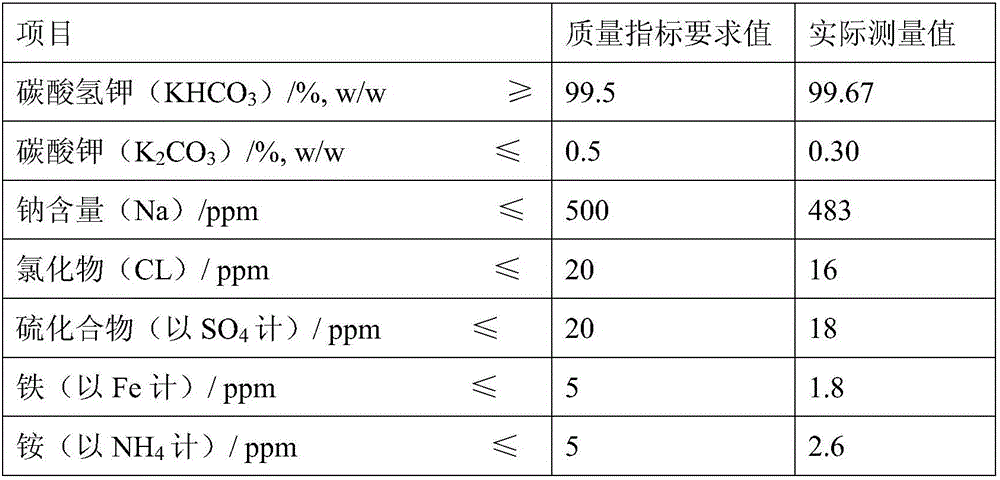
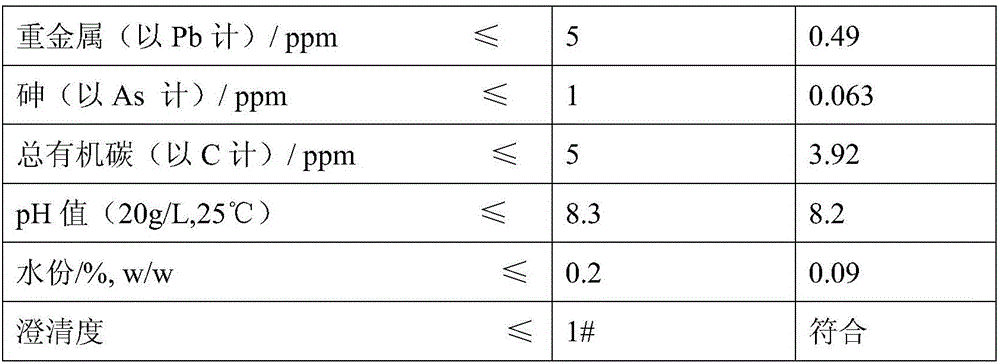
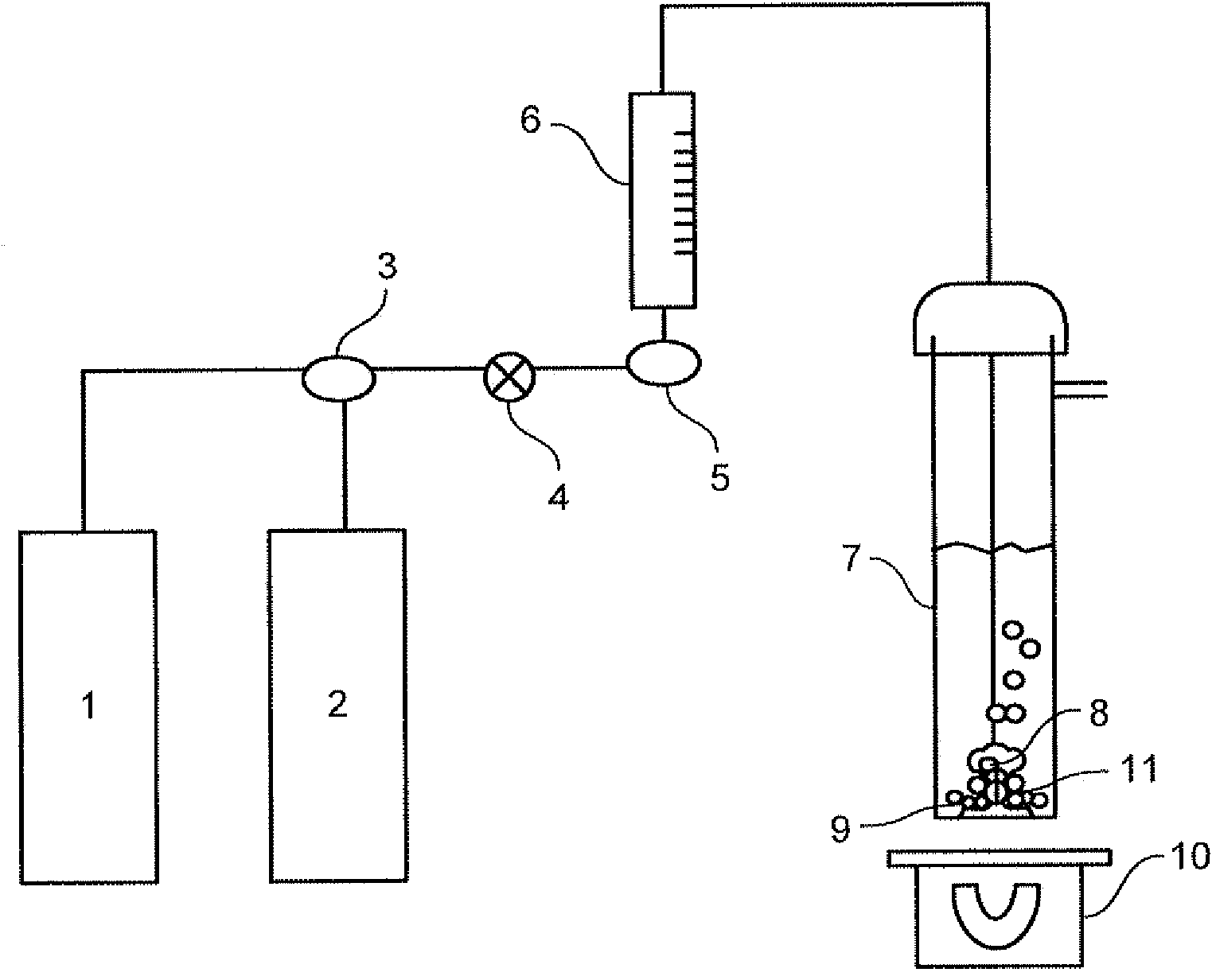
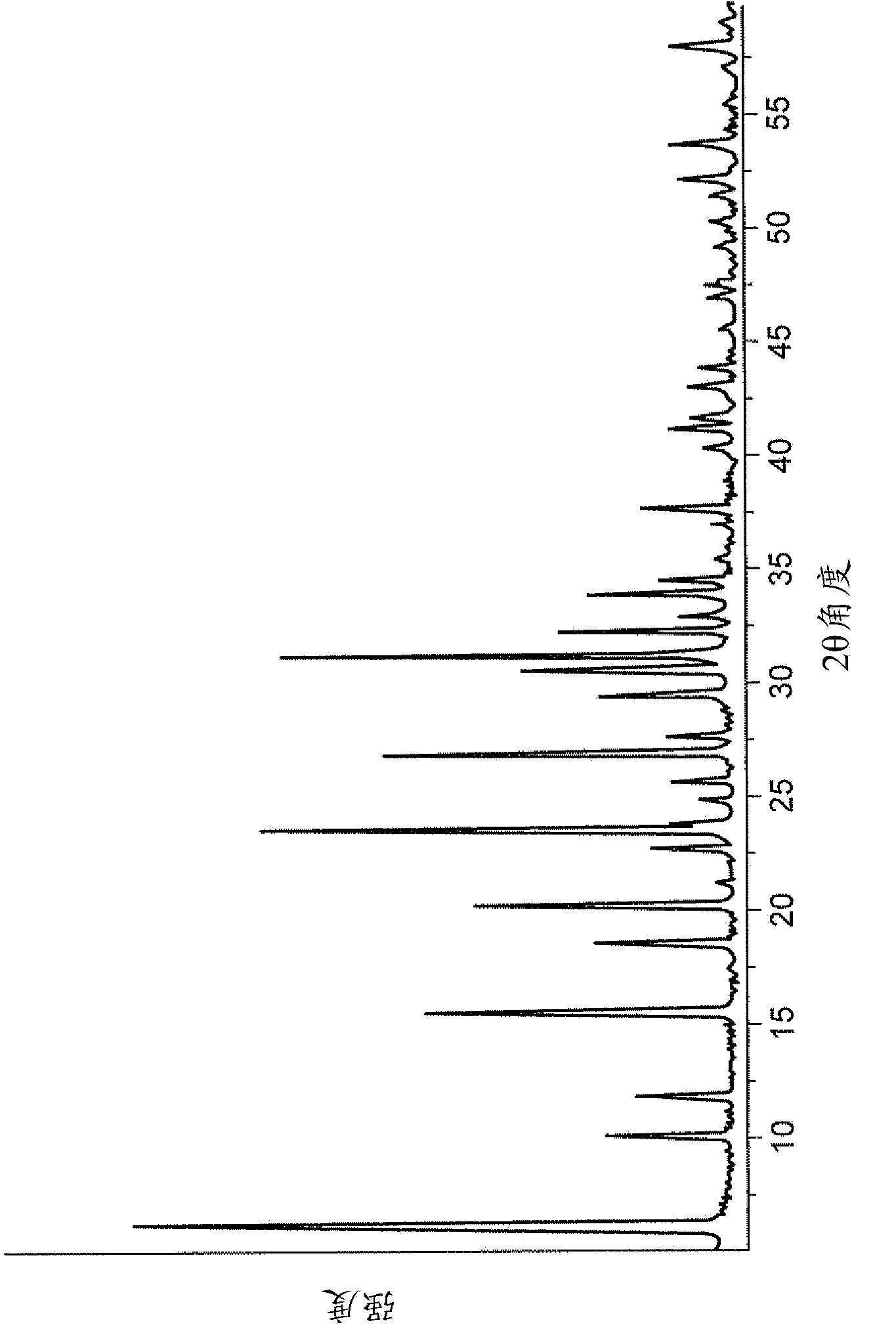
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
134results about "Bicarbonate preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Efficient Production of Fuels
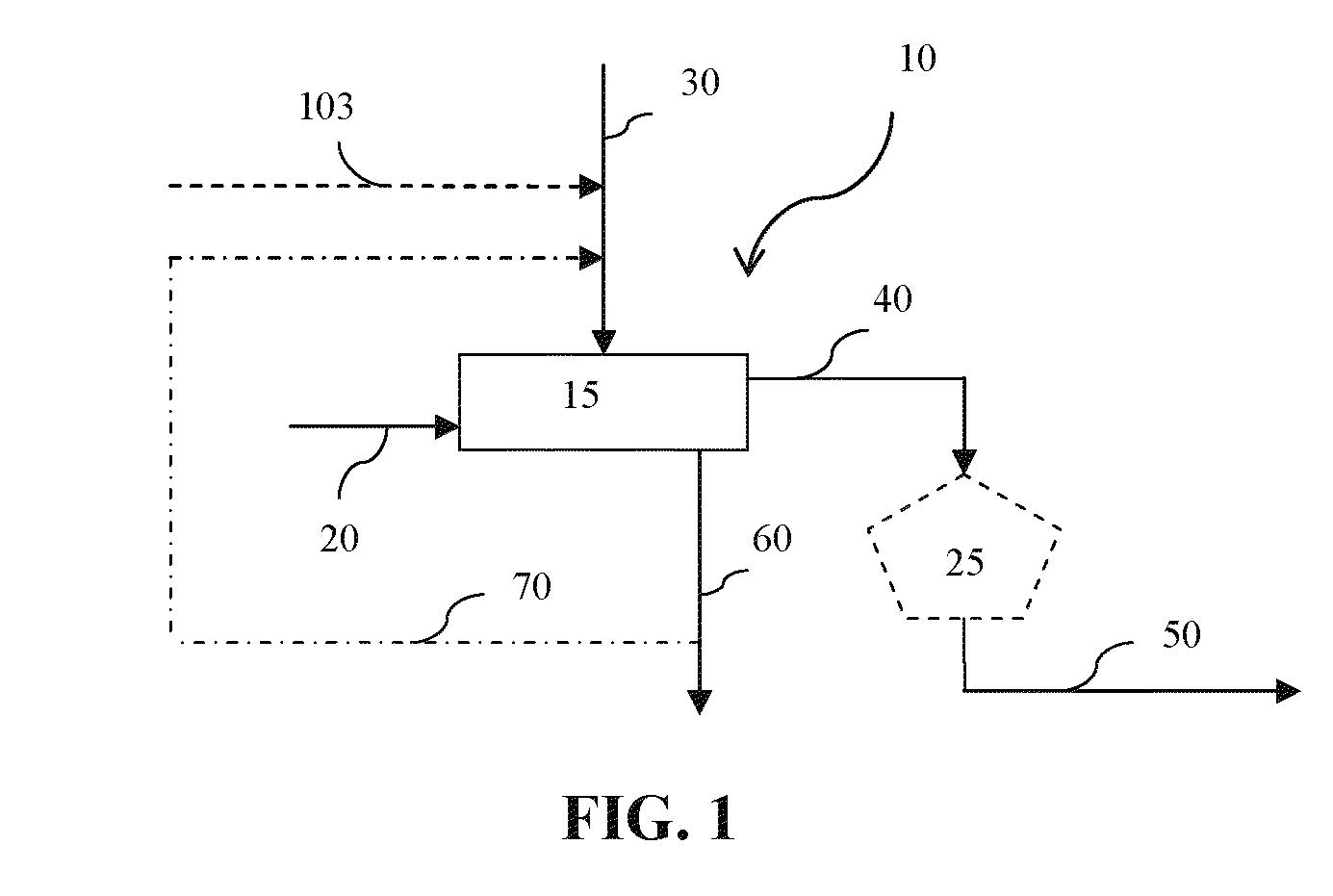
ActiveUS20090277799A1Efficient productionHydrogenBicarbonate preparationFluid phaseElectrical battery
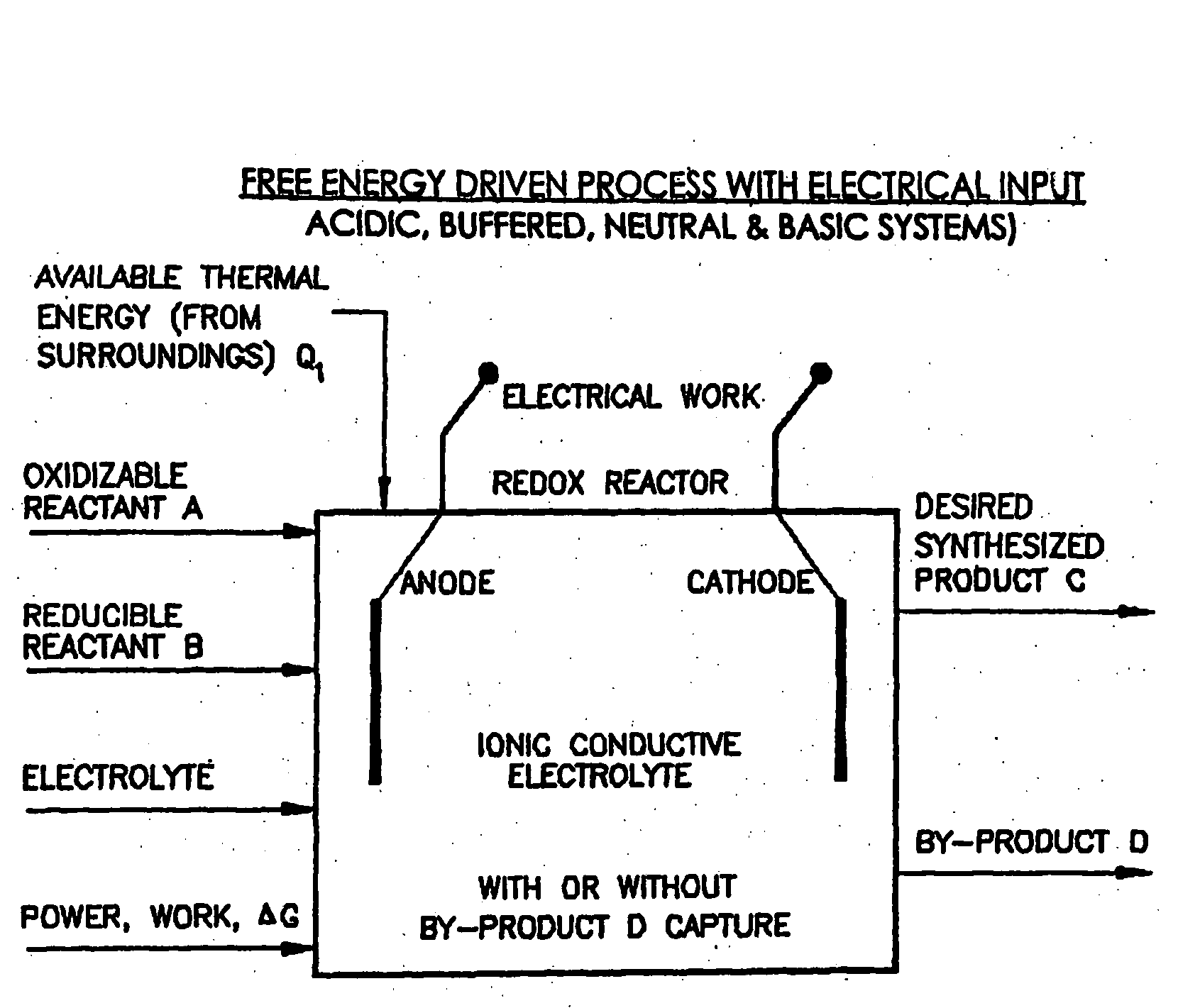
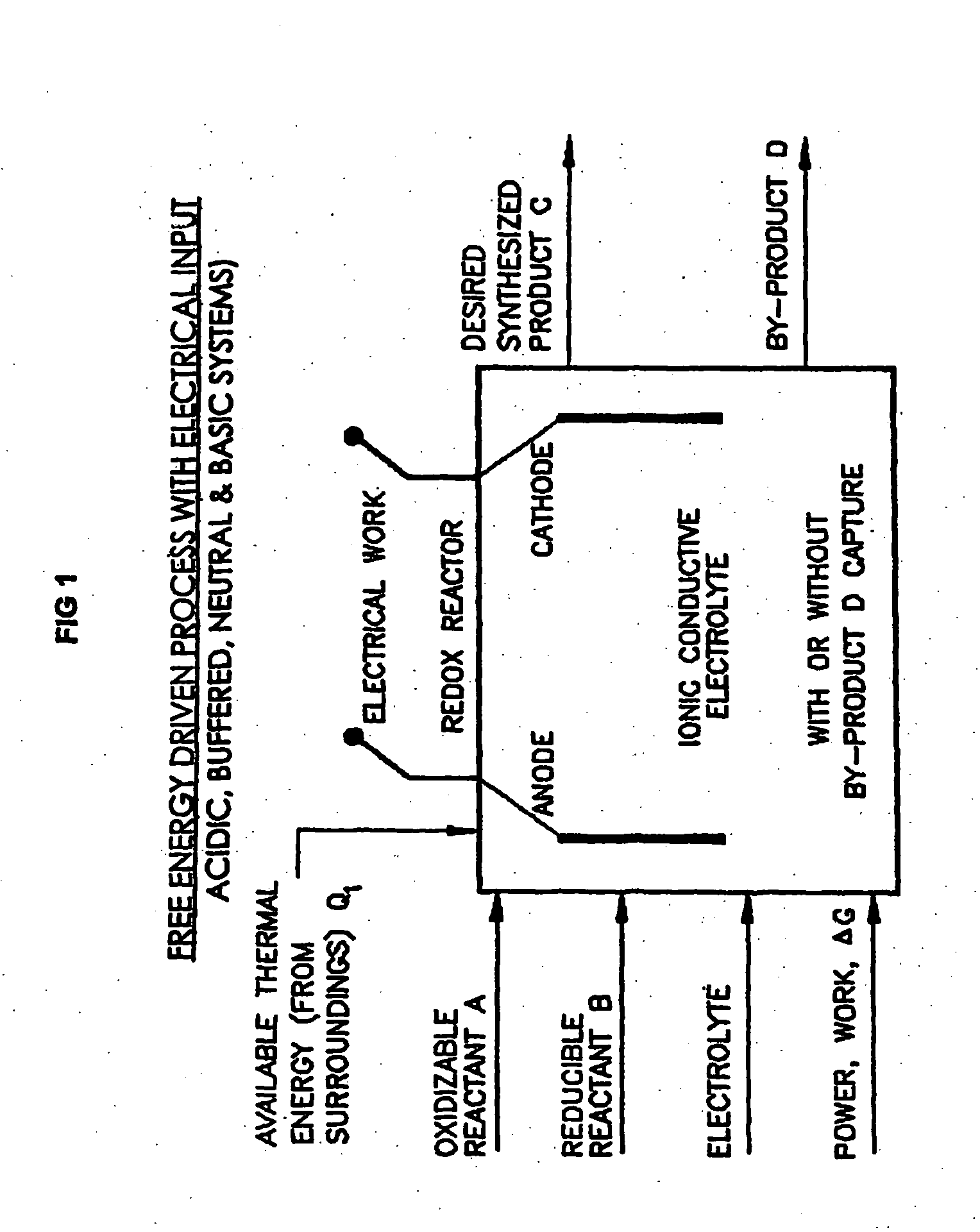
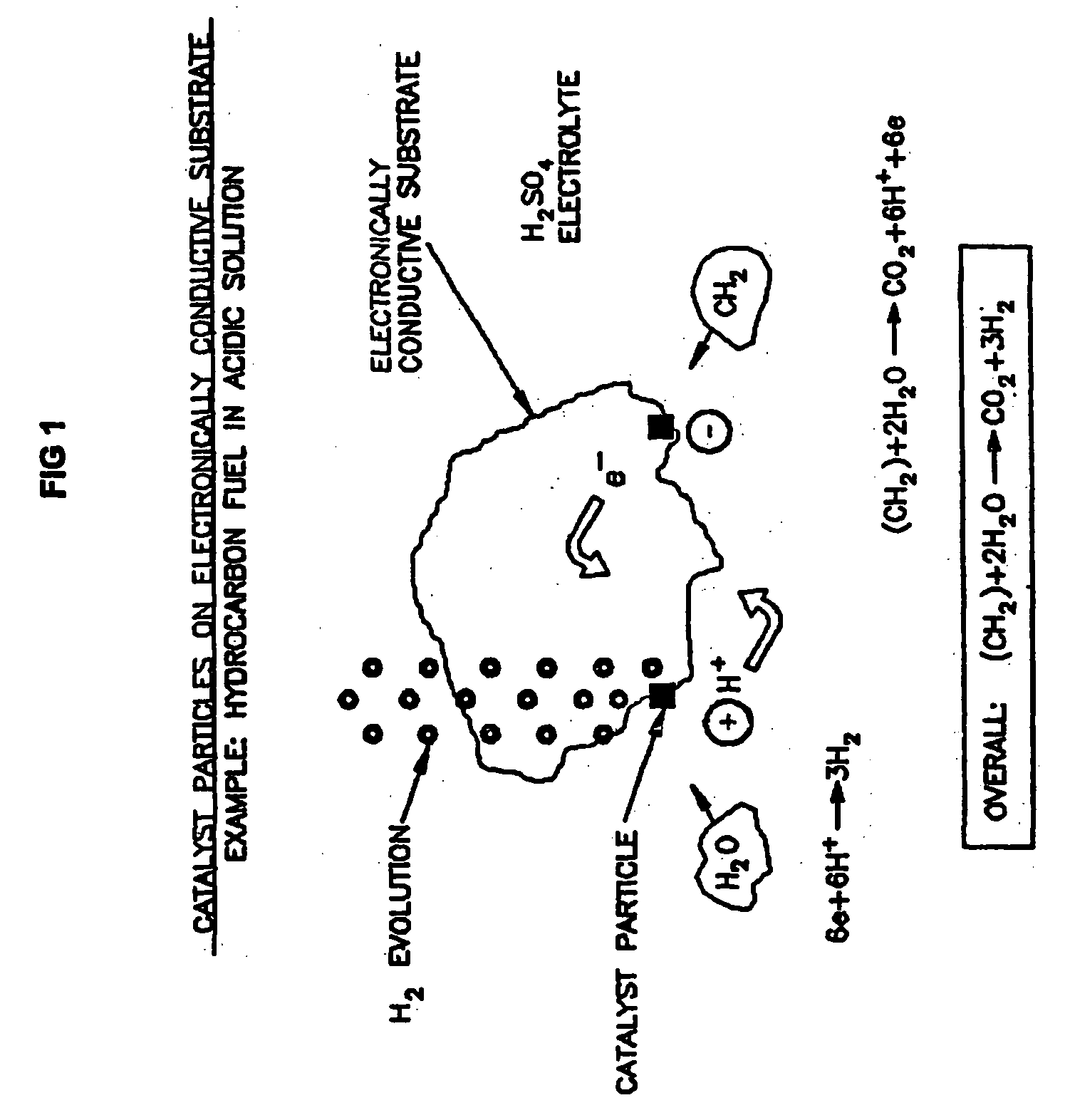
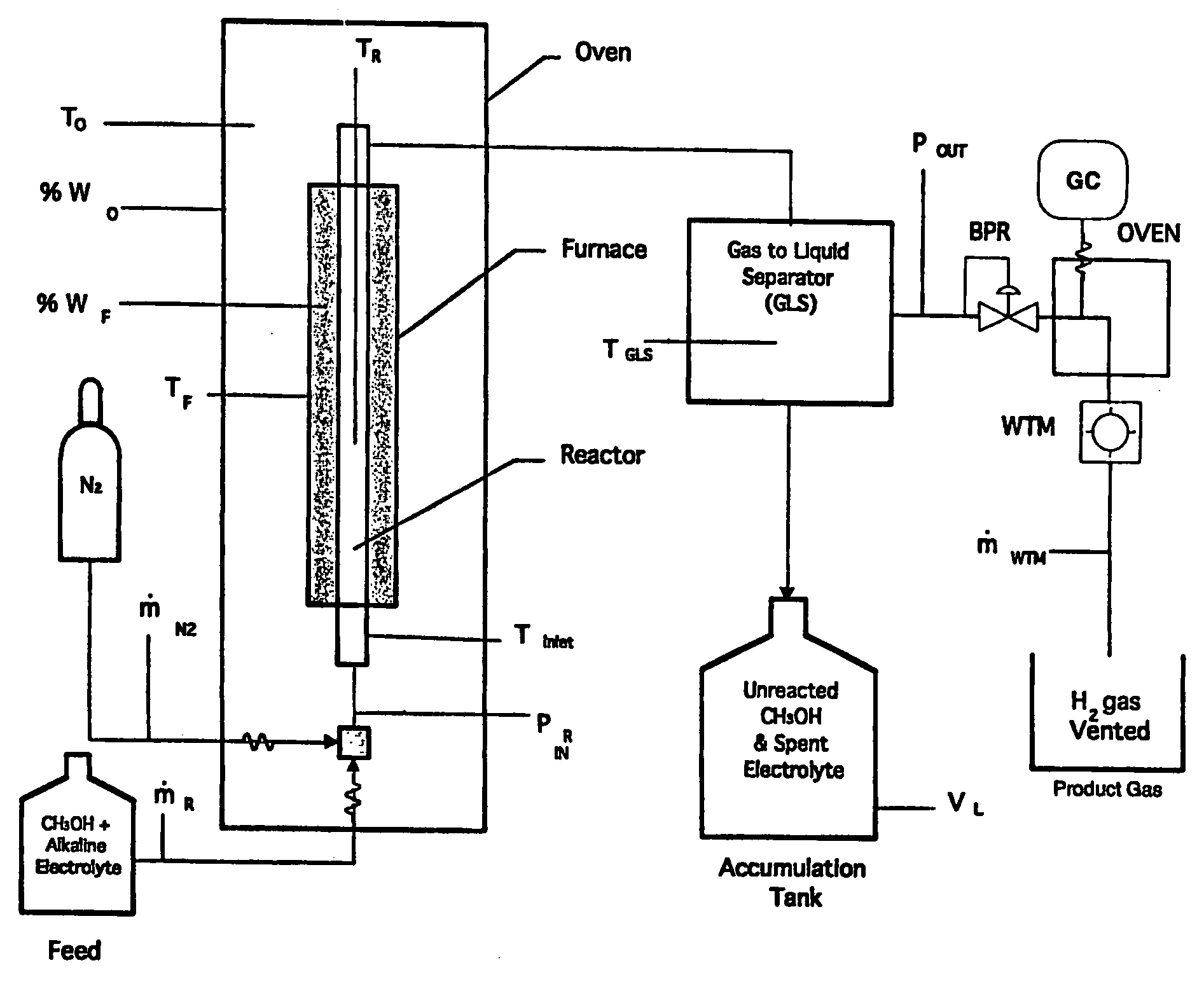
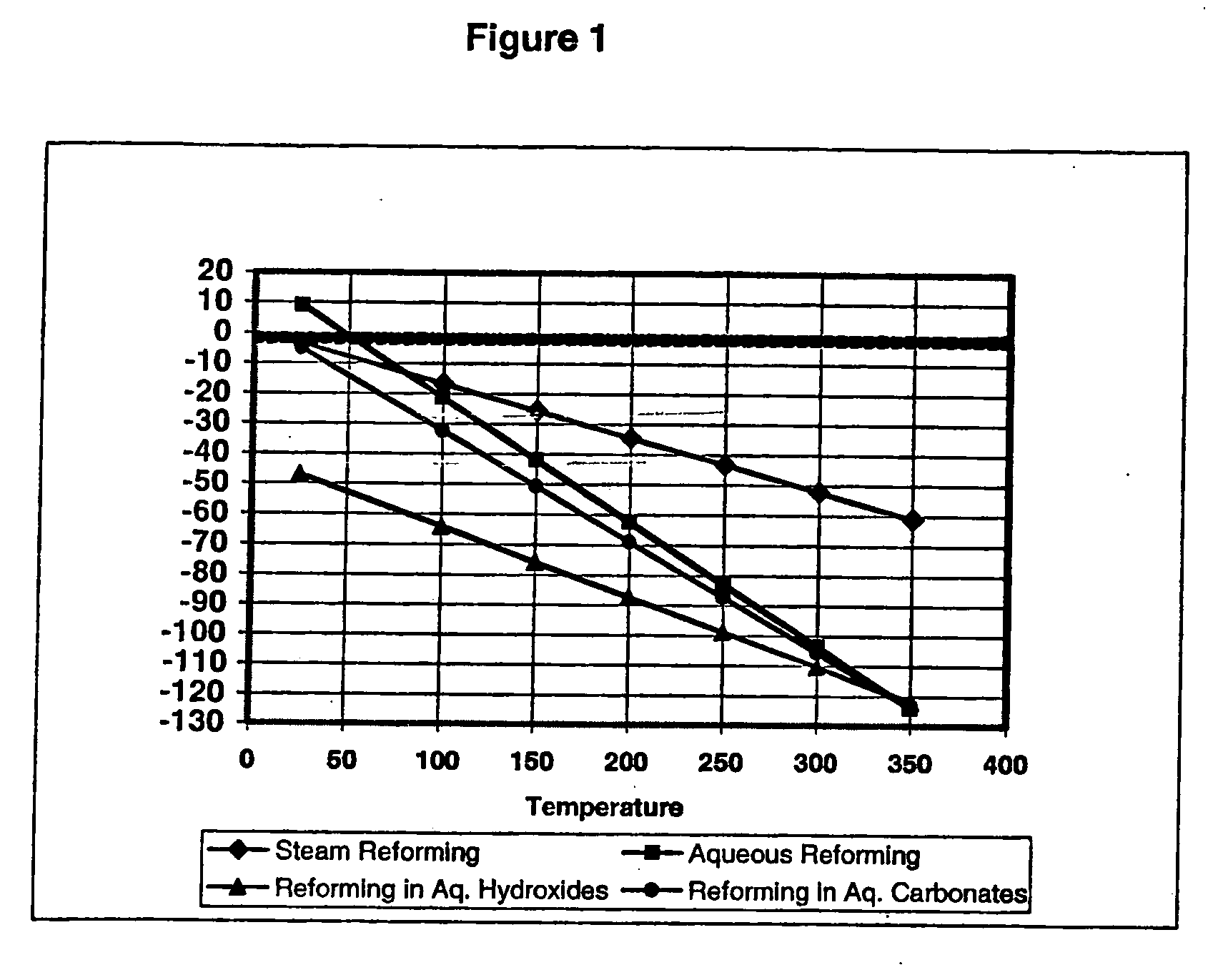
Liquid phase processes for producing fuel in a reactor comprising the step of combining at least one oxidizable reactant with liquid water and at least one electrolyte to form a mixture and conducting a fuel-producing reaction in the presence of an electron transfer material, wherein the mixture permits the movement or transport of ions and electrons to facilitate the efficient production of the fuel. An alternative embodiment produces fuel in an electrochemical cell, the reaction characterized by an overall thermodynamic energy balance according to the half-cell reactions occurring at the anode and cathode. Energy generated and / or required by the system components is directed according to the thermodynamic requirements of the half-cell reactions, thereby realizing improved fuel production efficiency.
