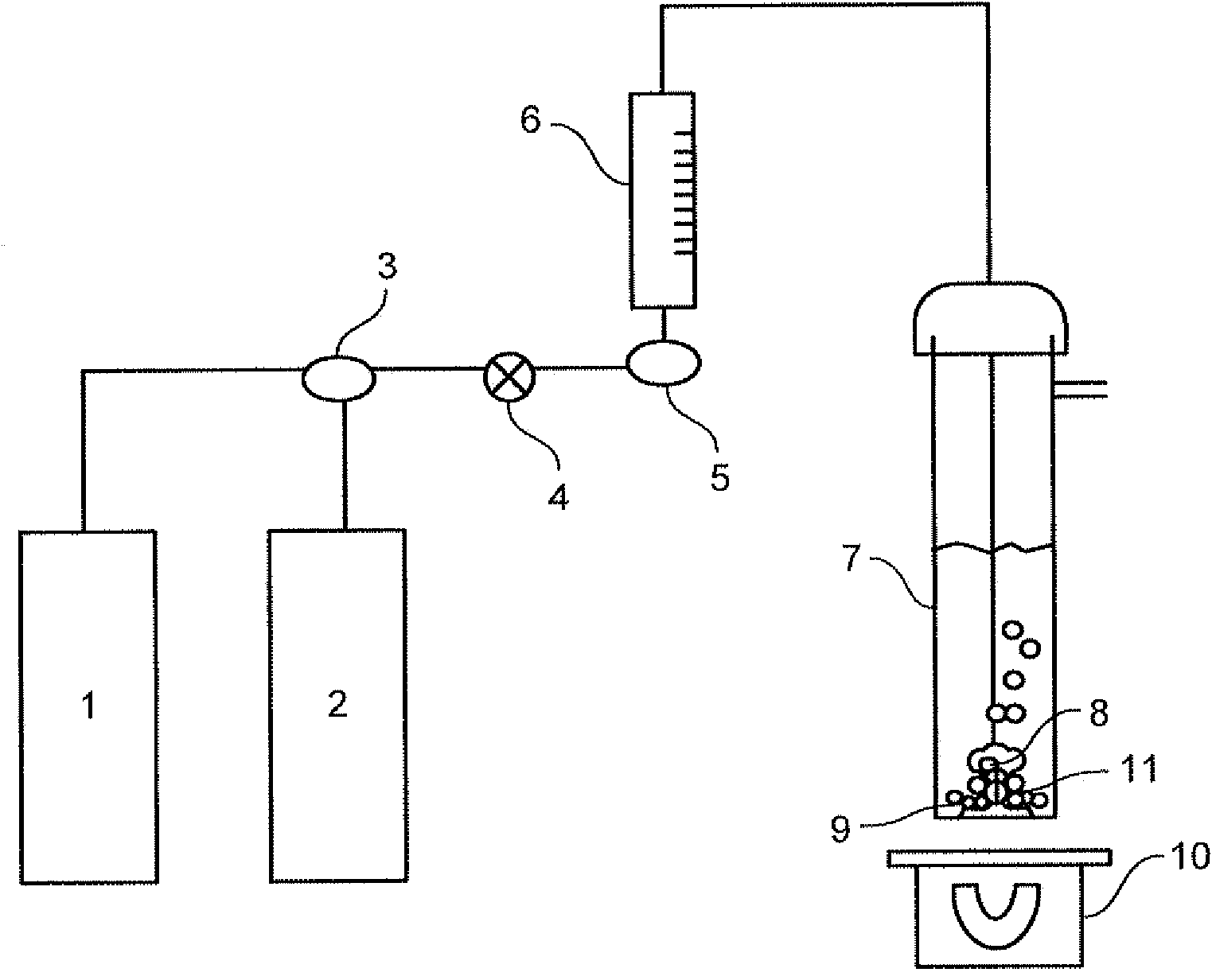
Conversion of gaseous carbon dioxide into aqueous alkaline and/or alkaline earth bicarbonate solutions
A bicarbonate, alkaline earth metal technology, applied in the directions of alkali metal carbonate, bicarbonate preparation, alkali metal compounds, etc., can solve the problems of poor mechanical resistance, low cation exchange capacity, poor chemical stability, etc., to improve effect of influence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] A blank or control run was done without any cation exchanger material. 150 mL of pre-boiled and demineralized water tempered at room temperature was added to a well cleaned 250 mL scrubbing flask 7 . On / off valve 4 is opened, nitrogen stream from source 2 is flowed at a rate of at least 250 ml / min at a pressure of 14 psig, and gas diffusing agent (8) is introduced into flask 7. The nitrogen flow time is at least 5 minutes. Then, change three-way valve 3 to flow 14 psig carbon dioxide gas from source 1 at a rate of at least 250 ml / min. The carbon dioxide gas flow time was 30 minutes at room temperature.
[0120] Using the previously mentioned analytical procedure, the amount of bicarbonate formed (as an anion) was 238 mg / L.
