Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
172 results about "Lithium nitride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lithium nitride is a compound with the formula Li₃N. It is the only stable alkali metal nitride. The solid is a redish pink color and has a high melting point.
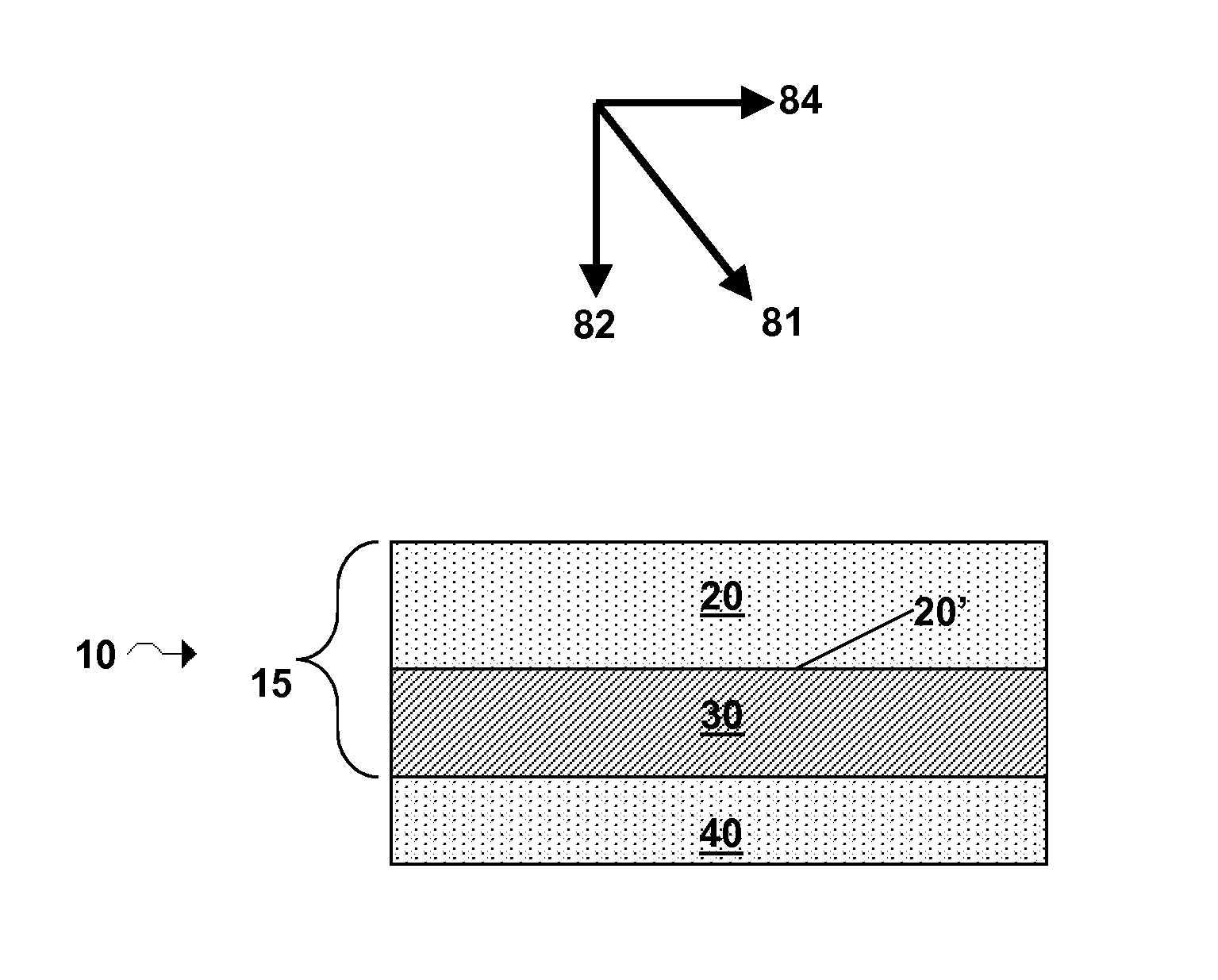
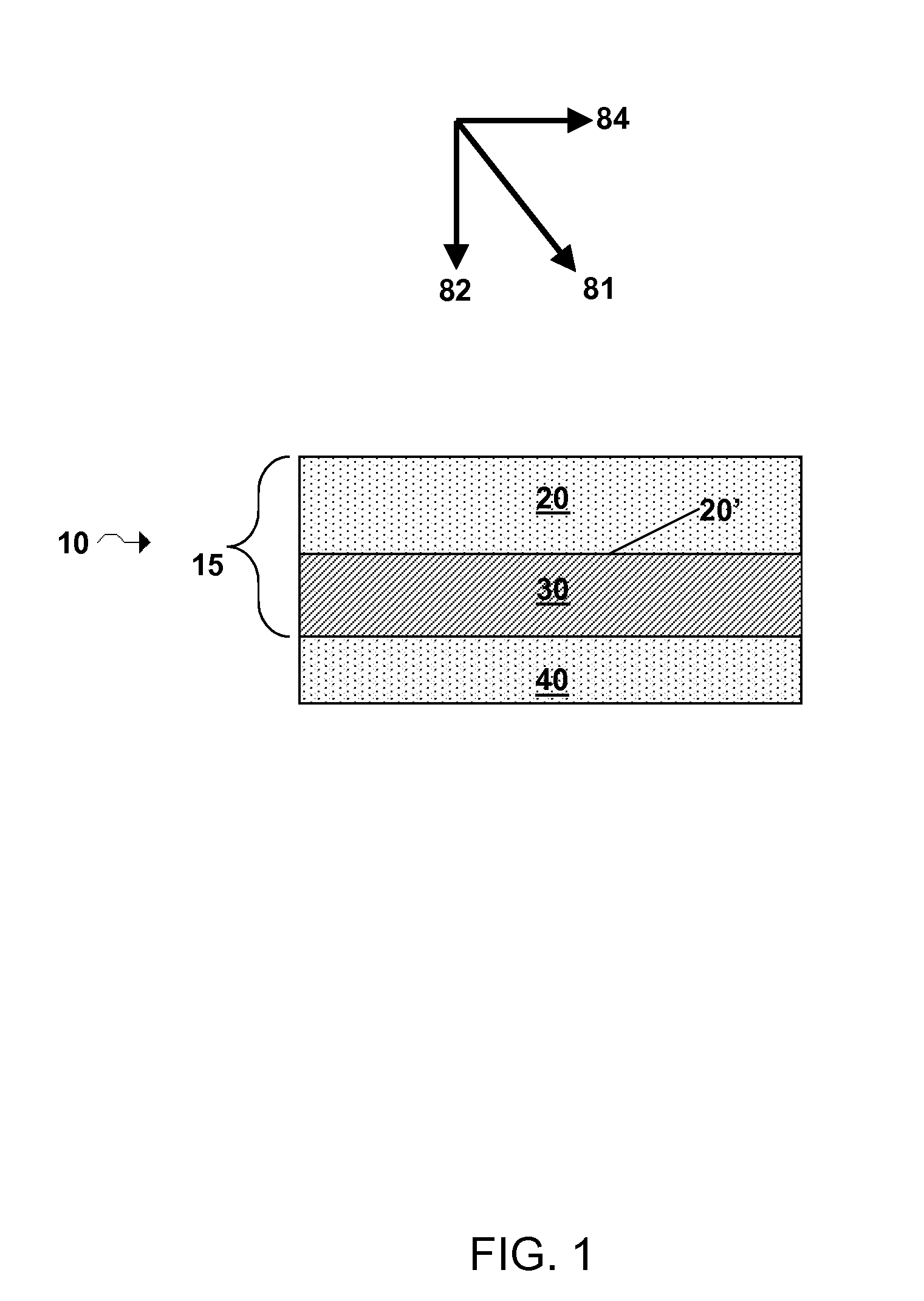
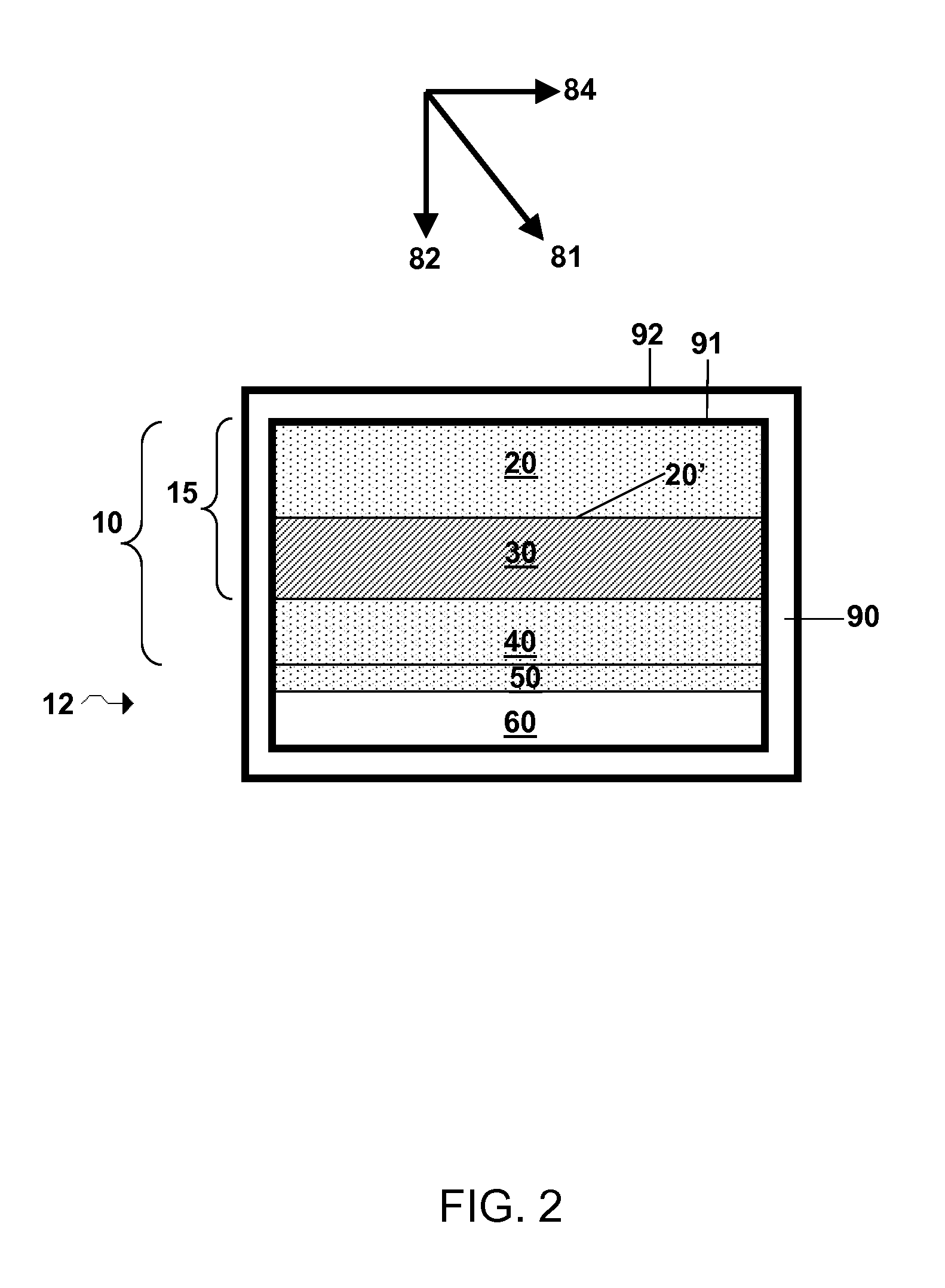
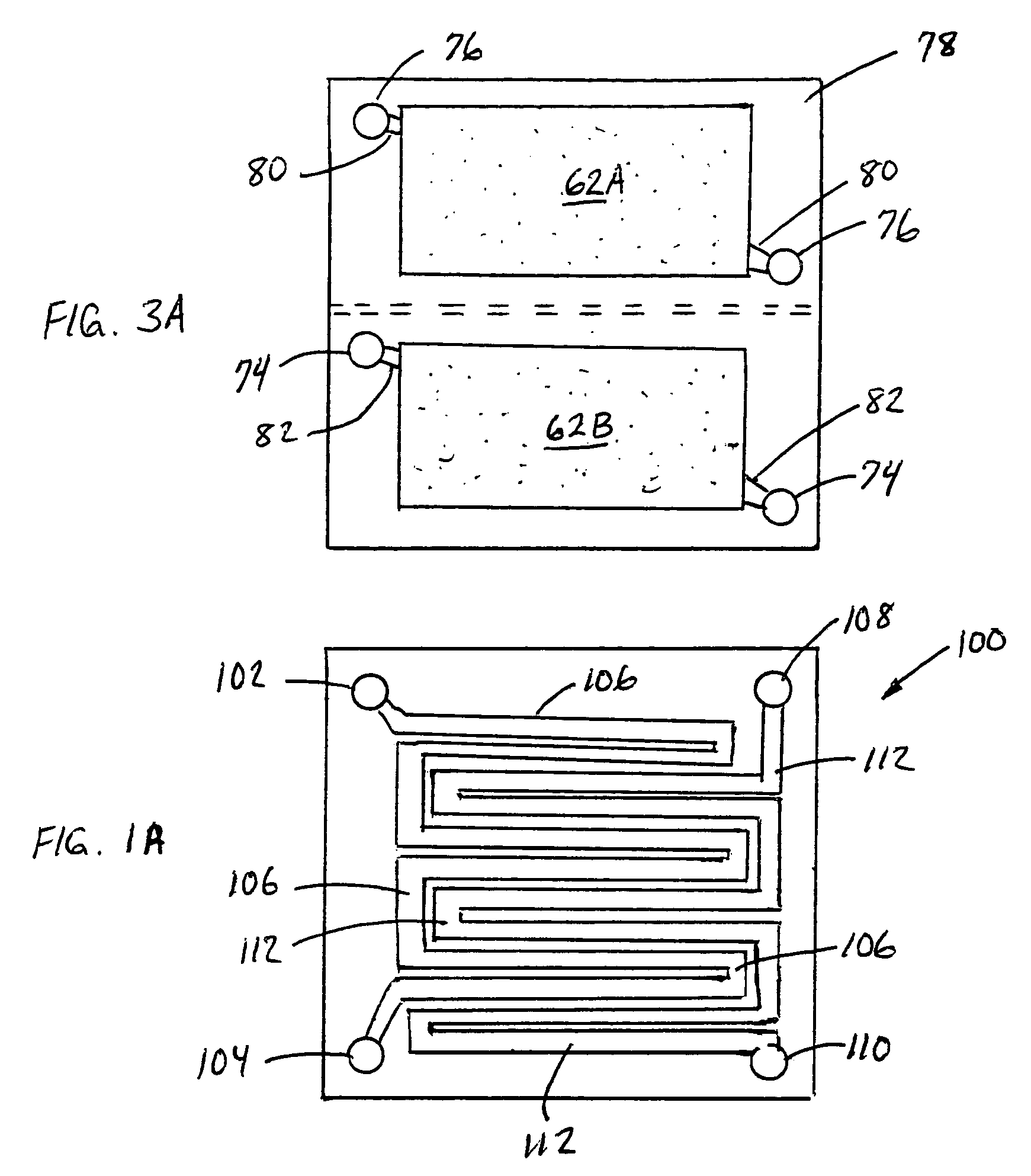
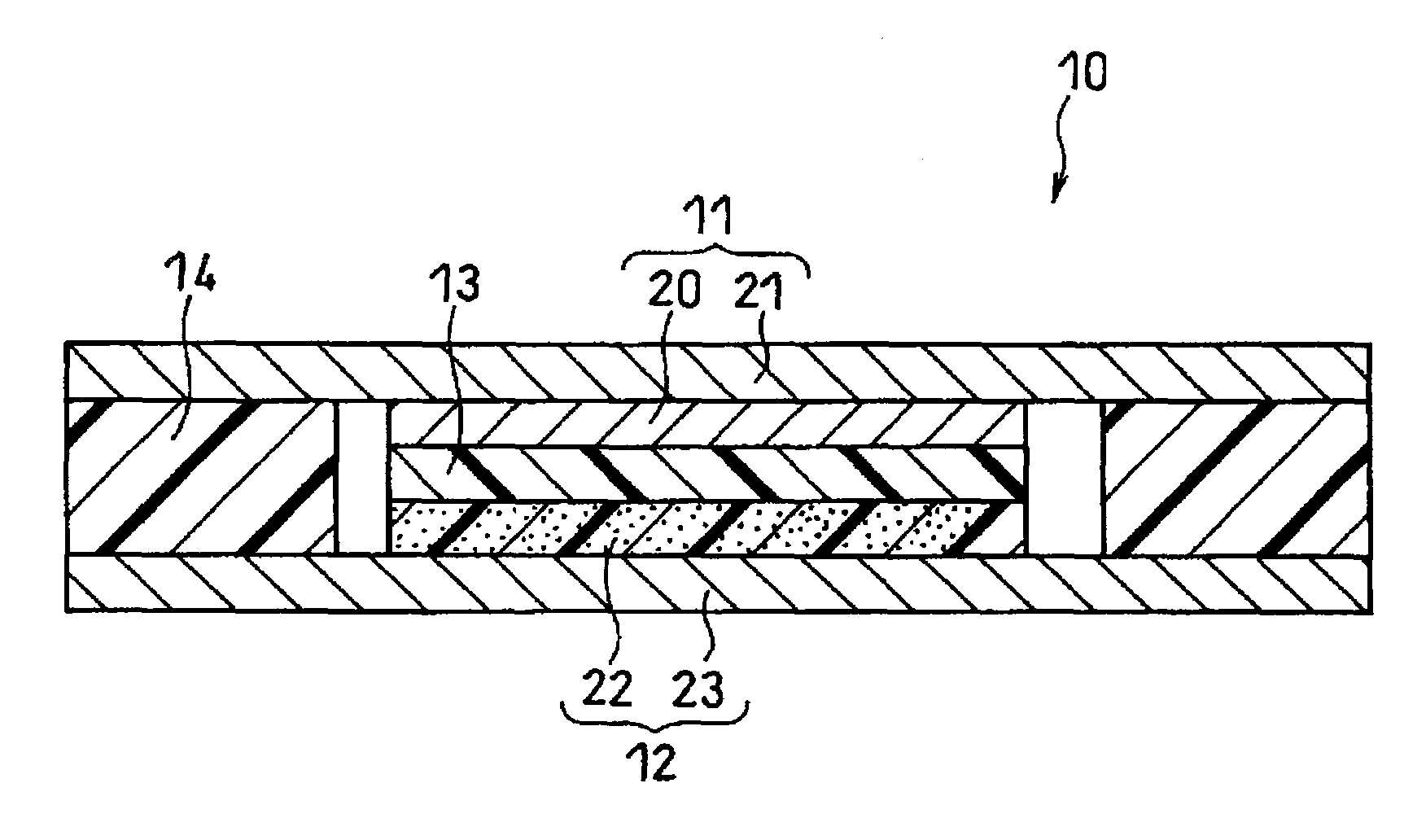
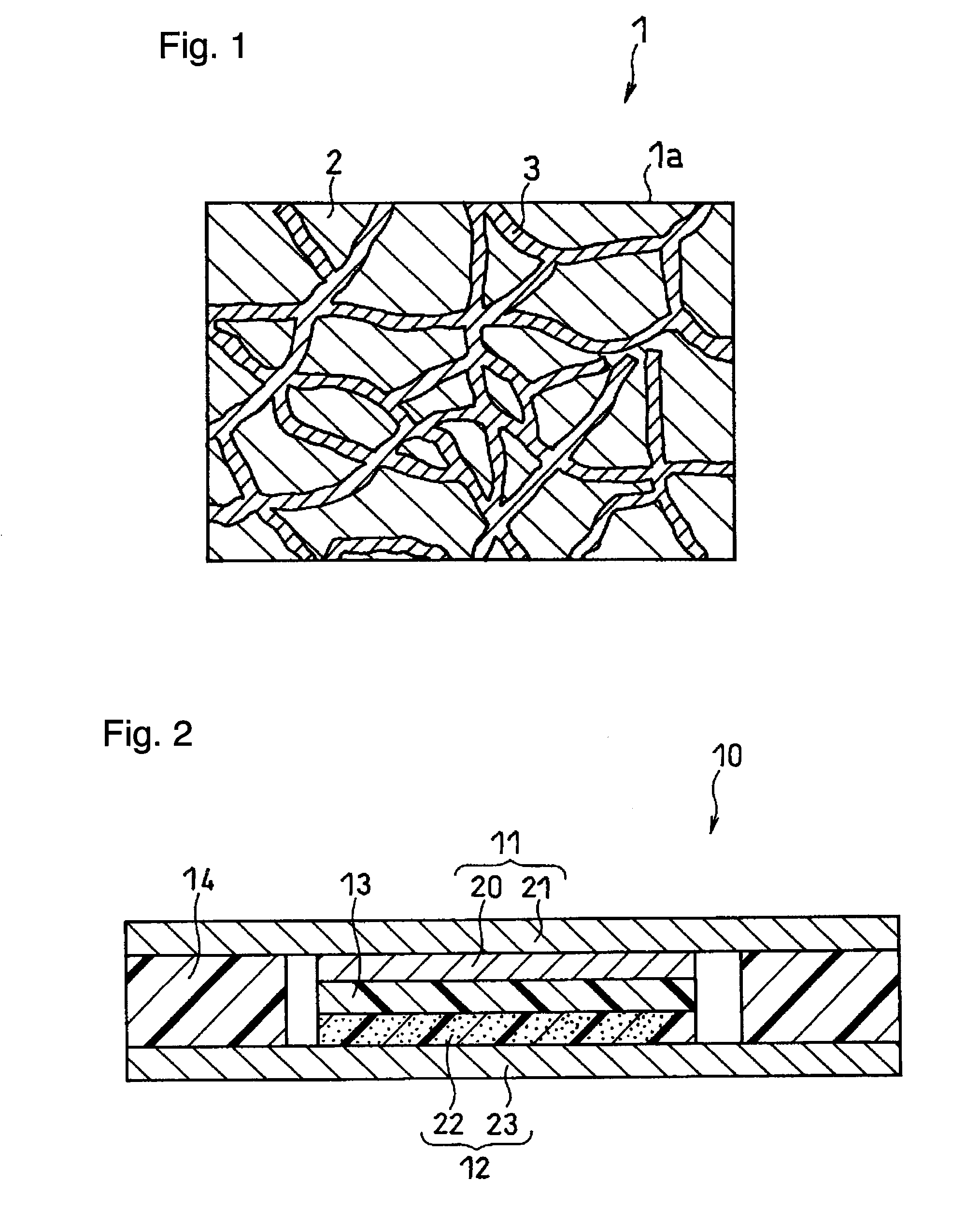
Electrode structure and method for making the same
Electrode structures, and more specifically, electrode structures for use in electrochemical cells, are provided. The electrode structures