Owner:GAS TECH INST
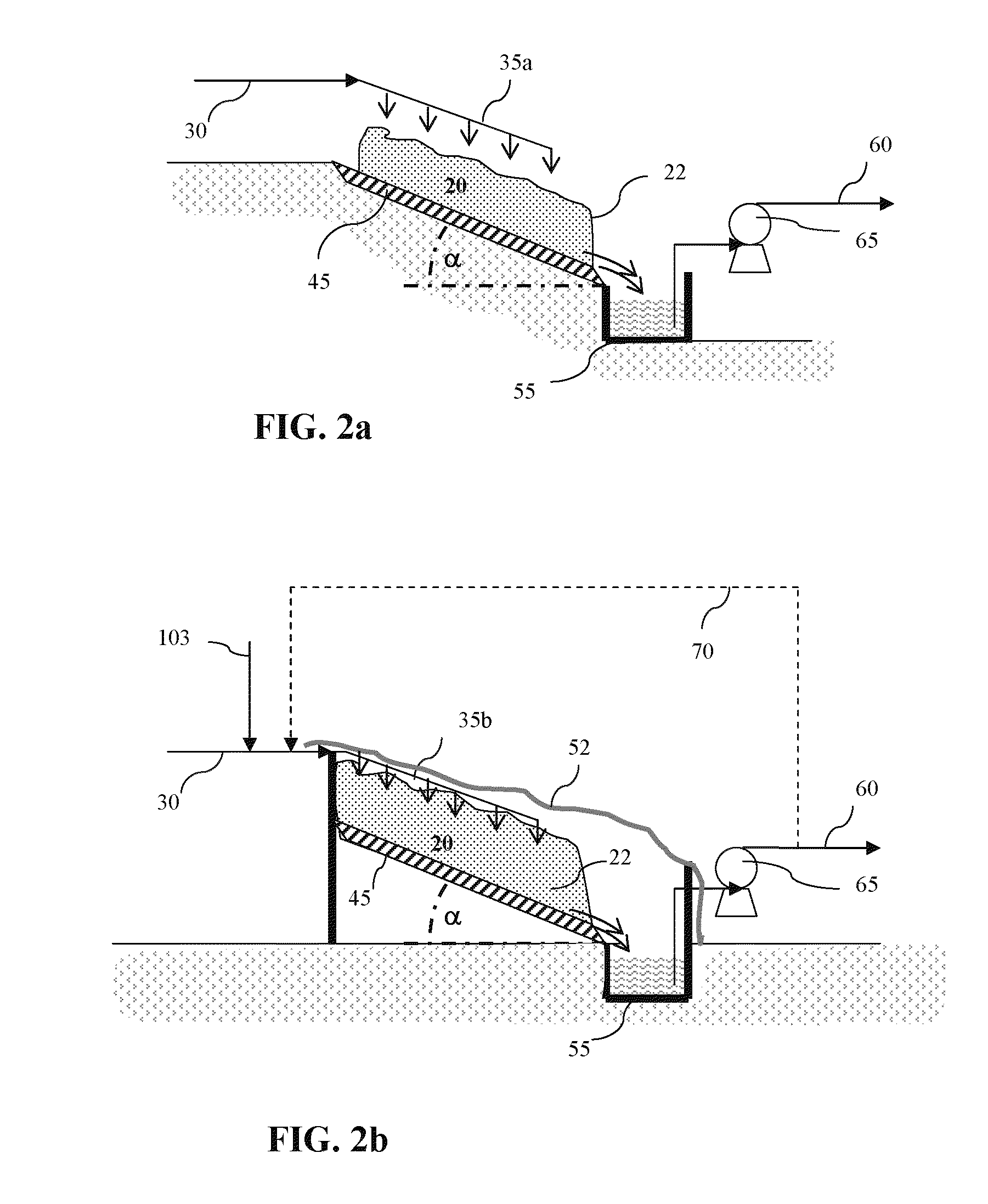
Process for production of dense soda, light soda, sodium bicarbonate and sodium silicate from solutions containing bicarbonate
ActiveUS7507388B2Processing requirementLow production costCrystallization separationAlkali metal silicatesSodium bicarbonateEvaporation
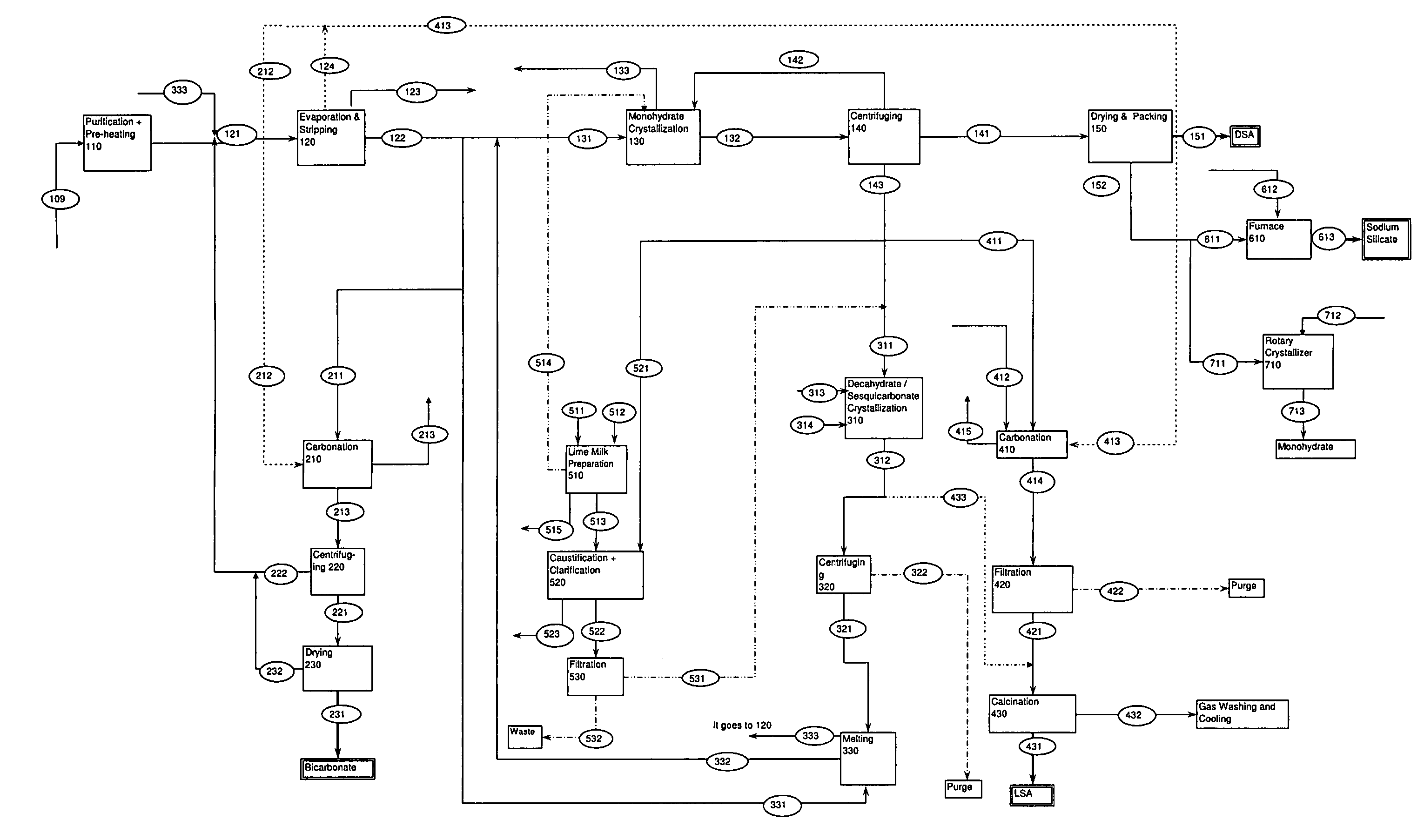
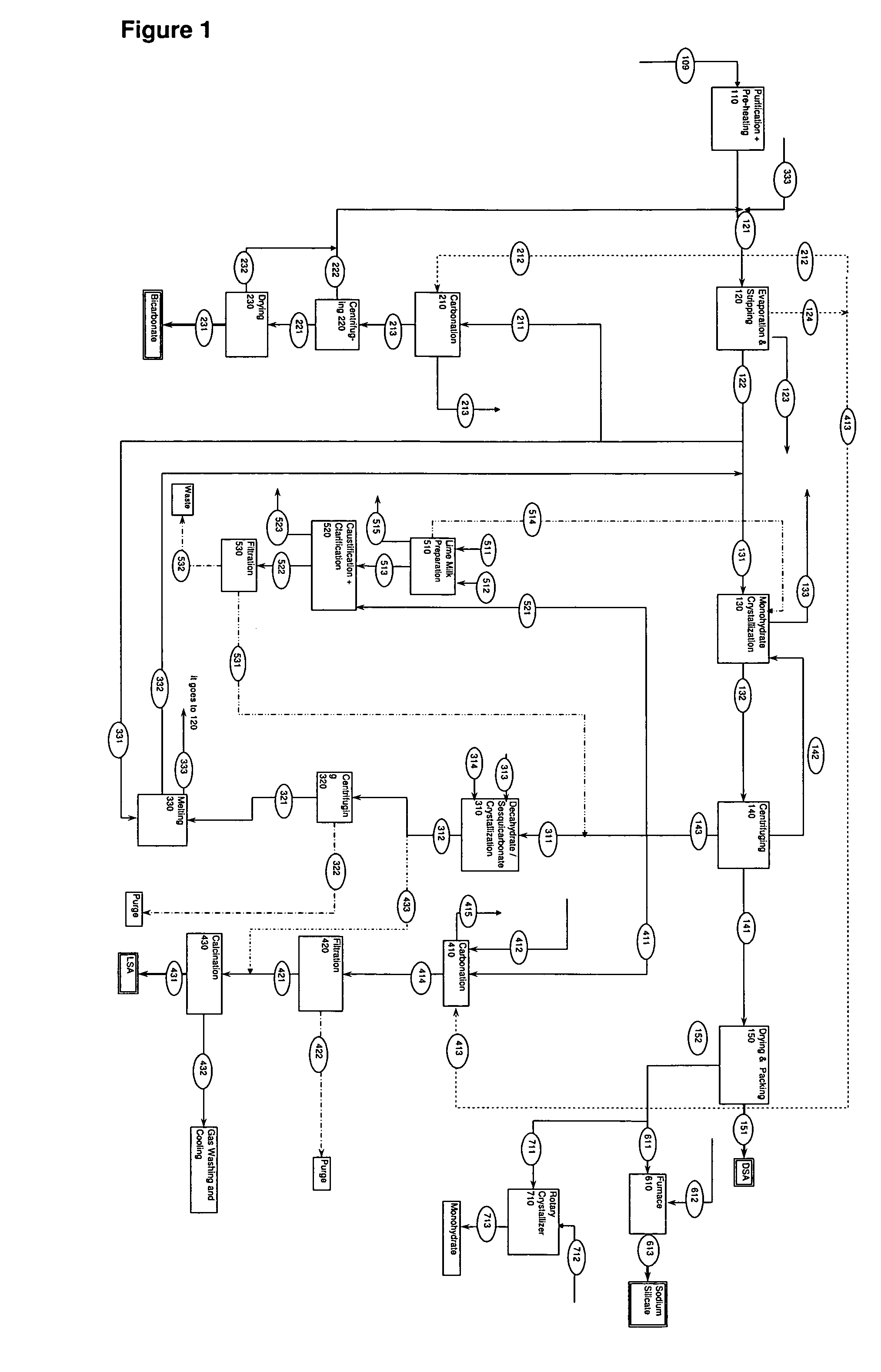
A process related to sodium chemicals production, including the processing of bicarbonate containing solutions obtained by solution mining of trona, nahcolite or wegscheiderite reserves and the lake waters containing bicarbonates, includes the steps of purification, evaporation-decarbonation, crystallization, centrifuging, and drying.
Owner:ETI SODA URETIM PAZARLAMA NAKLIYAT & ELEKTRIK URETIM SANAYI & TICARET A S
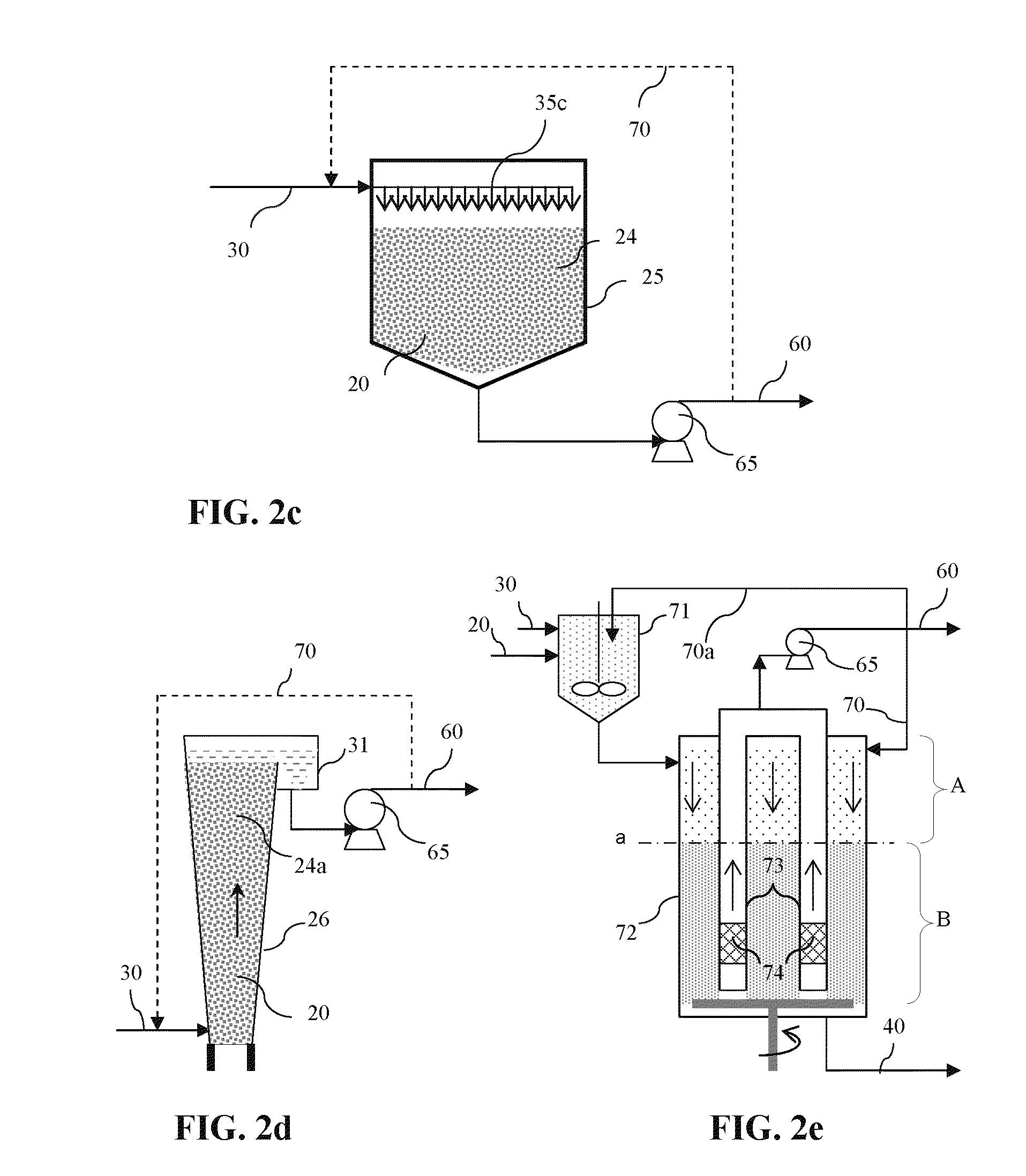
Process for producing sodium carbonate and/or sodium bicarbonate from an ore mineral comprising sodium bicarbonate
InactiveUS20100282614A1Solve the large energy consumptionEconomical and simpleSemi-permeable membranesBicarbonate preparationSodium bicarbonateSodium hydroxide
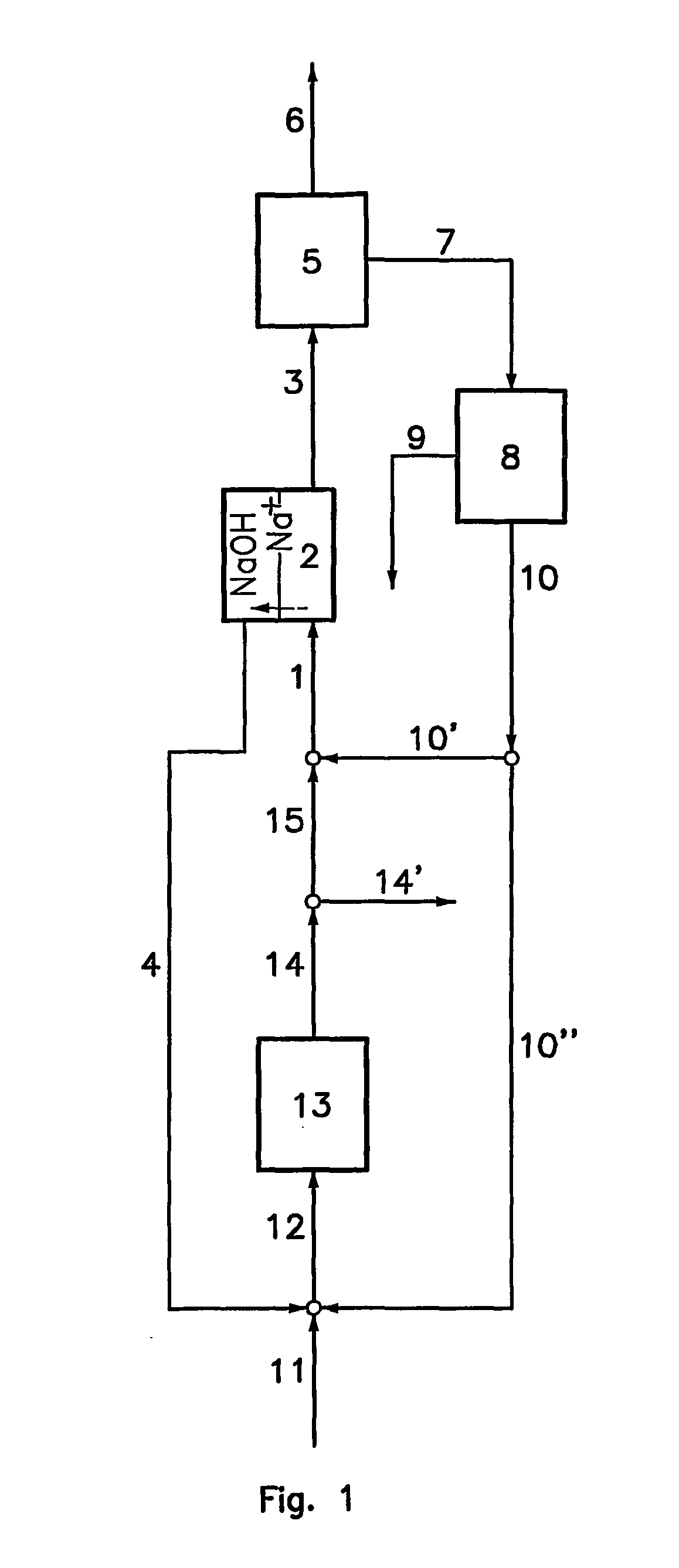
In a process to produce sodium carbonate and / or sodium bicarbonate from an ore mineral comprising sodium bicarbonate, a production solution comprising sodium carbonate is introduced into less basic compartments of an electrodialyser comprising alternating less basic and more basic adjacent compartments separated from each other by cationic membranes, the more basic compartments being delimited by anionic faces of bipolar membranes on one side and by the cationic membranes on the other side; a solution comprising sodium hydroxide is produced into the more basic compartments by combination of sodium ions flux crossing the cationic membrane and hydroxyl ions flux crossing the anionic face of the bipolar membranes, and is then extracted from the electrodialyser to be used as a reaction solution; the reaction solution is put into contact with the mineral ore to form a solution comprising sodium carbonate; and the solution comprising sodium carbonate is divided into a part used as the production solution and a remaining part used as a produced solution.
