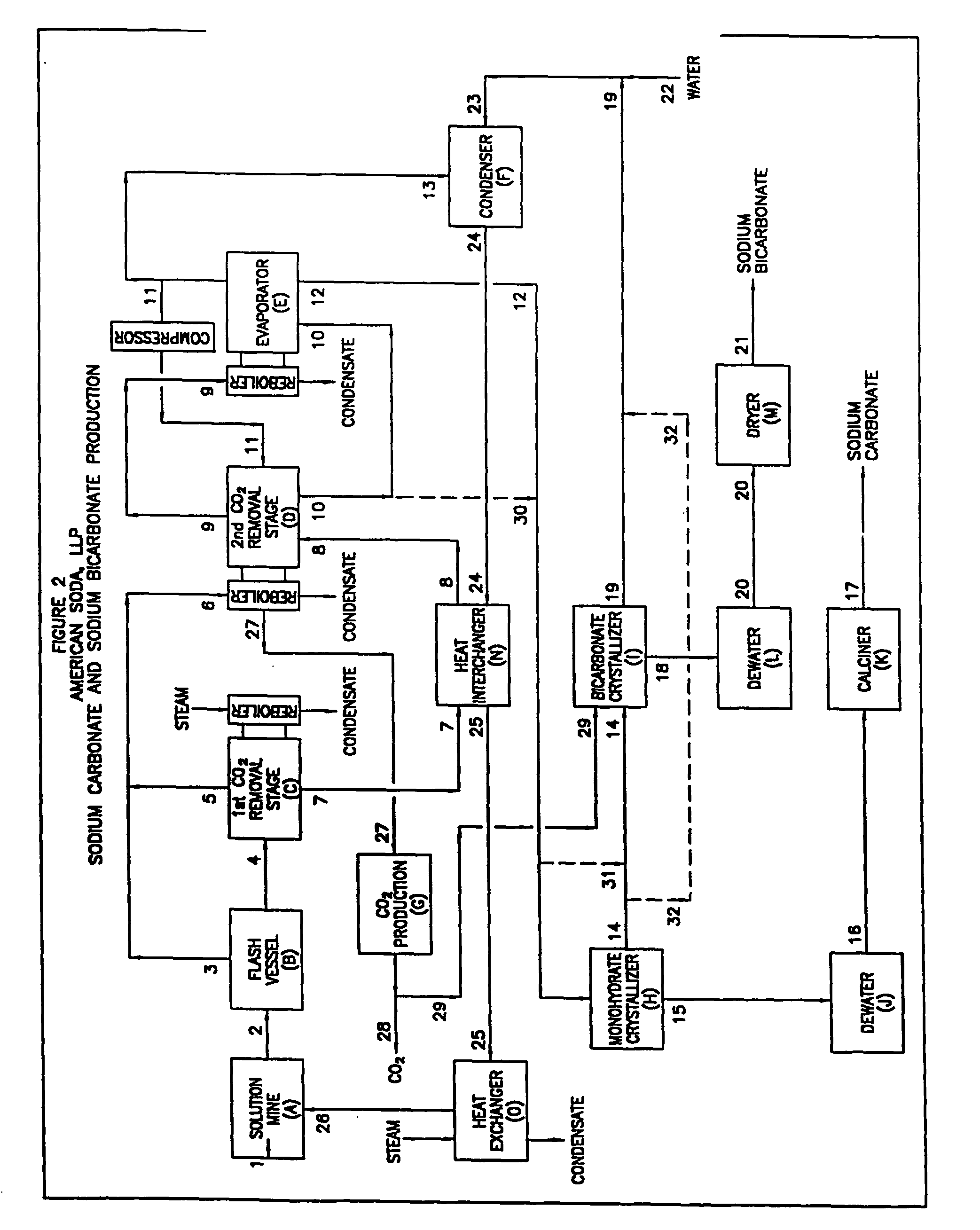
Sodium carbonate and sodium bicarbonate production from nahcolitic oil shale
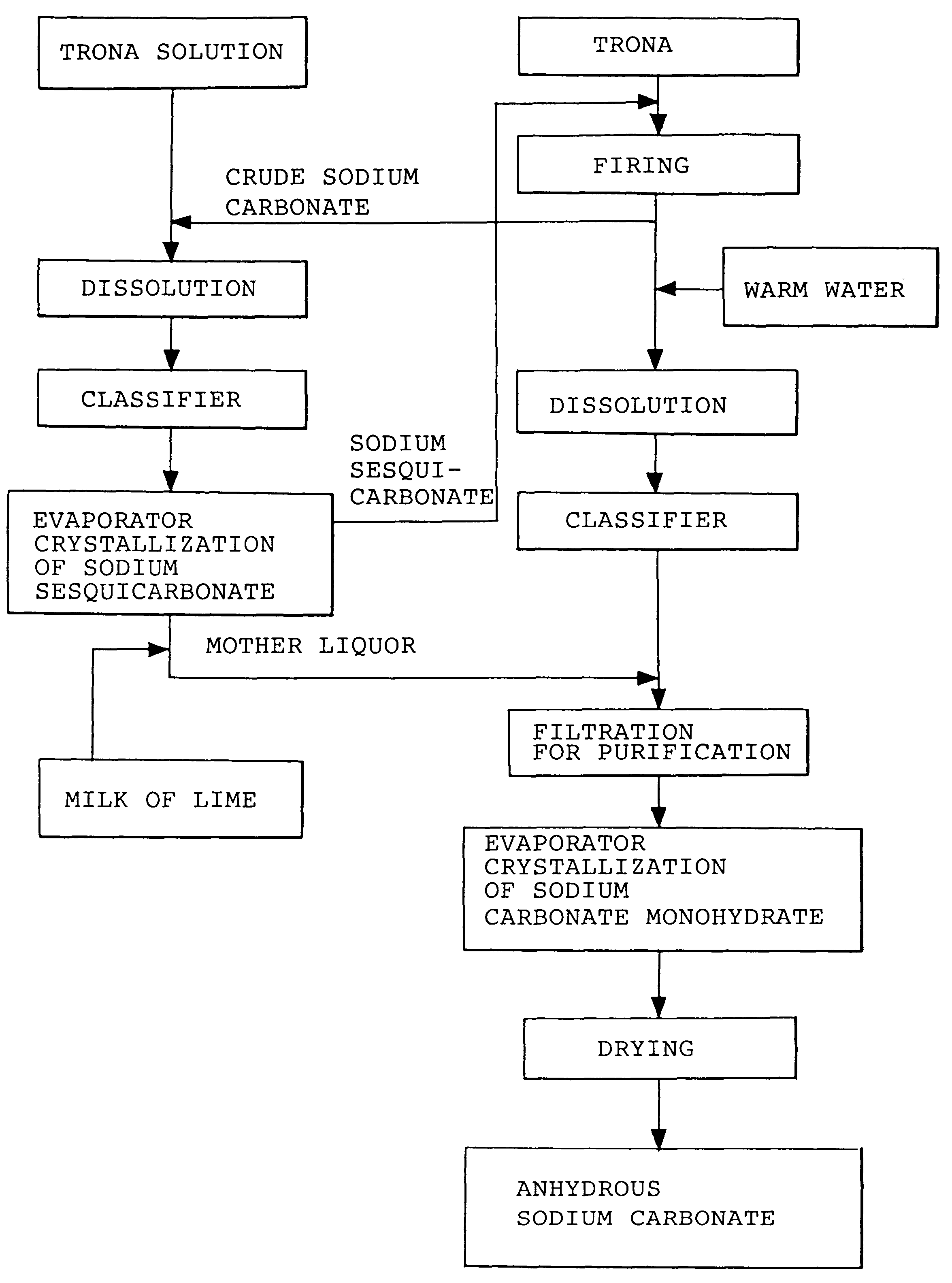
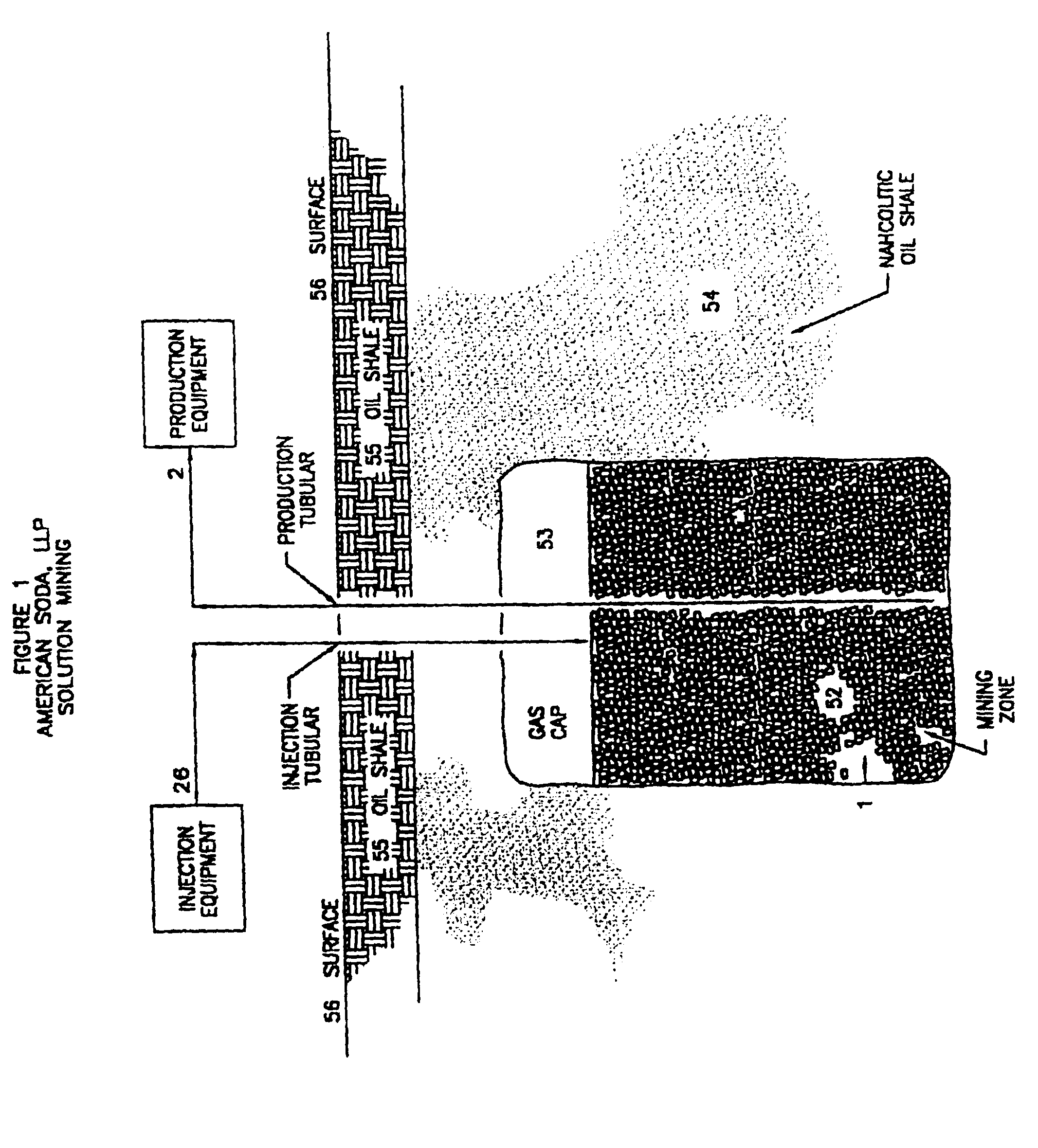
InactiveUS6854809B1Solve the blockageEfficient executionVarying alkali metal carbonate water contentUnderground miningSodium bicarbonateNahcolite
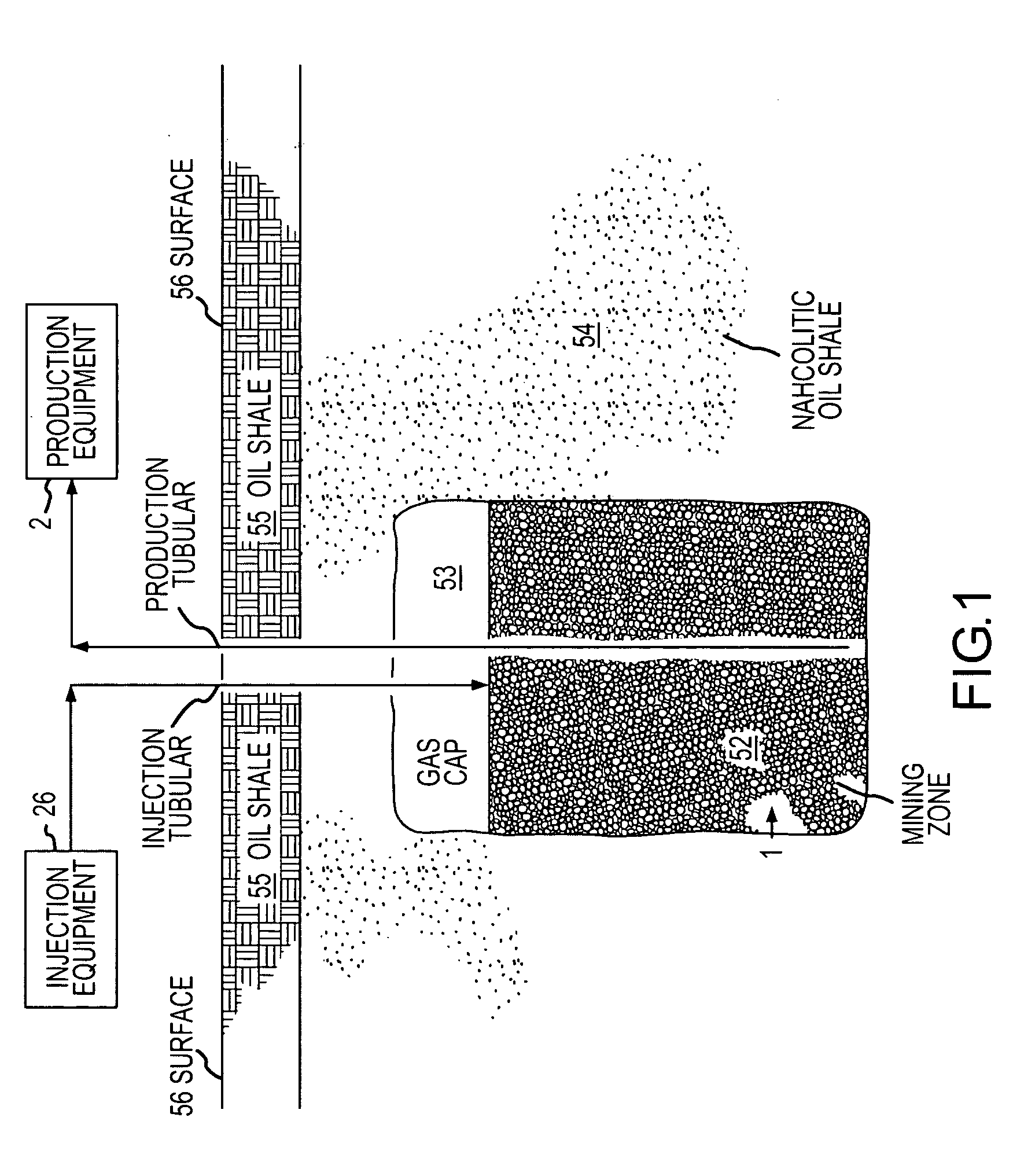
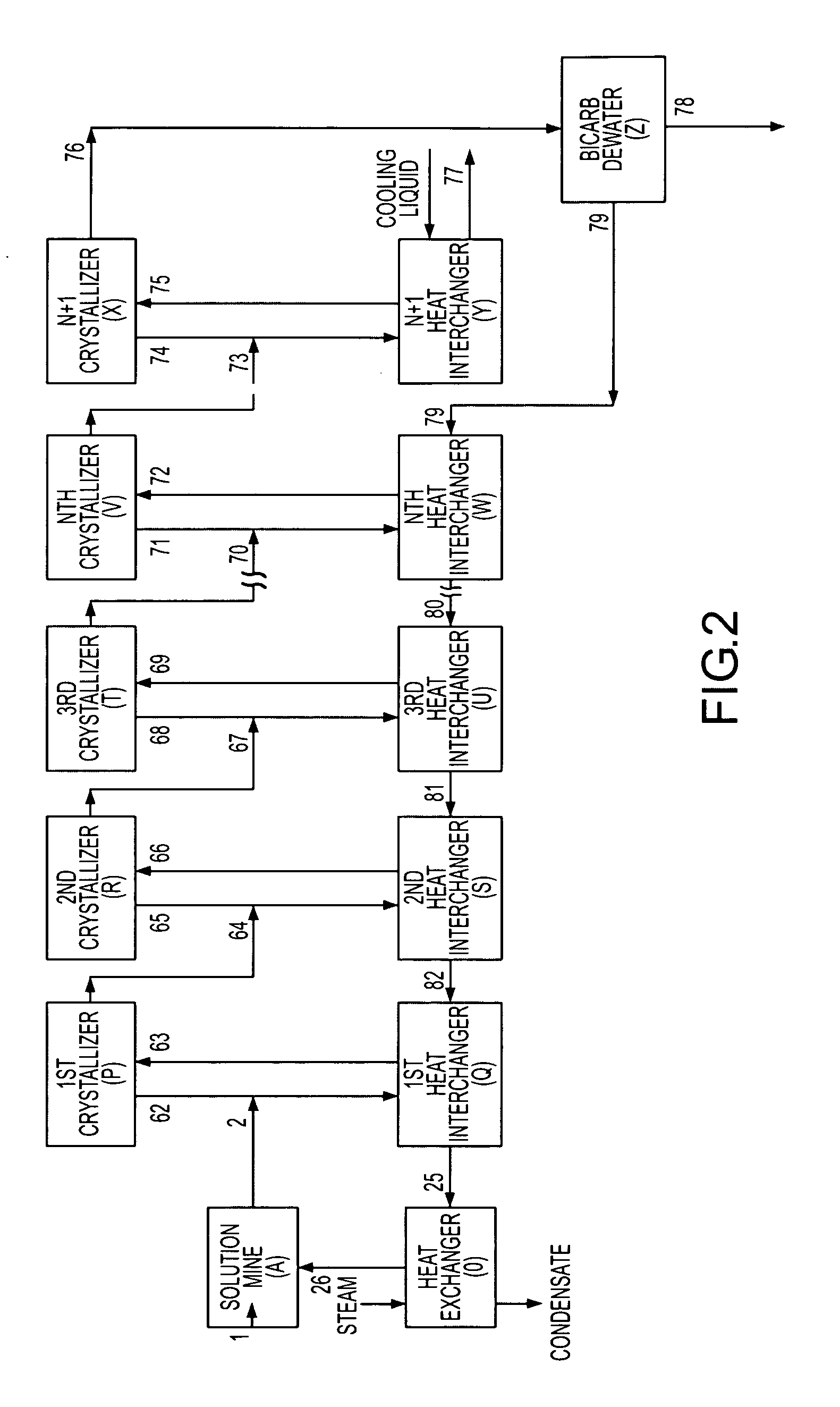
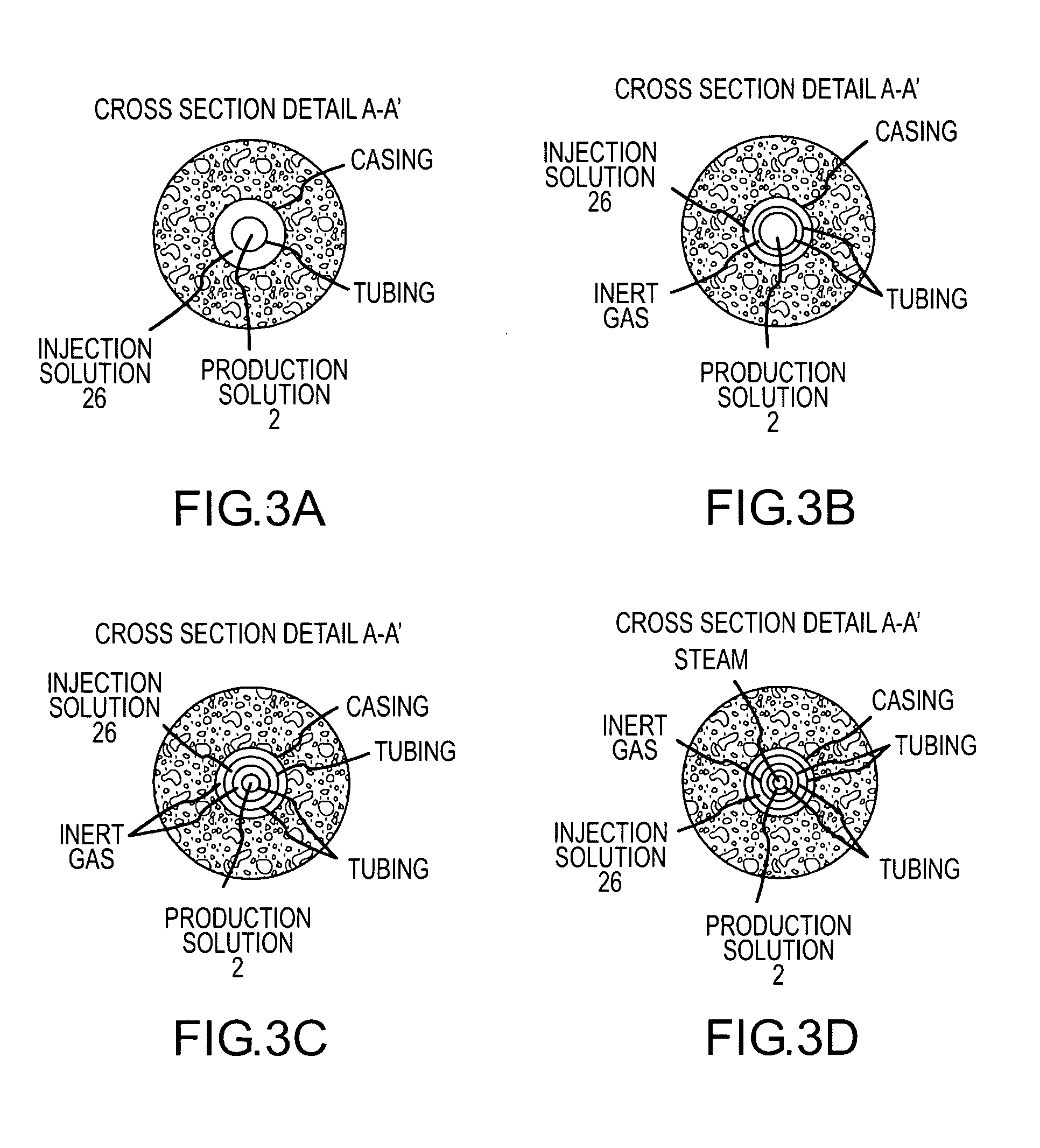
A method for solution mining nahcolite, capable of extracting nahcolite from geological formations lean in nahcolite comprising injecting high pressure water (which may include recycled aqueous solution of bicarb and sodium carbonate) at a temperature of at least 250° F. into the formation, dissolving nahcolite in the hot water to form a production solution and recovering the production solution. The invention also includes the processing of the production solution to provide sodium carbonate and, optionally, sodium bicarbonate, comprising: decomposing the sodium bicarbonate portion of the hot aqueous production solution to form a hot aqueous solution of sodium carbonate; evaporating water from the hot aqueous solution comprising sodium carbonate to form a concentrated solution of sodium carbonate; producing sodium carbonate monohydrate from the concentrated solution of sodium carbonate by crystallization; and dewatering and calcining the sodium carbonate monohydrate to produce anhydrous sodium carbonate.