described herein may include one or more protective layers. In one set of embodiments, a protective layer may be formed by exposing a lithium metal surface to a plasma comprising ions of a gas to form a ceramic layer on top of the lithium metal. The ceramic layer may be highly conductive to lithium ions and may protect the underlying lithium metal surface from reaction with components in the electrolyte. In some cases, the ions may be nitrogen ions and a lithium nitride layer may be formed on the lithium metal surface. In other embodiments, the protective layer may be formed by converting lithium to lithium nitride at high pressures. Other methods for forming protective layers are also provided.
Owner:SION POWER CORP
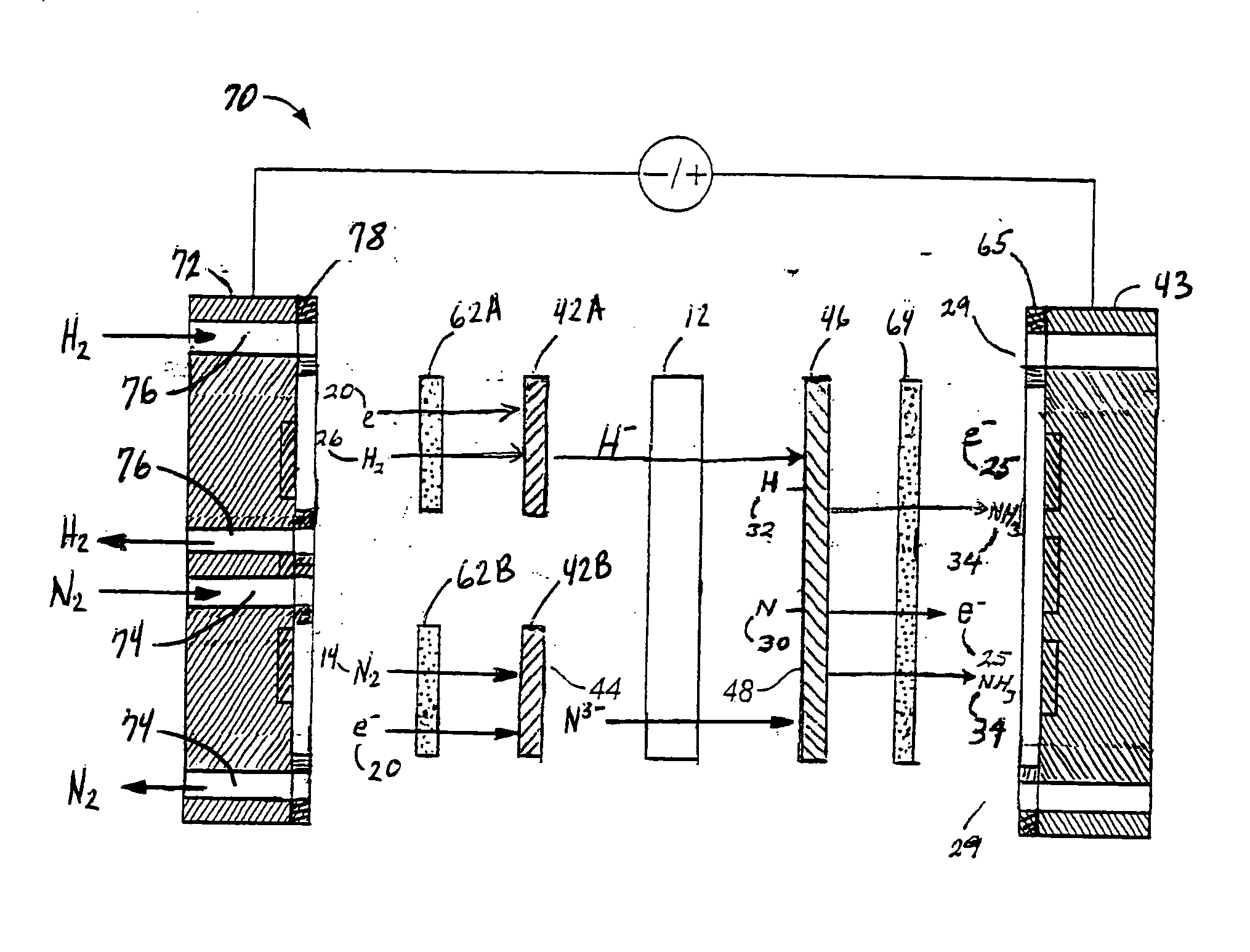
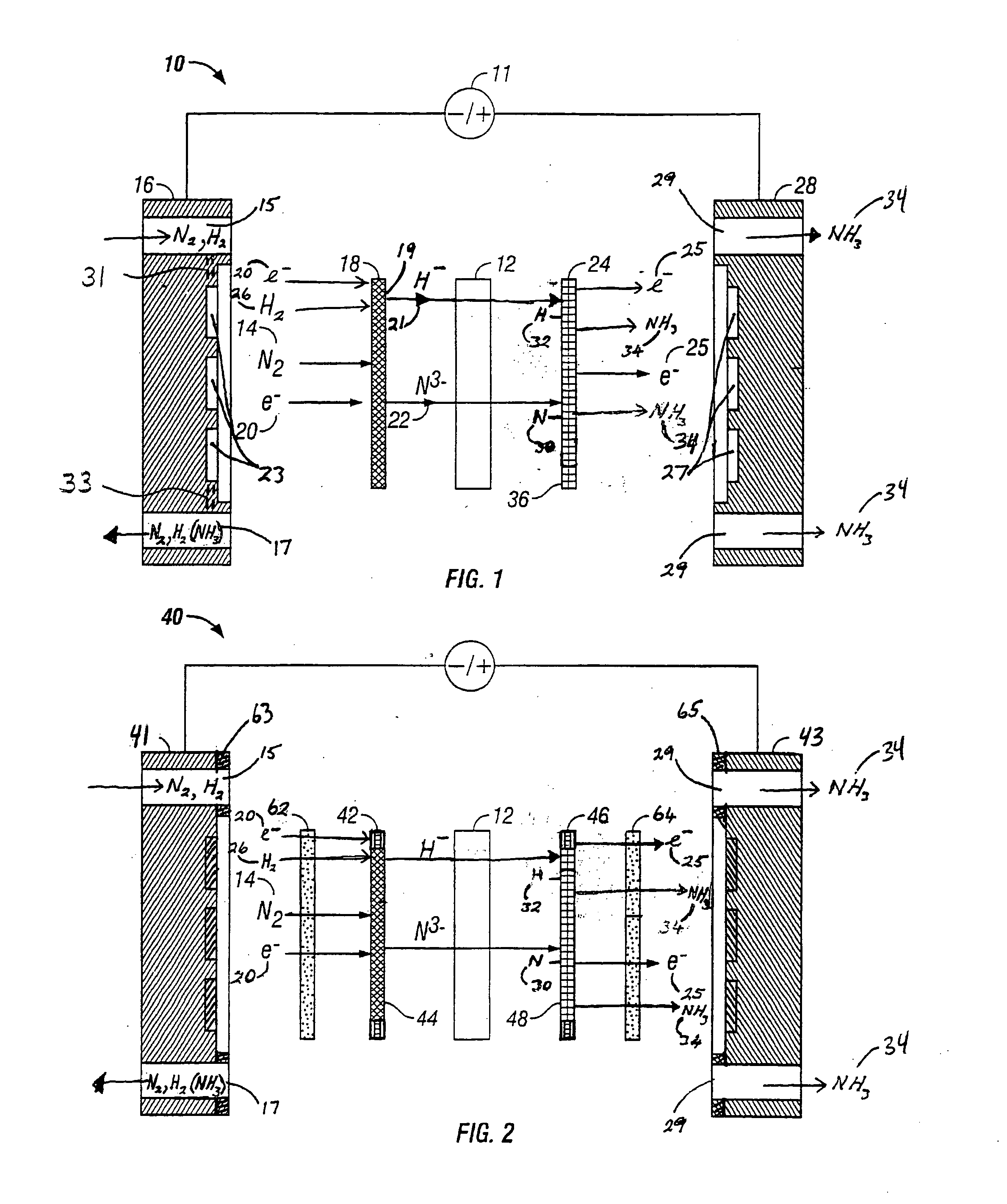
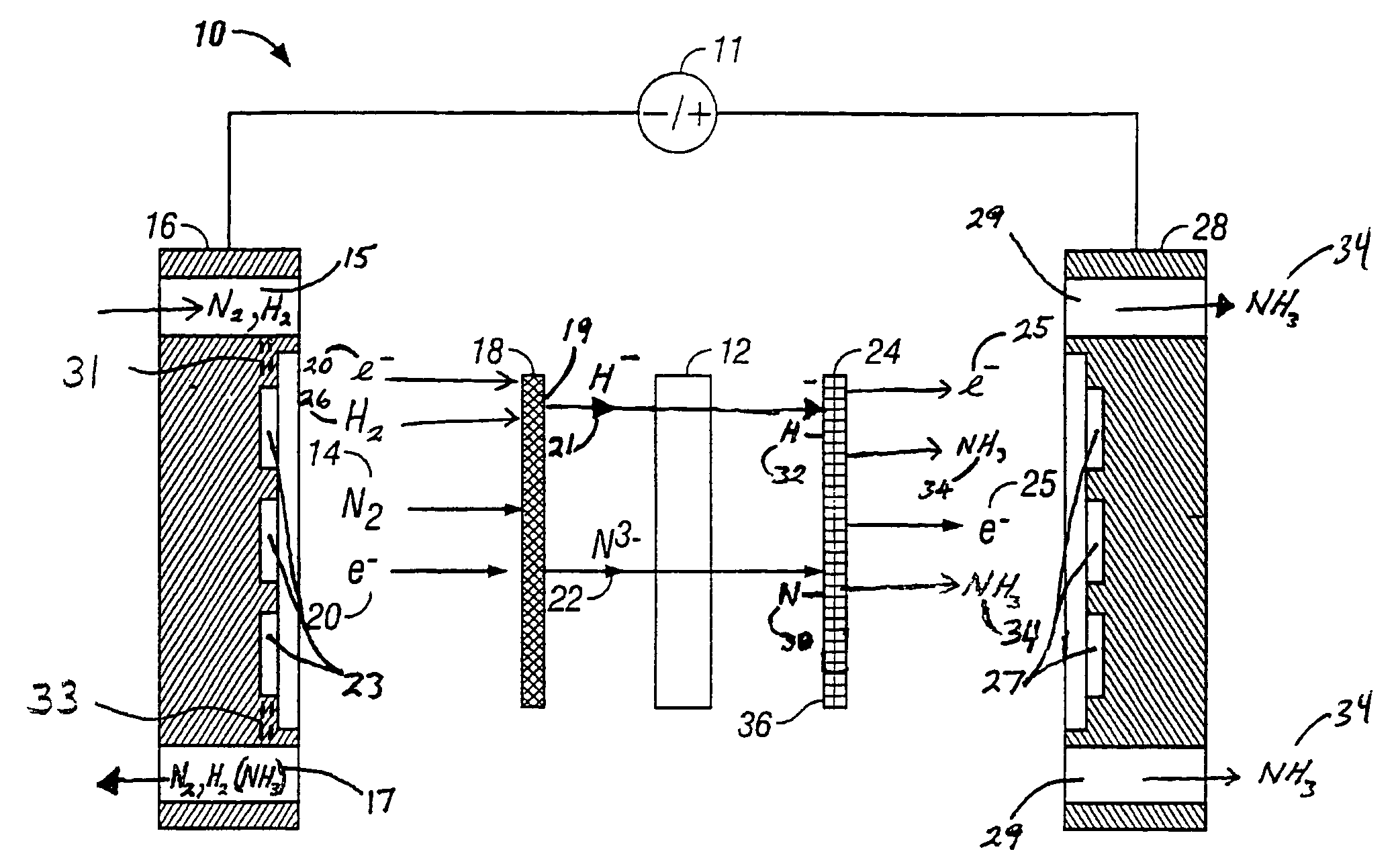
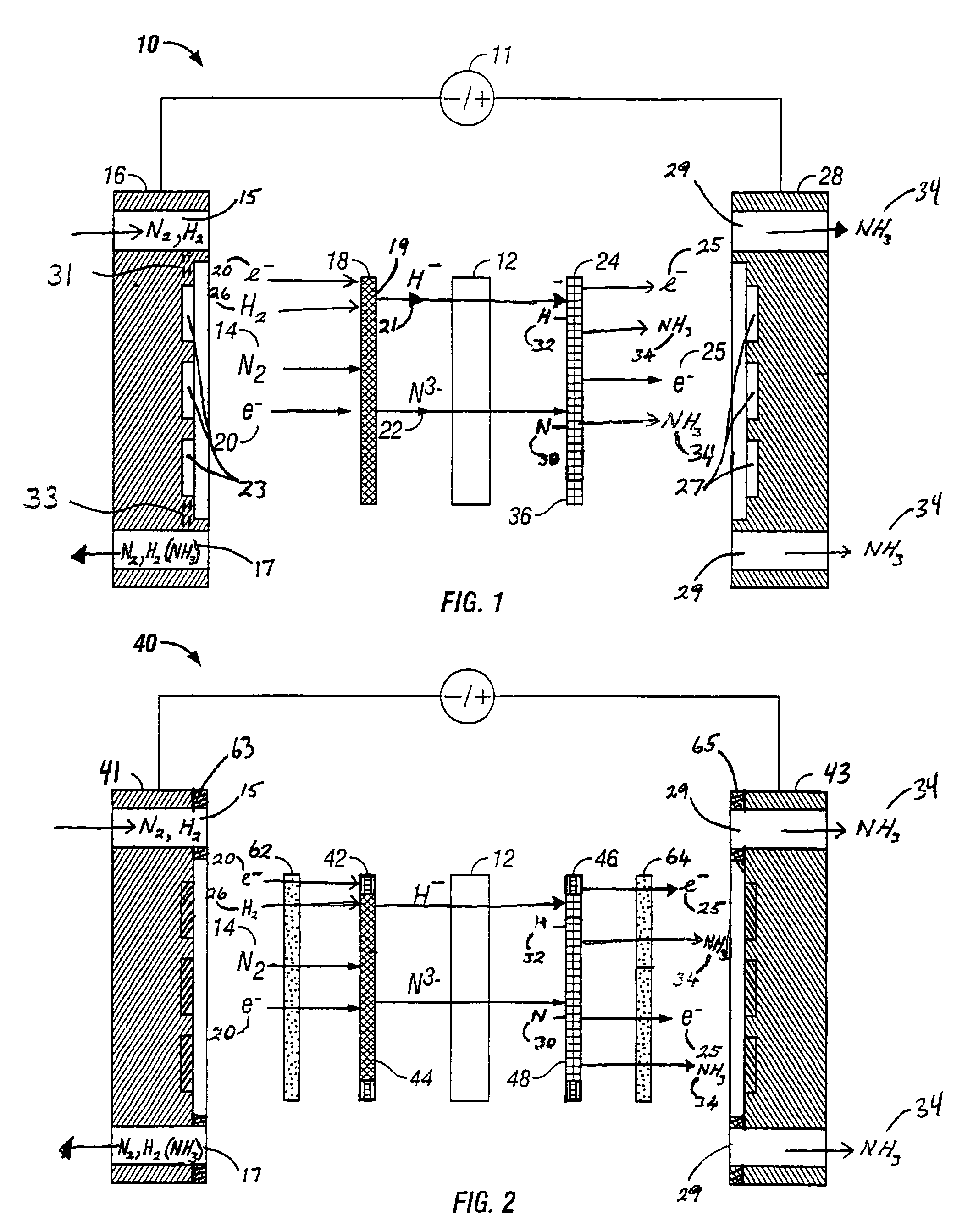
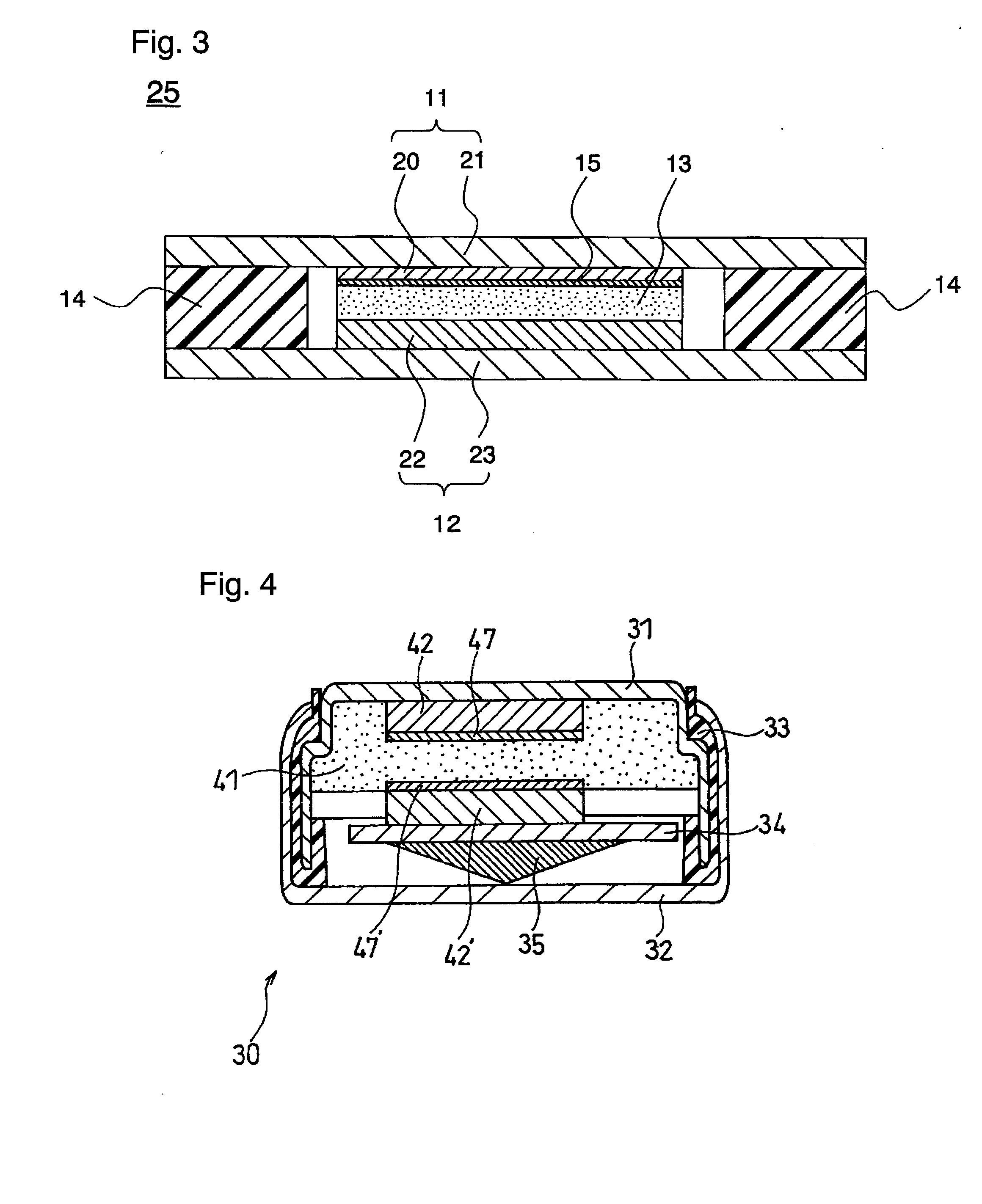
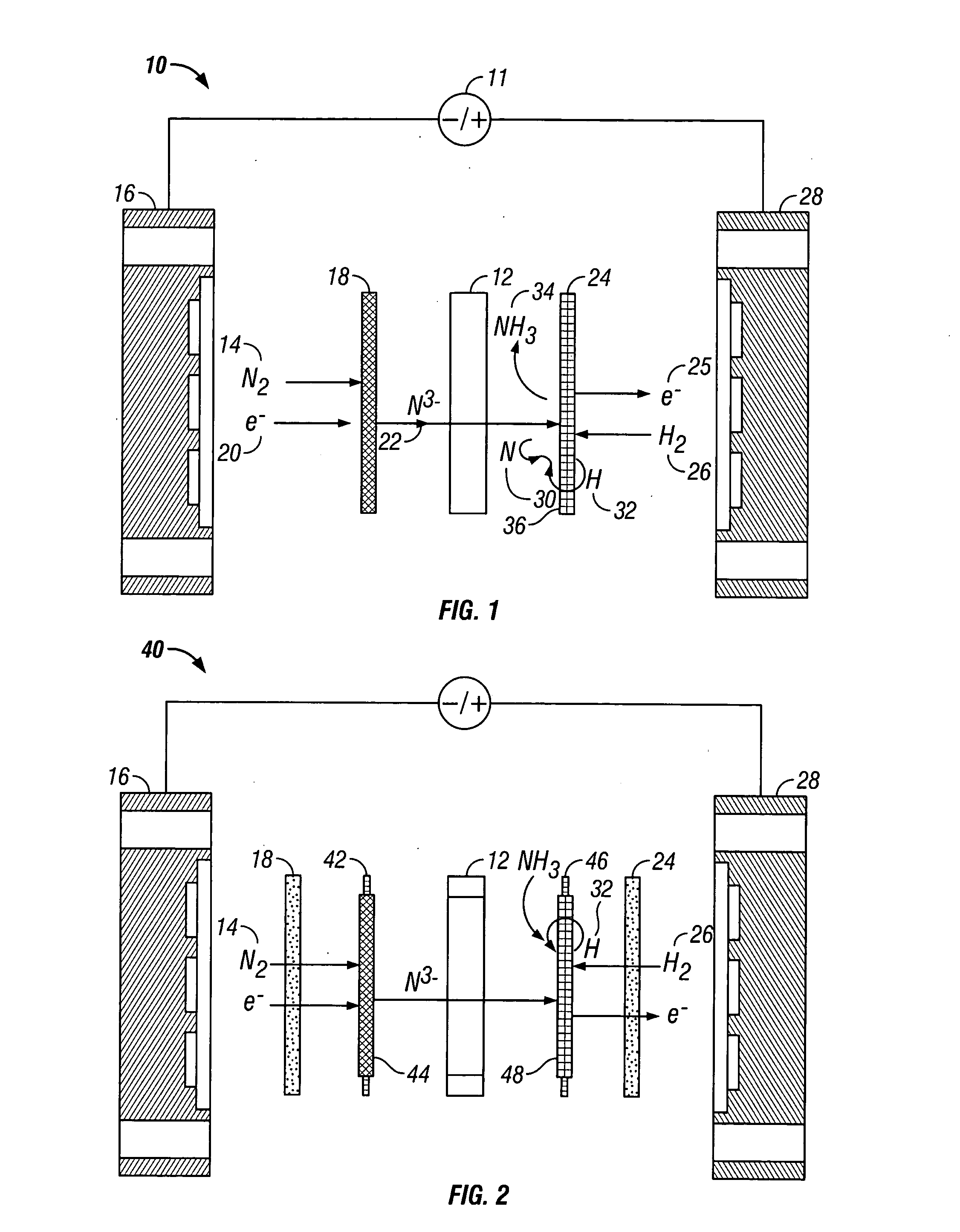
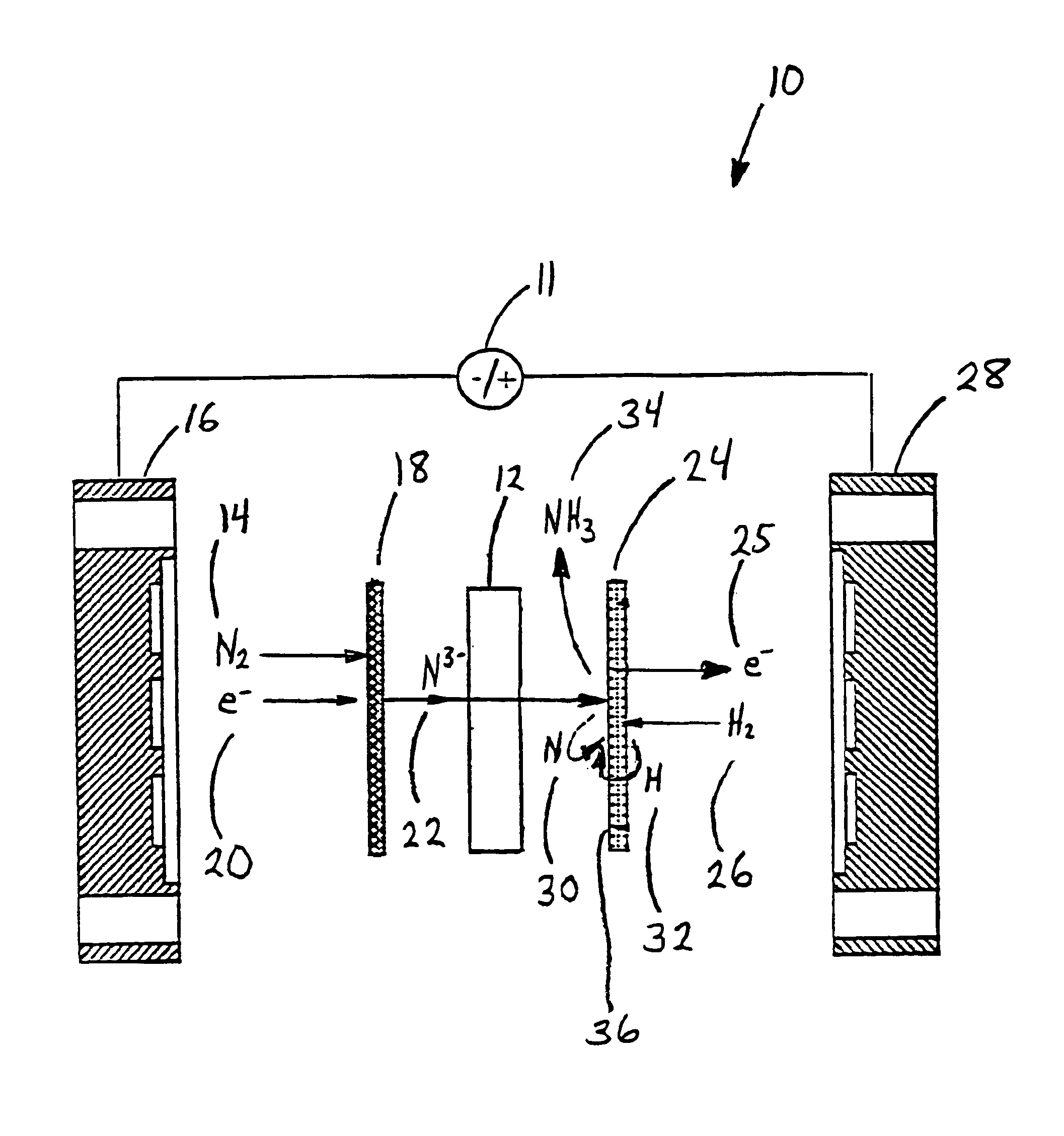
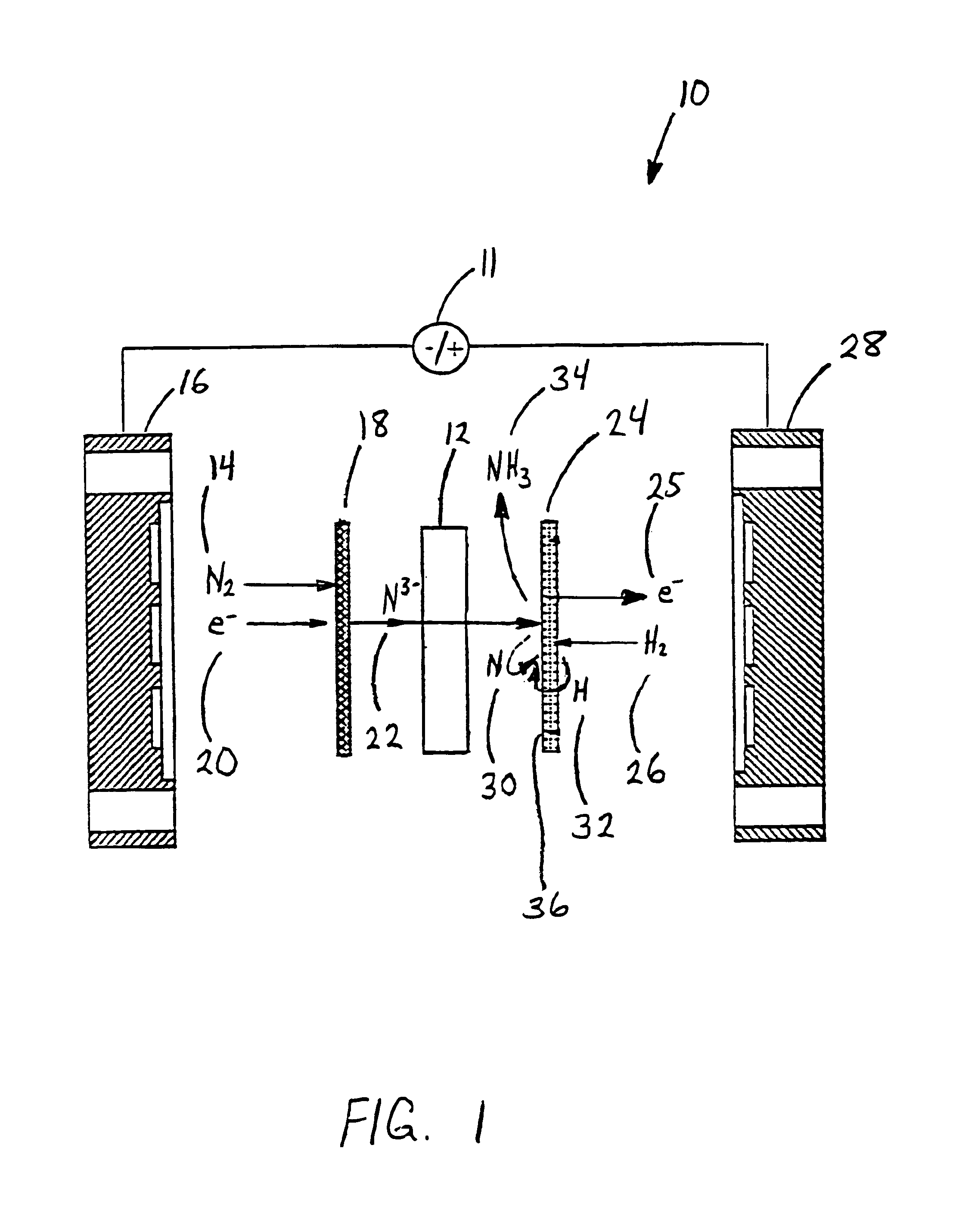
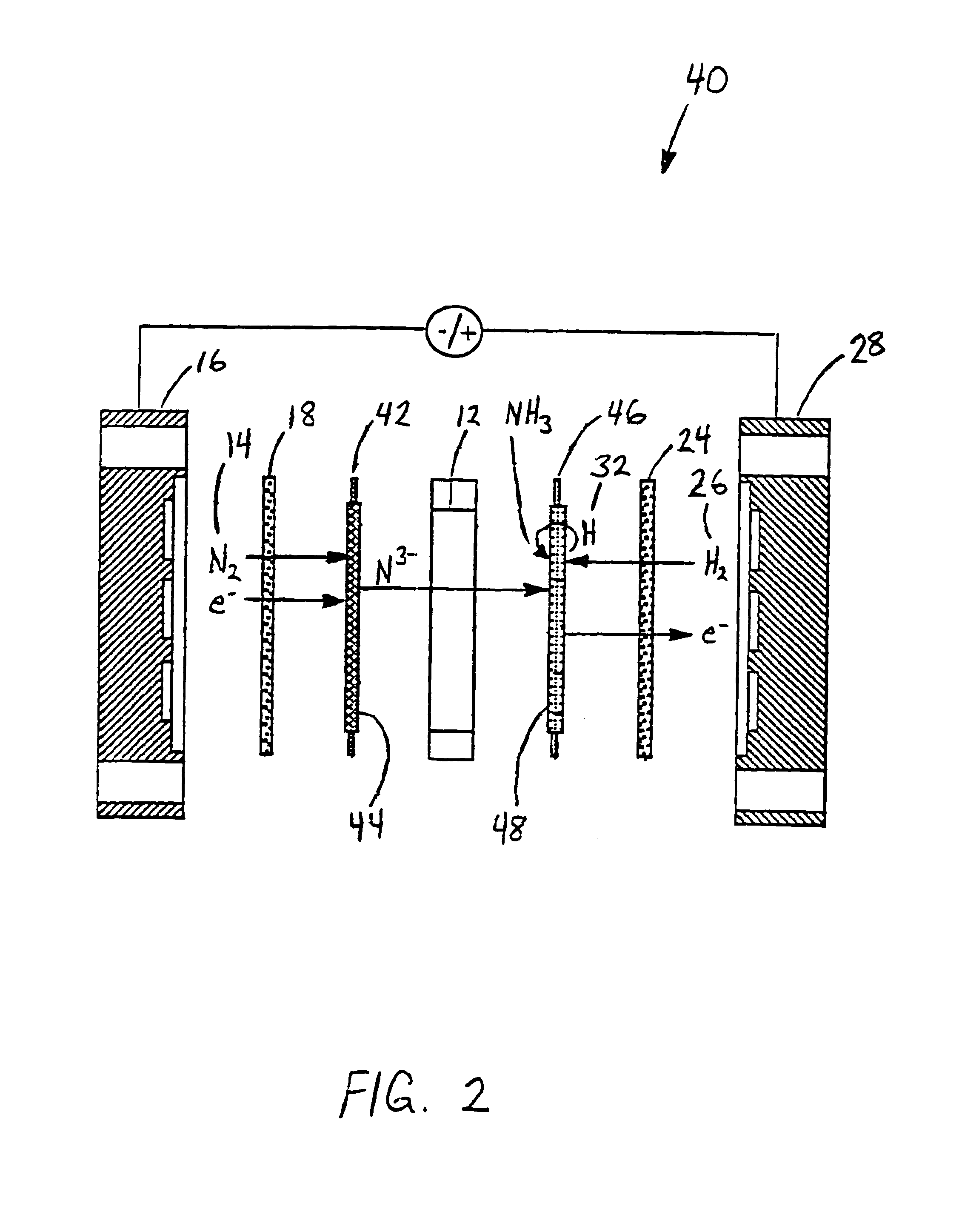
Electrochemical synthesis of ammonia
A method for the anodic electrochemical synthesis of ammonia gas. The method comprises providing an electrolyte between an anode and a cathode, providing nitrogen and hydrogen gases to the cathode, oxidizing negatively charged nitrogen-containing species and negatively charged hydrogen-containing species present in the electrolyte at the anode to form adsorbed nitrogen species and adsorbed hydrogen species, respectively, and reacting the adsorbed nitrogen species with the adsorbed hydrogen species to form ammonia. Nitrogen and hydrogen gases may be provided through a porous cathode substrate. The negatively charged nitrogen-containing species in the electrolyte may be produced by reducing nitrogen gas at the cathode and / or by supplying a nitrogen-containing salt, such as lithium nitride, into the molten salt electrolyte. Similarly, the negatively charged hydrogen-containing species in the electrolyte may be produced by reducing hydrogen gas at the cathode and / or by supplying a hydrogen-containing salt, such as lithium hydride, into the molten salt electrolyte.