Owner:SOLVAY SA
Sodium bicarbonate production method
InactiveUS20030017099A1Improvement in recovered sodium valuePromote recoveryBicarbonate preparationRubidium/caesium/francium compoundsEnvironmental engineeringSodium hydrogencarbonate
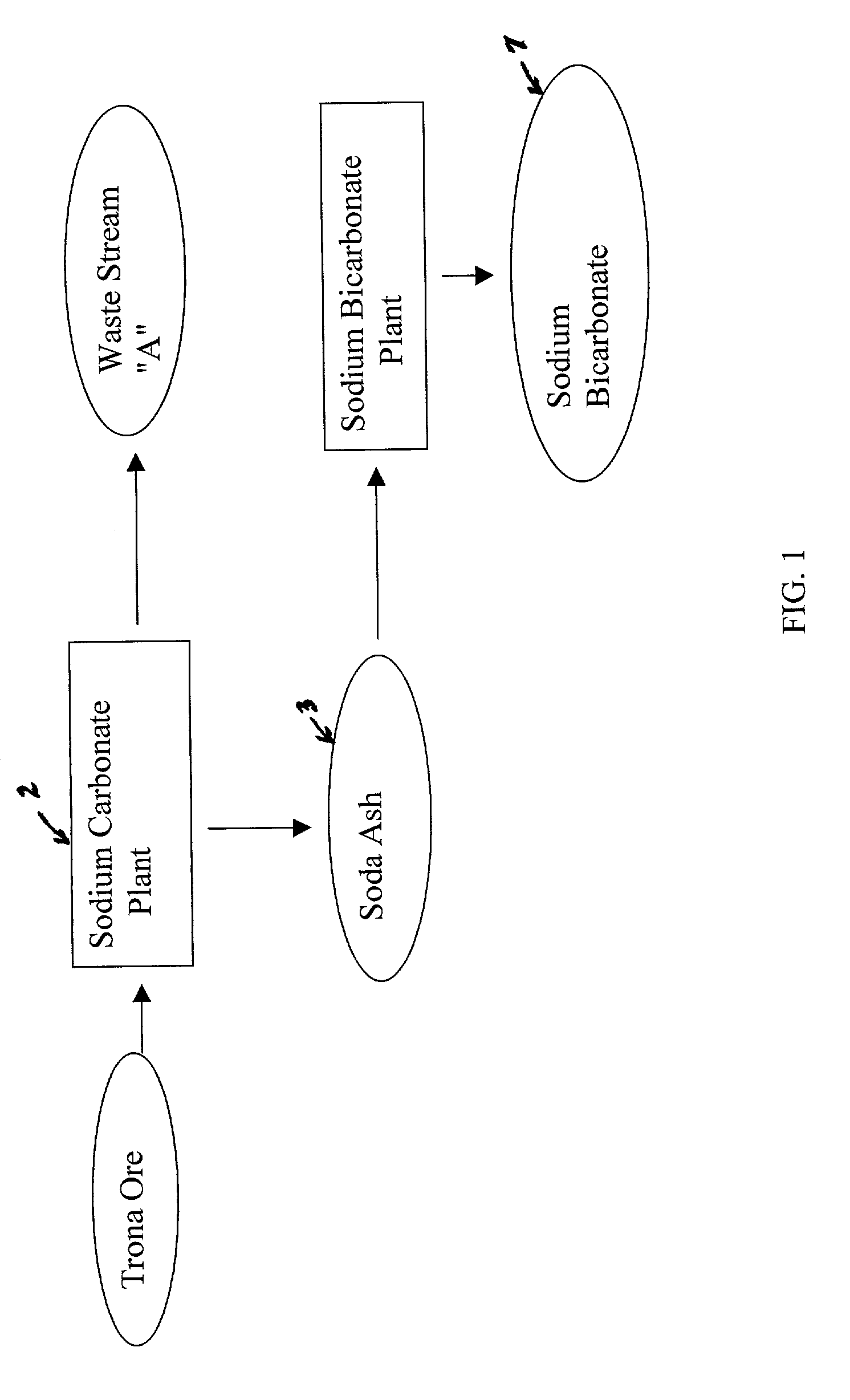
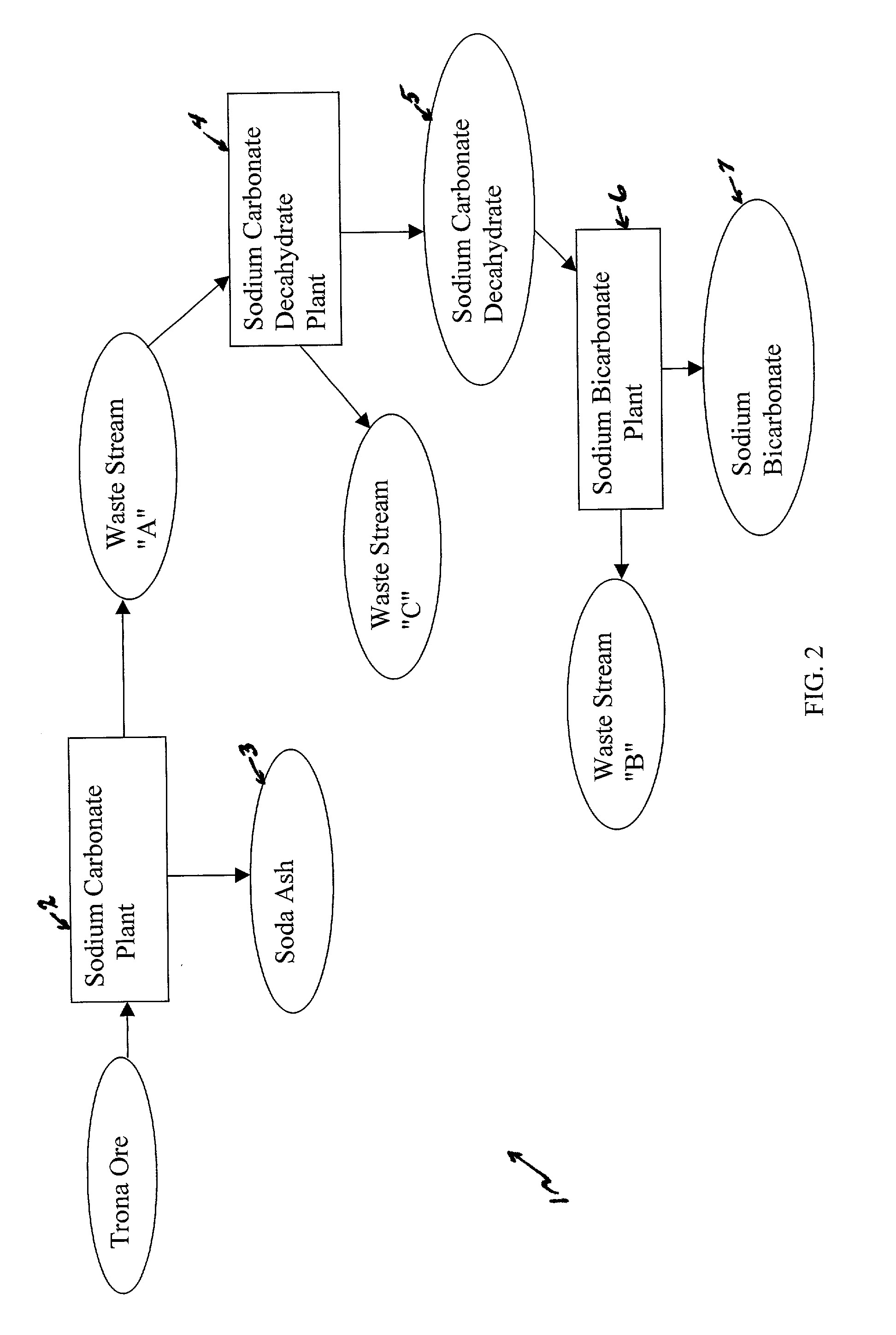
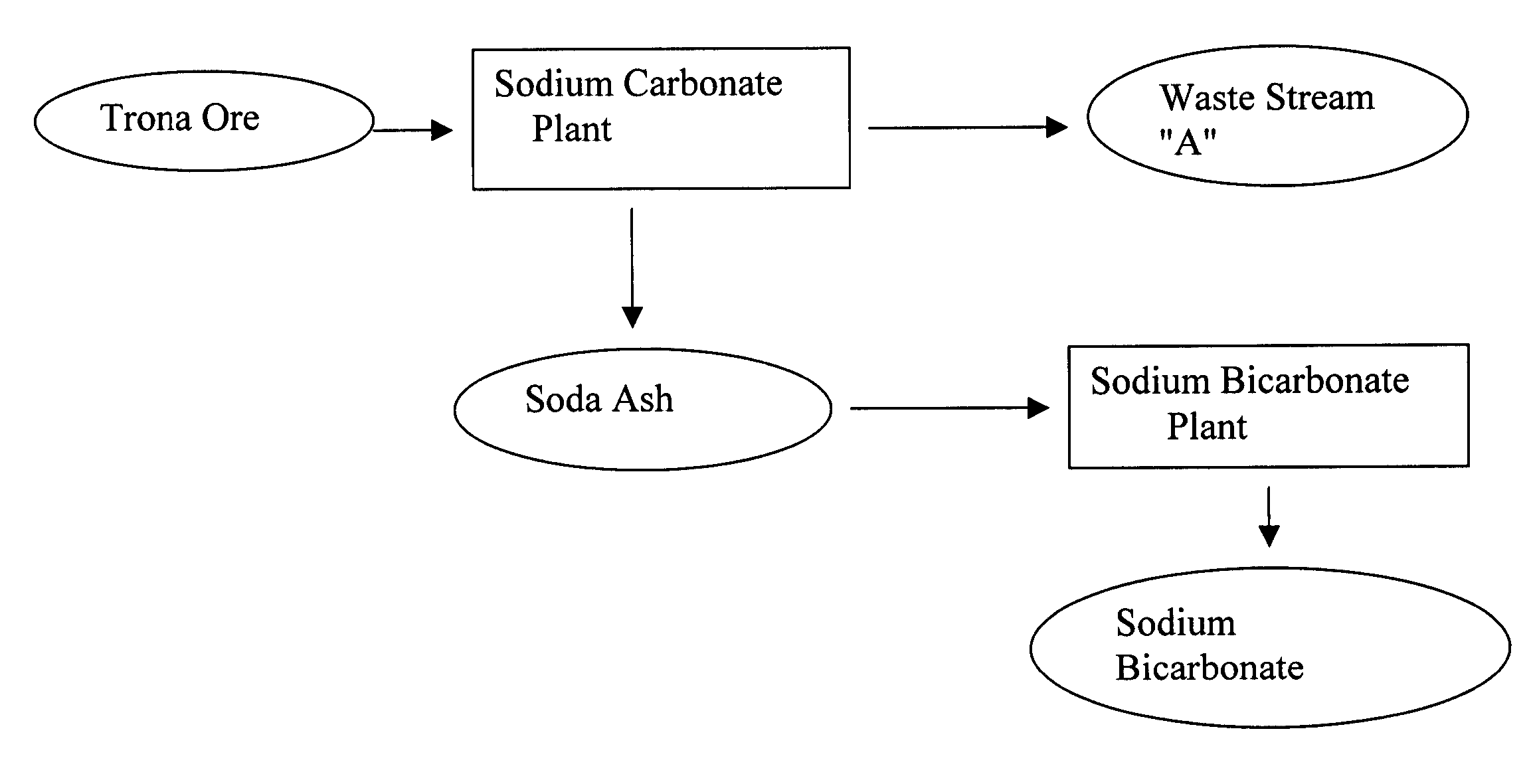
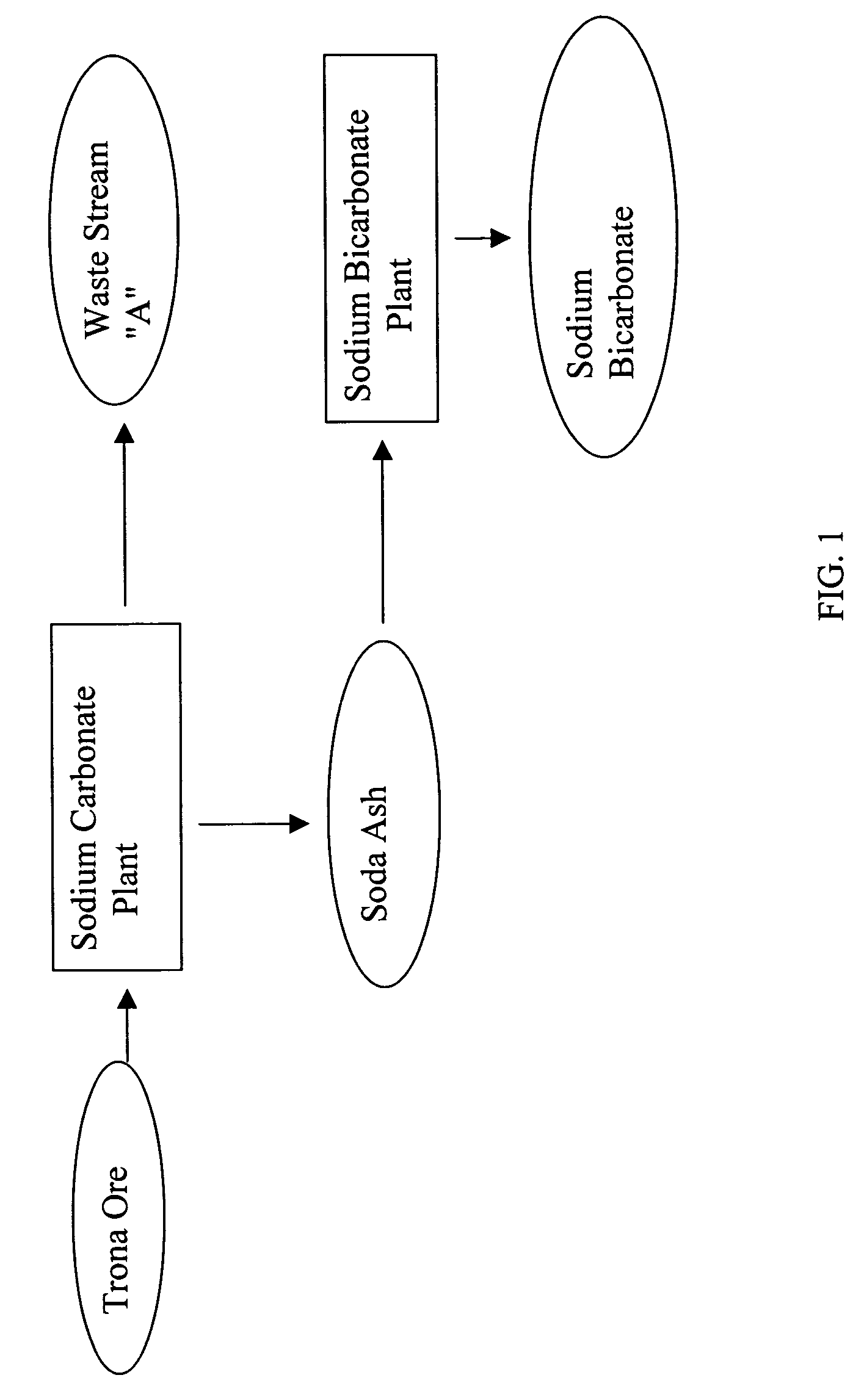
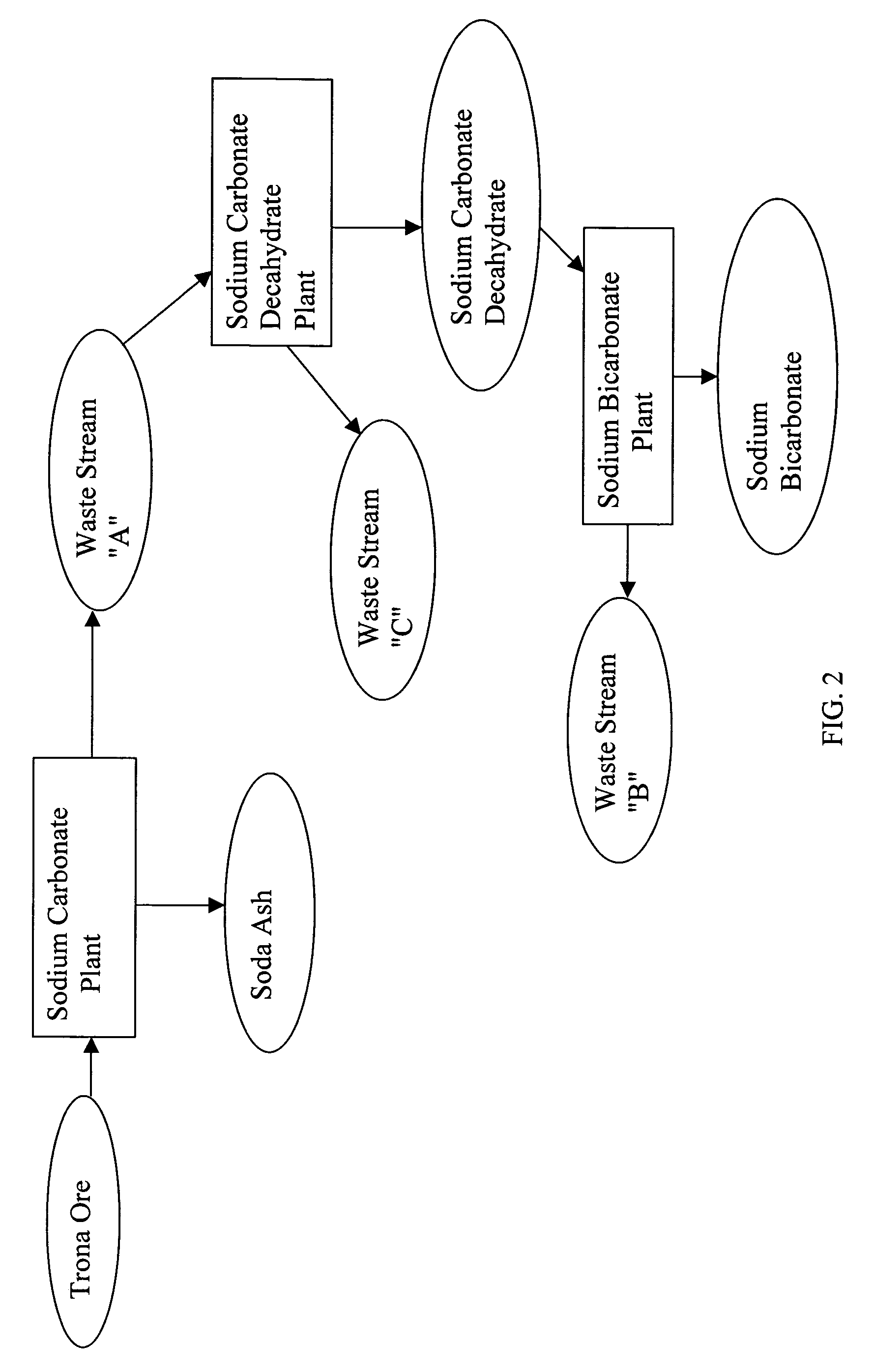
A method of producing additional sodium bicarbonate having a high degree of purity and obtaining a net reduction in effluent waste water, as compared to prior processes, when starting from trona ore is disclosed. The process entails utilizing the waste-water effluent stream from the conversion of trona ore to sodium carbonate as the feed for the conversion of sodium carbonate to sodium bicarbonate.
Owner:CHURCH & DWIGHT CO INC
Sodium bicarbonate production method
InactiveUS20040057892A1Improvement in recovered sodium valuePromote recoveryBicarbonate preparationVarying alkali metal carbonate water contentWastewaterEngineering
A method of producing sodium bicarbonate having a high degree of purity and obtaining a net reduction in effluent waste water, as compared to prior processes, when starting from trona ore is disclosed. The process entails utilizing the waste-water effluent stream from the conversion of trona ore to sodium carbonate as the feed for the conversion of sodium carbonate to sodium bicarbonate.
Owner:CHURCH & DWIGHT CO INC
Process for preparing super activated carbon from nut shell carbon and waste liquid activation treatment method of process
The invention discloses a process for preparing super activated carbon from nut shell carbon and a waste liquid activation treatment method of the process, belonging to the field of biochemical engineering and energy materials. The process comprises the following steps: soaking nut shell carbon which is subjected to pre-washing and thermal treatment A into an oxidization substance in a vacuum reaction kettle; performing thermal treatment B on the nut shell carbon soaked into the oxidization substance, and soaking into an alkali aquo-complex activating agent in the vacuum reaction kettle; in inert gas or reductive atmosphere, heating the nut shell carbon to perform thermal treatment C so as to obtain activated nut shell carbon; adding water to leach and separate the activated nut shell carbon so as to obtain alkali activated mother liquor A and a solid; adding water into the solid for leaching again so as to obtain carbonate mother liquor B and activated carbon slurry; performing multi-stage washing and acidization treatment on the activated carbon slurry, washing with water, and separating so as to obtain a crude activated carbon product; and performing thermal treatment D of different stages on the crude activated carbon product, thereby obtaining an activated carbon product. The super activated carbon with high yield and good properties can be prepared, and meanwhile useful byproducts such as bicarbonate and hydrated silica can be prepared.
Owner:BEIJING UNIV OF CHEM TECH
Impurities removal from waste solids in the production of soda ash, sodium bicarbonate and/or other derivatives
ActiveUS20110274599A1Low impurity contentIncrease valueBicarbonate preparationSolvent extractionSodium bicarbonateDissolution
A method for removing impurities from a waste solid to provide at least a portion of a suitable crystallizer feed to a process for making crystalline sodium carbonate, bicarbonate, and / or other derivatives. The method comprises: contacting the waste solid with a leach solution to dissolve at least one impurity and dissolving the resulting leached residue. Leaching may include heap percolation. The leach solution may comprise a crystallizer purge liquor, a process waste effluent, a mine water, or mixtures thereof. The method may further comprise adding a magnesium compound to the resulting leached residue during or after its dissolution to remove another impurity. The waste solid preferably comprises a pond solid containing such impurities. The pond solid may be recovered from a pond receiving crystallizer purge liquor(s) and / or other process waste effluent(s). The pond solid may contain sodium carbonate, any hydrate thereof, sodium bicarbonate, and / or sodium sesquicarbonate. The impurities to be removed may comprise sodium chloride, sodium sulfate, silicates, and / or organics.