Owner:AMERICAN SODA
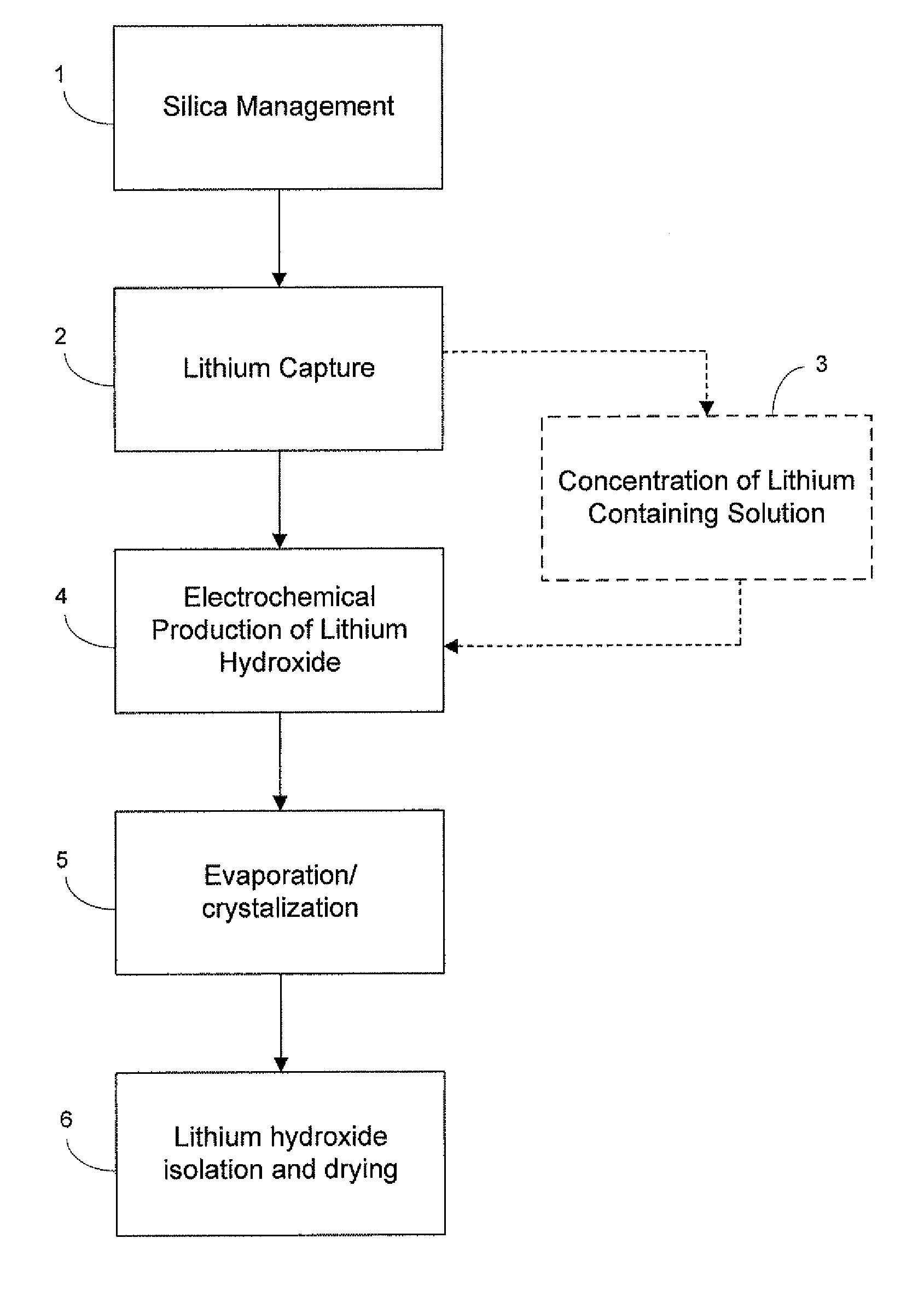
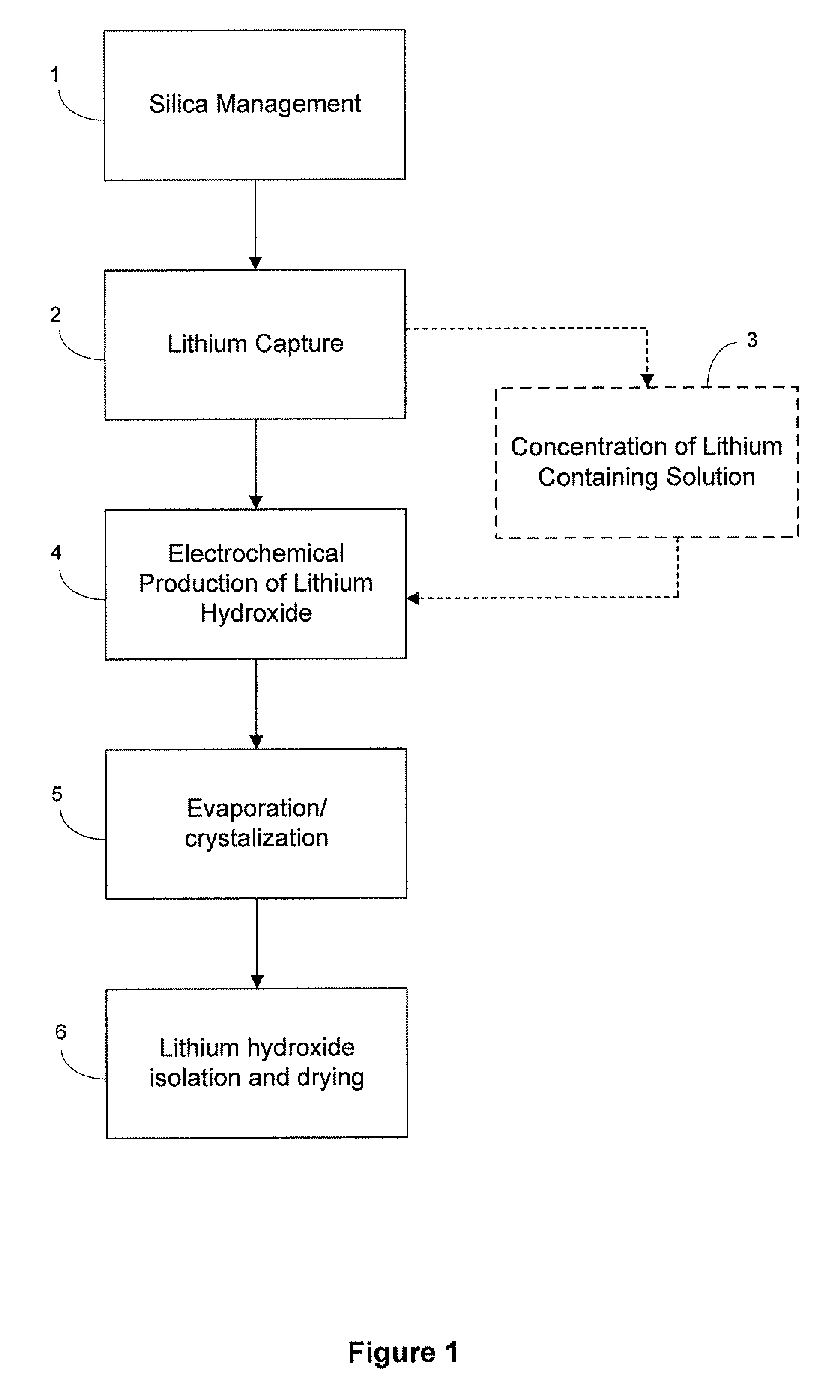
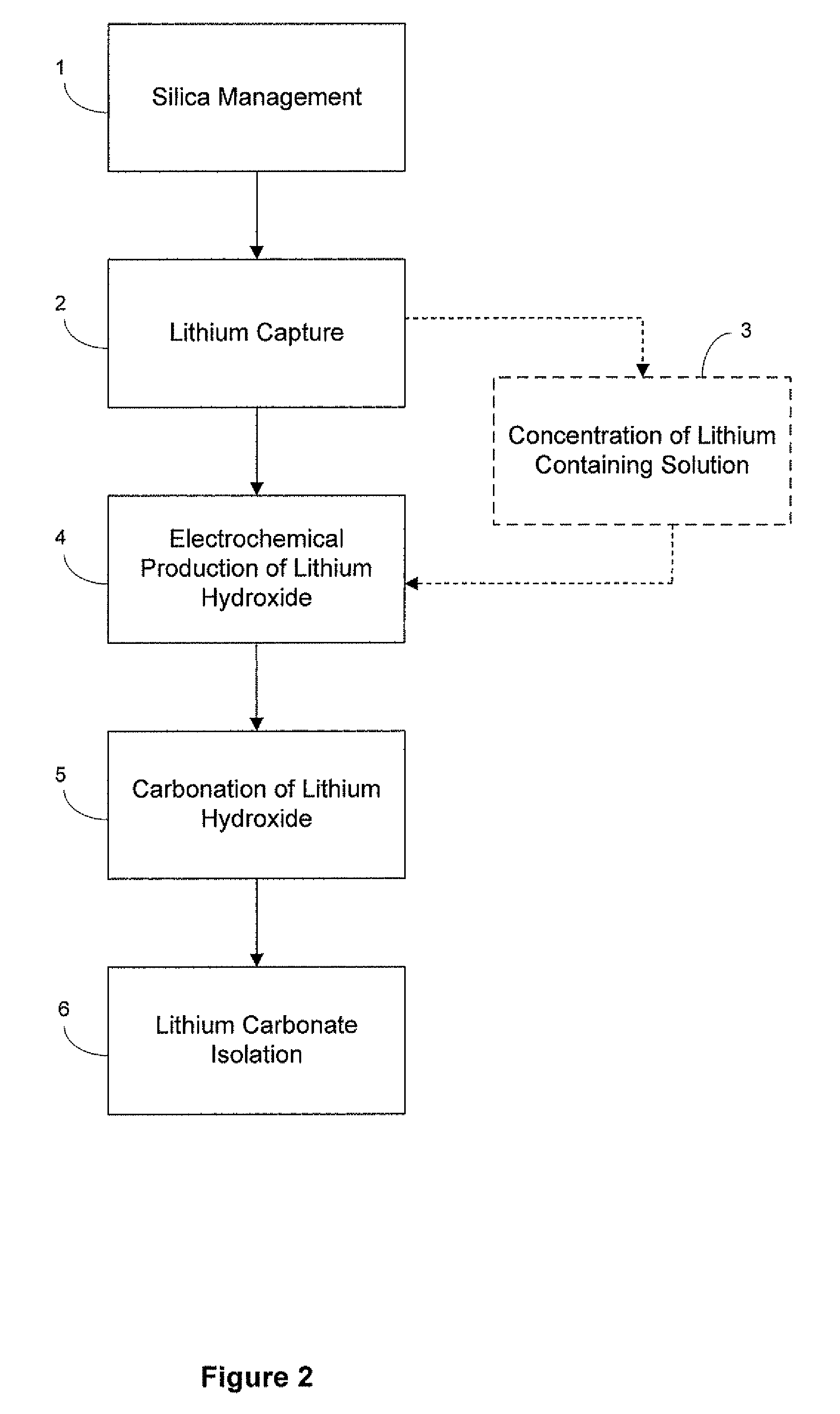
Preparation of lithium carbonate from lithium chloride containing brines
ActiveUS8741256B1Reduce concentrationMetal recyclingVarying alkali metal carbonate water contentLithium chlorideLithium hydroxide
This invention relates to a method for the preparation of lithium carbonate from lithium chloride containing brines. The method can include a silica removal step, capturing lithium chloride, recovering lithium chloride, supplying lithium chloride to an electrochemical cell and producing lithium hydroxide, contacting the lithium hydroxide with carbon dioxide to produce lithium carbonate.