Owner:LYNTECH INC
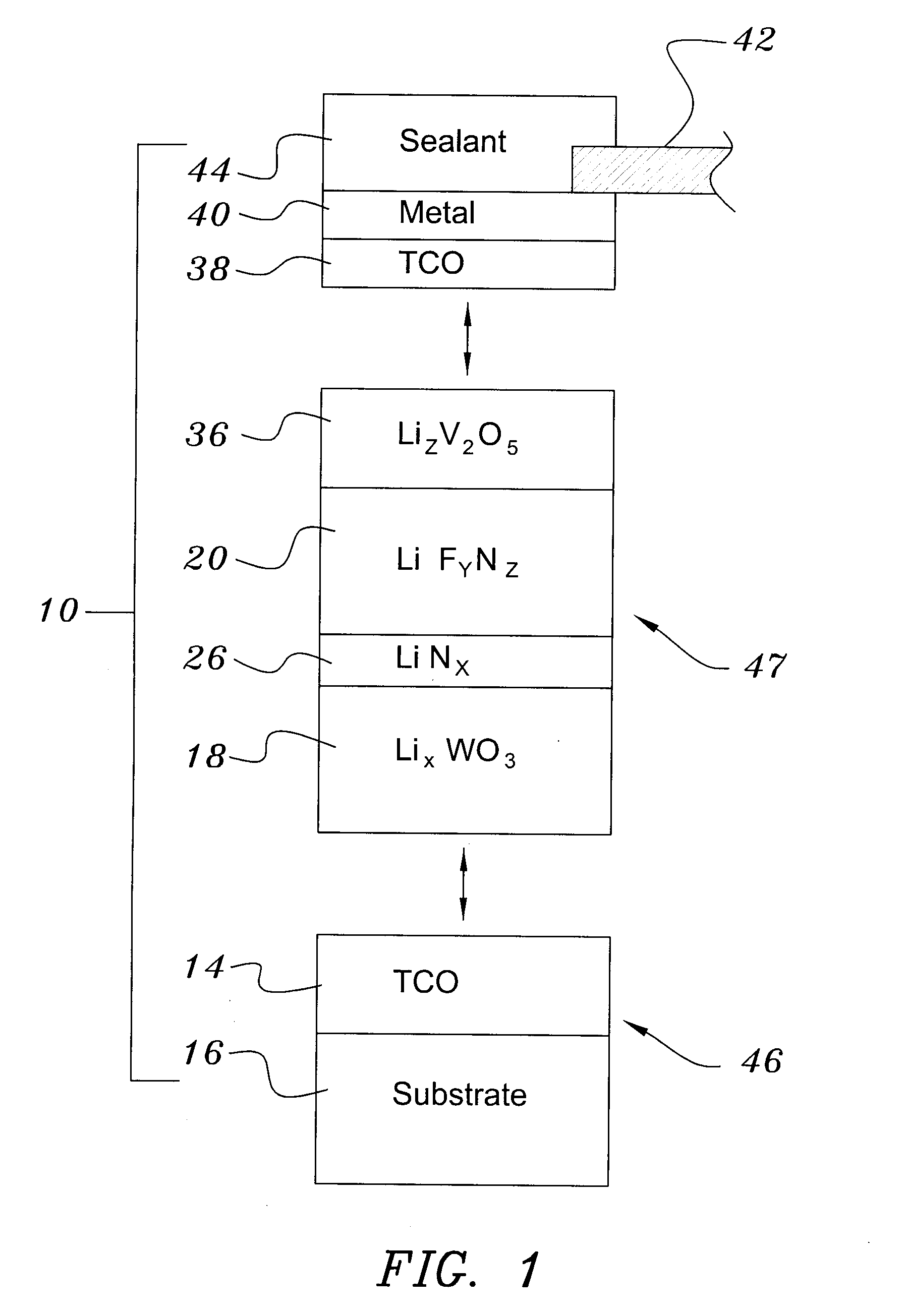
Electrochromic Device with Self-forming Ion transfer Layer and Lithium Fluoro-Nitride Electrolyte
ActiveUS20070292606A1Solve many processesReduce usageCoatingsSpecial surfacesEvaporationElectrochromism
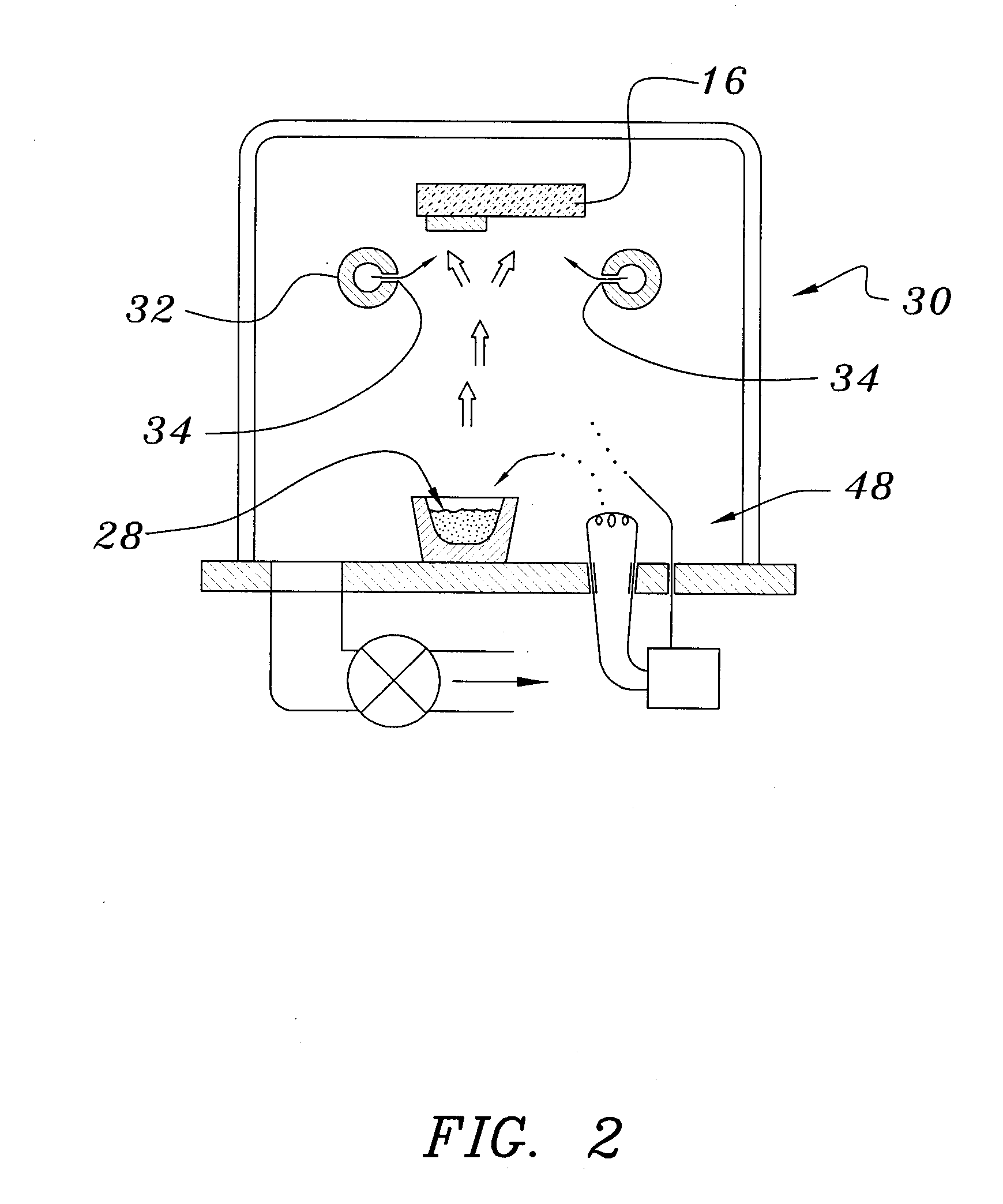
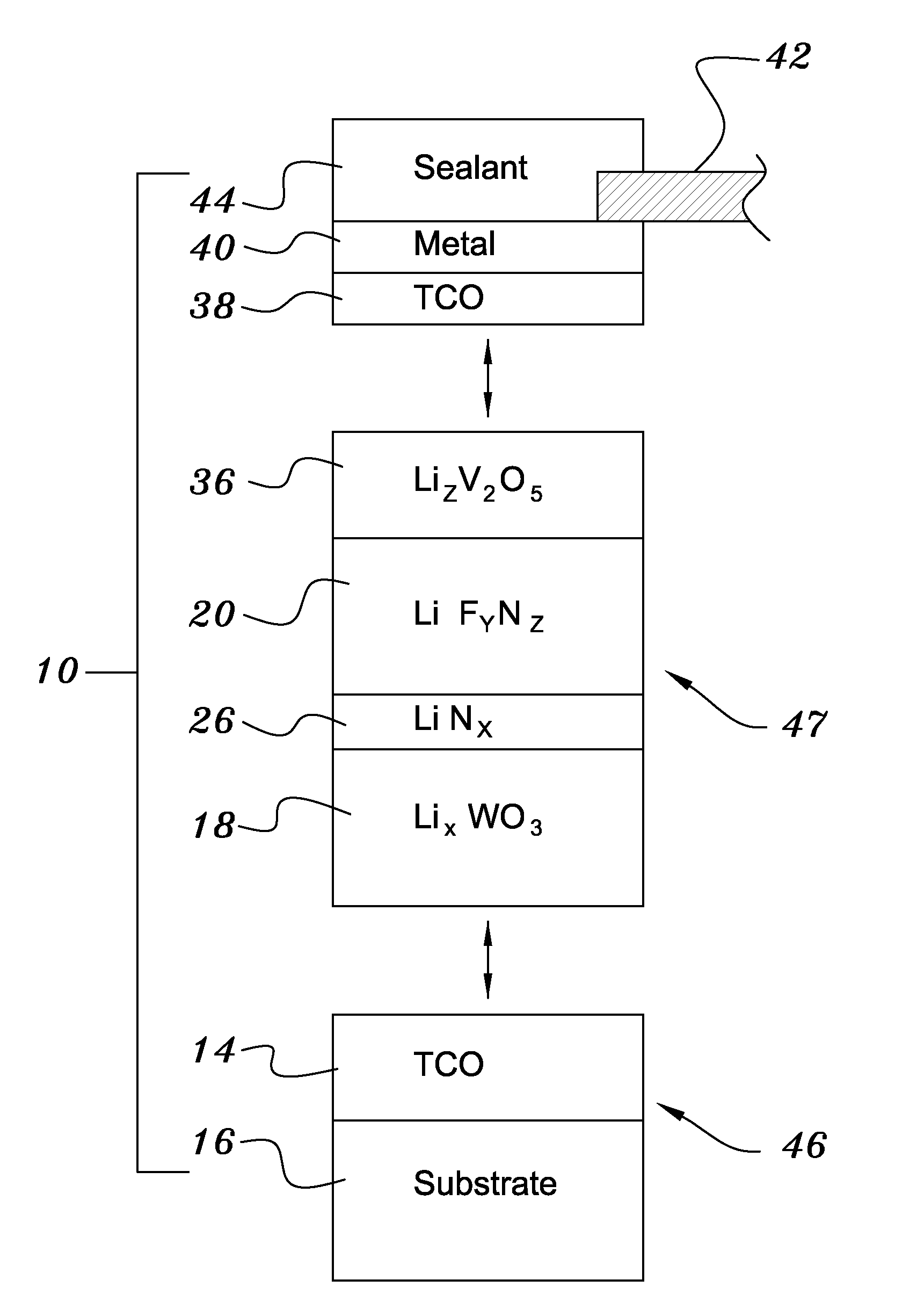
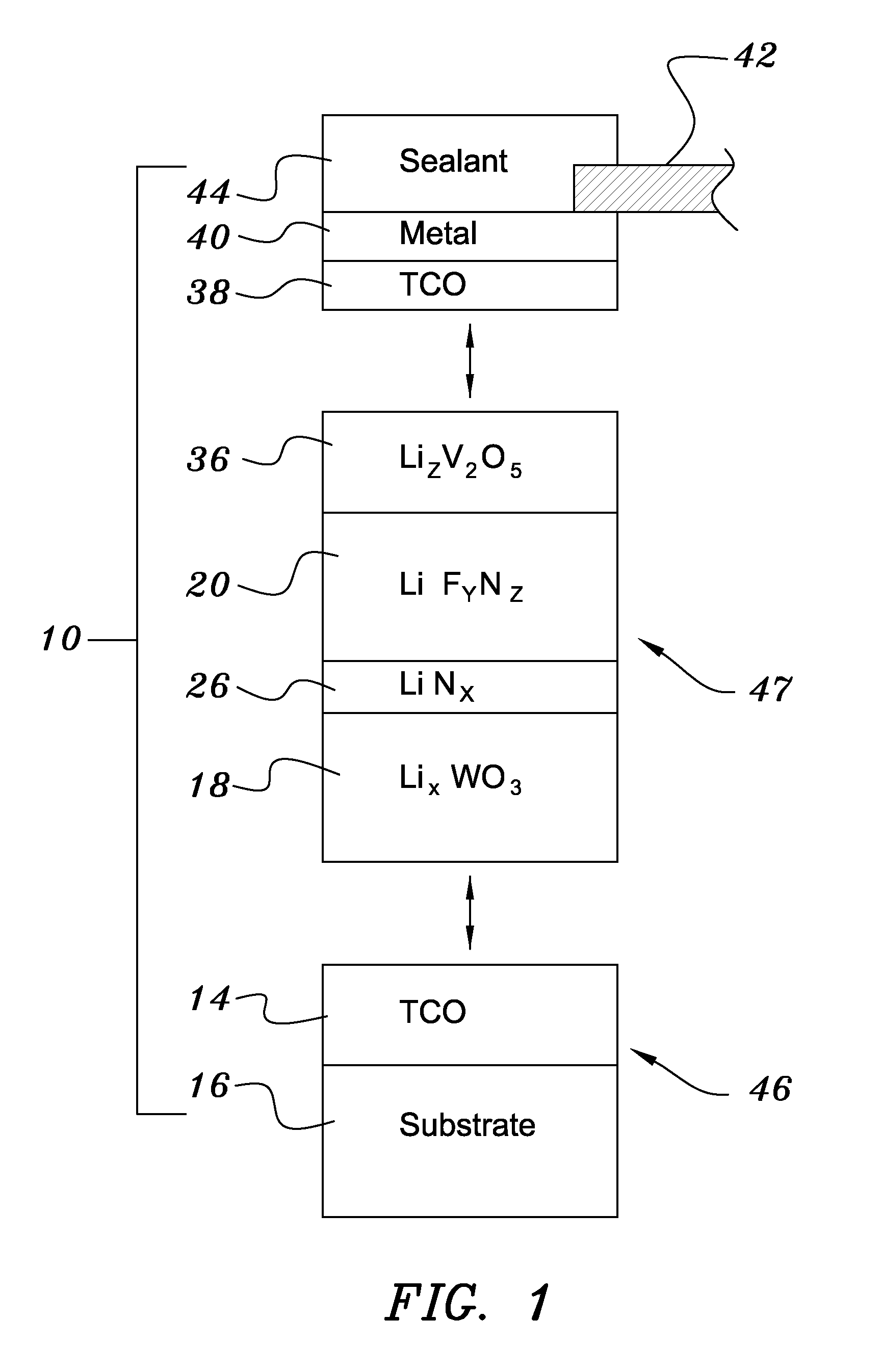
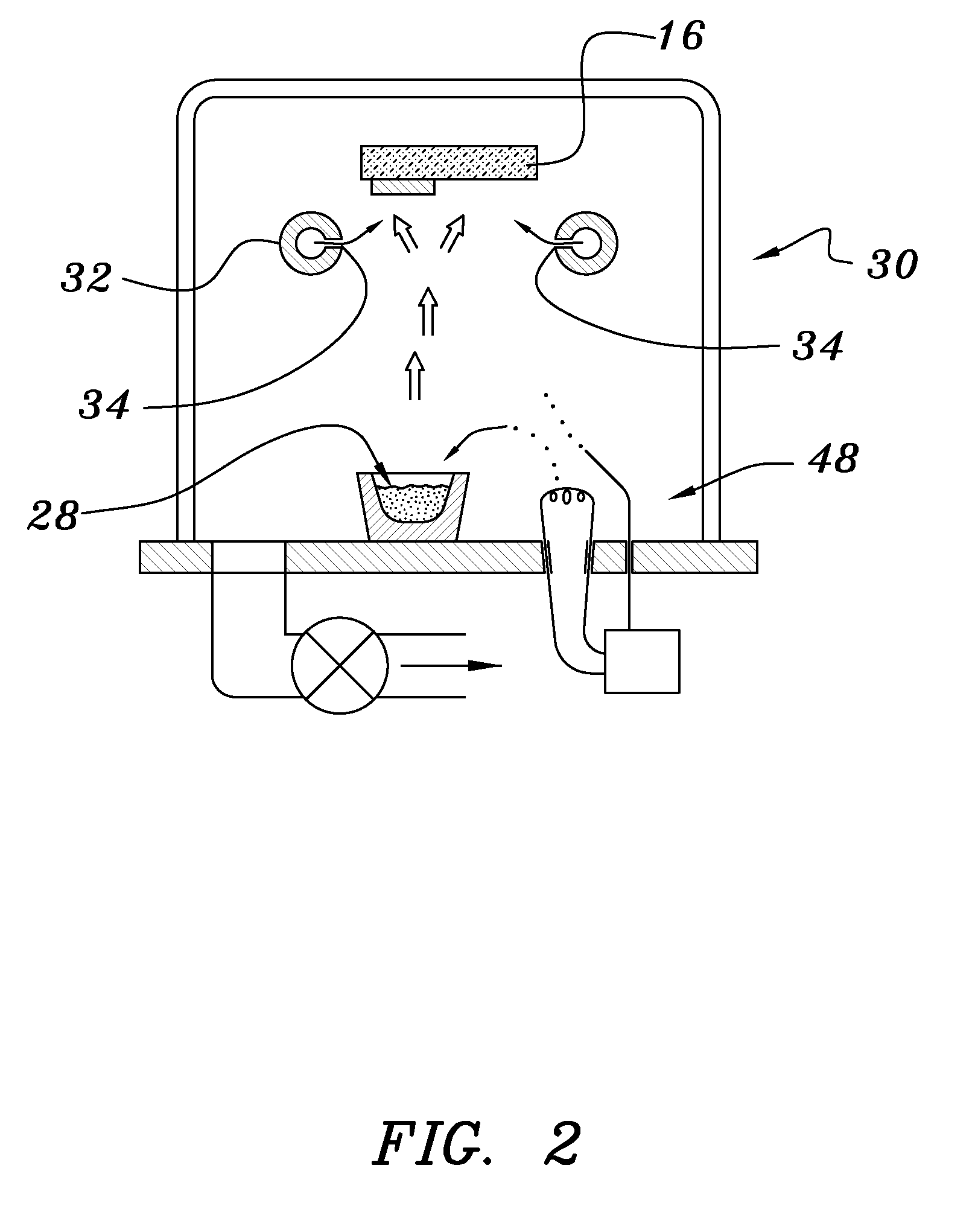
A method of preparing an electrochromic device involves forming multiple layers of selected materials on a substrate in a vacuum processing chamber. A first of these layers is an electrode layer deposited directly on the substrate and used for making contact to a subsequently deposited precursor film, preferably tungsten oxide, from which an electrochromic layer is formed by lithium loading in the presence of ionized nitrogen. This not only forms the electrochromic layer by diffusion of the lithium into the tungsten oxide, but also forms a thin lithium nitride ion transfer layer on the then exposed surface. Subsequently, a lithium fluoro-nitride electrolyte layer is formed on the ion transfer layer by evaporation from a lithium fluoride source in the presence of ionized nitrogen. An ion storage layer, which may be a vanadium oxide and a transparent second electrode layer are subsequently vacuum deposited.