Owner:SOLVAY CHEM INC
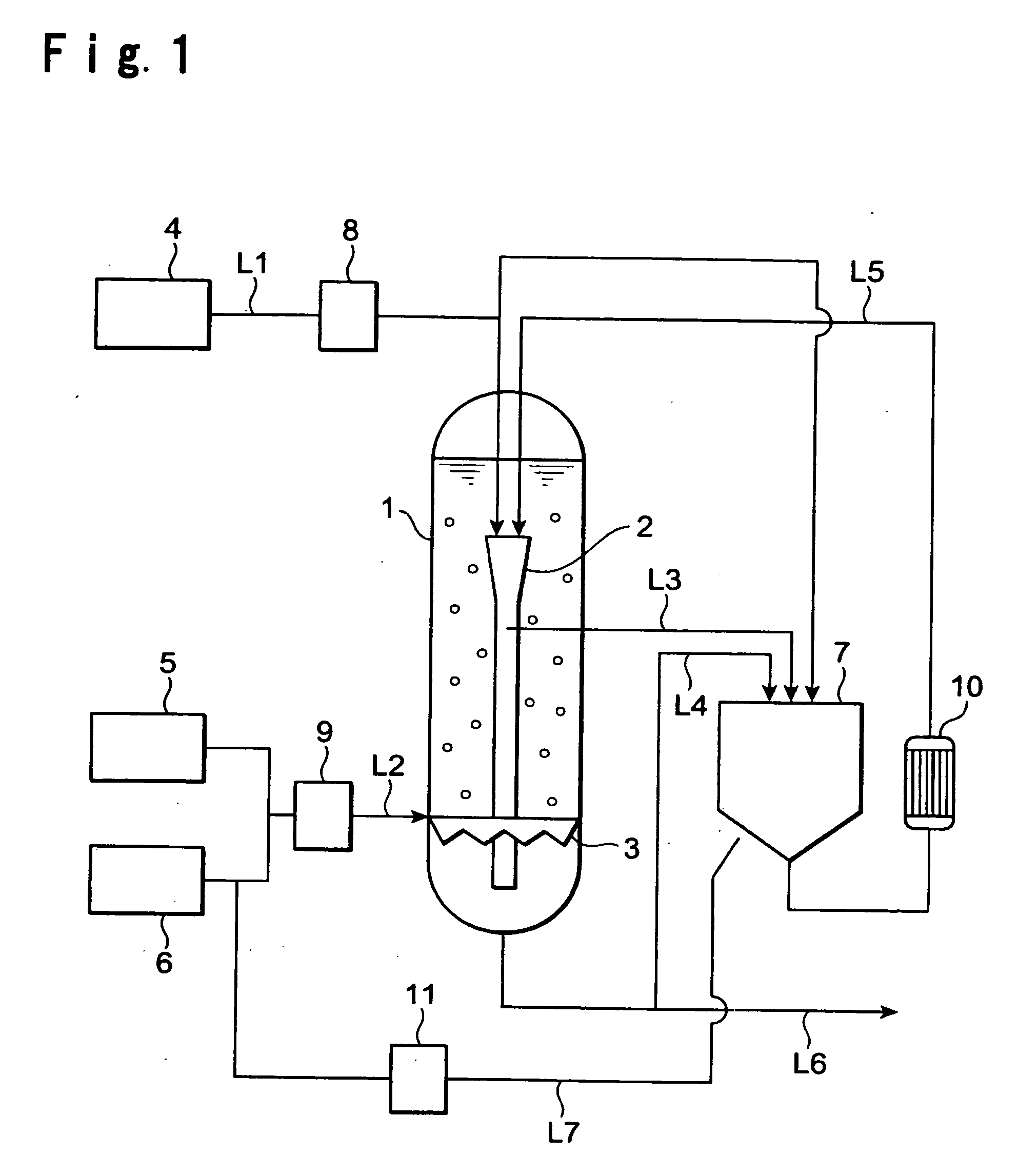
Process for producing alkali metal hydrogencarbonate
InactiveUS20060193765A1Easily and certainly control apparent numberImprove liquidityBicarbonate preparationRubidium/caesium/francium compoundsSlurryAqueous solution
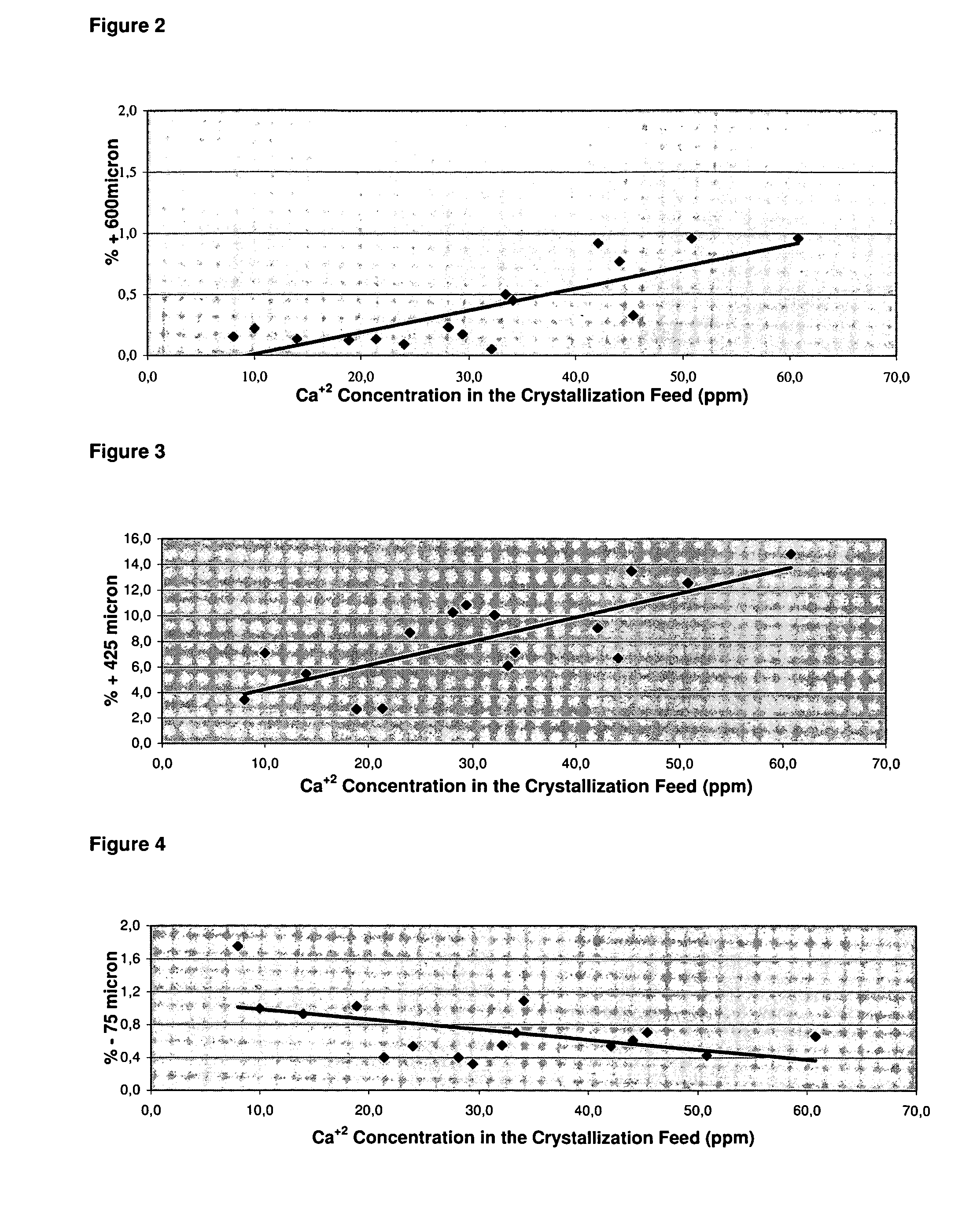
To provide a process for easily obtaining an alkali metal hydrogencarbonate having a large crystal particle size in good yield by an industrially convenient installation by a crystallization method from an aqueous solution. A process for producing an alkali metal hydrogencarbonate, which comprises reacting an aqueous solution containing alkali metal ions with carbon dioxide in a prescribed crystallizer to precipitate crystals of an alkali metal hydrogencarbonate, characterized in that a part of a slurry containing the above crystals in the above aqueous solution, is withdrawn from the above crystallizer and, after a part of the above crystals is dissolved, returned to the above crystallizer.
Owner:ASAHI GLASS CO LTD
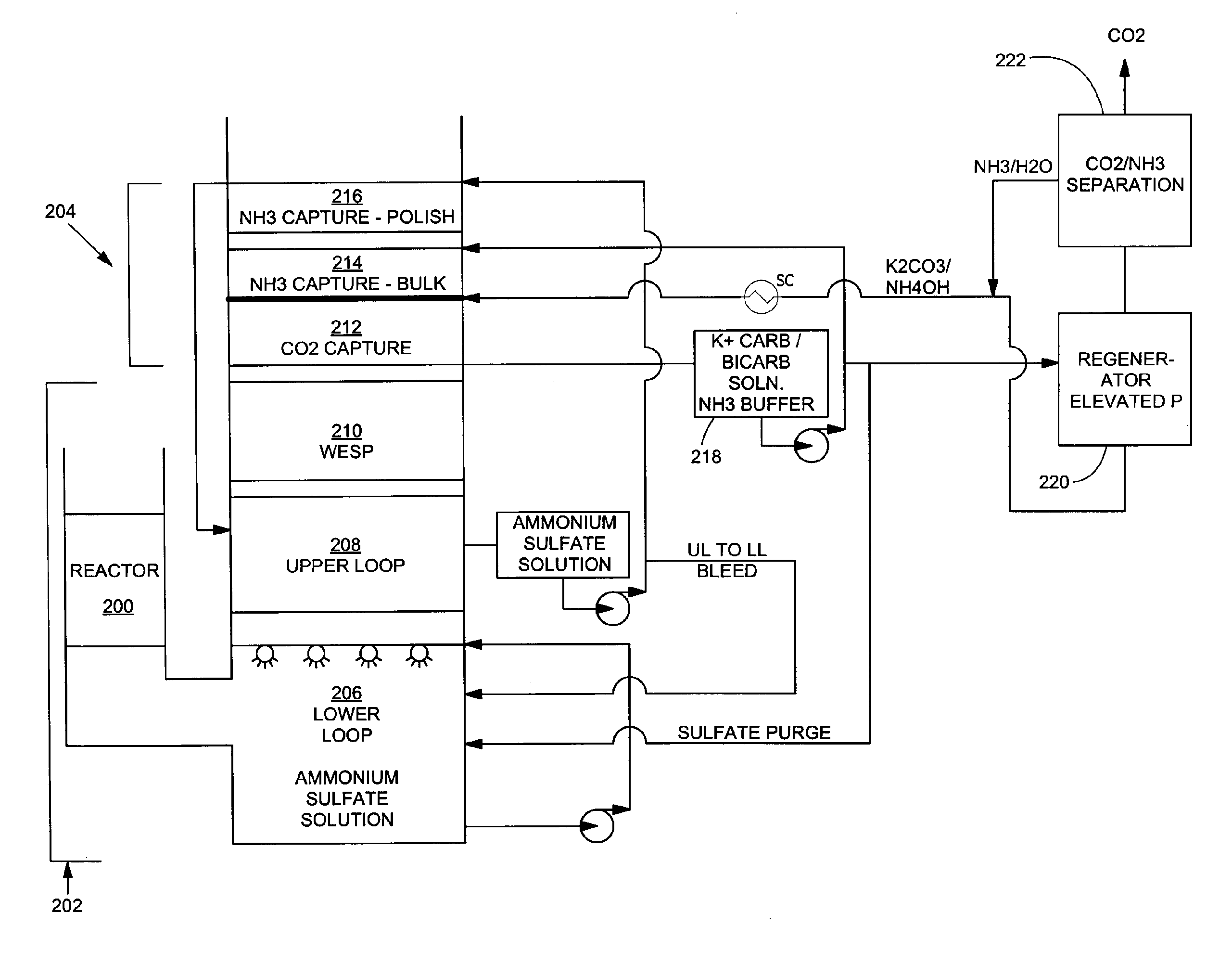
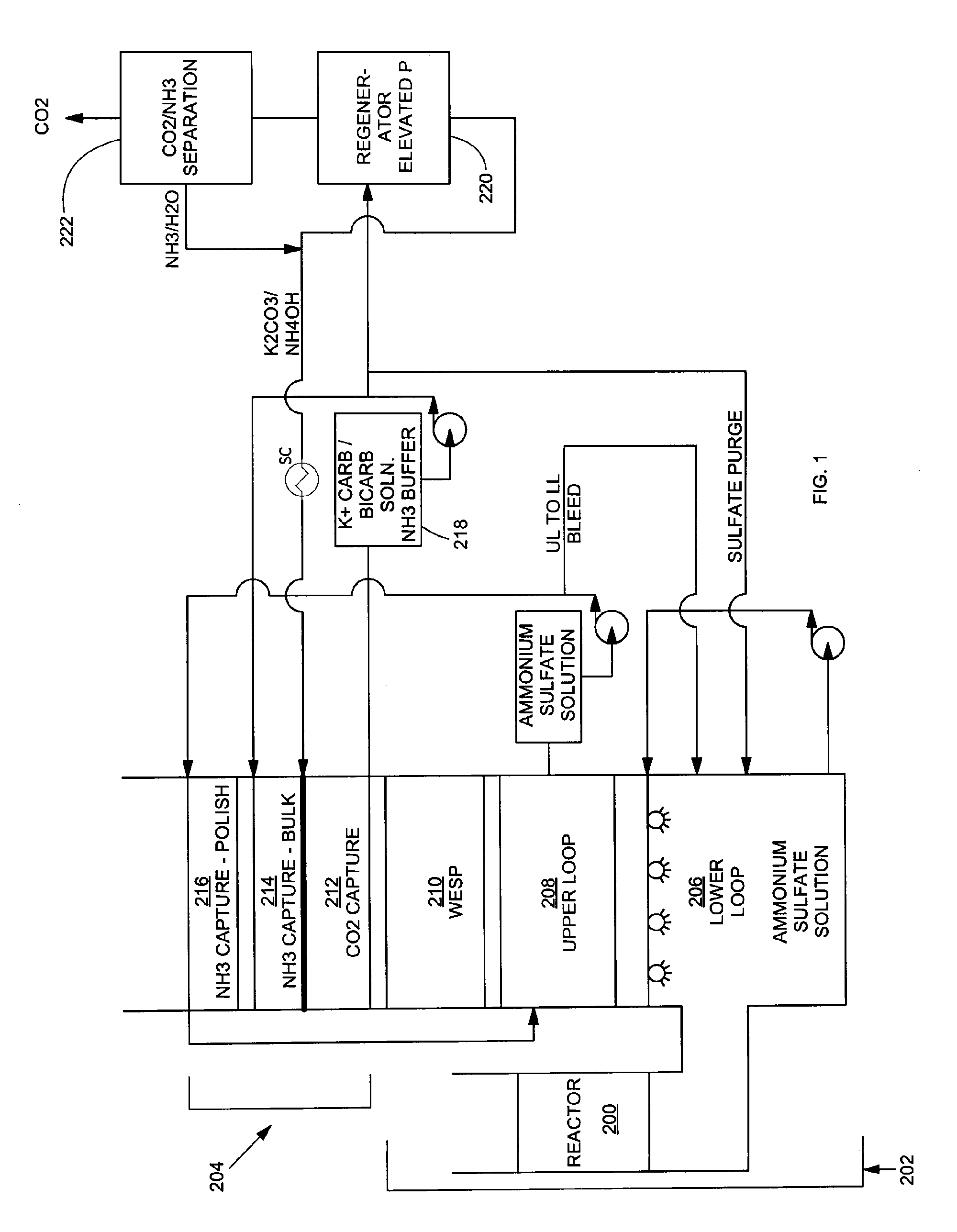
Removal of carbon dioxide from flue gas streams using mixed ammonium/alkali solutions
A process for removing carbon dioxide from a gas stream by scrubbing the carbon dioxide from the gas stream with a mixture of ammonium and alkali carbonates such as sodium carbonate and / or potassium carbonate. Using the mixed alkali carbonate solution as the CO2 scrubbing solution offers the opportunity for both low regeneration energy and low ammonia volatility while still maintaining a high rate of CO2 hydration.
Owner:POWERSPAN CORP
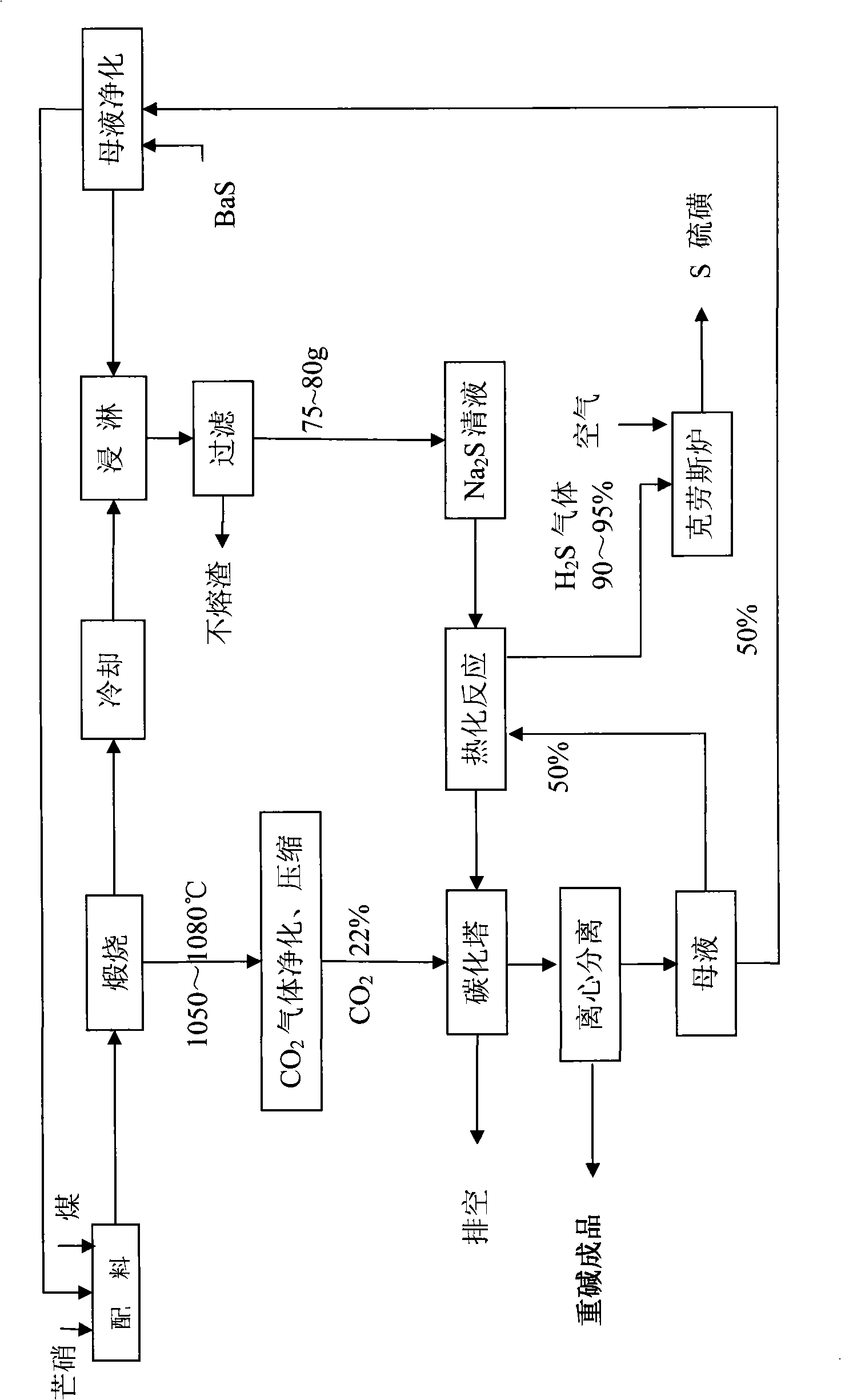
Method for producing sodium bicarbonate and sulfur from mirabilite by wet process
InactiveCN101264904AAchieving zero emissionsAchieve recyclingBicarbonate preparationSulfur preparation/purificationSodium bicarbonateCarbonization
The invention discloses a method for clean method for producing sodium bicarbonate and sulphur by means of Glauber salt wet method for reducing the emission of CO2 and avoiding the discharge of wastewater, which comprises the following steps: A, sodium sulphide preparing process, B, hot carbonatation process, C, sulphur preparing process, D, cold carbonization process, and E, mother liquor purifying. Glauber salt coal is reduced and calcined into sodium sulphide, and the sodium sulphide solution is heated and carbonized into a mixed solution with the soda as the main component by use of sodium bicarbonate, then the mixed solution is cold-carbonated by CO2 to separate out NaHCO3 crystal, which is dried to finally obtain the soda in conformity with relevant standard. The production method has the advantages of simple process, environmental friendliness, no pollution, full circulation of the mother liquor, and no wastewater discharged.
Owner:GANSU JINSHI CHEM
Efficient Production of Hydrogen
InactiveUS20090277800A1Efficient responseRaise the pHBicarbonate preparationElectrolysis componentsEnergy transferLiquid water
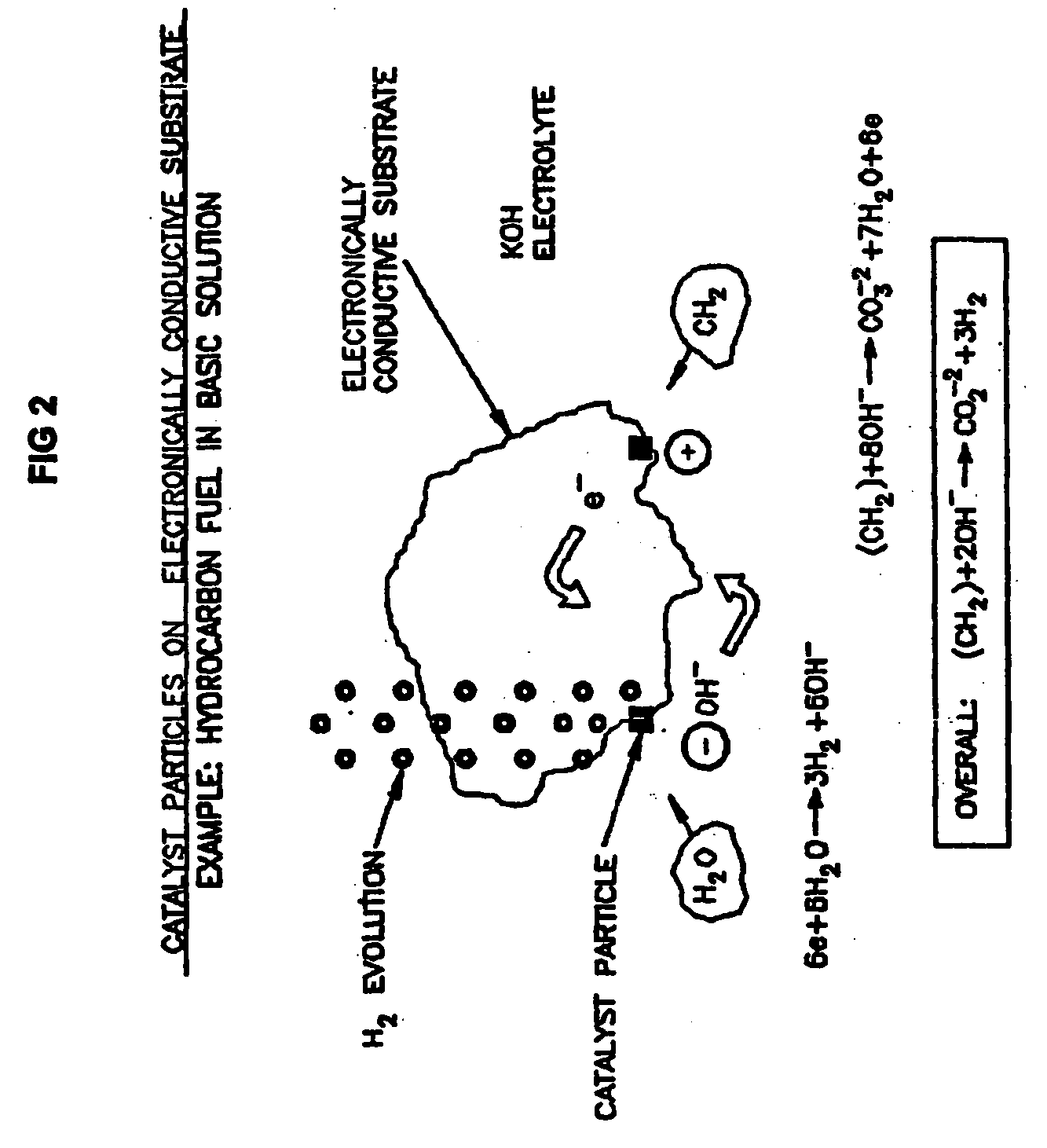
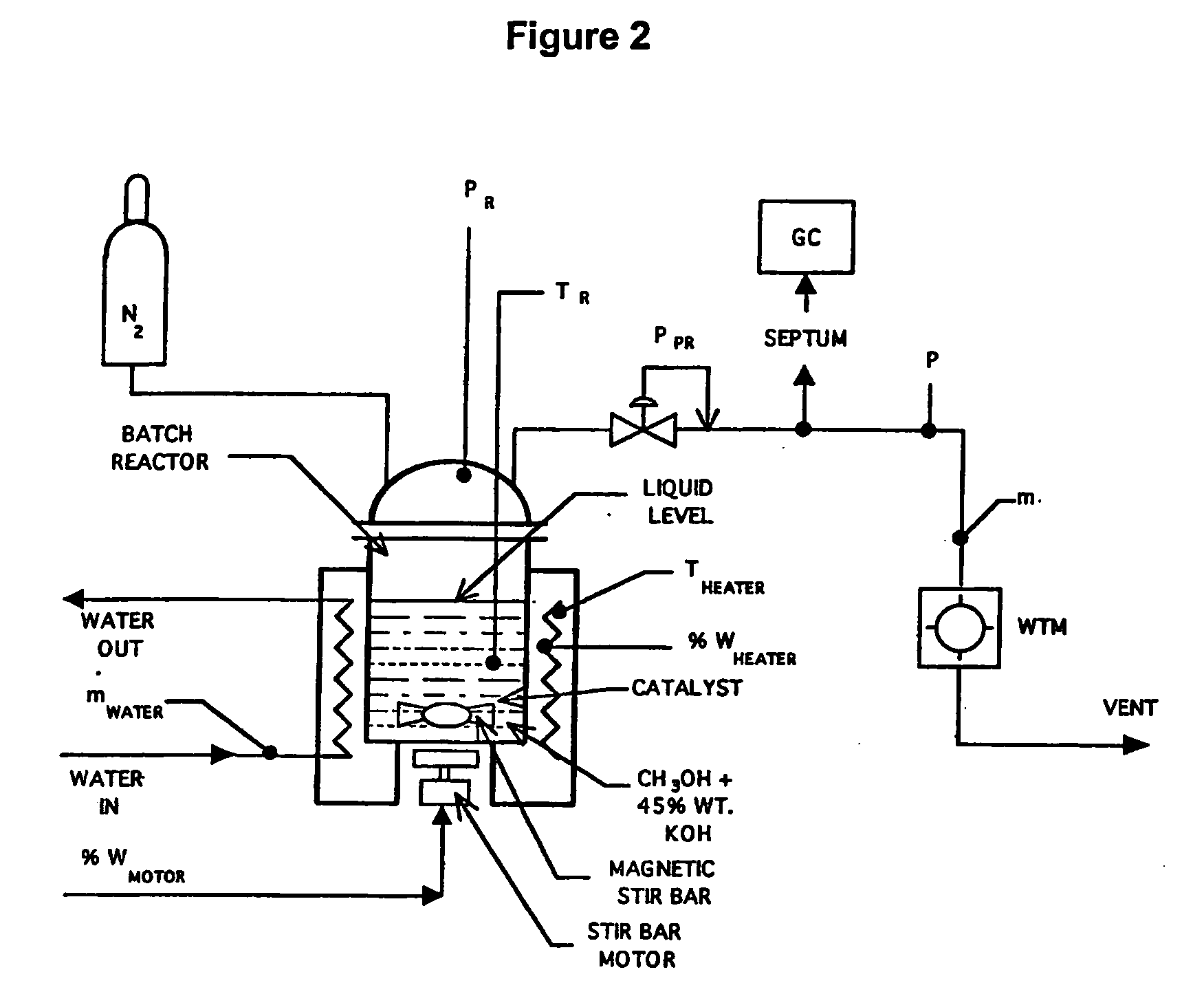
A liquid phase process for producing hydrogen gas in a reactor comprising the step of combining at least one oxidizing reactant with liquid water and at least one alkaline electrolyte to form a mixture having a pH, wherein the pH of the mixture is substantially maintained at a value of about 10.5 or greater and conducting a reaction in the presence of an electron transfer material that permits the movement of electrons. An alternative method produces hydrogen gas from a reaction in an electrochemical cell, the reaction characterized by an overall thermodynamic energy balance and half-cell reactions occurring at each of an anode and cathode. Energy transfers, such as thermal and electric, are analyzed and controlled in order to satisfy the thermodynamic energy balance of the reaction for efficient hydrogen production.