Owner:TERRALITHIUM LLC
Sodium bicarbonate production from nahcolite
InactiveUS20040231109A1Solve the blockageEfficient executionVarying alkali metal carbonate water contentAlkali metal sulfites/sulfatesSodium bicarbonateThermal water
Owner:NIELSEN KURT R +2
Sodium carbonate and sodium bicarbonate production from nahcolitic oil shale
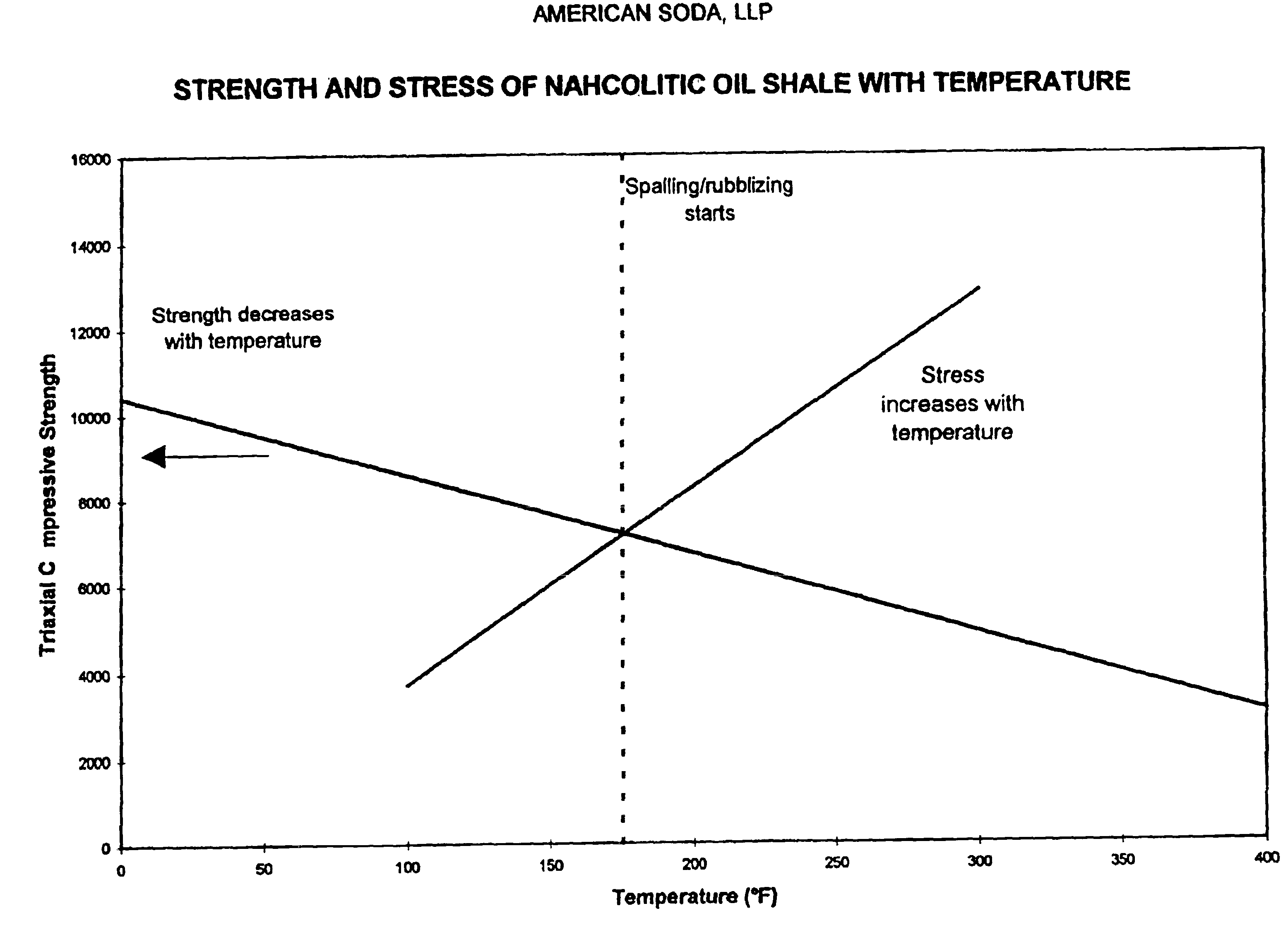
InactiveUS20040026982A1Reduced compressive strengthIncrease pressureVarying alkali metal carbonate water contentConstructionsSodium bicarbonateNahcolite
A method for solution mining nahcolite, capable of extracting nahcolite from geological formations lean in nahcolite comprising injecting high pressure water (which may include recycled aqueous solution of bicarb and sodium carbonate) at a temperature of at least 250° F. into the formation, dissolving nahcolite in the hot water to form a production solution and recovering the production solution. The invention also includes the processing of the production solution to provide sodium carbonate and, optionally, sodium bicarbonate, comprising: decomposing the sodium bicarbonate portion of the hot aqueous production solution to form a hot aqueous solution of sodium carbonate; evaporating water from the hot aqueous solution comprising sodium carbonate to form a concentrated solution of sodium carbonate; producing sodium carbonate monohydrate from the concentrated solution of sodium carbonate by crystallization; and dewatering and calcining the sodium carbonate monohydrate to produce anhydrous sodium carbonate.
Owner:SOLVAY CHEM INC
System and process for preparing low-salt dense soda ash by liquid-phase hydration
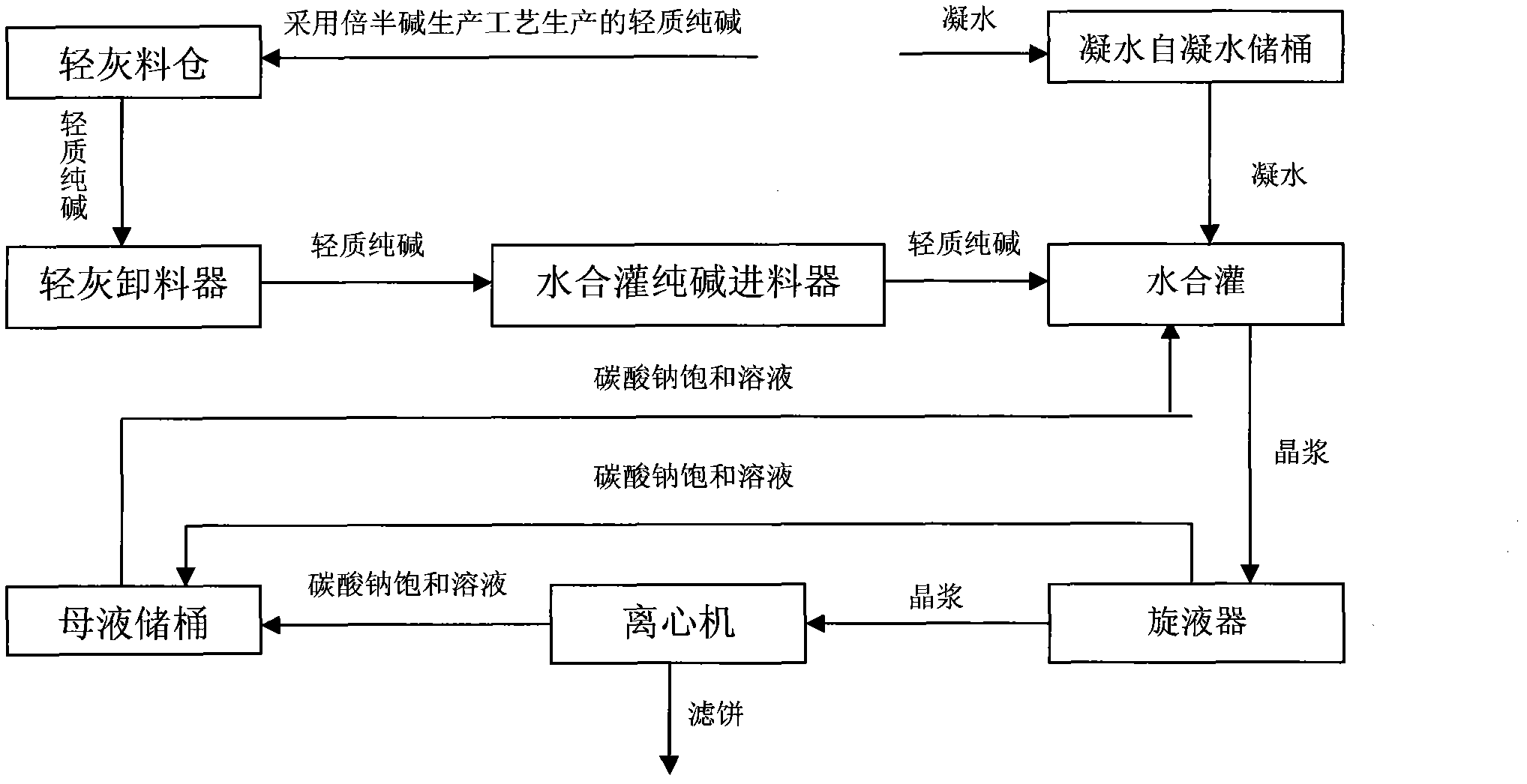
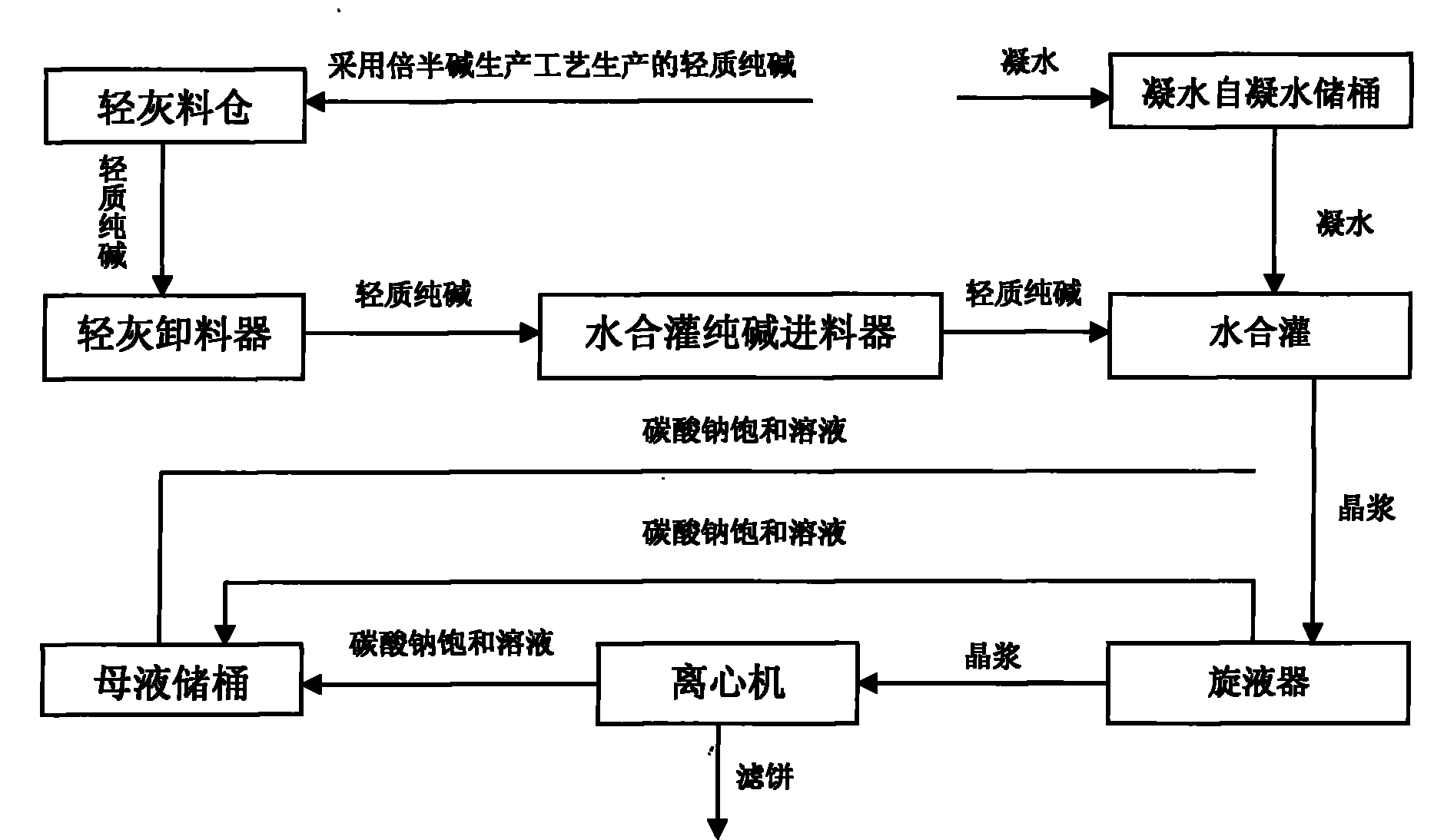
InactiveCN101928021AConstant material levelFine granularityVarying alkali metal carbonate water contentReaction temperatureSlurry
The invention discloses a system and a process for preparing low-salt dense soda ash by liquid-phase hydration. The method comprises the following steps of: adding light soda ash with the temperature of more than 93 DEG C, condensate water and saturated solution of sodium carbonate according to a mass ratio of 1:0.22:1.4-1:0.22:2.0 into a hydration tank for hydration, wherein the reaction temperature is controlled to be between 100 and 104 DEG C and the reaction time is 20 to 25 minutes, and crystallizing the soda ash in a form of a monohydrate soda, namely Na2CO3.H2O after the reaction; after separating the monohydrate soda crystal slurry with a hydrocyclone separator, feeding the top liquid into a mother liquor storing barrel and feeding the bottom liquid into a centrifuge; after dehydrating the bottom liquid with the centrifuge to obtain a monohydrate soda filter cake; and drying, cooling and screening the monohydrate soda filter cake to obtain the finished product, namely the low-salt