Embodiment 2
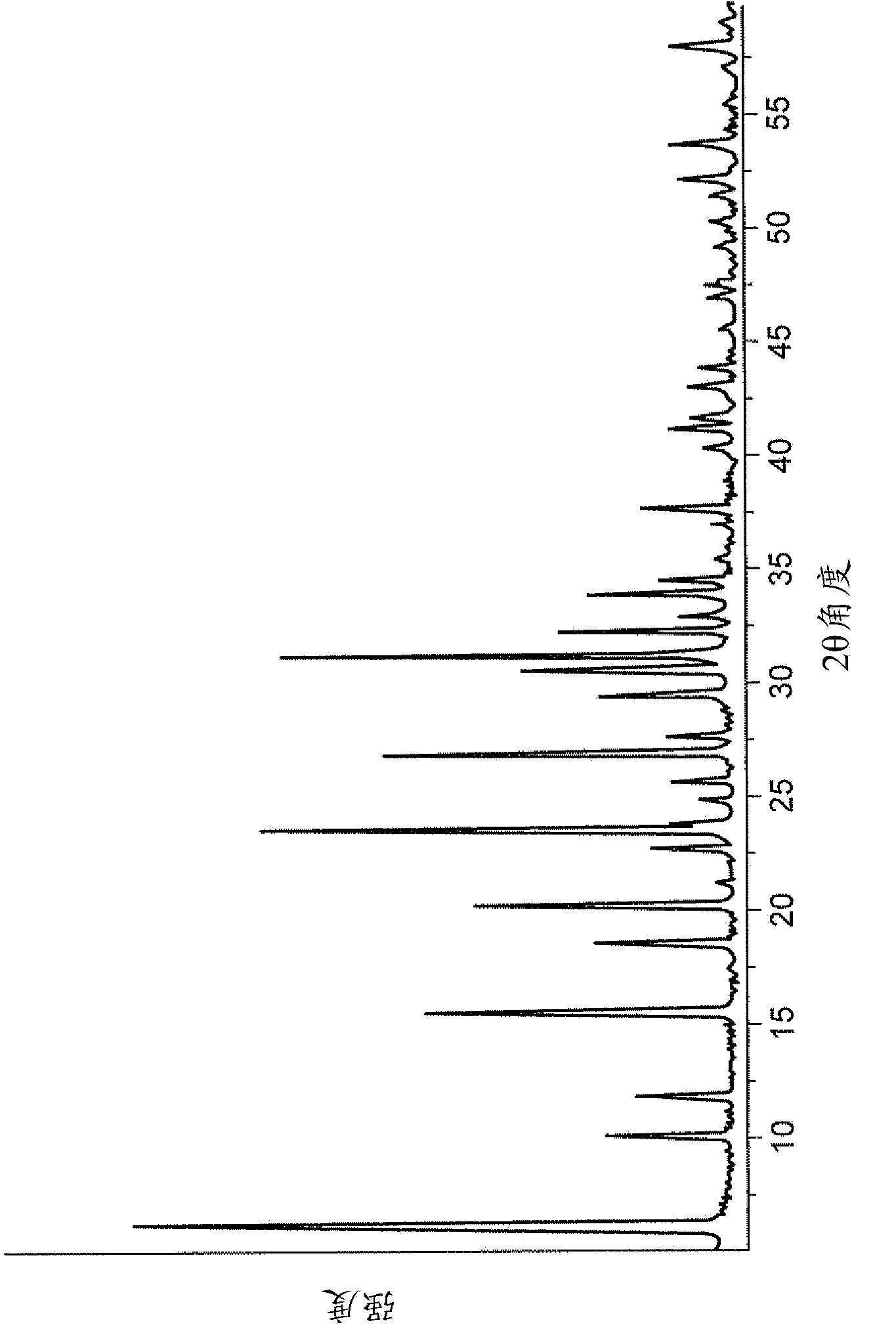
[0122] In this example, the XC sodium form of the cation exchanger material was tested. This is a commercial microsphere of faujasite type X zeolite whose X-ray diffraction pattern is shown in figure 2 middle. The ratio of silicon to aluminum (mol / mol) is given by chemical analysis, and the BET specific surface area (S) is shown in Table 1 of Example 3. image 3 Scanning electron micrographs are provided showing the overview of microspheres of the XC material, varying in size roughly between 5 μm and 100 μm. Figure 4 A scanning electron microscope photograph of microspheres of this XC cation exchanger material is shown.
[0123] A mass of cation exchanger material XC was charged to a well cleaned scrubbing flask 7 and the procedure described in Example 1 was carried out. The time for flowing the carbon dioxide gas was also 30 minutes.
[0124] Using the previously mentioned analytical procedure, the amount of bicarbonate formed (as an anion) was 427 mg / L and the phenolph...
Embodiment 3
[0127] This example examines various sodium form cation exchanger materials of the aluminosilicate type. In addition to the cation exchangers described in Example 2, the properties of other cation exchangers are also shown in Table 1 below. Figure 5 The X-ray diffraction pattern of the AC material is shown, which is representative of a typical type A zeolite. AC materials consist of a set of image 3 Microspheres with the size distribution shown in . Both AA material and AX material are X-ray amorphous materials, and their patterns are shown in Figure 6 and Figure 7 middle. These materials are obtained from the gels normally used for the synthesis of zeolites A and X, respectively, but their synthesis is terminated before the crystal growth stage in order to obtain a small core and thus a larger outer surface. Figure 8 shows AA material and AX material in the zeolite framework range 380cm -1 ~1300cm -1 in the infrared spectrum. They show some bands of the typical I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| aperture size | aaaaa | aaaaa |
| solid containing ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com