Owner:GRDC LLC
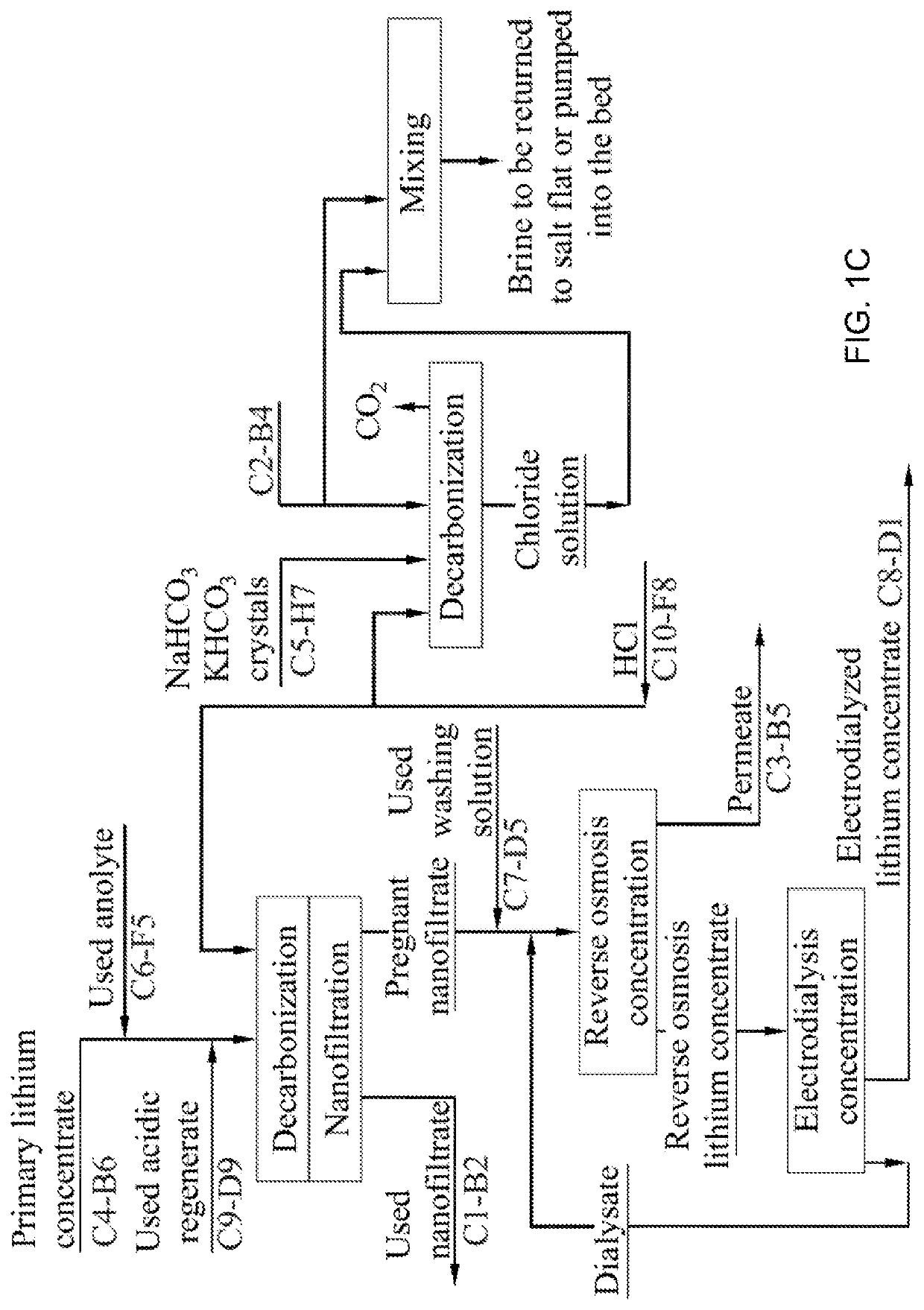
Method for producing lithium hydroxide monohydrate from brines
ActiveUS20210087697A1Reduce consumptionEliminate riskSeawater treatmentWater/sewage treatment apparatusSuspended particlesLithium chloride
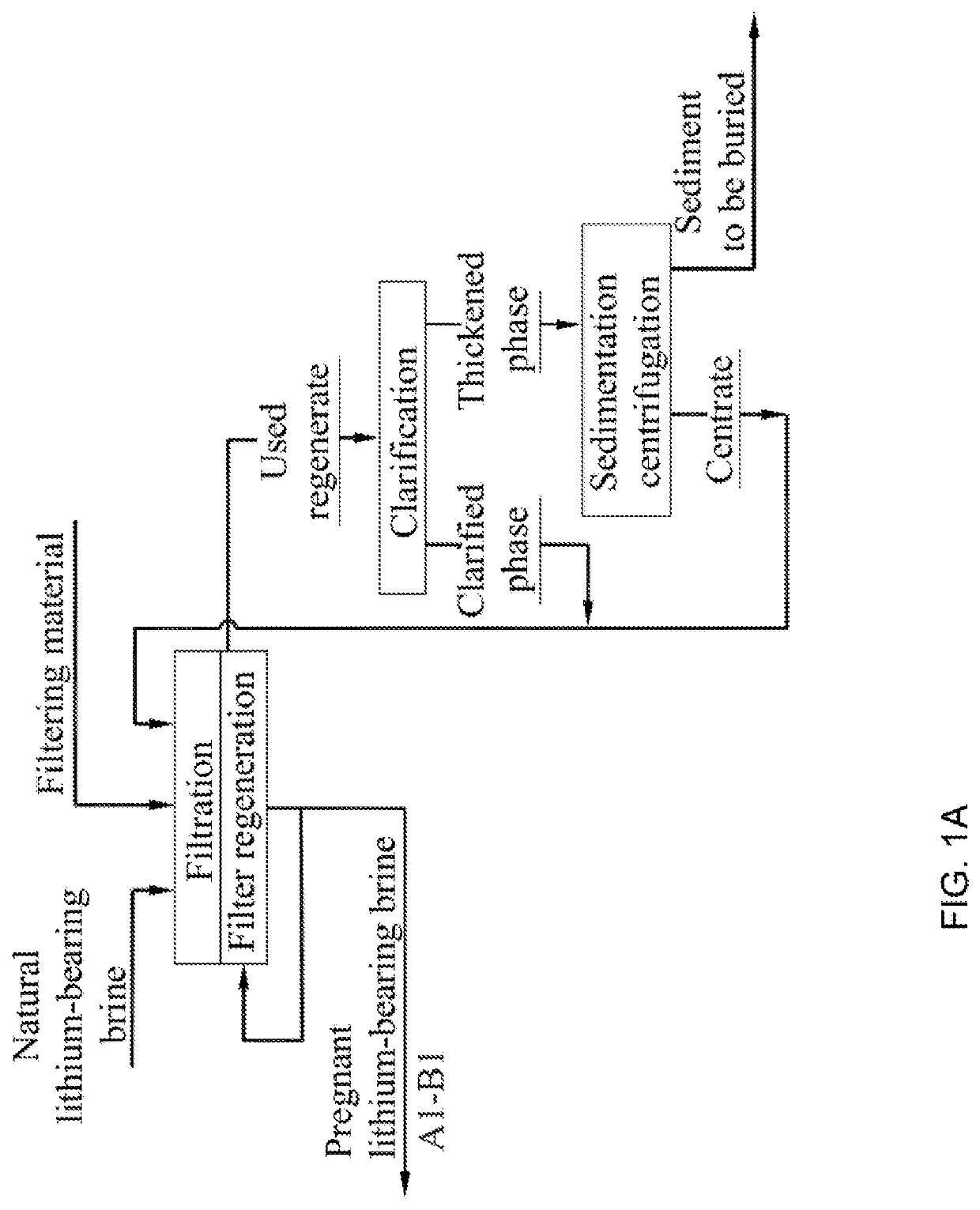
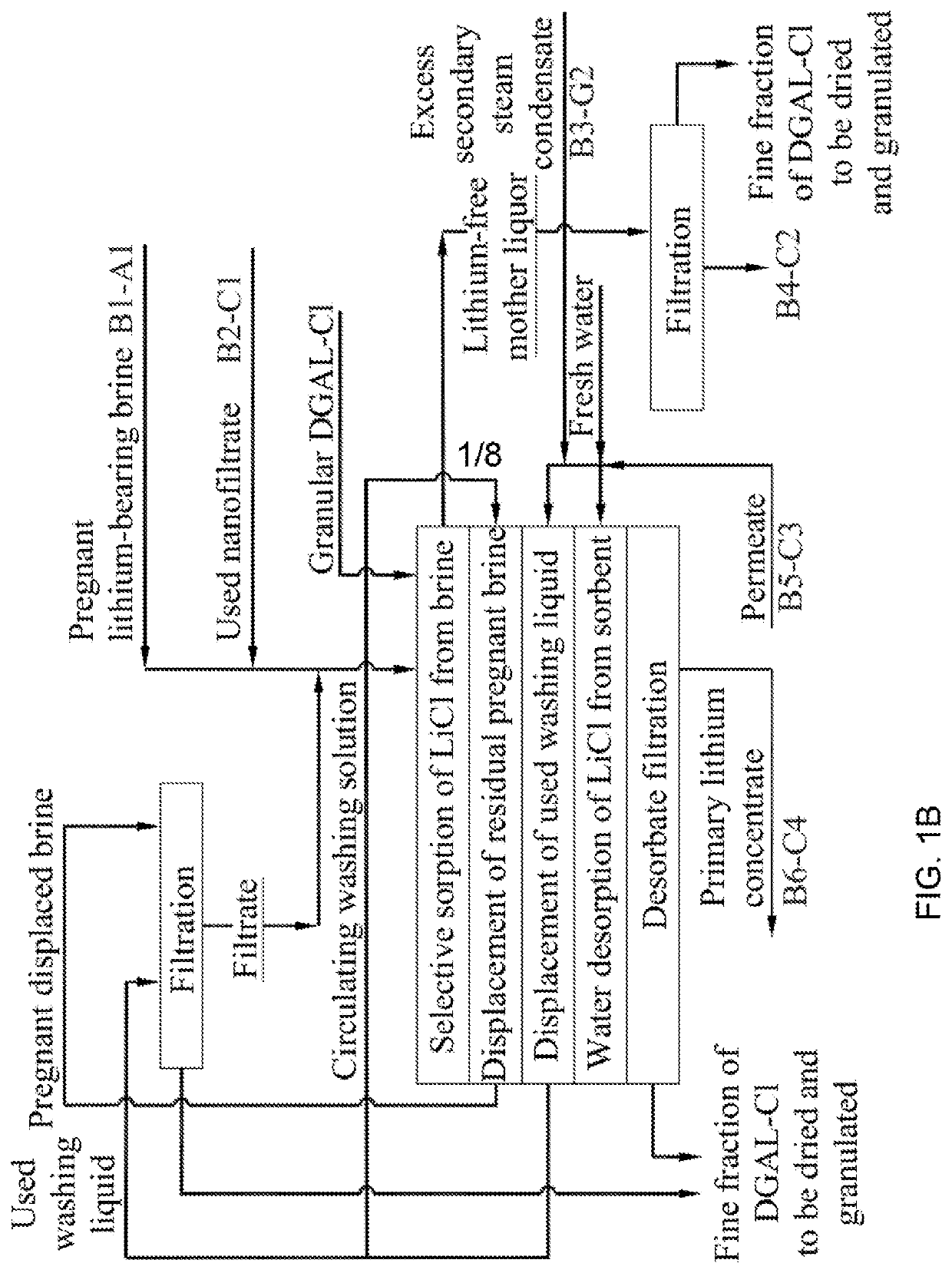
A method for LiOHH2O production from lithium-bearing multicomponent hydromineral raw materials includes filtering lithium-bearing brine contaminated with suspended particles with regeneration of filters and processing of used regenerate, and obtaining pregnant lithium-bearing brine, isolation of lithium chloride from the brine in the form of a primary concentrate in sorption-desorption modules, and nanofiltration of the primary lithium concentrate from magnesium, calcium and sulfate ions. By means of reverse osmosis, electrodialysis concentration and ion-exchange purification from impurities followed by thermal concentration, the primary lithium concentrate is converted into a pregnant lithium chloride concentrate which is converted into a LiOH solution by membrane electrolysis. The LiOH solution is boiled down, resulting in LiOH.H2O crystallization.
Owner:ECOSTAR NAUTECH CO LTD
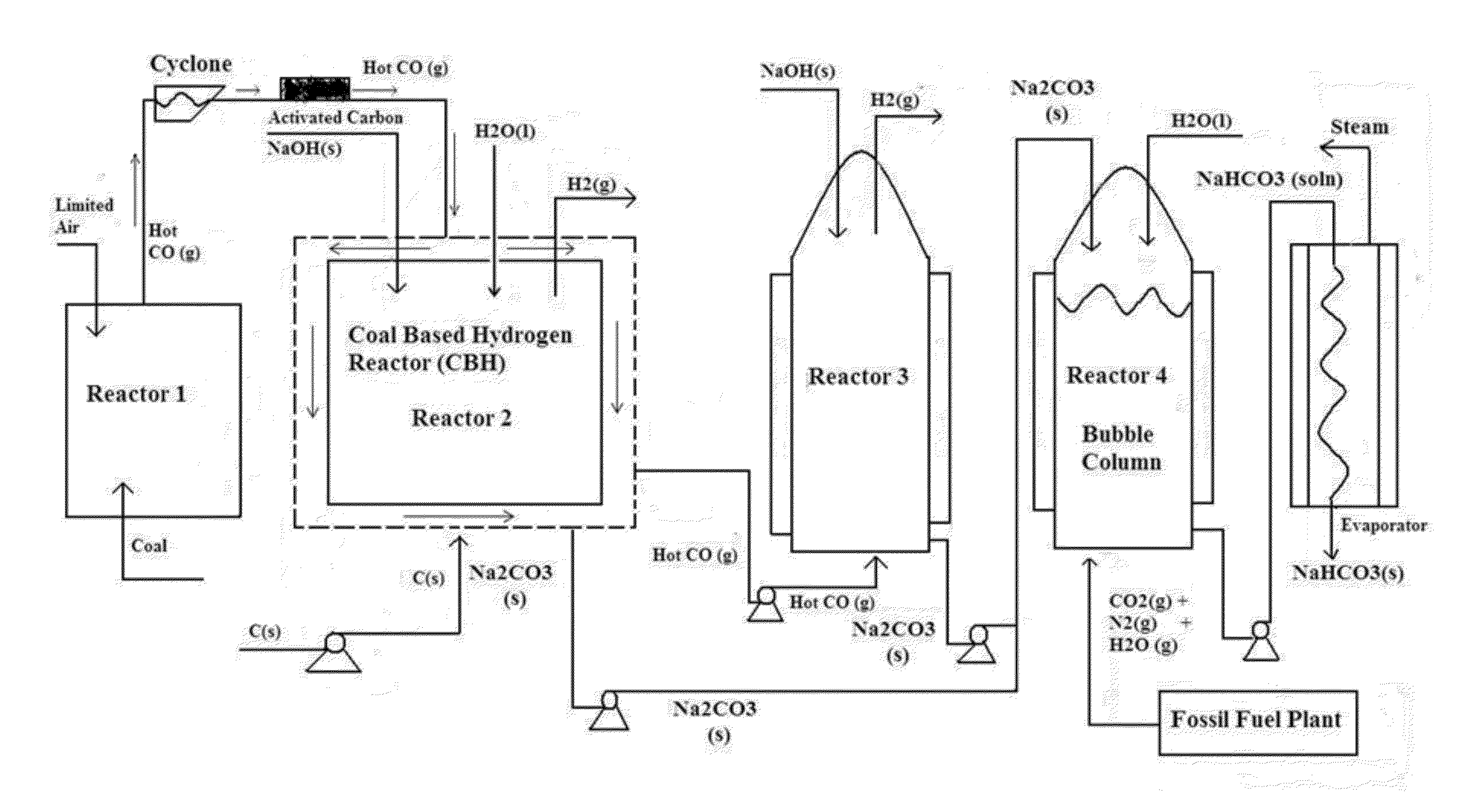
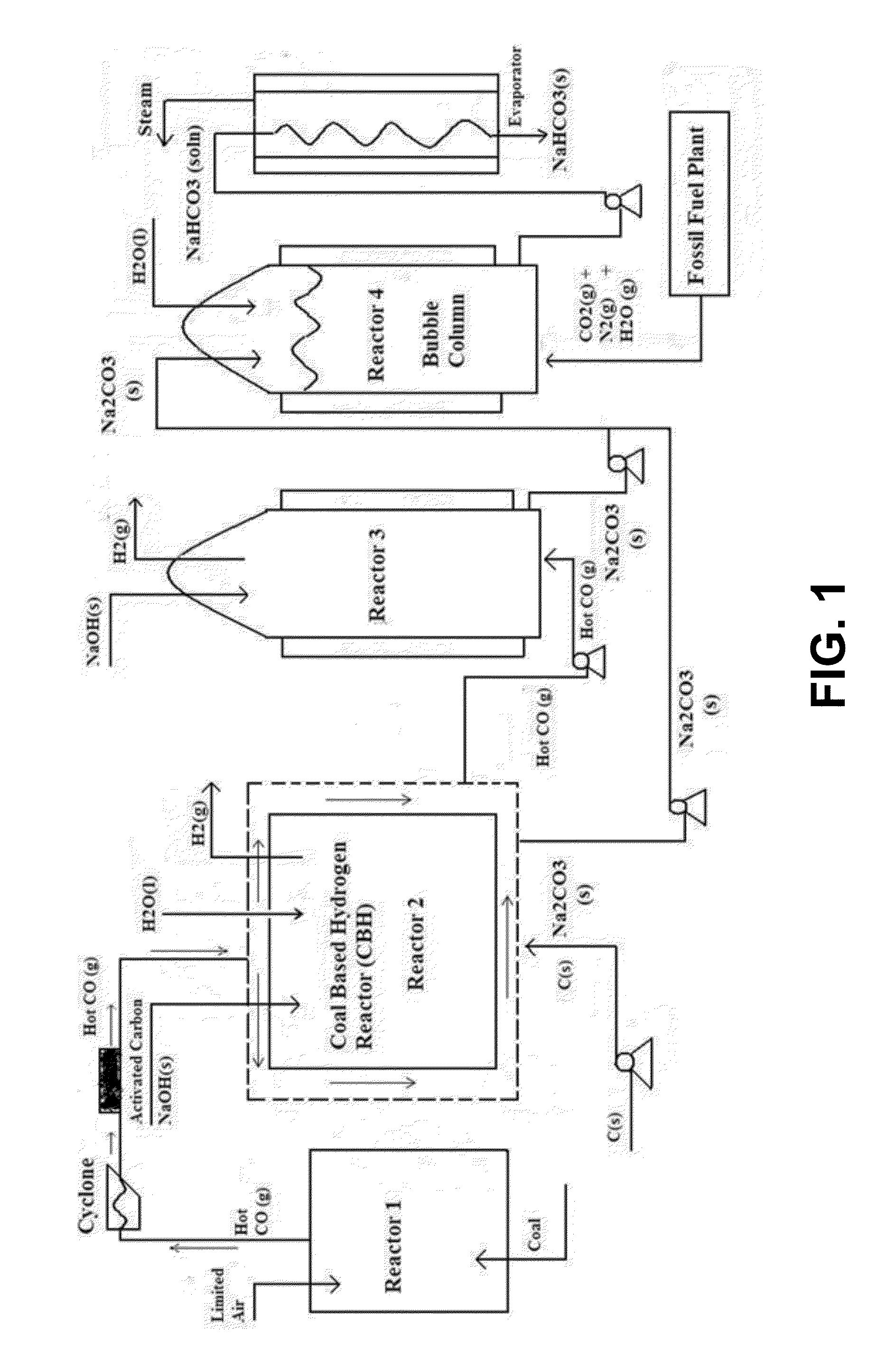
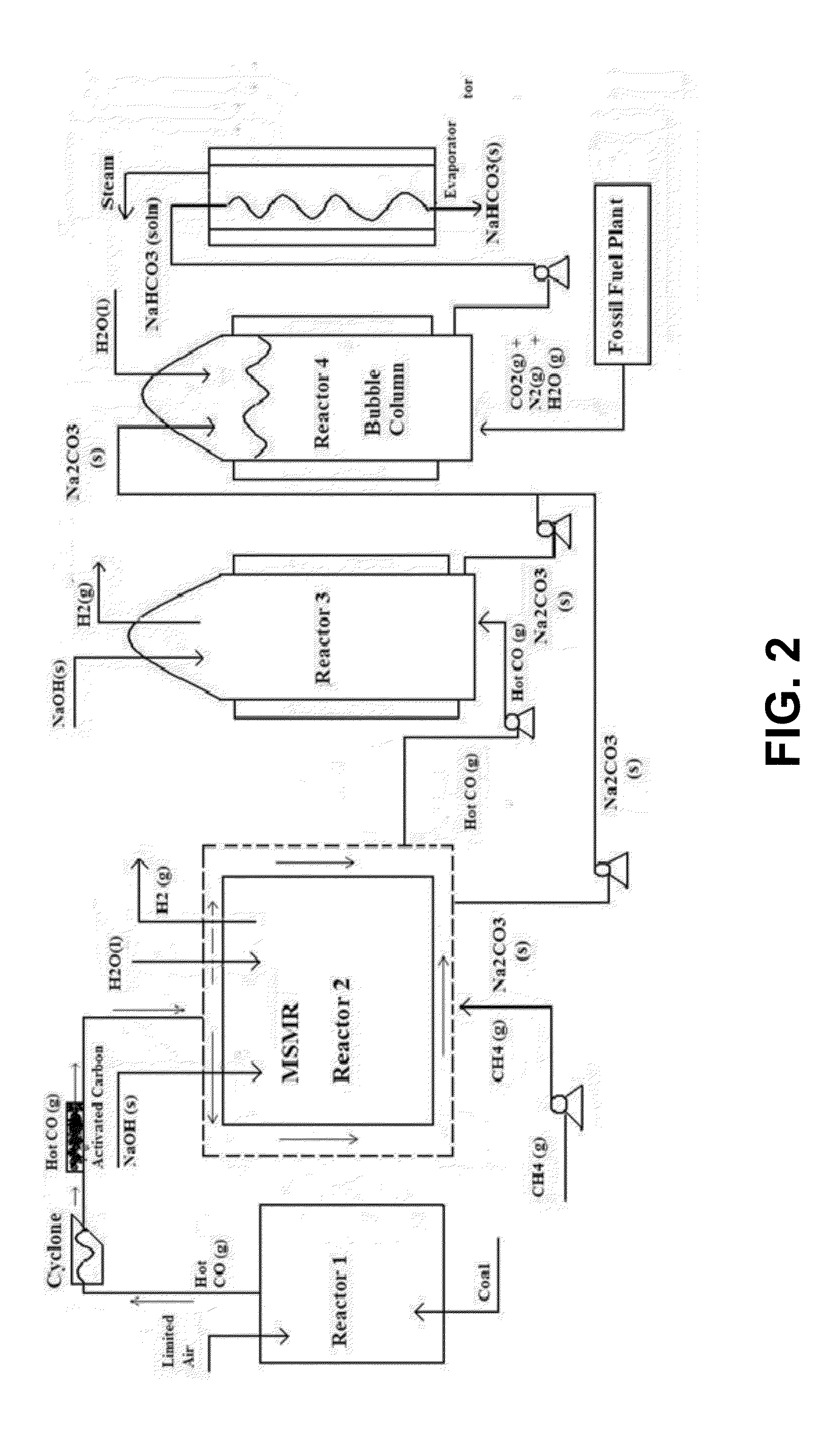
Carbon Sequestration in Municipal Solid Waste to Energy Plants
InactiveUS20130019785A1Limited applicationReduce carbon emissionsDirect carbon-dioxide mitigationHydrogen/synthetic gas productionRefuse-derived fuelExhaust fumes
This invention describes a process for a complete sequestration of carbon (CO2) from Municipal Solid Waste (MSW) to energy plants which produce Refuse Derived Fuel and the associated exhaust gases. The described process results in production of energy from the waste and disposal of the MSW with zero carbon emission.
Owner:SAXENA SURENDRA
Method for preparing medicinal sterile sodium bicarbonate raw powder
InactiveCN101613119AQuality assuranceGuaranteed outputBicarbonate preparationSodium bicarbonateFiltration
The invention relates to a method for preparing medicinal sterile sodium bicarbonate raw powder, which comprises the following steps: (1) subjecting industrial sodium carbonate serving as a raw material to purification, dissolution in water, decarburization and membrane filtration; (2) introducing industrial carbon dioxide gas subjected to membrane treatment into solution of sterile sodium bicarbonate to carry out acidification, and controlling parameters such as flow rate, reaction time, terminal pH value and stirring speed; (3) adding ethanol liquid subjected to membrane treatment into solution in which reaction is finished to allow sodium bicarbonate crystals to precipitate gradually, adjusting stirring speed, and controlling crystallization time to allow the crystallization to be completed fully; and (4) filtering the solution by a crystal filter to obtain the product, and drying the product by using a vibratory drying machine to obtain a finished product that meets medicinal standards.