dense soda ash, wherein when the centrifuge is used for dehydrating, water is added to wash off a part of salts. The system can guarantee the constant material level of a material bin so that the feeding of the soda ash is stable. The obtained soda ash product has the advantages of large crystallization granule size, high first-rate product rate and high added value, wherein the product with crystallization granule size of more than 180 mu m is up to over 75 percent; the qualification rate is up to 100 percent; and the first-rate product rate is up to 95 percent.
Owner:内蒙古博源工程有限责任公司
Production of lithium compounds directly from lithium containing brines
InactiveUS7214355B2Reduce in quantityPromote absorptionCalcium/strontium/barium carbonatesVarying alkali metal carbonate water contentSalt waterSlurry
Metal carbonates that are insoluble in water but that have a corresponding metal biocarbonate salt that is more than 75% by weight soluble in water are purified by preparing an aqueous slurry of the metal carbonate: introducing carbon dioxide gas to form a corresponding metal biocarbonate solution and heating that solution to form a purified metal carbonate and precipitate it.
Owner:ROCKWOOD LITHIUM INC
Method for preparing anhydrous sodium carbonate
InactiveCN101712480AHigh puritySolve the problem of low pass rateVarying alkali metal carbonate water contentSodium bicarbonateAqueous solution
The invention relates to a method for preparing anhydrous sodium carbonate. The preparation method comprises the following steps: (1) dissolving industrial sodium carbonate in water to prepare aqueous solution of the industrial sodium carbonate, and adding sodium hydroxide into the solution to remove Mg2+ and Ca2+; (2) adding hydrogen peroxide into aqueous solution of the sodium carbonate in which the Mg2+ and the Ca2+ are removed to remove Fe2+; (3) introducing CO2 into the aqueous solution of the sodium carbonate in which the Fe2+ is removed; (4) filtering the aqueous solution of the sodium carbonate to obtain sodium carbonate crystals; and (5) washing, dehydrating and drying the sodium carbonate crystals to obtain an anhydrous sodium carbonate finished product serving as a reference reagent. The method can effectively remove the Mg2+, the Ca2+ and the Fe2+ in the industrial anhydrous sodium carbonate, remarkably improve the purity of the product, and meanwhile effectively solve the problem of lower product qualification rate caused by low clarity of the anhydrous sodium carbonate finished product because of concentrating the solution of the sodium carbonate for long term.
Owner:TIANJIN CHEM REAGENT RES INST
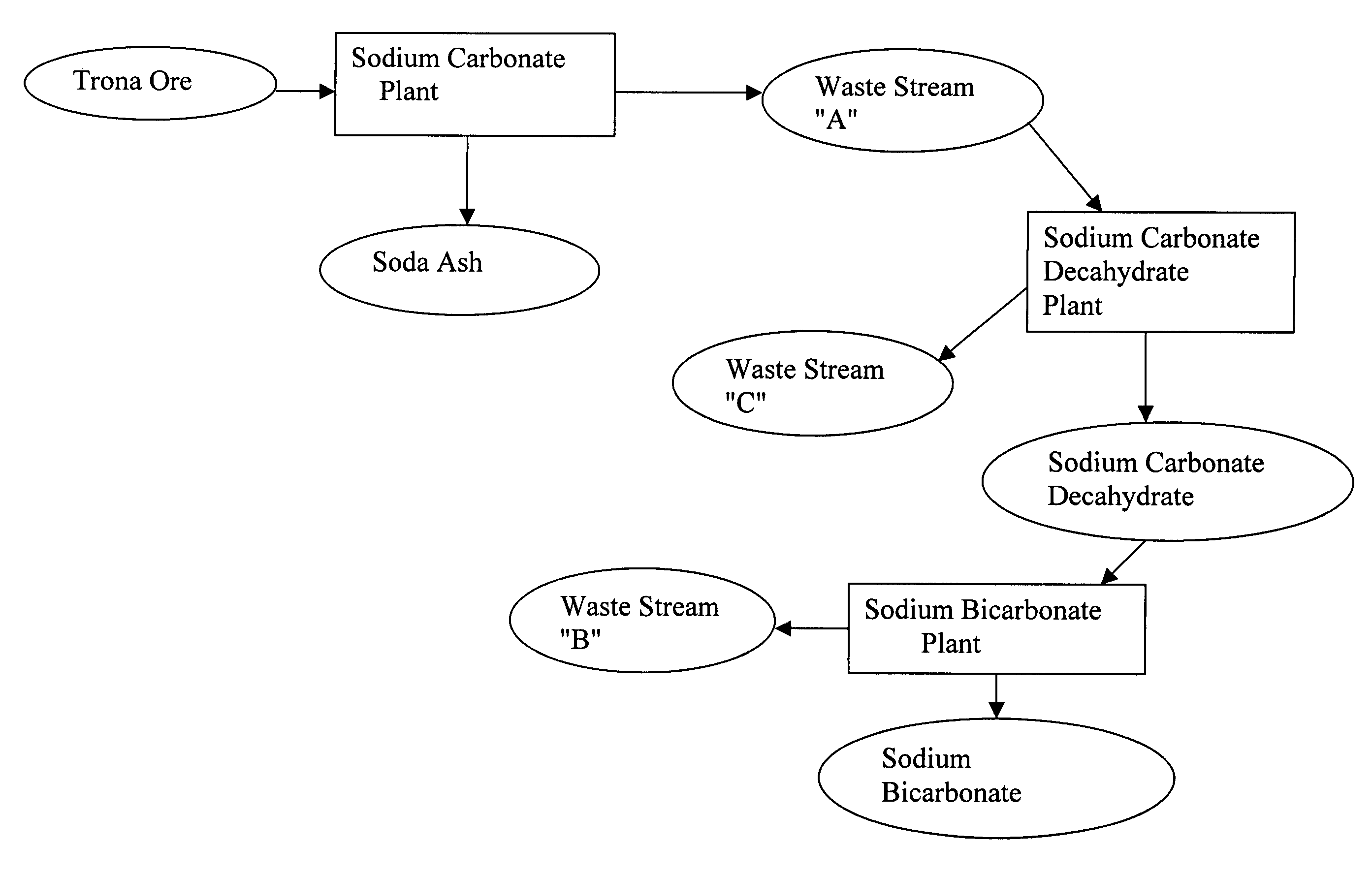
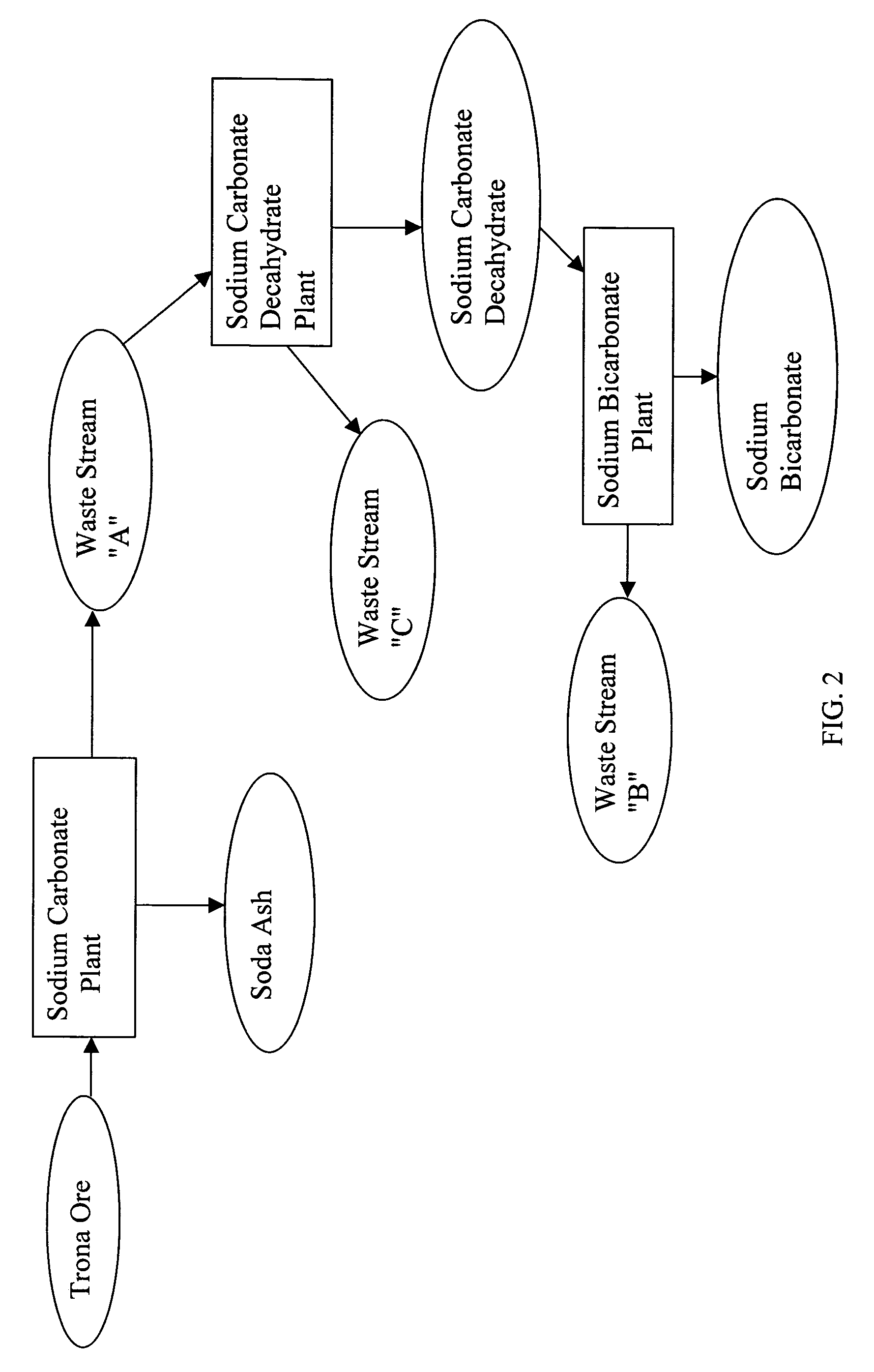
Sodium carbonate recovery from waste streams and impounded sodium carbonate decahydrate deposits