Owner:ECLIPSE ENERGY SYST
Electrochromic device with self-forming ion transfer layer and lithium-fluoro-nitride electrolyte
ActiveUS7265891B1Solve many processesReduce usageElectrolytic capacitorsNon-linear opticsEvaporationElectrochromism
An electrochromic device is prepared by forming multiple layers of selected materials on a substrate in a vacuum processing chamber. A first of these layers is an electrode layer deposited directly on the substrate and used for making contact to a subsequently deposited precursor film, preferably tungsten oxide, from which an electrochromic layer is formed by lithium loading in the presence of ionized nitrogen. This not only forms the electrochromic layer by diffusion of the lithium into the tungsten oxide, but also forms a thin lithium nitride ion transfer layer on the then exposed surface. Subsequently, a lithium fluoro-nitride electrolyte layer is formed on the ion transfer layer by evaporation from a lithium fluoride source in the presence of ionized nitrogen. An ion storage layer, which may be a vanadium oxide and a transparent second electrode layer are subsequently vacuum deposited.
Owner:ECLIPSE ENERGY SYST
Electrode structure and method for making the same
Electrode structures, and more specifically, electrode structures for use in electrochemical cells, are provided. The electrode structures described herein may include one or more protective layers. In one set of embodiments, a protective layer may be formed by exposing a lithium metal surface to a plasma comprising ions of a gas to form a ceramic layer on top of the lithium metal. The ceramic layer may be highly conductive to lithium ions and may protect the