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY
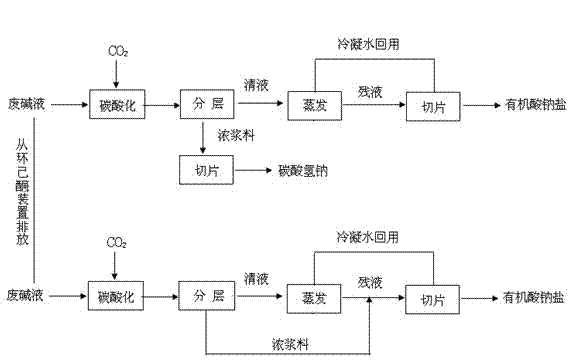
Method for recovering sodium bicarbonate and organic acid sodium salt from cyclohexanone waste alkali solution
InactiveCN102874848AAchieve emissionsShort processBicarbonate preparationCarboxylic acid salt preparationSodium bicarbonateCyclohexanone
The invention provides a method for recovering sodium bicarbonate and organic acid sodium salt from a cyclohexanone waste alkali solution. The method comprises the following steps of: performing gas-liquid contact carbonation reaction on CO2 and the waste alkali solution, precipitating and demixing to obtain clear liquid and concentrated sodium bicarbonate slurry; concentrating and sectioning the concentrated slurry to obtain a crude sodium bicarbonate; and evaporating, concentrating and sectioning the clear liquid to obtain crude organic acid sodium salt, or stirring and mixing raffinate obtained after the clear liquid is evaporated and the concentrated slurry, concentrating and sectioning to obtain the crude organic acid sodium salt. By the method, available resources in the cyclohexanone waste alkali solution are recovered, so that inorganic or organic chemical products such as the sodium bicarbonate and the organic acid sodium salt are obtained, and secondary pollutants are not discharged in the recovering process. Compared with the prior art, the method has the advantages that the whole technological process is short and low in energy consumption, equipment investment and running cost, the added value of the obtained recycled products is high, and social and economic benefits are obvious.
Owner:赵志军
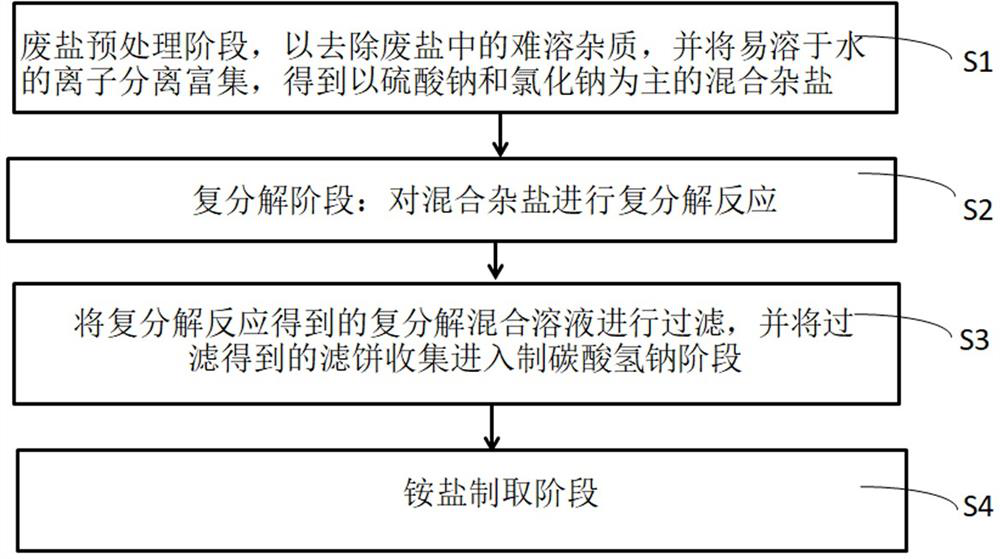
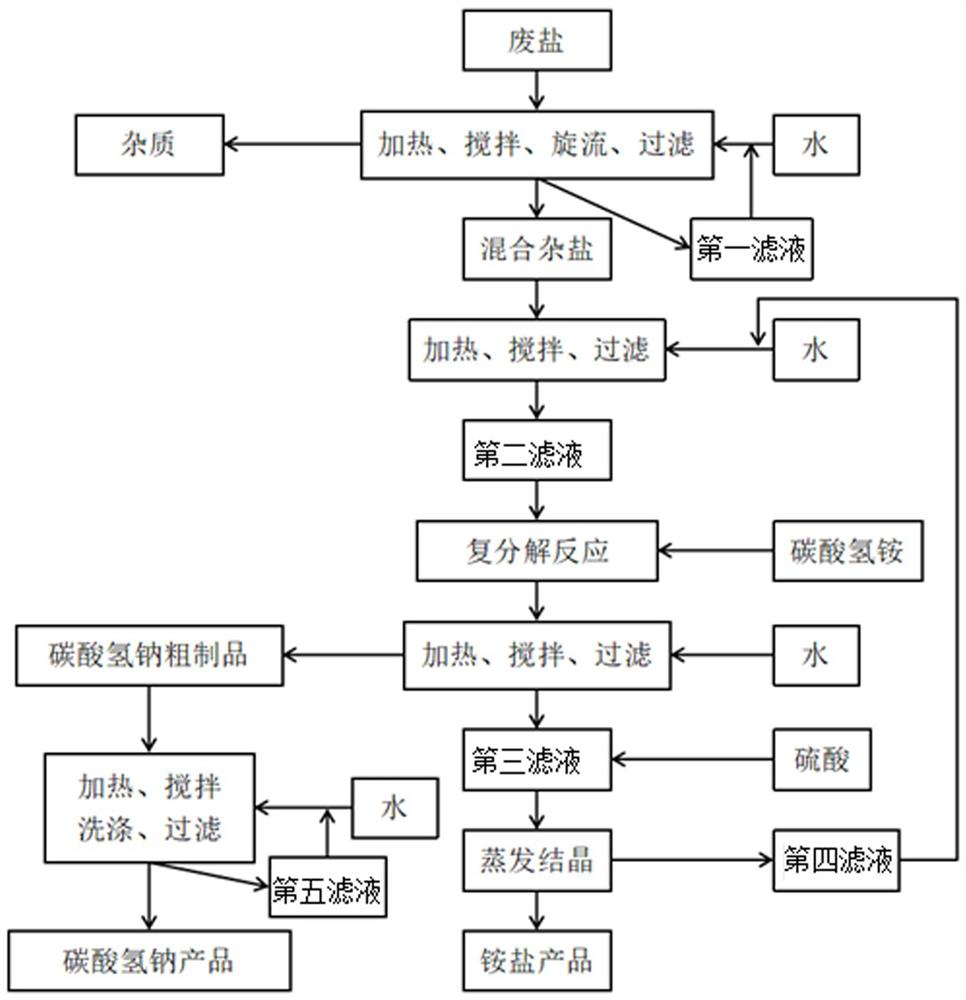
Resourceful treatment method of waste salt
ActiveCN113336246APromote resource utilizationThe method flow is simpleBicarbonate preparationAmmonium fluorideSodium bicarbonateEnvironmental engineering
The invention discloses a resourceful treatment method of waste salt. The method comprises the following steps: S1, a waste salt pretreatment stage: removing insoluble impurities in waste salt, and separating and enriching water-soluble ions to obtain mixed hybrid salt mainly comprising sodium sulfate and sodium chloride; S2, a double decomposition stage: carrying out double decomposition reaction on the mixed hybrid salt; S3, filtering a metathesis mixed solution obtained by the metathesis reaction, and collecting filter cake obtained by filtering to enter a sodium bicarbonate preparation stage; and S4, an ammonium salt preparation stage. The waste salt mainly containing sodium chloride and sodium sulfate is subjected to resourceful treatment, industrial sodium bicarbonate and ammonium salt products are obtained, so that resourceful treatment of the waste salt is greatly improved. The method is simple in process and low in cost, the utilization rate of the waste salt is 84%-89%, the purity of the prepared product sodium bicarbonate is 94%-96%, and the nitrogen content (on a dry basis) in the ammonium salt is greater than or equal to 23.8%.
Owner:BEIJING GUODIAN LONGYUAN ENVIRONMENTAL ENG
Dry sorbent injection (DSI) recovery system and method thereof
InactiveUS20140205521A1Reduce operating costsLow sodiumProductsGas treatmentSodium bicarbonateSorbent
The invention generally relates to system and method for recovering sodium bicarbonate from a solid waste, and more particularly to a method and system for recovering sodium bicarbonate from fly ash of a coal fired plant collected downstream of an injection process for pollution reduction from the industrial process.
Owner:NEUMANN SYST GROUP
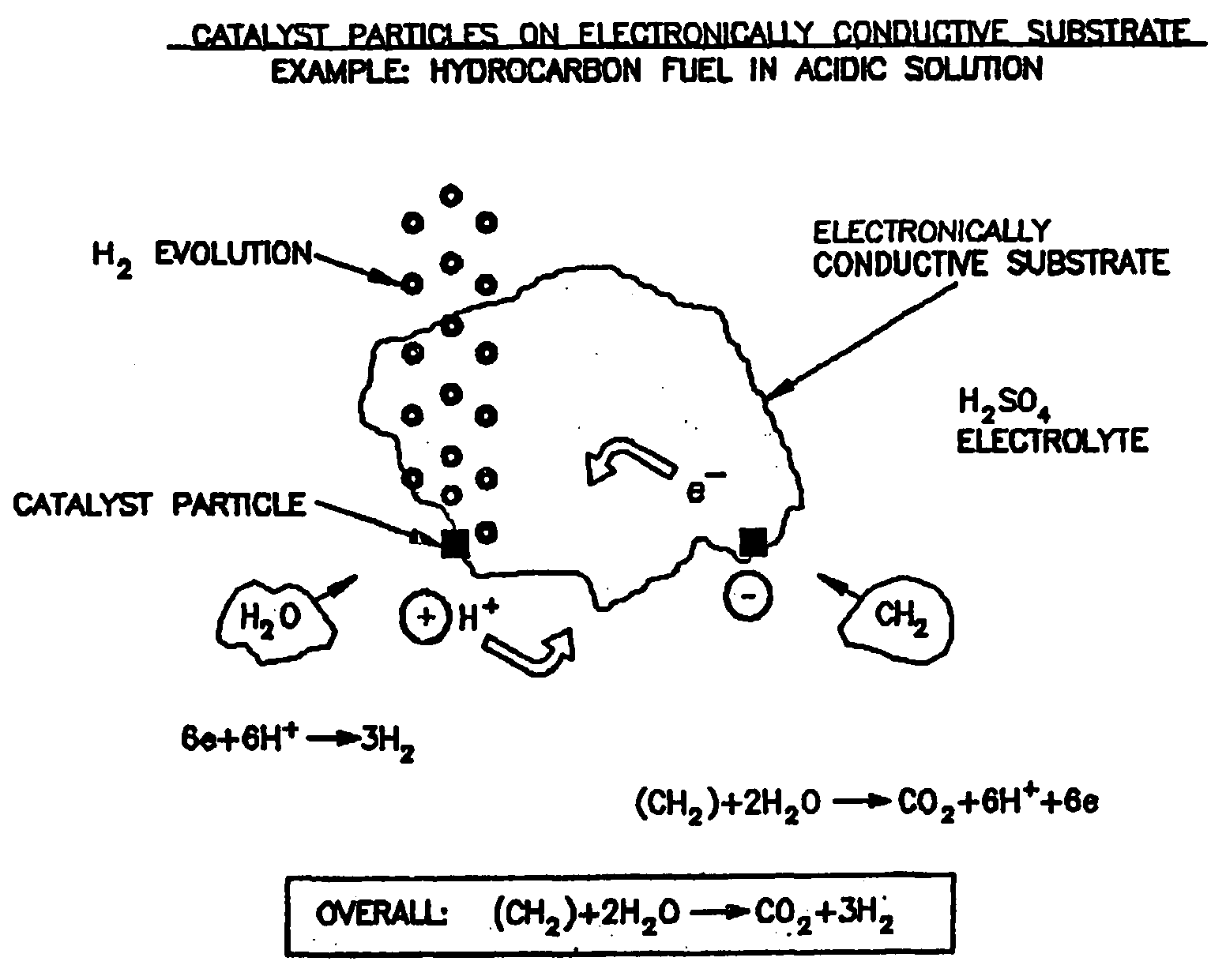
Hydrogen Production Using Electrochemical Reforming and Electrolyte Regeneration
ActiveUS20090266717A1Effective and economical regenerationLower in carbon monoxide and carbon dioxideBicarbonate preparationElectrolysis componentsDicarbonateStream flow
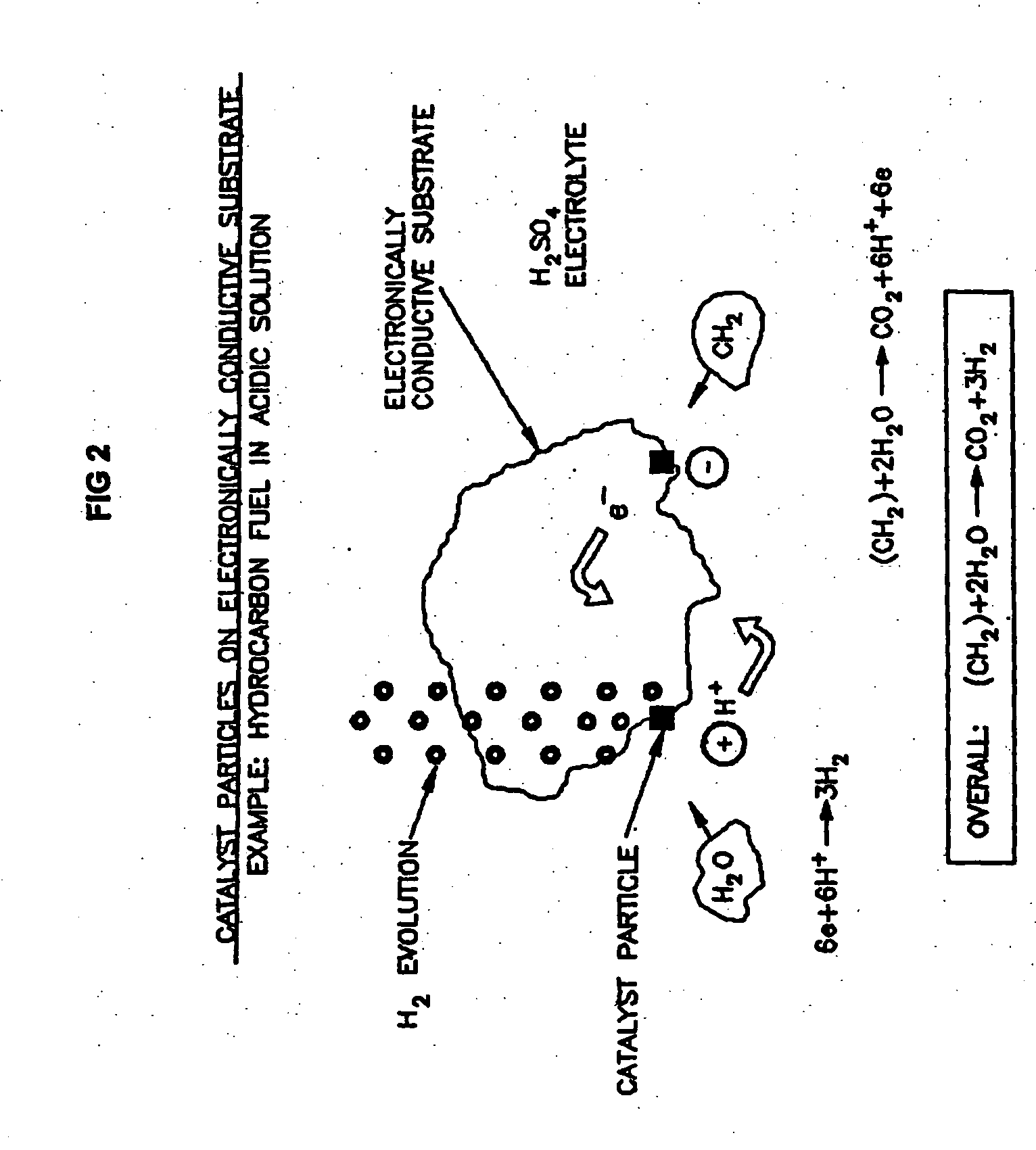
A process, preferably continuous, for producing hydrogen gas comprising contacting in the liquid phase at least one oxidizable organic substance in the presence of a mixture comprising at least one conductive catalyst and an aqueous alkaline carbonate electrolyte, wherein at least one bicarbonate composition produced by reaction of the electrolyte is regenerated and the at least one oxidizable organic substance comprises a oxygenated hydrocarbon, for example methanol and / or dimethyl ether. In a preferred embodiment the alkaline electrolyte is regenerated using steam. Various advantageous reaction schemes are described, utilizing, e.g., co-current and countercurrent stream flow and alternative tower sequence arrangements.
Owner:CONOCOPHILLIPS ENERGY TECH +1
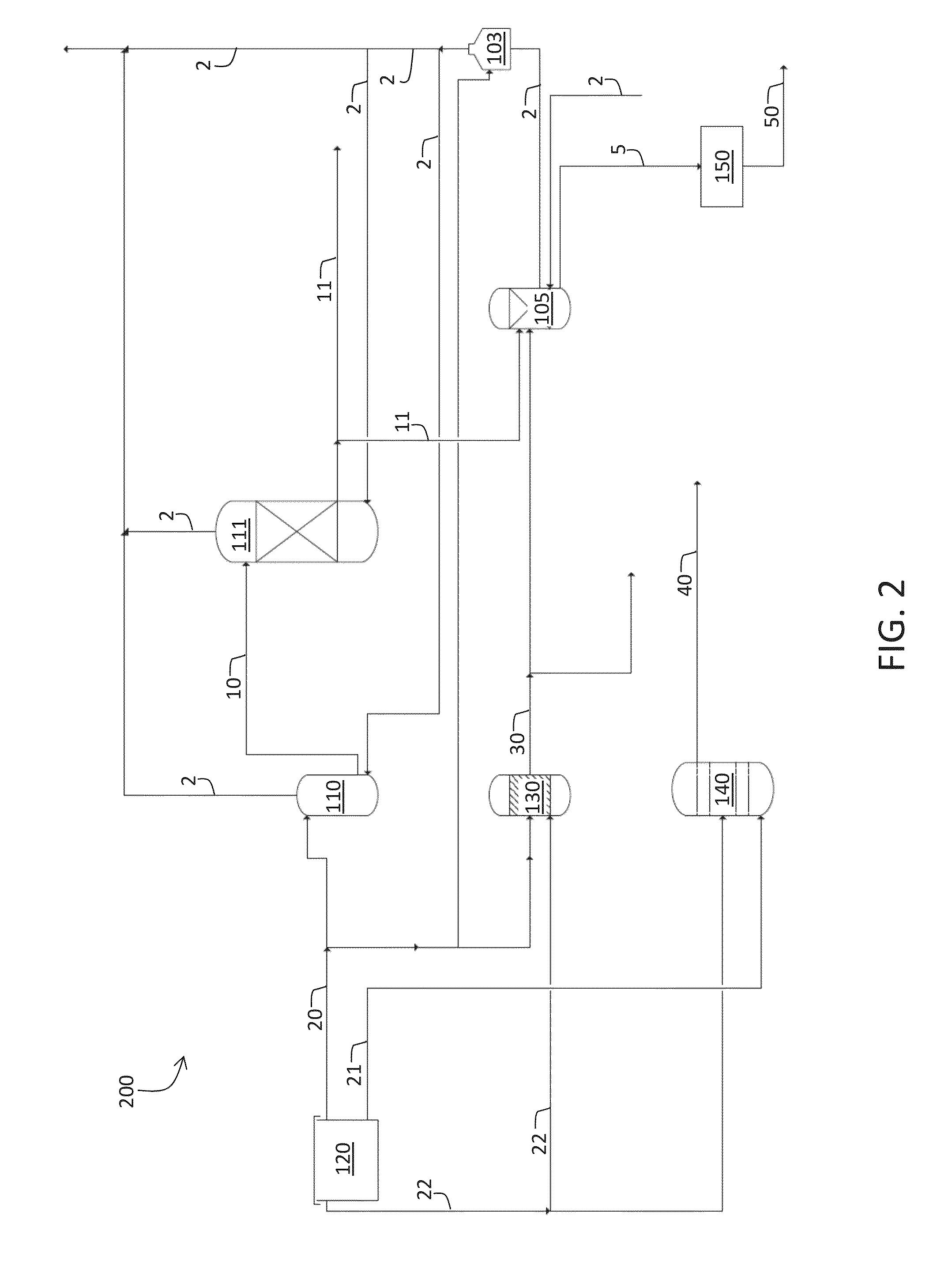
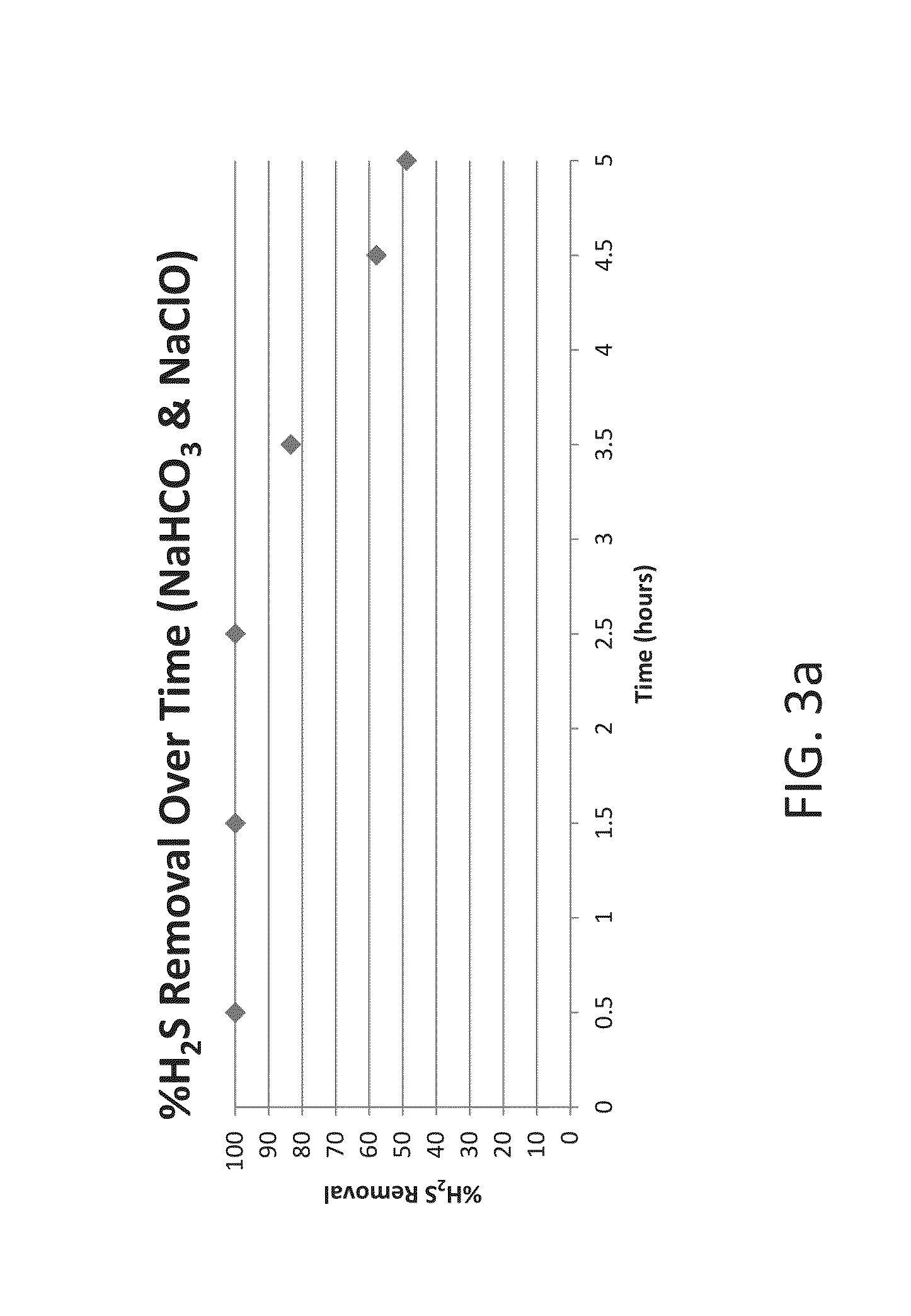
Systems and methods for acid gas removal from a gaseous stream
Apparatuses, systems, and methods for removing acid gases from a gas stream are provided. Gas streams include waste gas streams or natural gas streams. The methods include obtaining a hypochlorite and a carbonate or bicarbonate in an aqueous mixture, and mixing the aqueous mixture with the gas stream to produce sulfates or nitrates from sulfur-based and nitrogen-based acidic gases. Some embodiments of the present disclosure are directed to produce the carbonate and / or bicarbonate scrubbing reagent from CO2 in the gas stream. Still others are disclosed.