InactiveUS7645435B2Promote recoveryExtend the life cycleVarying alkali metal carbonate water contentCarbonate purificationSodium bicarbonateWaste stream
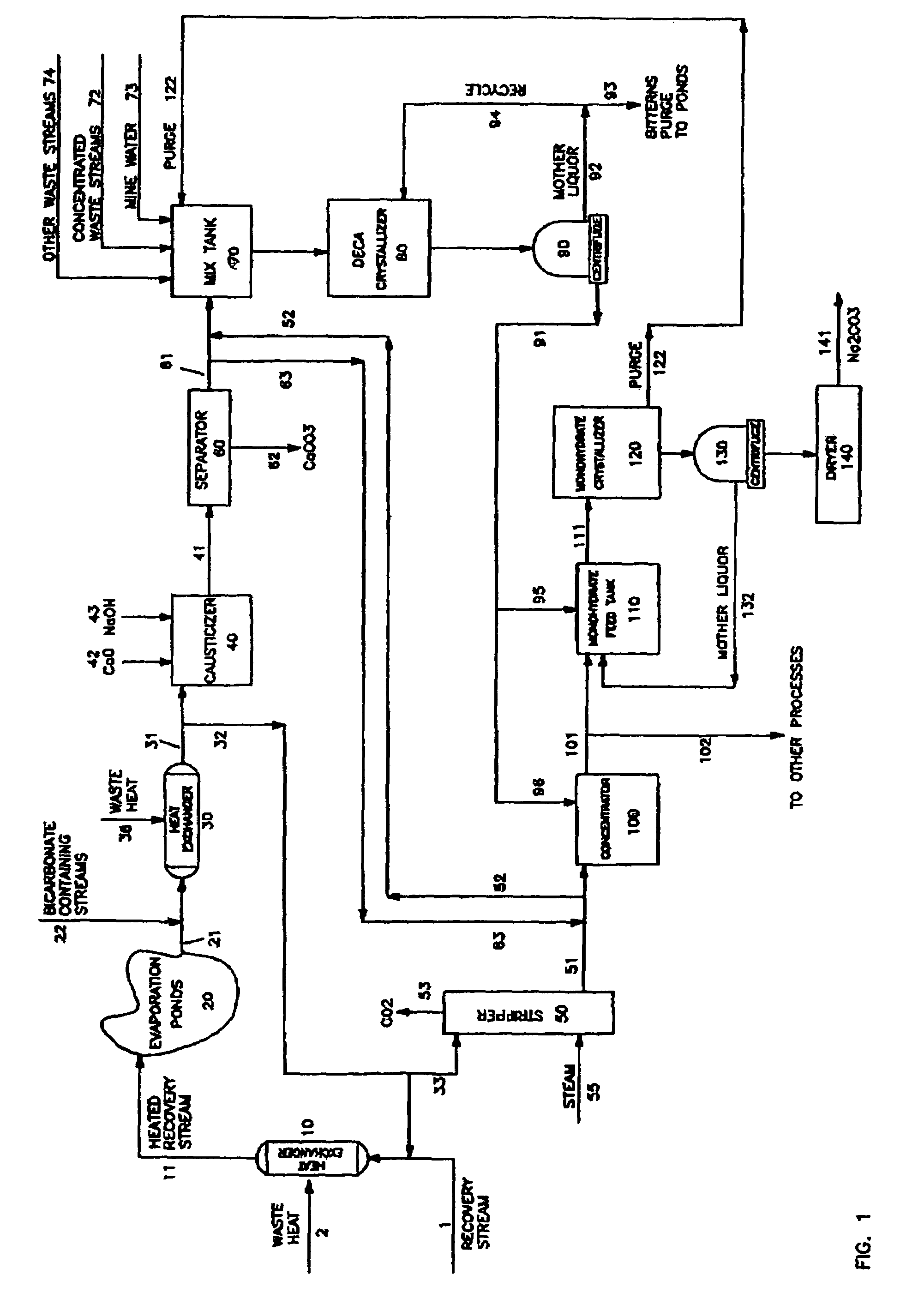
A process is described for recovering sodium carbonate or other sodium-based chemicals from sodium-bearing streams, including in particular mine water, evaporative pond water and sodium carbonate decahydrate deposits, recycle and purge streams, and other waste streams. In the process sodium bicarbonate-bearing streams are decarbonized to reduce the sodium bicarbonate concentration in a combination with other sodium-bearing streams, resulting in a liquor suitable as feed to a sodium carbonate decahydrate or sodium carbonate monohydrate process. The sodium bicarbonate stream is combined in a mix tank with other sodium carbonate bearing streams where the concentration is adjusted to form a liquor suitable to feed a sodium decahydrate or sodium carbonate monohydrate evaporation / crystallization step. In the process the combination of the various sodium-bearing streams is decarbonized to below 3.5% sodium bicarbonate when fed to a sodium decahydrate process and to below 1% sodium bicarbonate when fed to a sodium carbonate monohydrate process. The feed streams are adjusted in sodium carbonate concentration by higher concentrated sodium carbonate-bearing streams or by addition of sodium carbonate decahydrate produced from said streams or recovered form evaporation pond deposits, are then processed to produce sodium carbonate decahydrate or sodium carbonate monohydrate or further processed to form other sodium carbonate salts.
Owner:TATA CHEM NORTH AMERICA INC
Preparation method for reagent-grade anhydrous sodium carbonate
ActiveCN103601220AEfficient removalImprove product qualityVarying alkali metal carbonate water contentFiltration membraneCellulose acetate
The invention discloses a preparation method for reagent-grade anhydrous sodium carbonate. The method comprises the following steps with industrial anhydrous sodium carbonate as a raw material, dissolving the industrial anhydrous sodium carbonate in high-purity water; adding an organic amine immobilized catalyst; introducing carbon dioxide gas to adjust a pH value; and subjecting filtrate obtained after filtration to filtration with a cellulose acetate microporous filtration membrane, crystallization and drying so as to obtain the reagent-grade anhydrous sodium carbonate. The reagent-grade anhydrous sodium carbonate prepared by using the method has purity of 99.9 wt%, less than 10 ppm of impurity anions and less than 5 ppm of impurity metal ions, according with standards for guaranteed reagents prescribed in GBT-639-2008 for anhydrous sodium carbonate. The method has the advantages of simple and convenient operation, a good impurity removal effect, good product purity, low cost and suitability for industrial production.