underlying lithium metal surface from reaction with components in the electrolyte. In some cases, the ions may be nitrogen ions and a lithium nitride layer may be formed on the lithium metal surface. In other embodiments, the protective layer may be formed by converting lithium to lithium nitride at high pressures. Other methods for forming protective layers are also provided.
Owner:SION POWER CORP
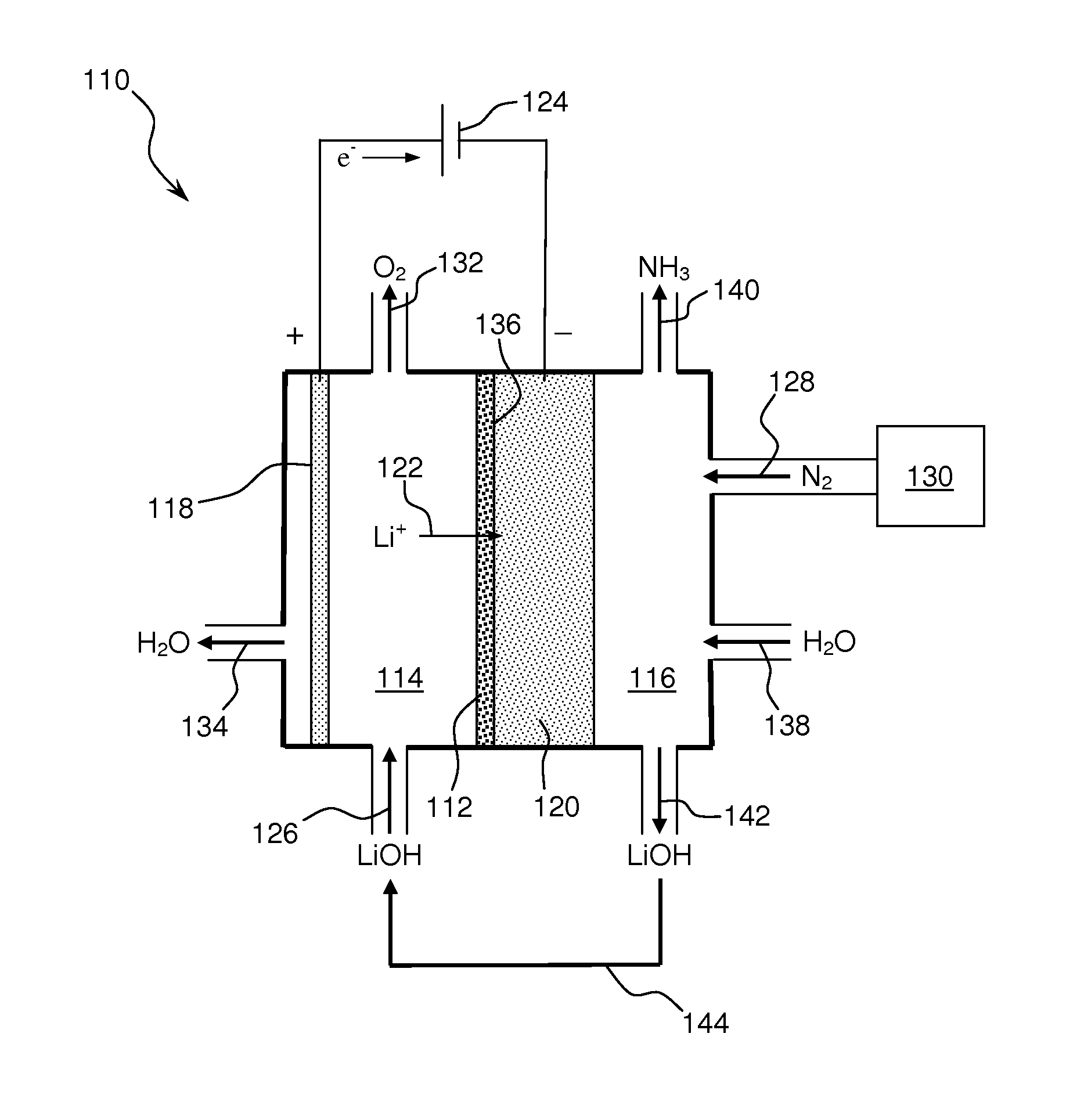
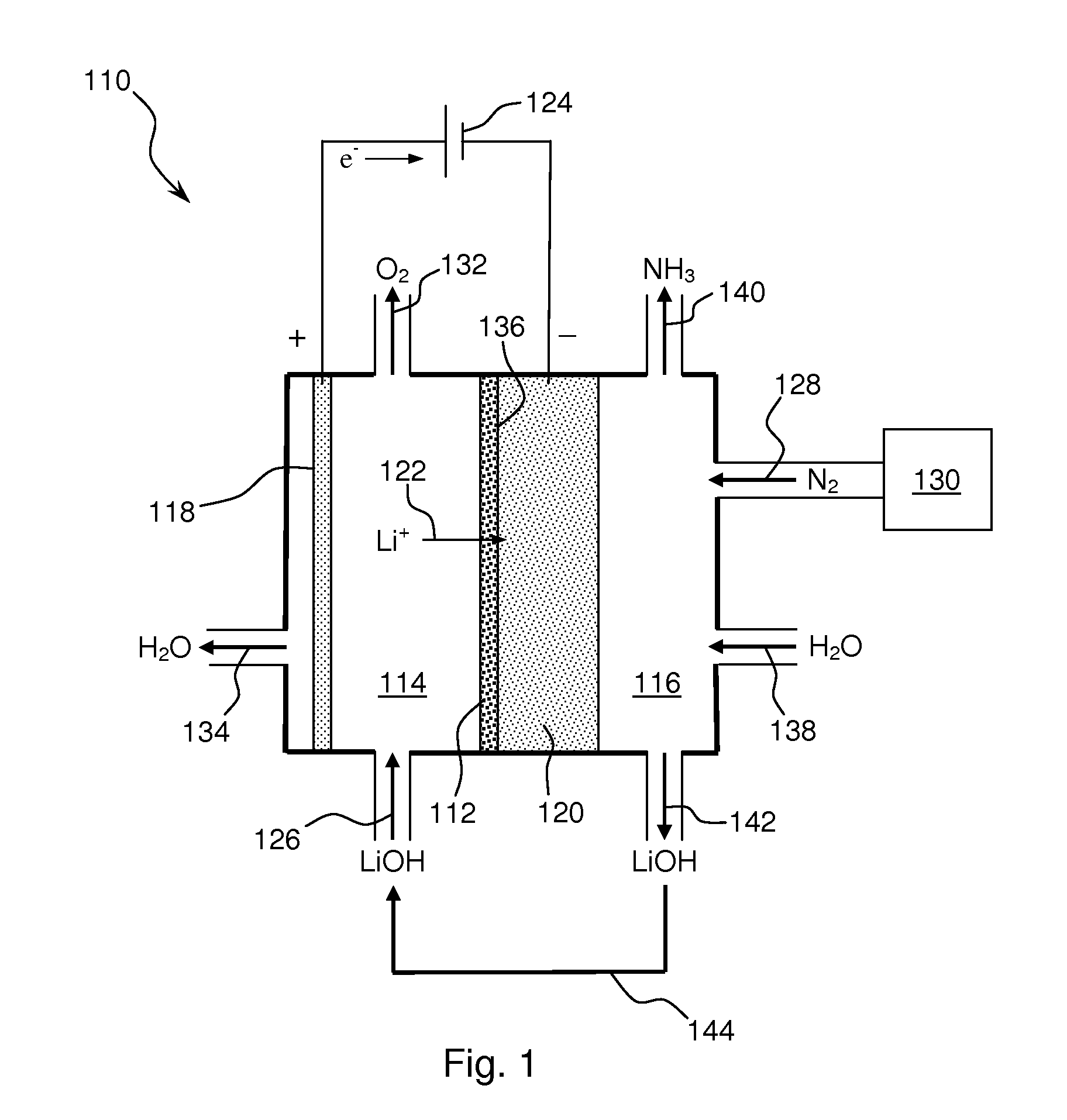
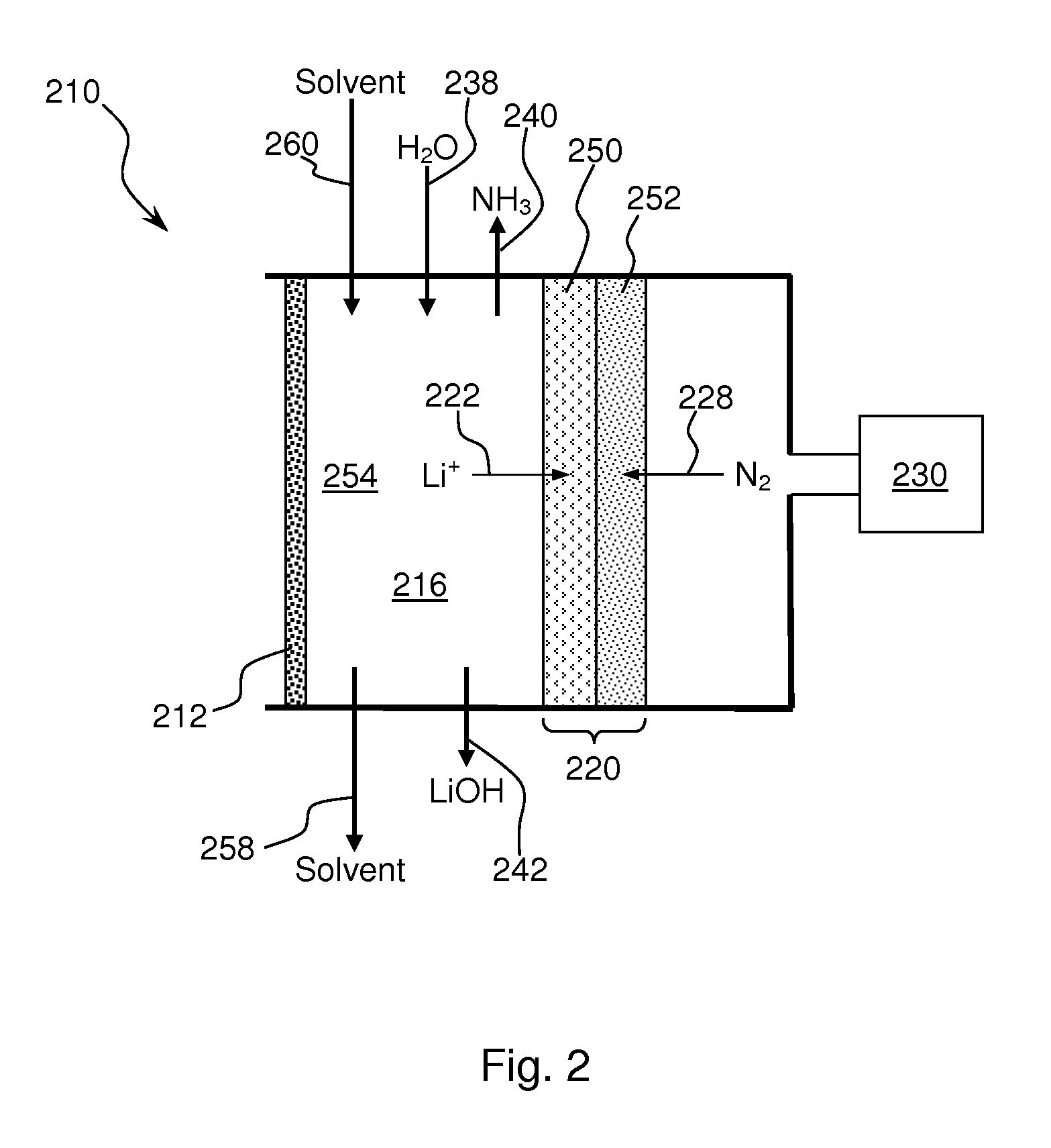
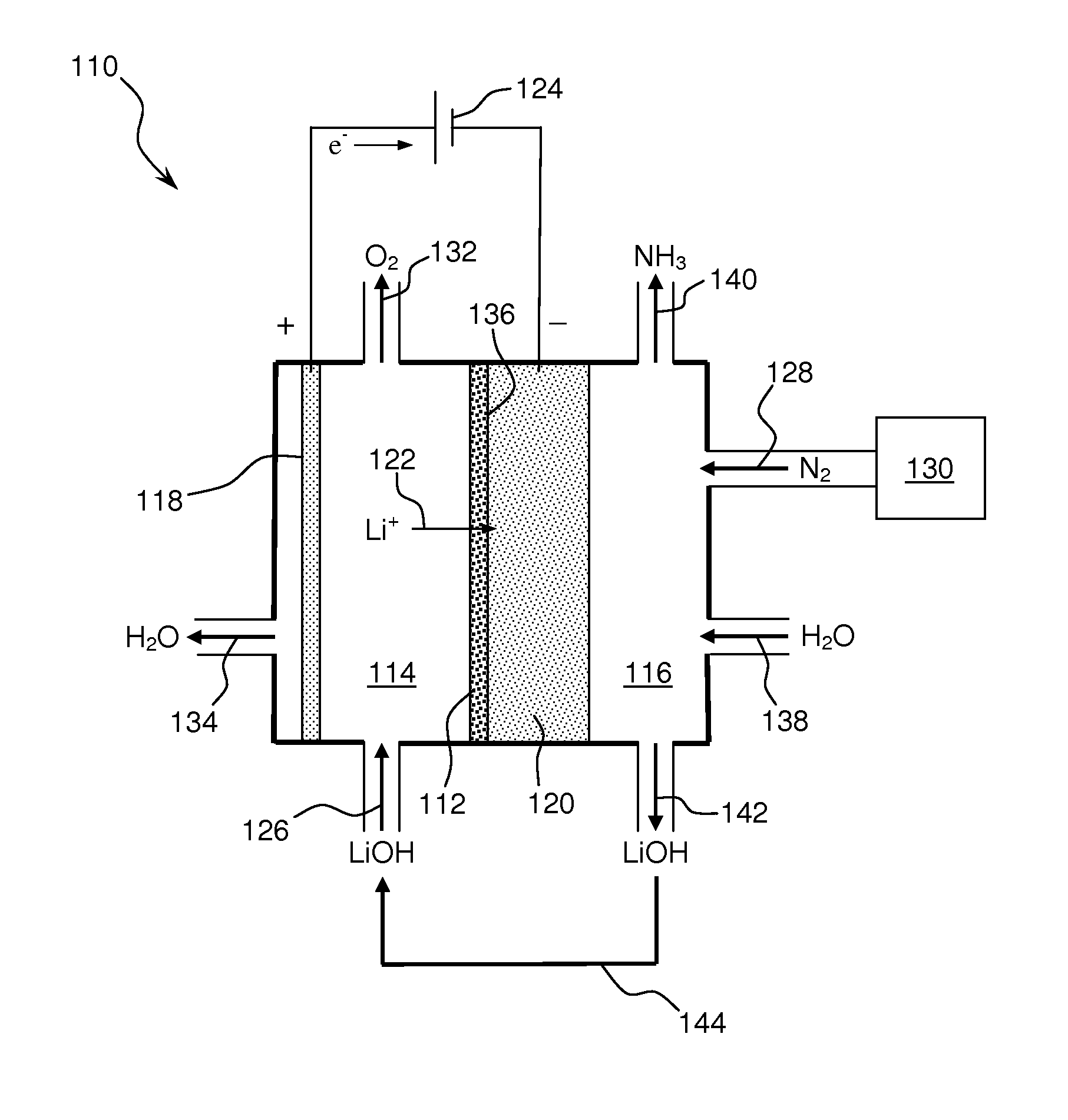
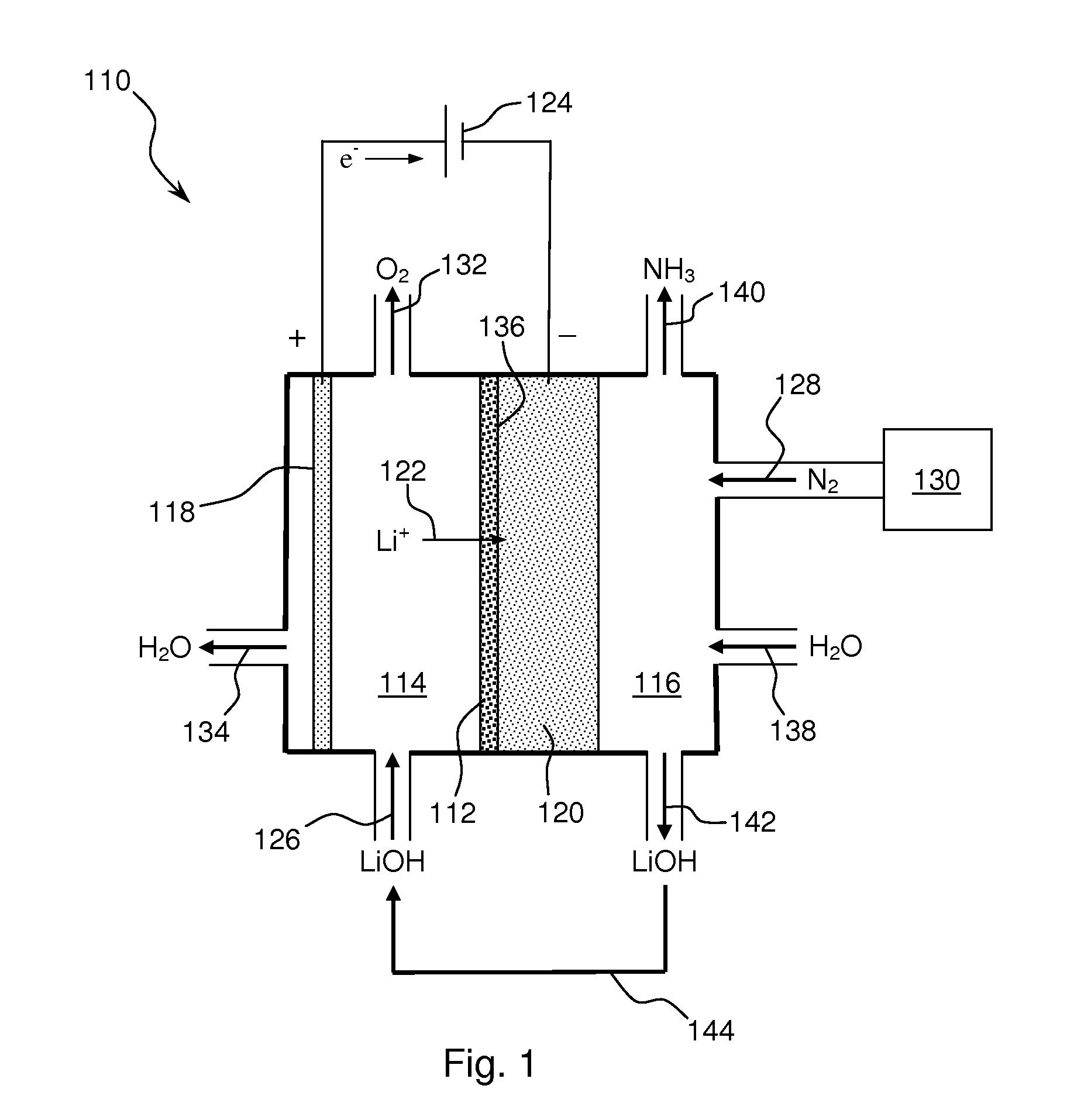
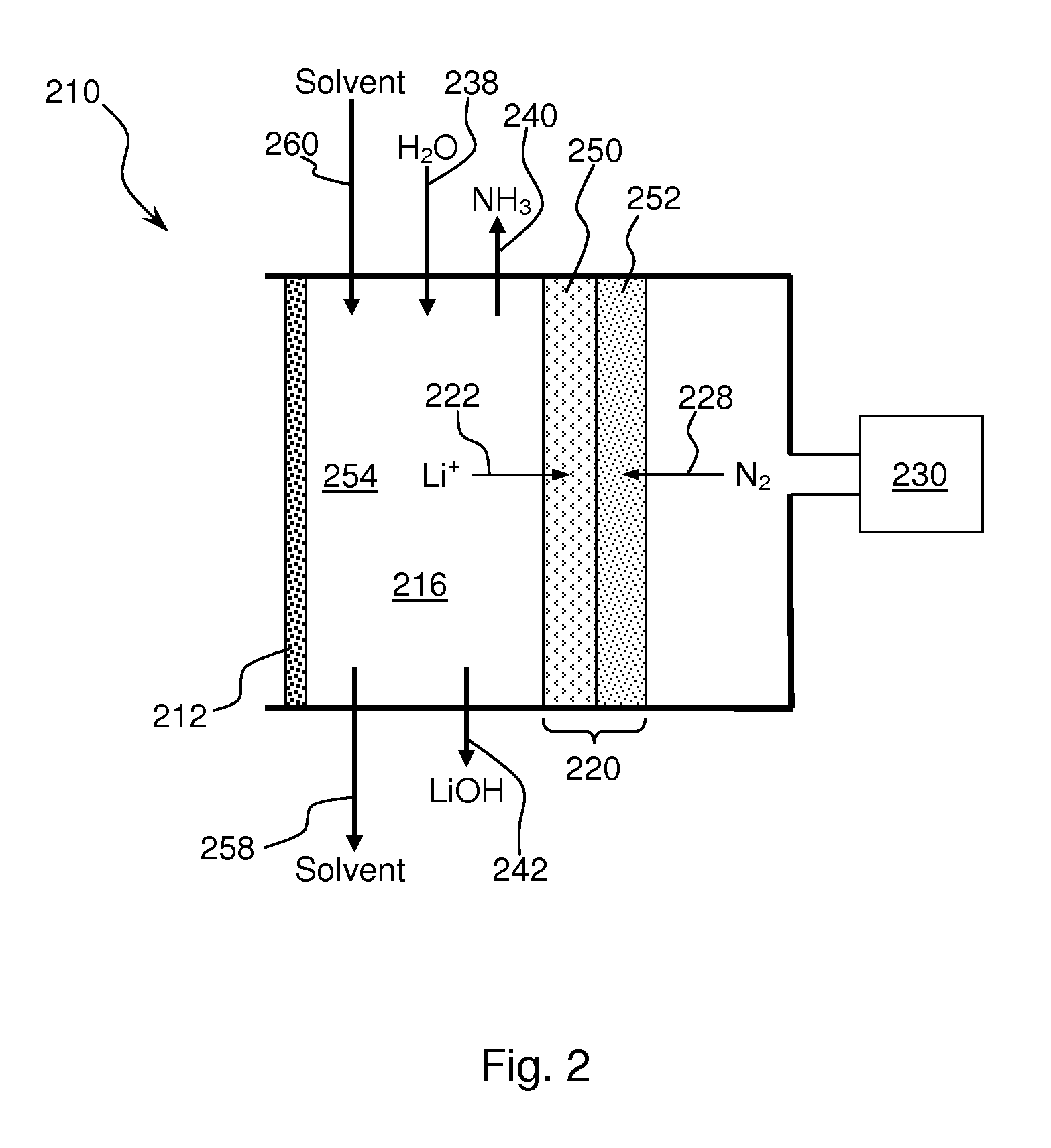
Ammonia synthesis using lithium ion conductive membrane
Ammonia is synthesized using electrochemical and non-electrochemical reactions. The electrochemical reactions occur in an electrolytic cell having a lithium ion conductive membrane that divides the electrochemical cell into an anolyte compartment and a catholyte compartment. The catholyte compartment includes a porous cathode closely associated with the lithium ion conductive membrane. The overall electrochemical reaction is: 6LiOH+N2→Li3N (s)+3H2O+3 / 2O2. The nitrogen may be produced by a nitrogen generator. The non-electrochemical reaction involves reacting lithium nitride with water and / or steam as follows: Li3N (s)+3H2O→3LiOH+NH3 (g). The ammonia is vented and collected. The lithium hydroxide is preferably recycled and introduced into the anolyte compartment. The electrolytic cell is shut down prior to reacting the lithium nitride with water. The cathode is preferably dried prior to start up of the electrolytic cell and electrolyzing Li+ and N2 at the cathode.