Owner:CARBONFREE CHEM HLDG LLC
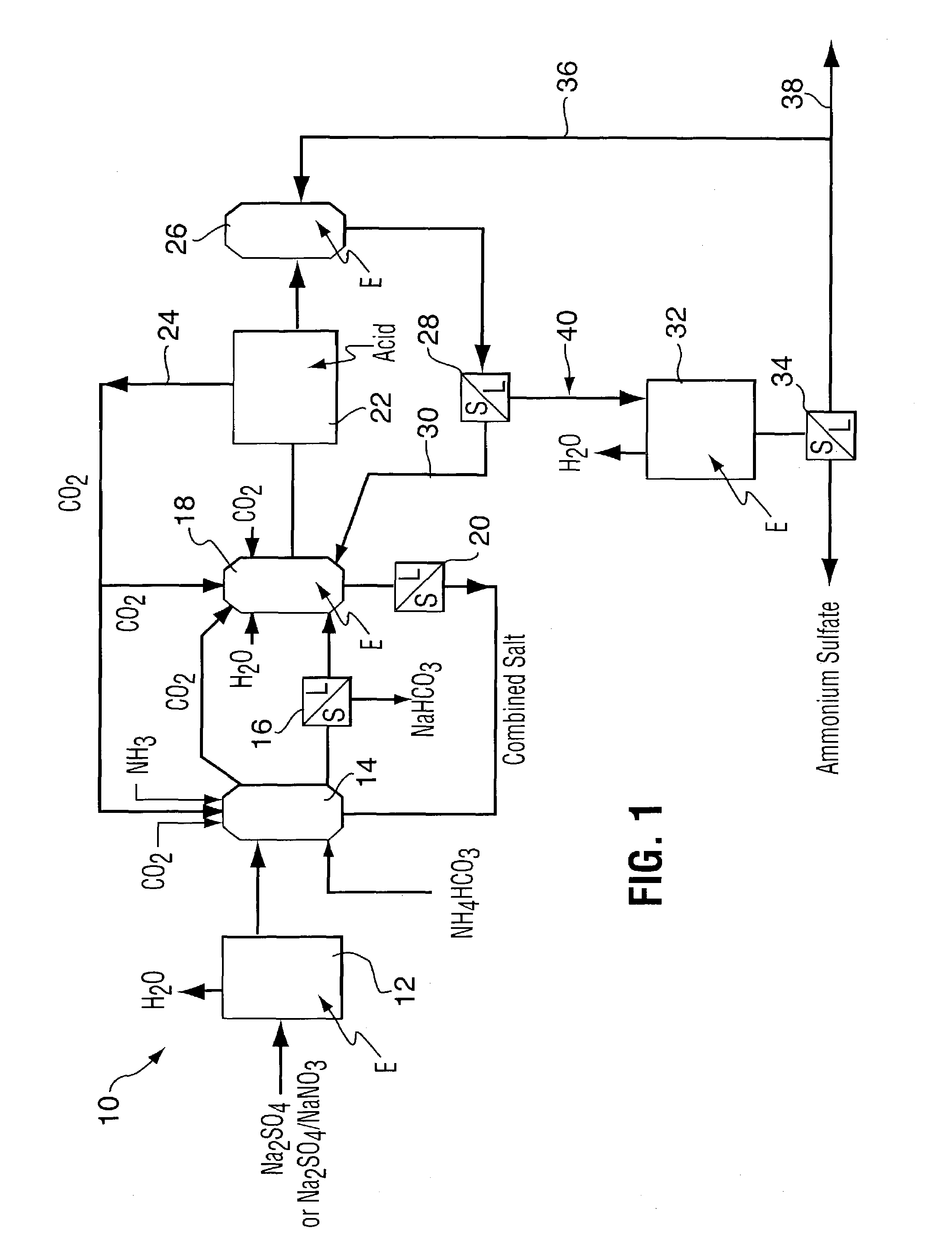
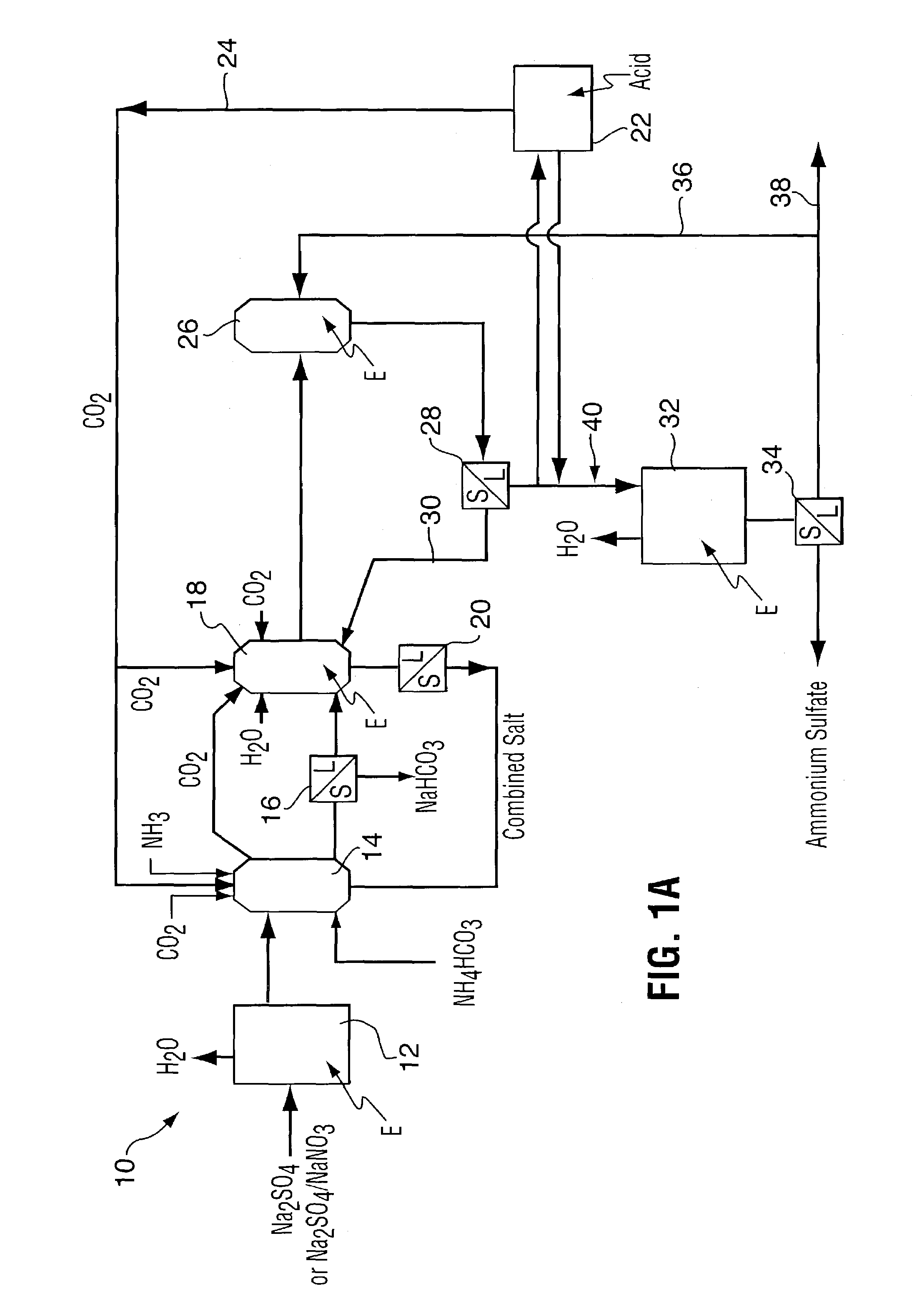
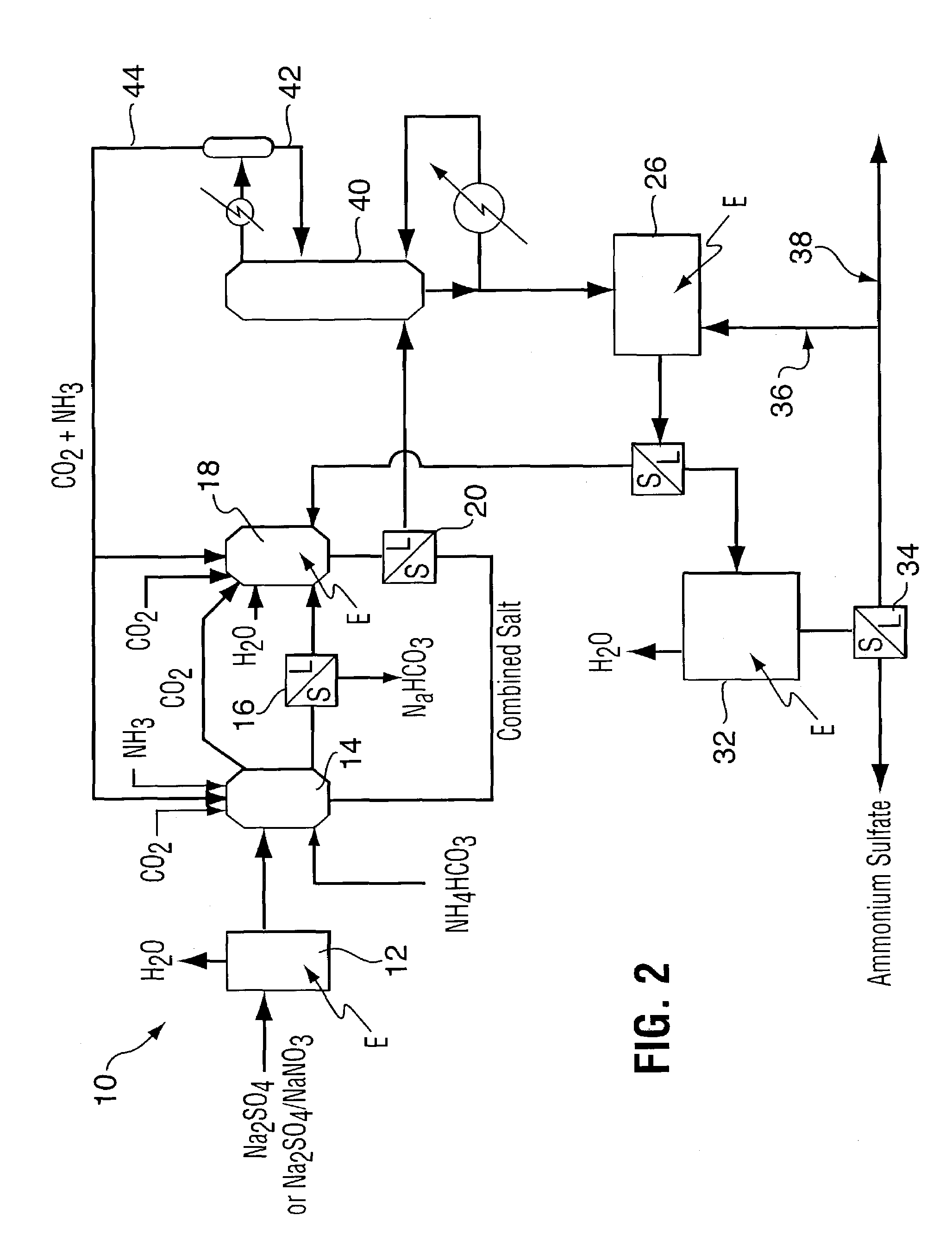
Method for recovering purified sodium bicarbonate and ammonium sulfate
A process for recovering sodium bicarbonate and ammonium sulfate from a sodium sulfate solution. The sodium sulfate solution can be pure or contain other compounds such as sodium sulfite, carbonate, chloride, fluoride, nitrate and nitrite as would be the case if the sodium sulfate solution were derived from a sodium bicarbonate flue gas purification process. Carbon dioxide and ammonia gases or solid ammonium bicarbonate are added to the sodium sulfate solution to precipitate sodium bicarbonate which is removed from solution. The remaining solution is treated in a unique series of precipitation steps in which reactants are first recycled back to the sodium bicarbonate crystallizer and then the amount of sodium in the solution is adjusted to an amount that allows high grade ammonium sulfate fertilizer product to be produced. The process is accomplished using evaporation and precipitation unit operations in a unique sequence that results in 100% conversion of the sodium salt feed stock to sodium bicarbonate and ammonium sulfate in a commercially viable manner.
Owner:AIRBORNE IND MINERALS INC CA
Energy-saving using method for circular batching of carbon mother liquor
ActiveCN101683995AReduce moistureLow costBicarbonate preparationAlkali-metal aluminates/aluminium-oxide/aluminium-hydroxide preparationHigh concentrationSodium bicarbonate
The invention relates to an energy-saving using method for circular batching of carbon mother liquor, which belongs to the technology of alumina production. The method is characterized by comprising the following steps: (1) introducing high concentration carbon mother liquor into a carbonizer to perform carbonization, separating out sodium hydrogen carbonate crystal after further reaction of the sodium carbonate, carbon dioxide and water so as to form sodium hydrogen carbonate slurry; and (2) after sedimentation and thickening, filtering and separating the sodium hydrogen carbonate slurry by using a vacuum filter to prepare solid sodium hydrogen carbonate and filtrate, returning the sodium hydrogen carbonate crystal to a batching system for batching; performing wet decomposition on the filtrate, converting the sodium hydrogen carbonate in solution into sodium carbonate through heat decomposition, evaporating the sodium carbonate, and using the evaporated mother liquor for circular batching. The method effectively reduces the moisture of raw slurry of a clinker calcining kiln, has good energy-saving effect, low cost, is simple and practical, and is easy to implement.
Owner:山东铝业有限公司
Method and device for producing baking soda
ActiveCN108439434AImprove production rateReduce solubilityProductsBicarbonate preparationSodium bicarbonateCooking & baking
The invention provides a method and a device for producing baking soda, wherein sodium bicarbonate is added to a dissolving tank to prepare raw material slurry containing a sodium bicarbonate solid, the raw material slurry enters an ammonia still, high temperature solution A1 containing high-concentration sodium bicarbonate and low-concentration sodium carbonate flowing out of the ammonia still fully enters a mixer, and is mixed with low temperature solution A2 containing low concentration sodium bicarbonate in the mixer, a clear liquid at the top of the mixer enters a cooler or a flasher to cool down to obtain the low temperature solution A2, and the low temperature solution (namely A2) enters the mixer; bottom crystal slurry of the mixer is subjected to solid-liquid separation to obtainsolid sodium bicarbonate crystal and filtrate; the solid sodium bicarbonate crystal is sent to a drying process to obtain the target product baking soda; the filtrate is sent to a filtrate barrel, thefiltrate of the filtrate barrel is sent to a carbonization tower, the sodium carbonate in the filtrate and carbon dioxide contained in an industrial gas are carbonized in the carbonization tower, anda reaction liquid flowing out from the carbonization tower is sent to the dissolution tank to complete the circulation of the liquid. The method has the advantages that the process flow is short, theenergy consumption is small, and the operation cost is low.
Owner:武汉德泽环保科技有限公司
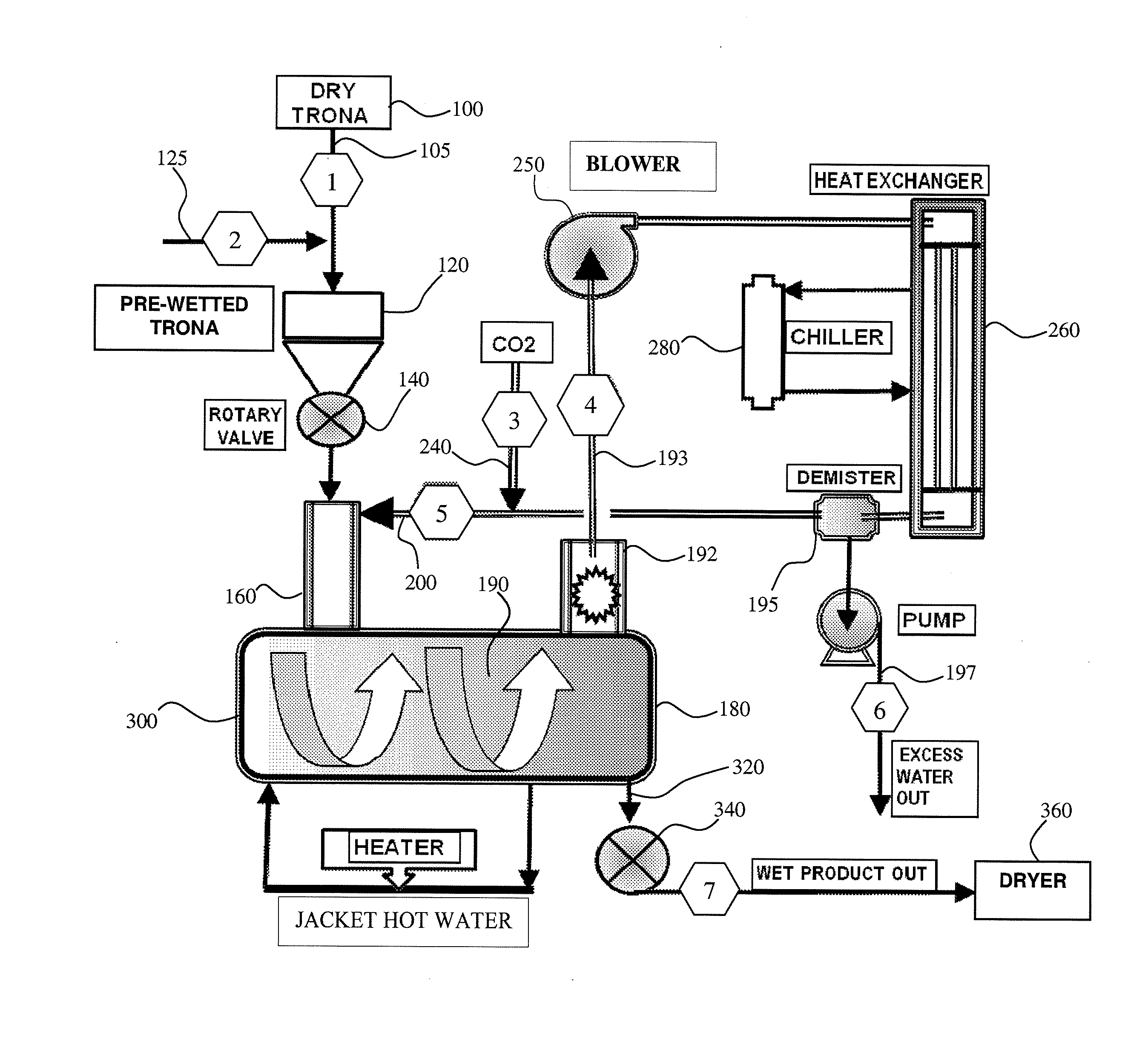
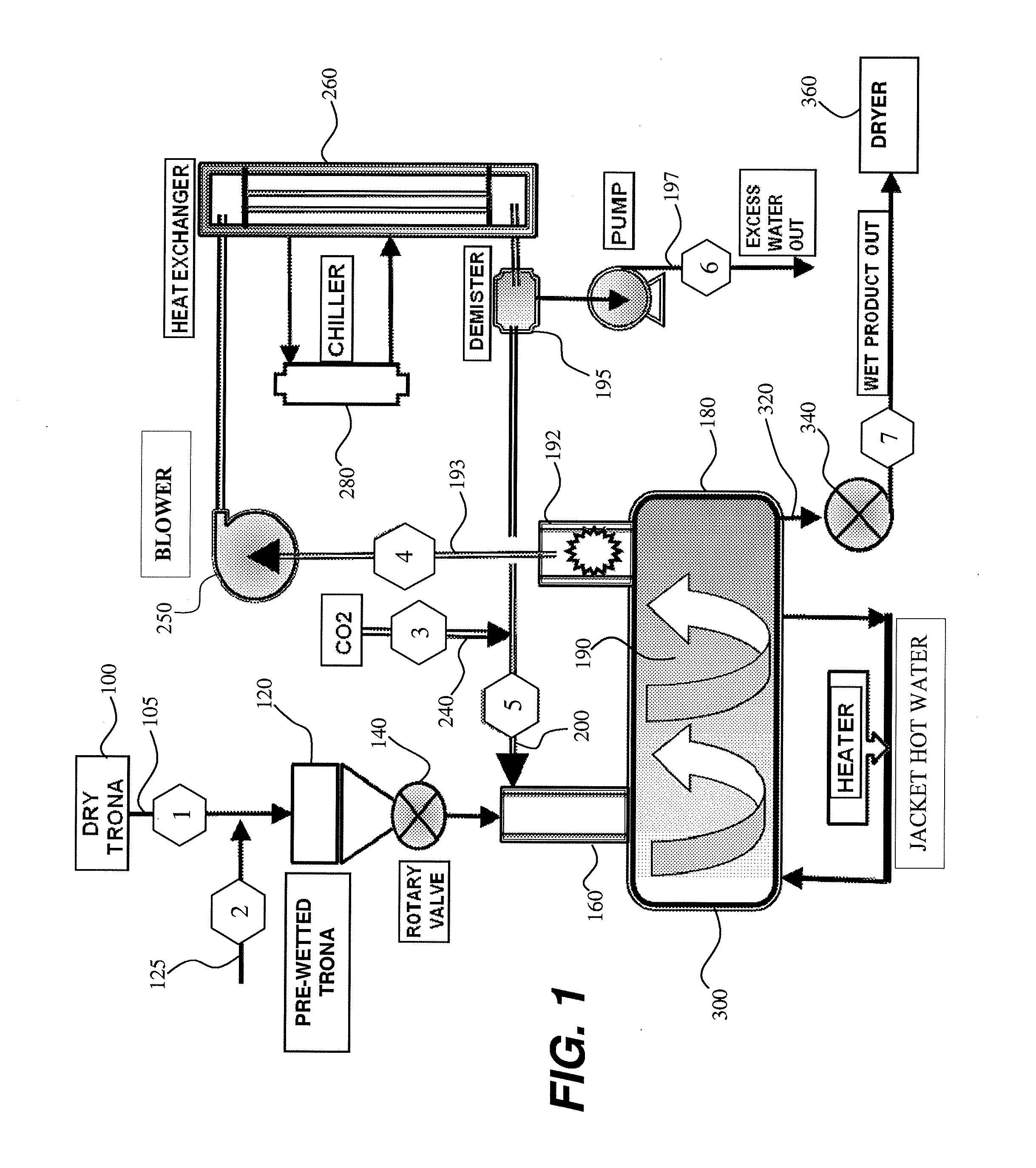
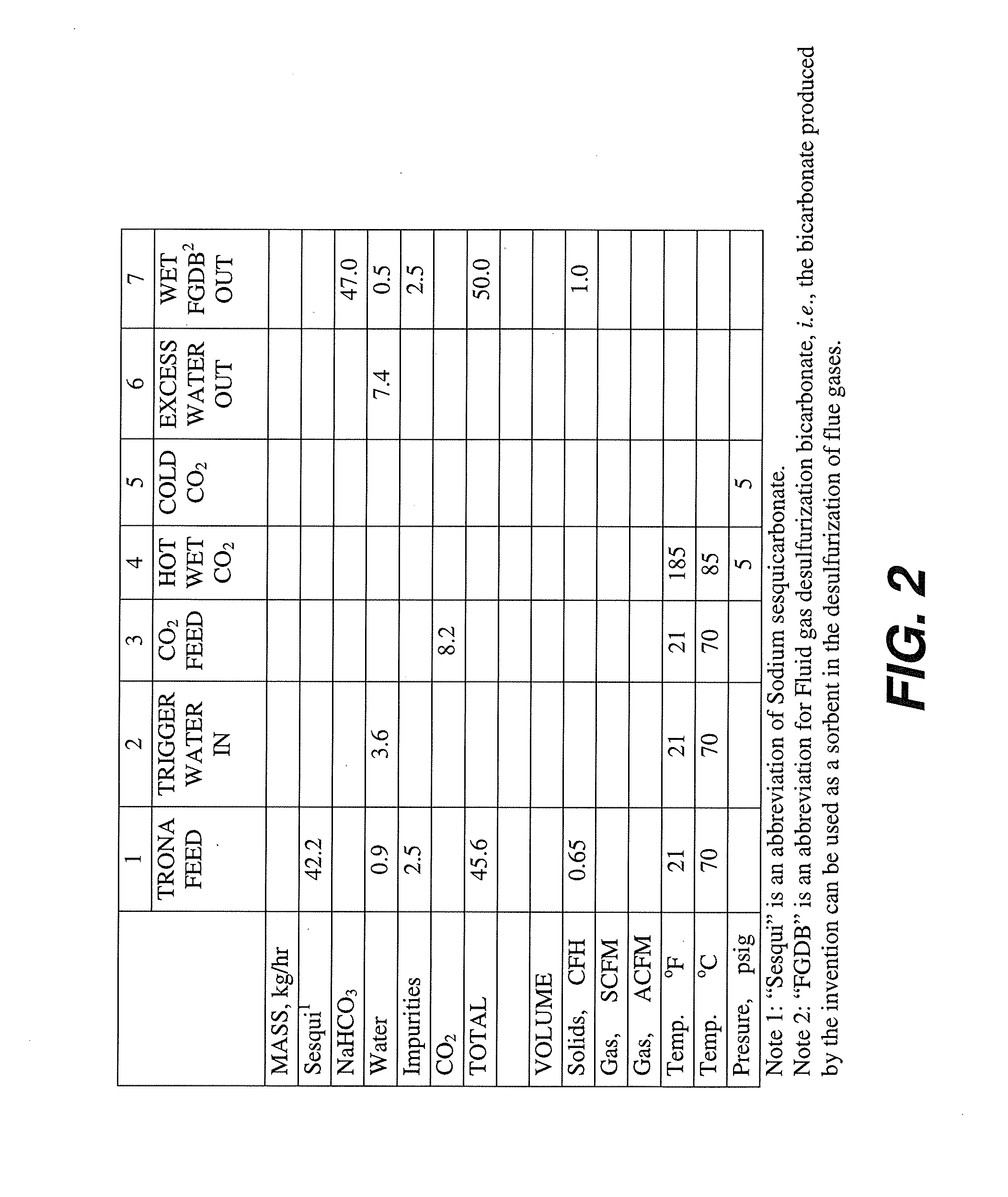
Boundary layer carbonation of trona
A boundary layer carbonation process for producing sodium bicarbonate crystals having specific surface area in the range 0.4 m2 / g to 2.5 m2 / g from Trona, wherein in one embodiment the process comprises the steps of: providing Trona particles having a particle size range of −4 +120 mesh; pre-wetting the Trona particles with water to provide a plurality of pre-wetted Trona particles each having a liquid water solution boundary layer deposited thereon; and carbonating the pre-wetted Trona particles across the water boundary layer to provide a product comprising sodium bicarbonate crystals.