Owner:SINOPHARM CHEM REAGENT
Method of producing soda ash and calcium chloride
InactiveUS20130216467A1Varying alkali metal carbonate water contentCalcium/strontium/barium fluoridesSodium bicarbonateSalt water
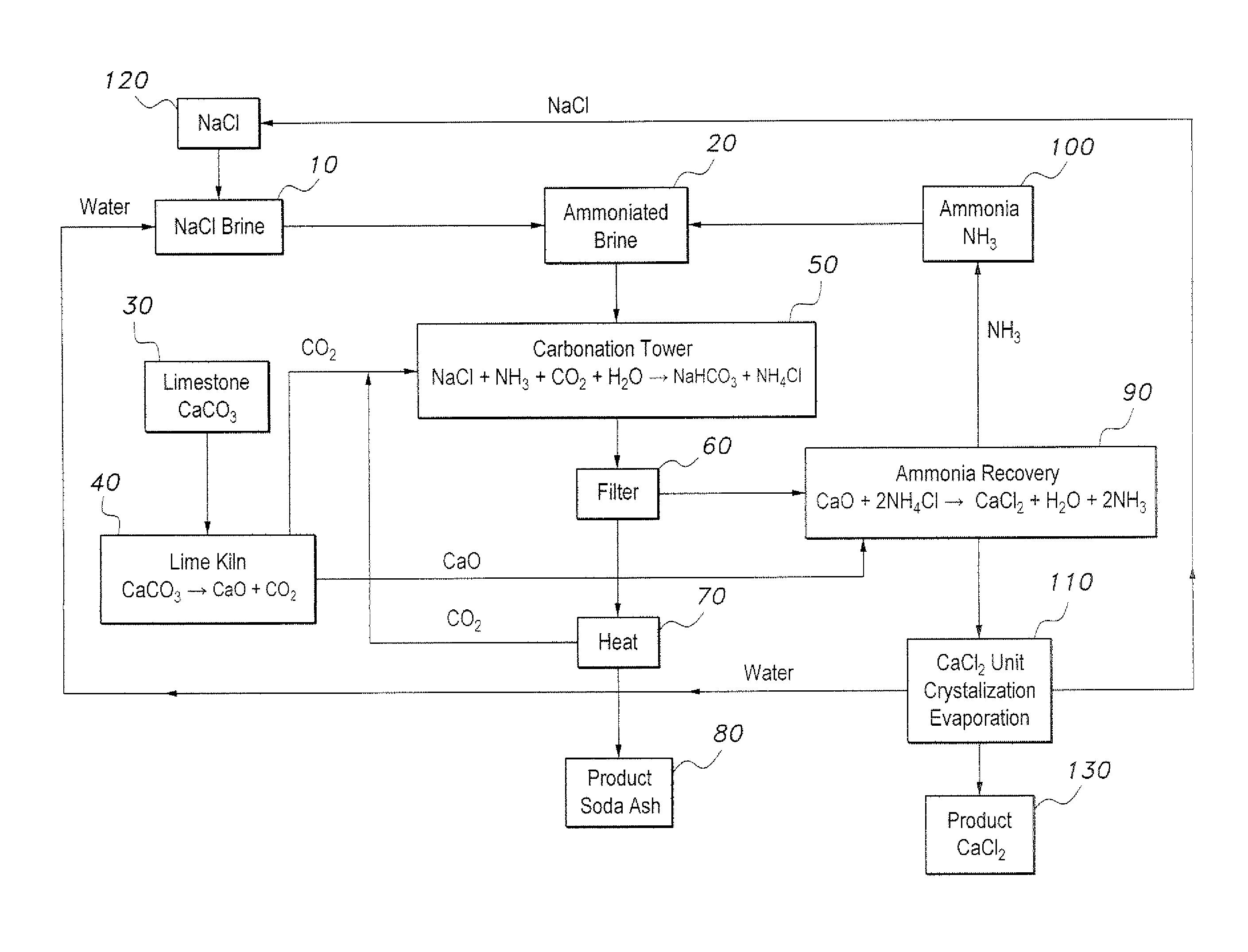
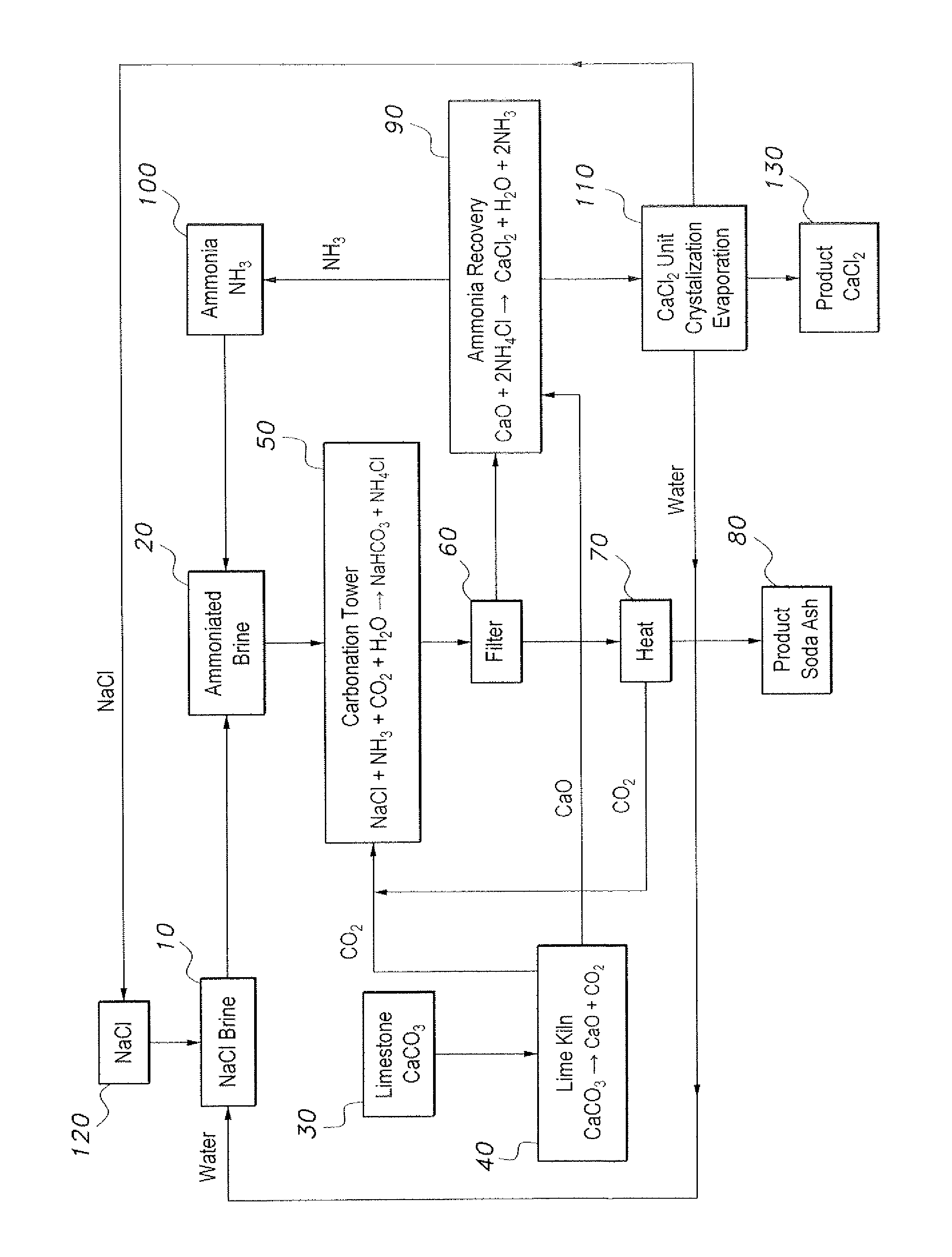
The method of producing soda ash and calcium chloride provides an environmentally friendly method of producing soda ash and calcium chloride without the production of waste and hazardous byproducts. The method of producing soda ash and calcium chloride is initiated with a volume of brine, which is ammoniated with gaseous ammonia to form ammoniated brine. Limestone is heated to produce calcium oxide and carbon dioxide. The ammoniated brine is reacted with the carbon dioxide to produce sodium bicarbonate, ammonium chloride and a brine effluent. The sodium bicarbonate is then calcined and decomposed to produce soda ash and gaseous carbon dioxide. The calcium oxide is reacted with the ammonium chloride to produce calcium chloride, water and ammonia. The ammonia is recycled to be used in the initial step of ammoniating the brine. The water and the brine effluent are also recycled and used to provide the brine in the initial step.
Owner:IDEA INT INVESTMENT & DEV
Process for producing sodium bicarbonate
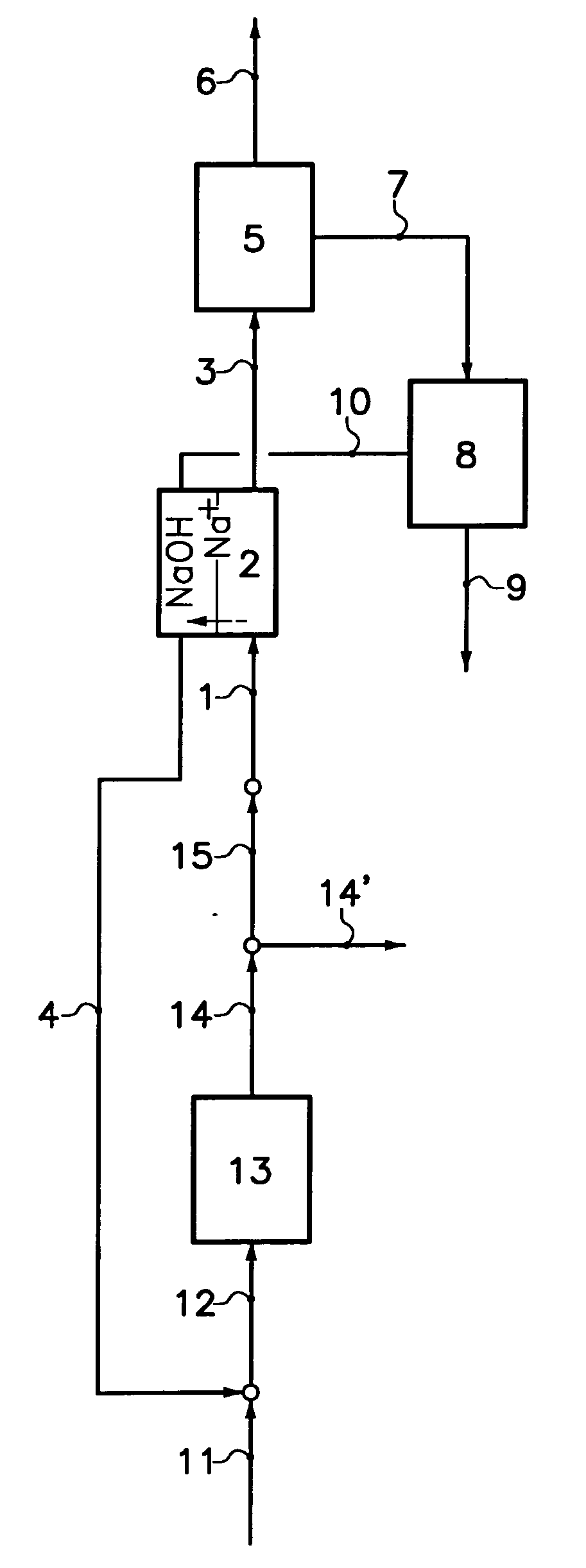
ActiveUS20100284891A1Large complexityLarge energy consumptionSemi-permeable membranesVarying alkali metal carbonate water contentSodium bicarbonateSodium hydroxide
In a process to produce sodium carbonate,a first production solution comprising sodium carbonate is introduced into less basic compartments of an electrodialyser comprising alternating less basic and more basic adjacent compartments separated from each other by cationic membranes, the more basic compartments being delimited by anionic faces of bipolar membranes on one side and by the cationic membranes on the other side;a second production solution comprising sodium carbonate is introduced into the more basic compartments of the electrodialyser;a solution comprising sodium hydroxide is produced into the more basic compartments by combination of sodium ions flux sodium ions crossing the cationic membrane and hydroxyl ions flux crossing the anionic face of the bipolar membranes, and is then extracted from the electrodialyser to be used as a reaction solution; andthe reaction solution is reacted with sodium bicarbonate in order to form a produced solution comprising sodium carbonate.
Owner:SOLVAY SA
Sodium bicarbonate production method
InactiveUS7255841B2Promote recoveryIncrease valueBicarbonate preparationVarying alkali metal carbonate water contentSodium bicarbonateWastewater
Owner:CHURCH & DWIGHT CO INC
Process for producing sodium hydrogencarbonate crystal particles having low caking property
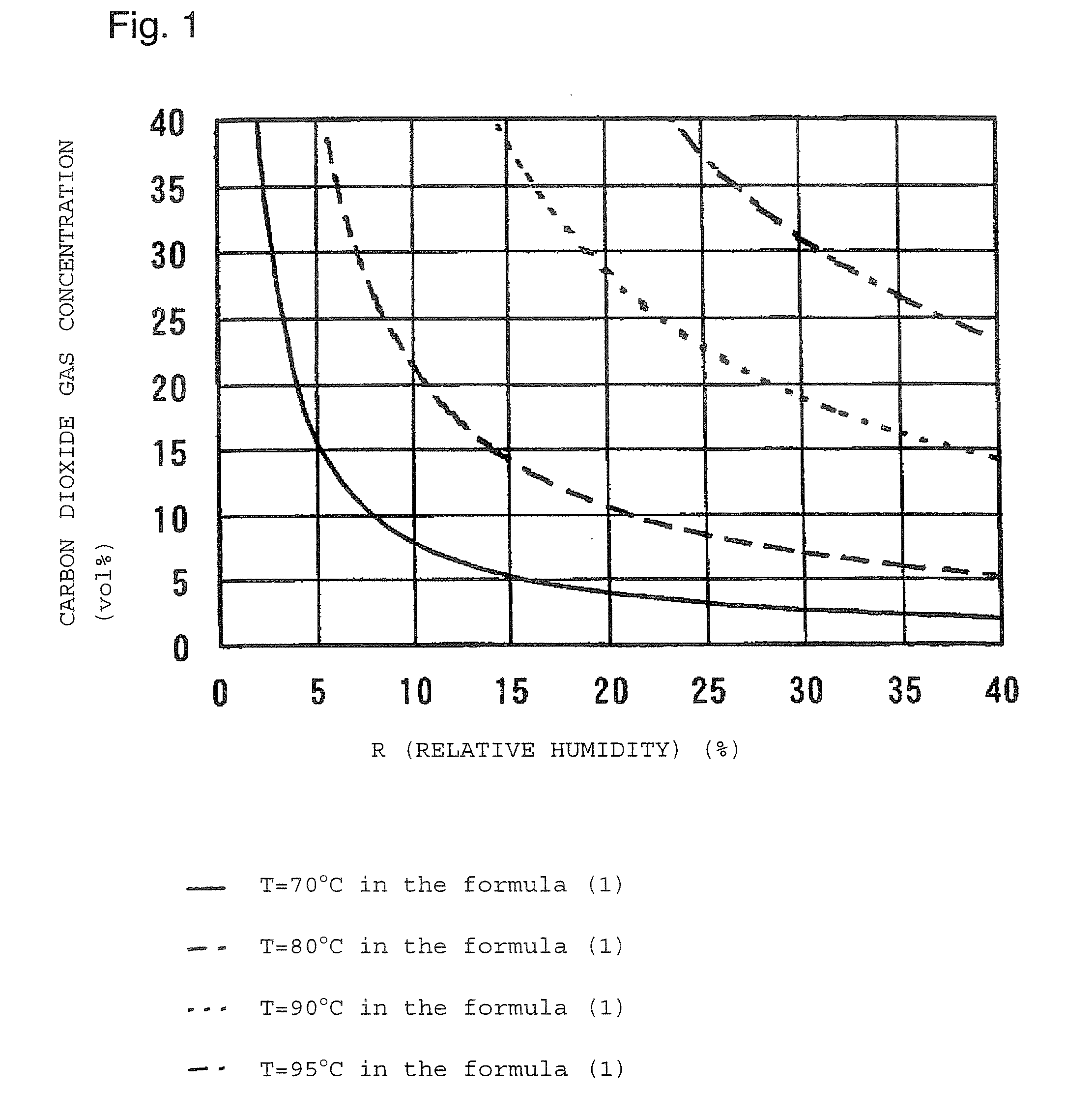
InactiveUS20070178037A1Longer treatment timeReduce cakingVarying alkali metal carbonate water contentAlkali metal carbonates moisture absorption preventionSodium bicarbonateGas concentration
A process for producing sodium hydrogencarbonate crystals, which comprise subjecting sodium hydrogencarbonate crystal powder having an average particle size of from 50 to 500 μm based on the mass to heat treatment at a temperature of from 70 to 95° C. by a heating gas having a carbon dioxide gas concentration of at most the concentration calculated by the following formula (1): Carbon dioxide gas concentration=0.07l×e(0.1×T)×R(−0.0005×T−0.9574) (1)(wherein T is the temperature (%) of sodium hydrogencarbonate crystals, and R is the relative humidity (%), provided that the upper limit of the carbon dioxide gas concentration is 100 vol %) with reference to the graph of FIG. 1 wherein the horizontal axis (X-axis) represents the relative humidity and the vertical axis (Y-axis) represents the carbon dioxide gas concentration, to form anhydrous sodium carbonate on the surface of the sodium hydrogencarbonate crystal particles with a content of anhydrous sodium carbonate of from 0.03 to 0.40 mass % in the sodium hydrogencarbonate crystals. A process for producing sodium hydrogencarbonate crystal particles having a low caking property, which are useful in the field of food products, pharmaceuticals, bath additives, etc., which require no necessity to contain an anticaking agent, can be provided.
Owner:ASAHI GLASS CO LTD
Method for preparing high-purity anhydrous sodium carbonate by using membrane method
InactiveCN104445290AImprove filtering effectImprove corrosion resistanceVarying alkali metal carbonate water contentMagmaSlurry
The invention discloses a method for preparing high-purity anhydrous sodium carbonate by using a membrane method. The method comprises the following steps: (1) dissolving industrial sodium carbonate with distilled water of 55-65 DEG C, and stirring to ensure that calcium impurities in the industrial sodium carbonate are fully deposited; (2) adding a sodium hydroxide solution into the solution, and stirring at 55-65 DEG C to ensure that magnesium ions in the solution are fully deposited until the magnesium ion content of the solution is lower than 5ppm; (3) filtering the solution by using a ceramic membrane; (4) evaporating a filtrate after filtration, putting an obtained magma solution into a centrifuge for separation, and adding deionized water into the centrifuge to wash filter cakes until no chloride ion is generated in the filter cakes; and (5) drying the product obtained by centrifuging to obtain high-purity anhydrous sodium carbonate, wherein in the high-purity anhydrous sodium carbonate, Ca<2+> is less than or equal to 1ppm, Mg<2+> is less than or equal to 1ppm, SS is less than or equal to 1ppm, and Cl<-> is less than or equal to 2ppm. In the high-purity anhydrous sodium carbonate prepared by the method disclosed by the invention, Ca<2+> is less than or equal to 1ppm, Mg<2+> is less than or equal to 1ppm, SS is less than or equal to 1ppm, and Cl<-> is less than or equal to 2ppm.
Owner:TIANJIN BOHUA YONGLI CHEM IND
Combined solid waste, carbon dioxide quicklime sparging, brine water, and reverse osmosis/ion exchange processes for the production of soda chemicals
The proposed invention uses a classical chemical equation where carbon dioxide CO2 is reacted with quick lime Ca(OH)2 to produce soda carb NaHCO3 and concentrating it to 6% using advanced membrane and resin technology. The invention requires three chemicals CO2, Ca(OH)2, and sodium chloride NaCl to produce NaHCO3. The output of many industrial processes lacks waste heat and in many instances CO2 and the present invention combines a solid waste processing unit to the above processes which allows the production of solid products or high % liquors. Availability of waste heat sources can lead to high efficiency in NaHCO3, Na2CO3, and NaOH production. The process is not chloro-alkali electrochemical or Solvay column ammonia processing technique. Advanced membrane uses technologies of reverse osmosis and nanofiltration systems while resin technology uses ion exchange systems. Therefore, we conveniently call it the solid waste-quicklime membrane SWQM process.
Owner:ENGSL FZE +1
Method for preparing high-purity anhydrous sodium carbonate
InactiveCN101885495AAdvanced production technologySimple stepsVarying alkali metal carbonate water contentAlcoholFiltration
The invention relates to a method for preparing high-purity anhydrous sodium carbonate. The method comprises the following steps of: (1) dissolving the anhydrous sodium carbonate; (2) preparing soda crystals, which is to slowly pour solution A into a sieve for filtration, allow the filtrate to flow into a alcohol basin, continuously stir the mixed solution and allow the mixed solution to cool naturally till the sodium carbonate is separated out; and (3) preparing the high-purity anhydrous sodium carbonate, which is to fill product B obtained by step (2) into a polyethylene plastic barrel, add distilled water to dissolve the product B with stirring, heating, stirring and crystallizing the mixed solution, performing crystallization to obtain crystals, and drying the crystals to obtain the high-purity anhydrous sodium carbonate. The method for preparing the high-purity anhydrous sodium carbonate comprises the step that the purified sodium carbonate is filtered to separate out crystals in the ethanol. The whole production process is advanced, the steps are simple, and the process parameters and all operation are easily controlled.
Owner:TIANJIN CHEM REAGENT RES INST
Method for separating crystal slurry in synthetic heavy soda ash procedure
InactiveCN1413909AIncrease production capacityLarge grainVarying alkali metal carbonate water contentSlurryTower
A process for separating crystal from slurry in the procedure of synthesizing heavy sodium carbonate includes such steps as synthesizing heavy sodium carbonate in carbonizing tower, loading the reaction liquid in the thickening equipment, continuously growing crystal grains, loading the solid-liquid mixture in alkali filter for separation, calcining the solid to obtain finished product, and returning the clean liquid back to system.
Owner:JIANGSU DEBANG CHEMICAL INDUSTRY GROUP CO LTD
Heavy soda ash fluidized calcoining coling system and process thereof
ActiveCN1785812AReduce volumeSmall sizeVarying alkali metal carbonate water contentEngineeringCalcination
The present invention discloses a heavy ash fluidizing calcinations and cooling system and its technological process. Said system includes the following several portions: material conveying device, alkali-returning device, calcinations cooling device, dust-removing device and air-supplying device, etc. Besides, said invention also provides their connection mode and concrete steps of said technological process.
Owner:SHANDONG TIANLI DRYING TECH & EQUIP
Method for comprehensively utilizing solid waste in methyl carbonate production
InactiveCN101671038ASignificant comprehensive benefitsIncrease productionVarying alkali metal carbonate water contentOrganic compound preparationMethyl carbonateSolvent
The invention discloses a method for comprehensively utilizing solid waste in the methyl carbonate production, comprising the following steps: adding solvent in the solid waste, stirring and evenly mixing the mixture according to the volume ratio of 1:1.5; carrying out filter pressing separation to obtain organic phase solution and sodium carbonate solidoid substance containing crystallization water; distillating the organic phase solution at normal pressure under the condition that the temperature is lower than 70 DEG C to reclaim the solvent and simultaneously obtain about 40 percent of organic phase solution relative to the weight of waste residue; carrying out vacuum distillation on organic matters to obtain 5 percent of methyl carbonate relative to the organic phase, which is more than 85 percent of 1,2-propanediol and 1 percent of ice carbon; and baking the solidoid substance to volatilize to obtain 50 percent of industrial sodium carbonate relative to the weight of solid waste residue. The method overcomes the defects that the prior method causes the environment pollution and influences the safe running of a boiler, sodium carbonate, propanediol and methyl carbonate in the waste are burnt out and cannot be recovered so as to cause waste. The method can energy sources and change wastes into valuables.
Owner:张青
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com