Owner:CHURCH & DWIGHT CO INC
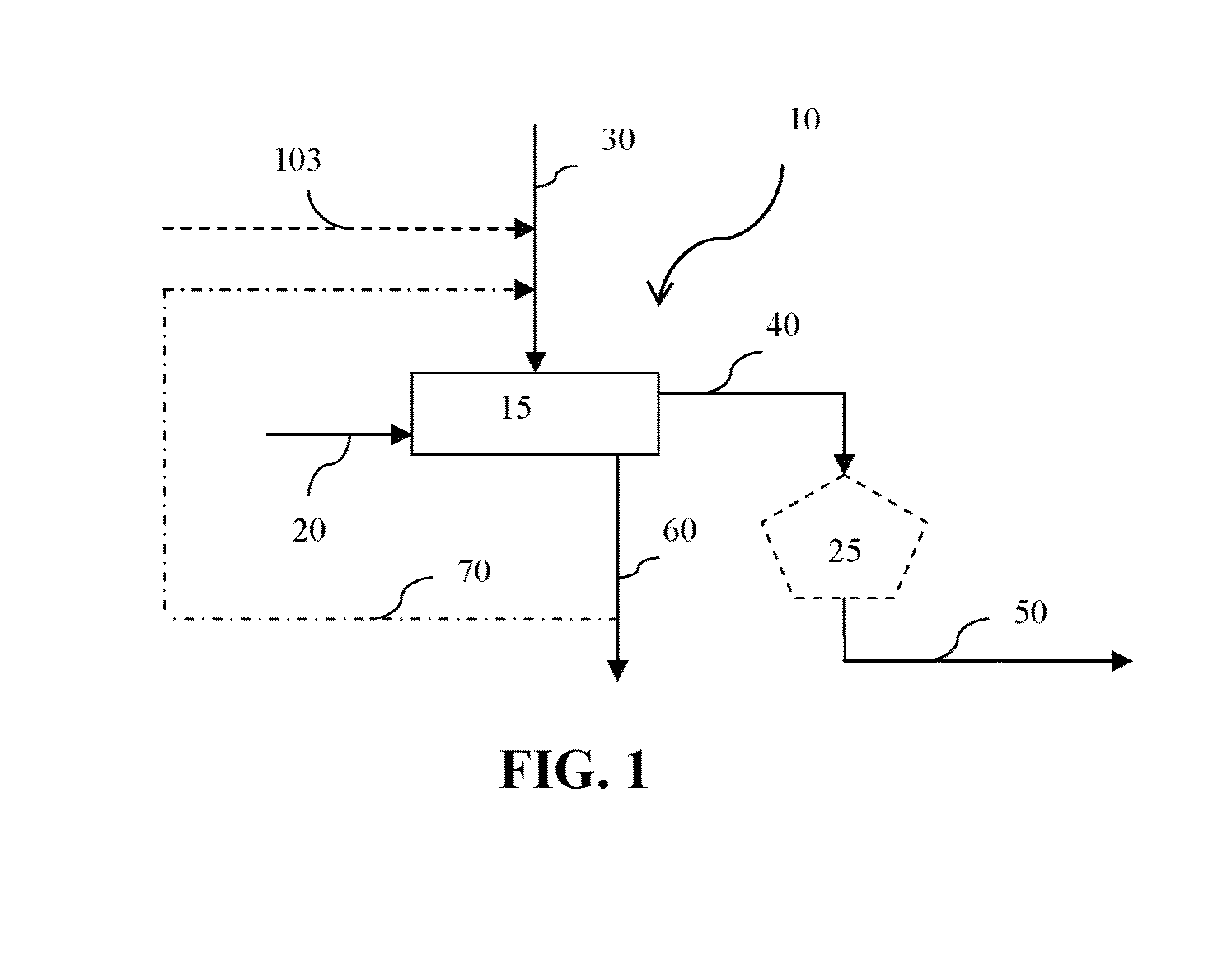
Carbonation and crystallization equipment, application thereof and preparation method for sodium bicarbonate
PendingCN108862332AAvoid cloggingRealize large-scale developmentBicarbonate preparationAlkali metal carbonates shape formationSodium bicarbonateCarbonization
The invention provides carbonation and crystallization equipment, application thereof and a preparation method for sodium bicarbonate, and relates to the field of chemical equipment. The carbonation and crystallization equipment comprises a crystallization barrel and a carbonization reactor, the carbonization reactor is arranged outside the crystallization barrel and is communicated with the crystallization barrel, and reaction final liquid in the carbonization reactor can enter the crystallization barrel for crystallization treatment. The carbonation nad crystallization equipment has the advantages that the technical problems that an existing carbonation tower is low in production efficiency and large in energy loss due to cleaning can be solved, and the technical effects that the operation efficiency and the production efficiency of the equipment are improved, and the energy consumption is reduced can be achieved.
Owner:BEIJING EDGEIN TECH +1
Preparation method of large-particle heavy baking soda
ActiveCN108996526AReduce solubilityLarge particlesBicarbonate preparationAlkali metal carbonates shape formationSodium bicarbonateCarbonization
The invention relates to a preparation method of large-particle heavy baking soda. The method comprises the following steps: performing a carbonization reaction on a sodium carbonate solution or a mixed solution of sodium carbonate and sodium bicarbonate and CO2, so as to obtain a mixture; and adding sodium carbonate and / or a solid and / or a solution of a mixture of sodium carbonate and sodium bicarbonate into the obtained mixture for crystallization and solid-liquid separation, so as to obtain the large-particle heavy baking soda. The sodium carbonate solution or the mixed solution of sodium carbonate and sodium bicarbonate can be prepared from heavy alkali in soda production. The preparation method is suitable for different baking soda manufacturing plants; and the baking soda is high inyield and large in particle size.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
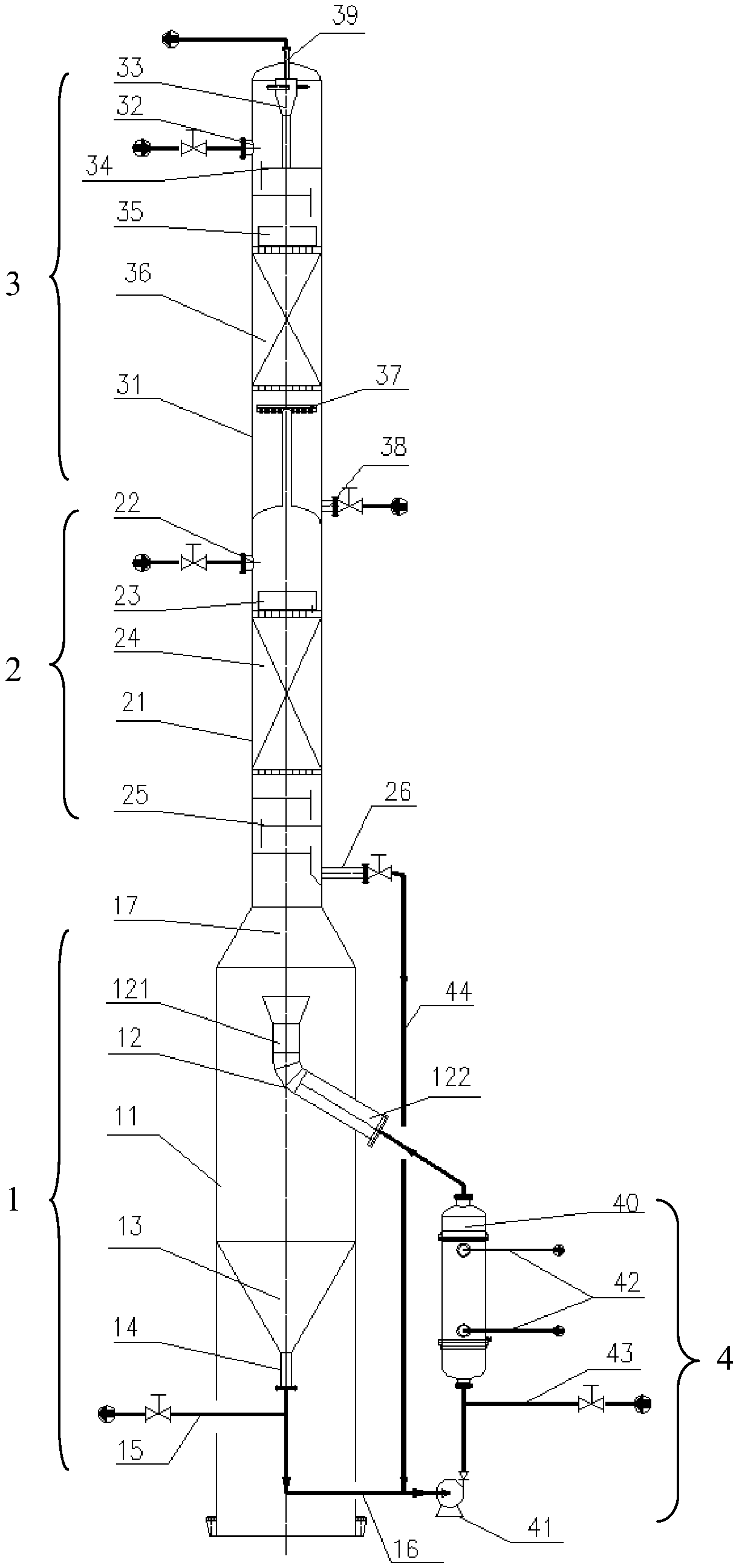
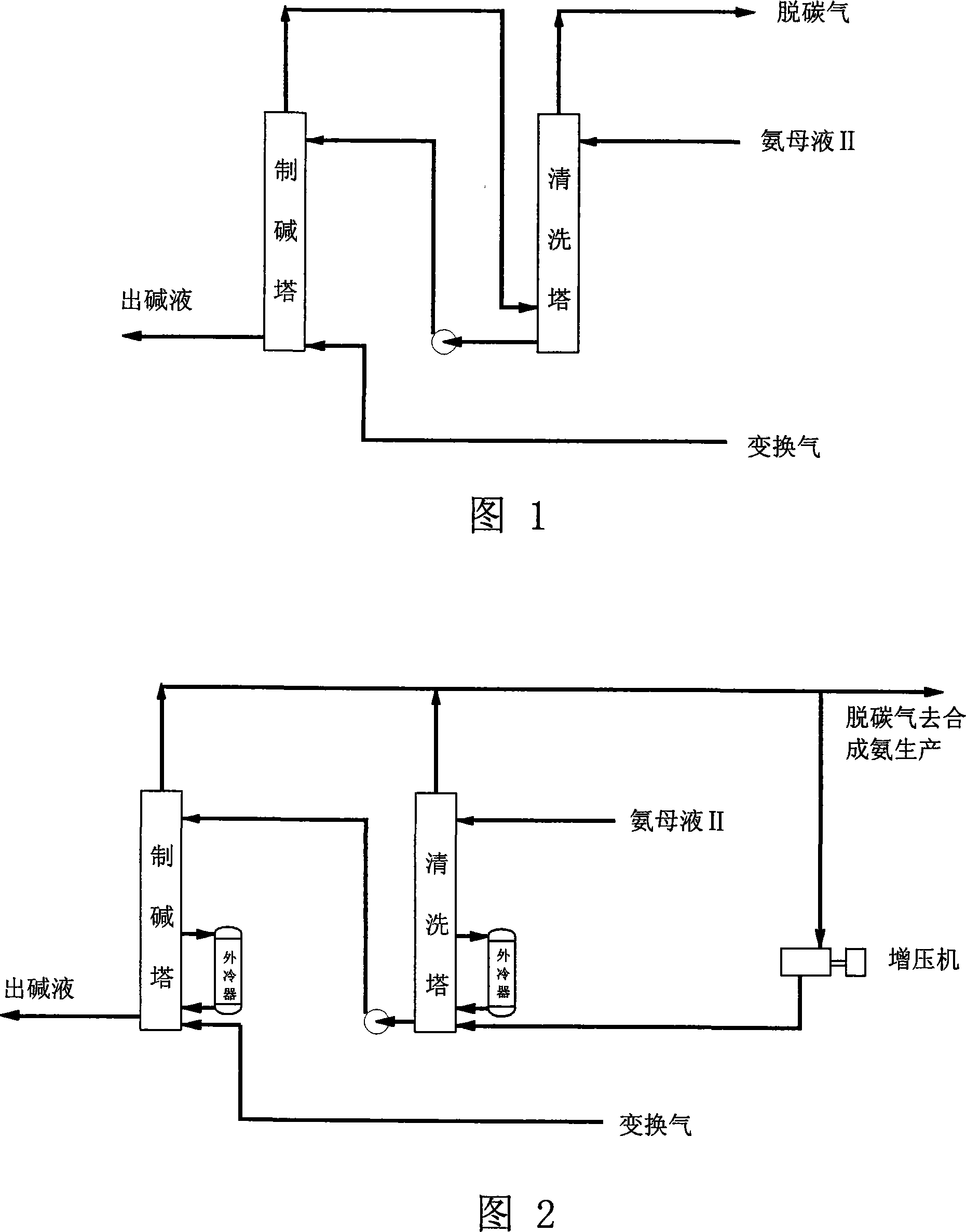
Cleaning process for outer cooling shift gas alkali preparation
ActiveCN101157462ANo pollutionTo achieve the purpose of environmentally friendly productionBicarbonate preparationDispersed particle separationComing outAmmonia production
The invention discloses a cleaning process in external cooling gas-transforming alkali preparation. At least two external cooling type carbonizing towers are paralleled into a set; each carbonizing tower is connected with a set of external coolers; after working for days, each carbonizing tower is cleaned alternately, and the connected external coolers are also cleaned at the same time. In the cleaning period, other carbonating towers but the one being cleaned continue the alkali preparing work; in cleaning, ammonia mother liquid 2 is introduced into the cleaned tower, and at the same time, one part of carbonized exhaust coming out from the alkali preparing towers is also introduced into the cleaned tower after being pressurized to accelerate dissolution of scars in the cleaned tower; the exhaust coming out from the cleaned tower is combined into the other part of the carbonized exhaust coming out from the alkali preparing towers and, used as raw material decarburizing gas for synthetic ammonia production, is sent with the ammonia mother liquid 2 discharged out of the cleaned tower into the alkali preparing towers for the alkali preparation. The cleaning process of the invention has no sewage discharge and does not pollute the environment; in addition, two to six or even more towers can be paralleled into a set for the alkali preparation, thereby significantly improving the working efficiency of the towers and reducing the adverse effect caused to the production by cleaning operation.
Owner:CHINA CHENGDA ENG +1
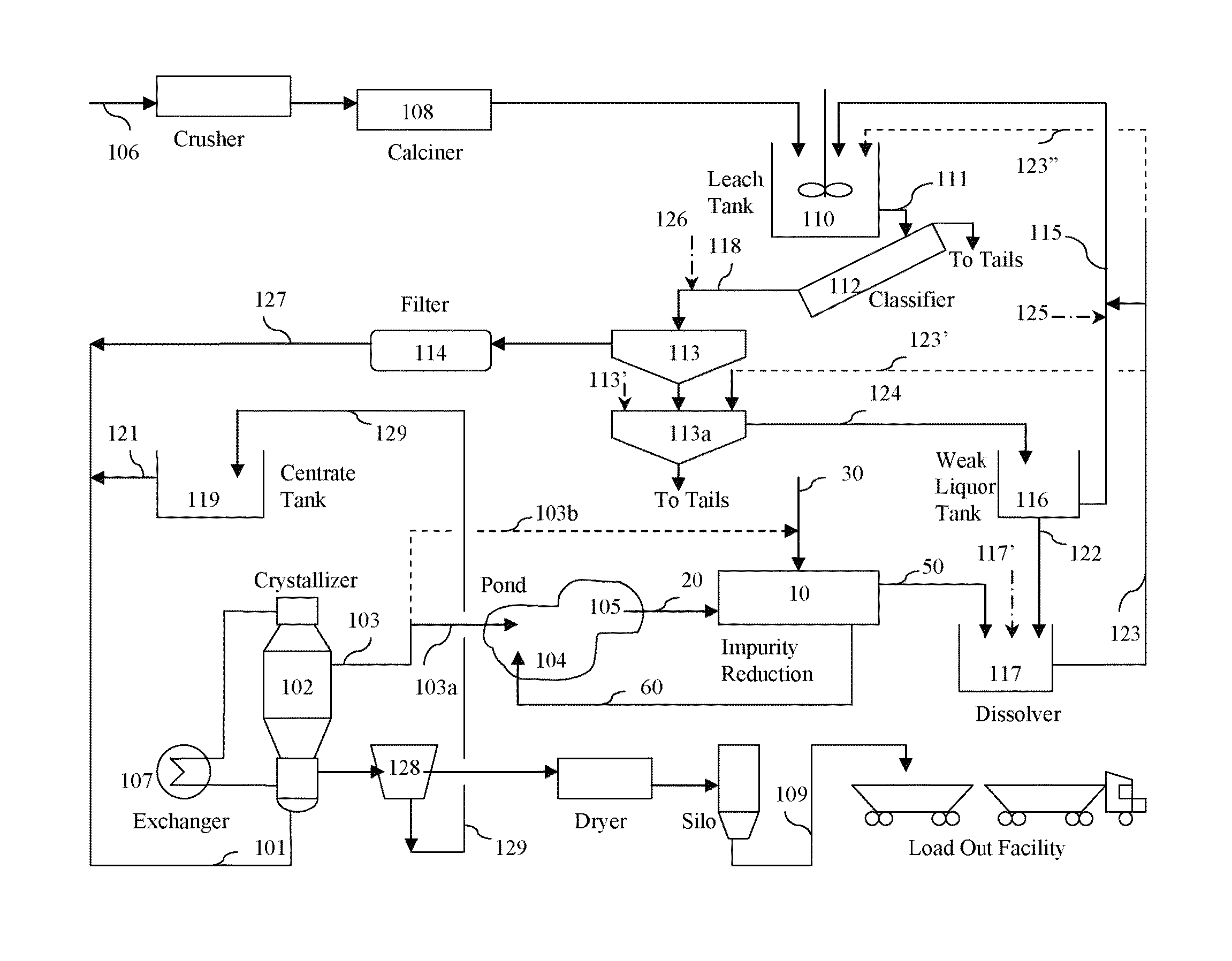
Impurities removal from waste solids in the production of soda ash, sodium bicarbonate and/or other derivatives
ActiveUS8771622B2Low impurity contentIncrease valueBicarbonate preparationAlkali metal sulfite/sulfate purificationSodium bicarbonateDissolution
A method for removing impurities from a waste solid to provide at least a portion of a suitable crystallizer feed to a process for making crystalline sodium carbonate, bicarbonate, and / or other derivatives. The method comprises: contacting the waste solid with a leach solution to dissolve at least one impurity and dissolving the resulting leached residue. Leaching may include heap percolation. The leach solution may comprise a crystallizer purge liquor, a process waste effluent, a mine water, or mixtures thereof. The method may further comprise adding a magnesium compound to the resulting leached residue during or after its dissolution to remove another impurity. The waste solid preferably comprises a pond solid containing such impurities. The pond solid may be recovered from a pond receiving crystallizer purge liquor(s) and / or other process waste effluent(s). The pond solid may contain sodium carbonate, any hydrate thereof, sodium bicarbonate, and / or sodium sesquicarbonate. The impurities to be removed may comprise sodium chloride, sodium sulfate, silicates, and / or organics.
Owner:SOLVAY CHEM INC
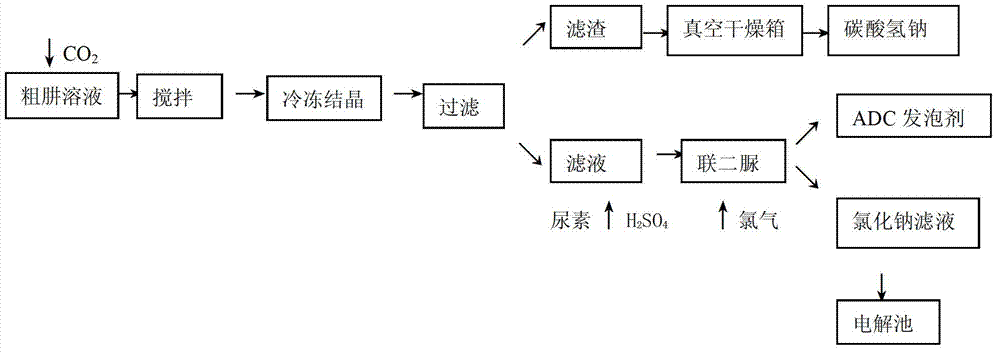
Recycling method of byproduct saline during preparation of hydrazine hydrate by using urea method
The invention discloses a recycling method of a byproduct saline during preparation of hydrazine hydrate by using a urea method. The method includes adding excess CO2 into a coarse hydrazine solution, and keeping the negative pressure of a steel cylinder containing the CO2 to be 100pa to 150pa and the flow to be 0.5-0.8mol / L; stirring the mixture continuously for 0.5 hour when the negative pressure in the steel cylinder is zero; freezing and crystallizing the solution through general freezing brine, and controlling the temperature in a range from 2 DEG C to 6 DEG C and the freezing time to be 2 hours; performing cold filtering to obtain filter residues, drying the filter residues in a vacuum drying oven for 4 hours, and keeping the temperature of the drying oven to be 35 DEG C to 45 DEG C; adding urea into the filtrate obtained through cold filtering, adding sulfuric acids to regulate the pH to be 3 to 4, controlling the temperature to be 106 DEG C to 120 DEG C, and reacting the mixture for 2 hours; and feeding chlorine into a biurea reaction still to perform oxidation to obtain the resultant ADC foaming agents, wherein the filtrate is sent to an electrolytic tank. The recycling method of byproduct saline during preparation of hydrazine hydrate by using the urea method has the advantages that the byproduct sodium salt of the hydrazine hydrate is fully recycled, the cost is reduced, and the method is a green chemical process.
Owner:HANGZHOU HI TECH FINE CHEM
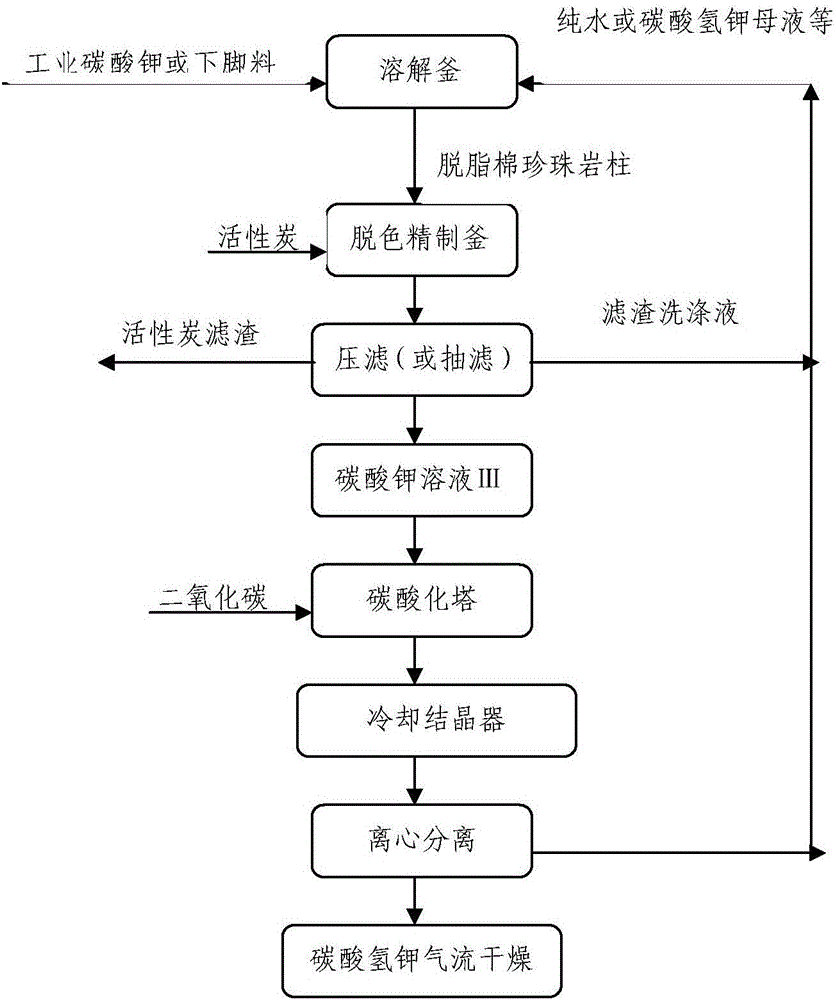
Production method of high-purity non-caking ammonium-free potassium bicarbonate
The invention discloses a production method of high-purity non-caking ammonium-free potassium bicarbonate, which comprises the following steps: dissolving leftovers with the content of above 98% in a production process of industrial potassium carbonate or potassium carbonate into deionized purified water (or vapor condensation recycled water), or a cleaning solution obtain in Step 3), or potassium bicarbonate mother liquor obtained in Step 5); removing oil with an adsorbent, performing decoloration refining with a de-coloring agent and performing a carbonation reaction on refined potassium carbonate solution, so that potassium carbonate is translated into potassium bicarbonate; separating potassium bicarbonate out by crystallization and purifying potassium bicarbonate, so as to obtain a potassium bicarbonate wet product; and drying the potassium bicarbonate wet product with high-temperature airflow, so as to obtain a potassium bicarbonate finished product.
Owner:ZHEJIANG DAYANG BIOTECH GROUP
Conversion of gaseous carbon dioxide into aqueous alkaline and/or alkaline earth bicarbonate solutions
InactiveCN103930372AHigh yieldCalcium/strontium/barium carbonatesBicarbonate preparationLe Chatelier's principleAlkaline earth metal
A material with cationic exchanger properties is introduced into aqueous media, where the equilibriums of carbon dioxide dissolution take place. A cationic exchanger material x / nMw E,- is used to capture hydronium cations (H3O<+>) according to: x / nM<+n>Ex<->(s) + xH3O<+>(aq) = xH3O<+>Ex<->(s) + x / nM<+n>(aq) where "x" stands for molar amount of the anionic centers of charge of the cationic exchanger material E<x-> balanced by x / n molar amount of metal M, "n" stands for the metal valence, and M is selected from the group consisting of 1A and / or 2A of the periodic table of elements. This capture of the hydronium cations, H3O<+>, shifts certain reaction equilibriums to the right, according to Le Chatelier's principle, producing more bicarbonate, HCO3<->, and / or carbonate, CO3<=>, than would otherwise be obtained.
Owner:SILICA DE PANAMA
Popular searches
Photography auxillary processes Electrolytic organic production Chemical/physical/physico-chemical processes Petrochemical industry Pressure vessels for chemical process Alkali metal oxides/hydroxides Alkali metal halide purification Alkali metal sulfites/sulfates Glucose production Zinc oxides/hydroxides
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com