Owner:ENLIGHTEN INNOVATIONS INC
Electrochemical synthesis of ammonia
A method for the anodic electrochemical synthesis of ammonia gas. The method comprises providing an electrolyte between an anode and a cathode, providing nitrogen and hydrogen gases to the cathode, oxidizing negatively charged nitrogen-containing species and negatively charged hydrogen-containing species present in the electrolyte at the anode to form adsorbed nitrogen species and adsorbed hydrogen species, respectively, and reacting the adsorbed nitrogen species with the adsorbed hydrogen species to form ammonia. Nitrogen and hydrogen gases may be provided through a porous cathode substrate. The negatively charged nitrogen-containing species in the electrolyte may be produced by reducing nitrogen gas at the cathode and / or by supplying a nitrogen-containing salt, such as lithium nitride, into the molten salt electrolyte. Similarly, the negatively charged hydrogen-containing species in the electrolyte may be produced by reducing hydrogen gas at the cathode and / or by supplying a hydrogen-containing salt, such as lithium hydride, into the molten salt electrolyte.
Owner:LYNTECH INC
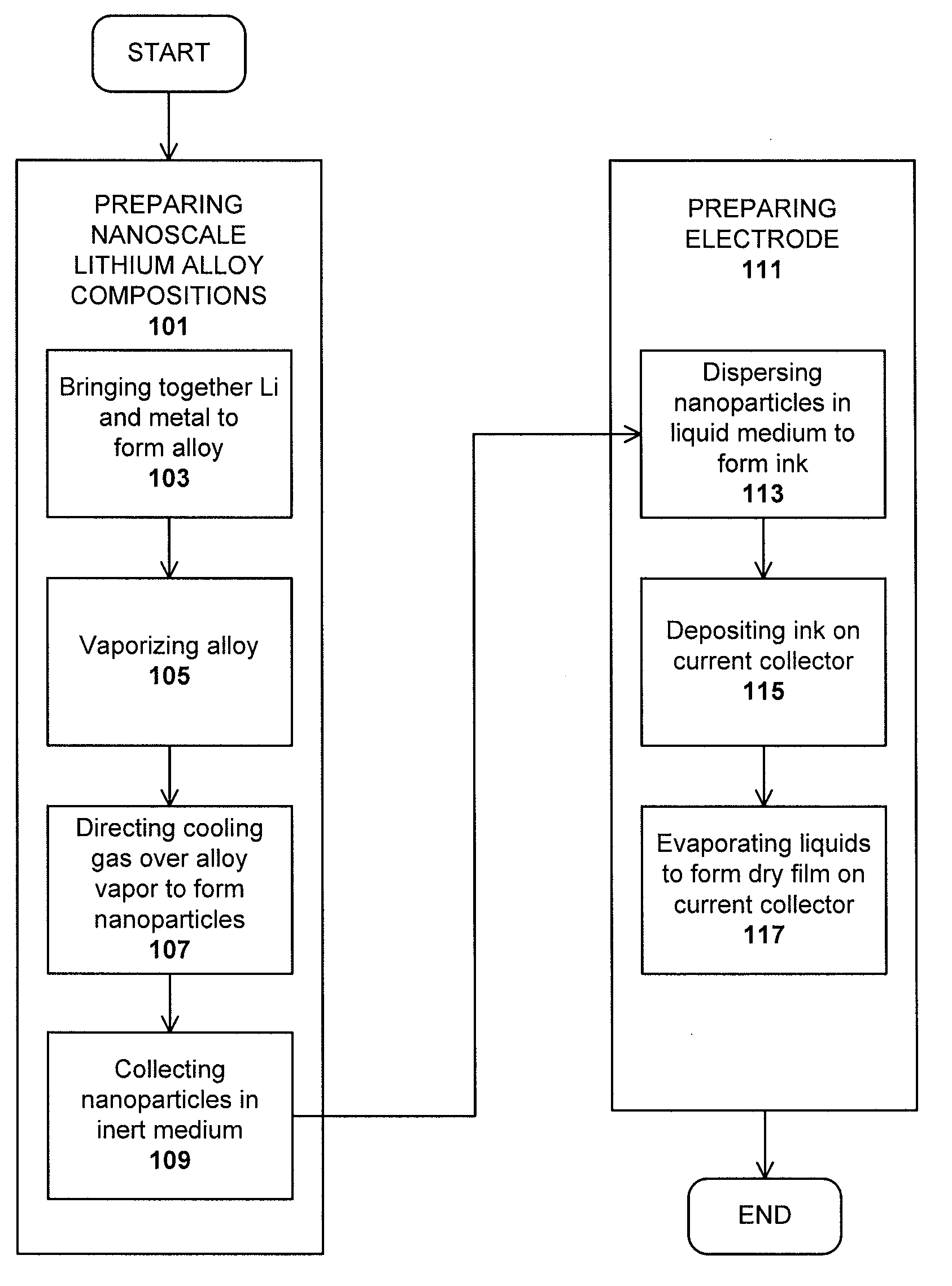
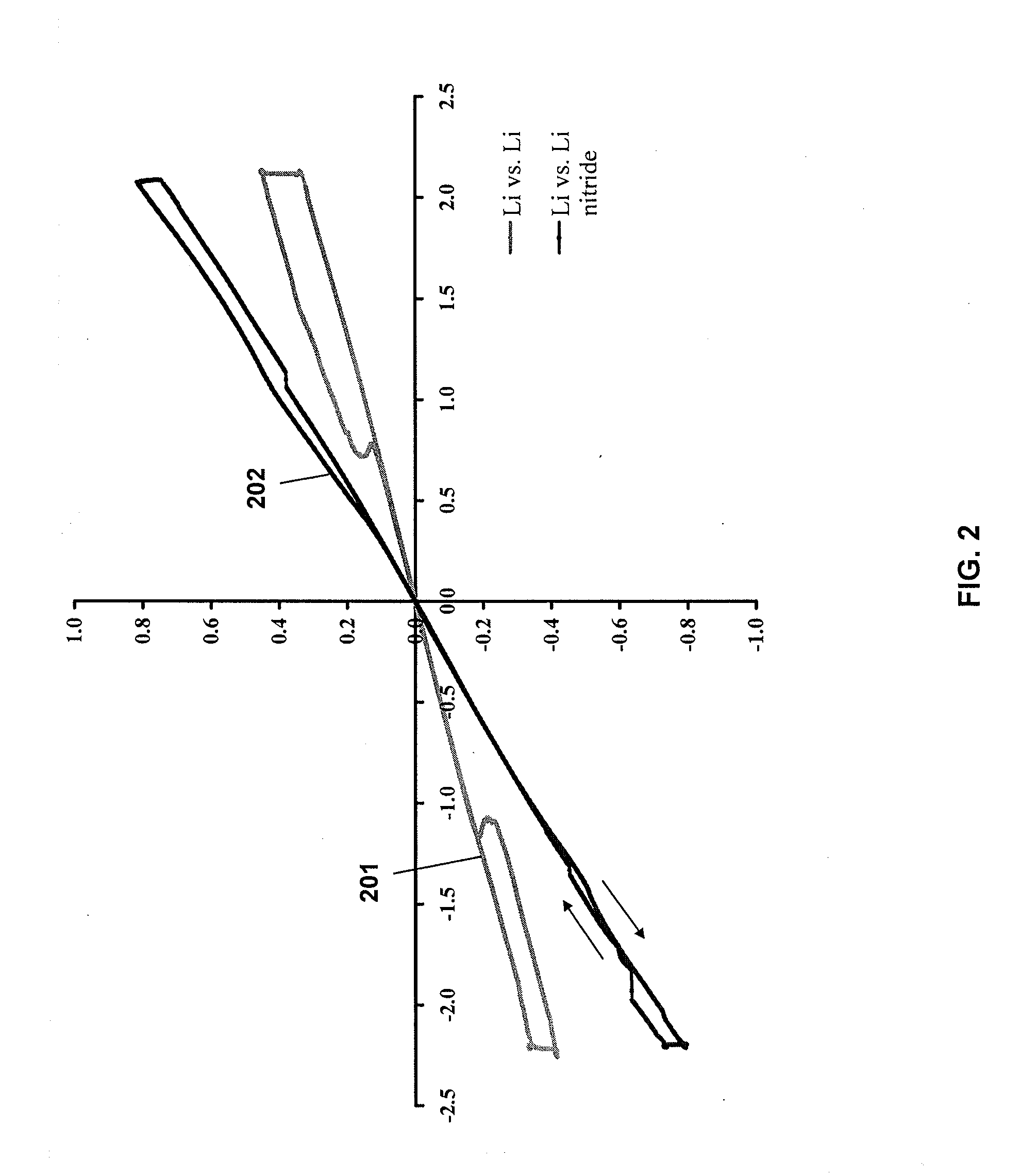
Lithium nanoparticle compositions for use in electrochemical applications
InactiveUS20100156353A1Uniform nanoscale particle sizeBatteries circuit arrangementsNon-aqueous electrolyte accumulator electrodesParticle compositionLithium nitride
Nanoscale lithium compositions are disclosed which are suitable for use in electrochemical applications such as electrodes and batteries. The compositions can include nanoparticles having lithium metal and / or lithium alloy cores. A shell material is contemplated comprising lithium nitride or another material that conducts lithium ions. Methods of preparing lithium compositions and methods of preparing electrodes comprising lithium compositions are further disclosed. The crystal structure of the nanoscale lithium compositions is preferably body centered cubic, allowing low volume expansion and high diffusivity of lithium from or into the core structures during discharge and charge processes, respectively.
Owner:BRICOLEUR PARTNERS LP
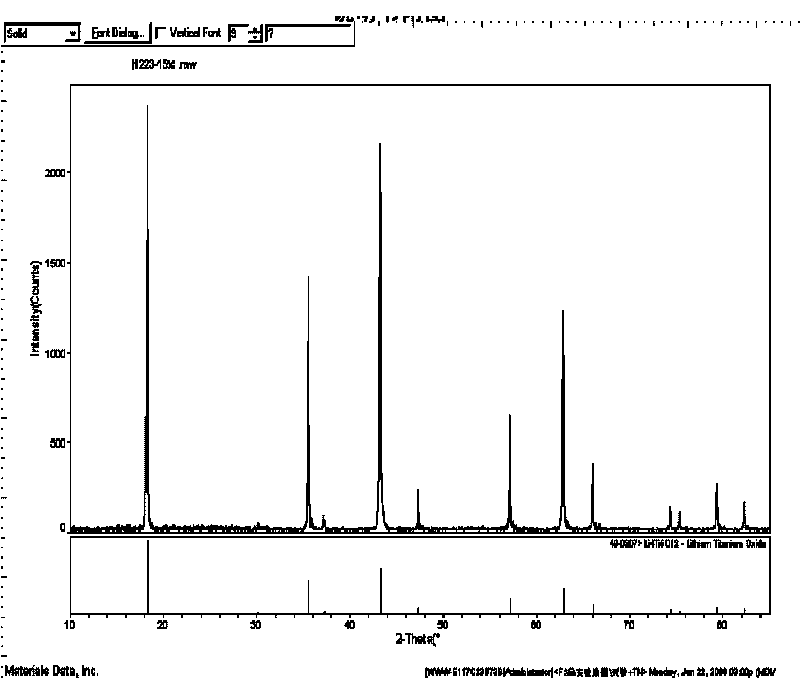
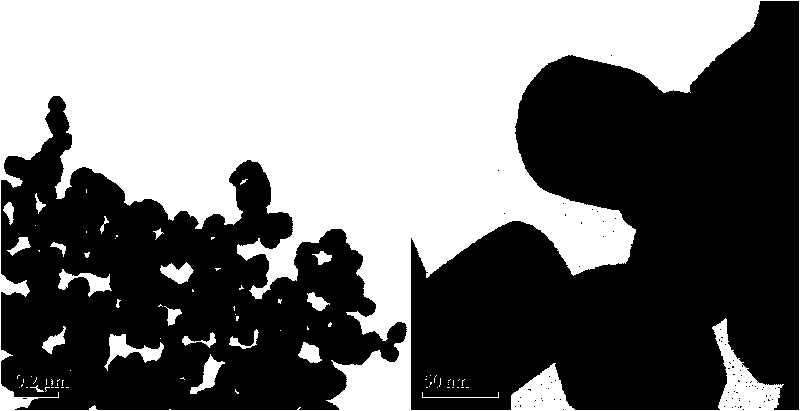
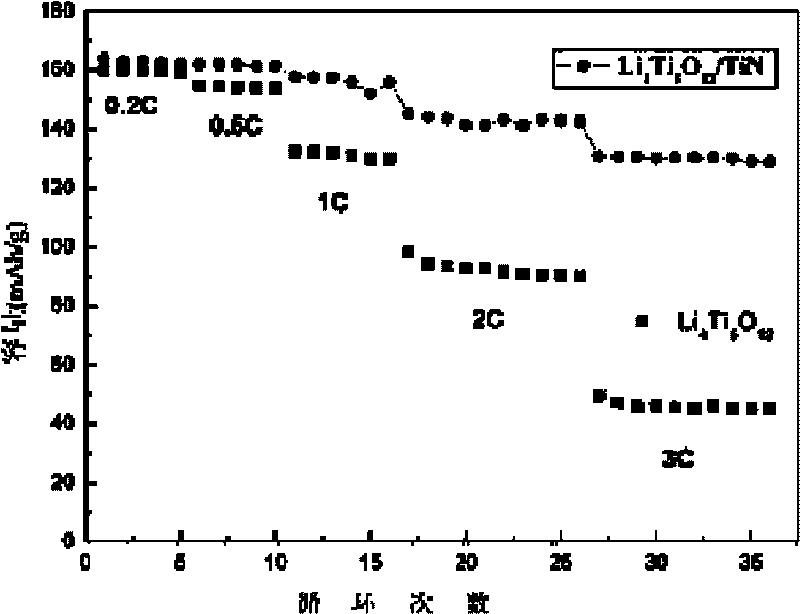
Method for preparing surface self-grown titanium nitride conducting film modified lithium titanate
The invention relates to a method for preparing a surface self-grown titanium nitride conducting film modified lithium titanate, which comprises the following steps of: uniformly mixing lithium titanate powder with solid nitrogen source in proportion in a dispersion medium in a mode of ultrasound or ball milling, drying the obtained slurry at the temperature of between70 and 120 DEG C, raising temperature to 600 to 900 DEG C in the inert protective atmosphere after grinding, preserving the heat for 10min to 1h, and then cooling together with a furnace to obtain the surface self-grown titanium nitride conducting film modified lithium titanate. Through heat nitrogen reaction, a layer of high-conductive film TiN is self-grown on the surface of the lithium titanate; the TiN film is firmly combined with the lithium titanate; and the prepared high-conductive Li4Ti5O12 / TiN material has high multiplying power of charge-discharge property and excellent circulating property. The preparation method has the advantages of low cost, simple operation and safety, and easy realization of mass production.
Owner:CENT SOUTH UNIV
All solid-state polymer battery
InactiveUS20100068628A1Increase resistanceReduce interfacial resistanceCell electrodesSolid electrolyte cellsPolymer scienceHigh rate
An all solid-state polymer battery uses: 1) a lithium based negative electrode active material including crystal grains and crystal grain boundaries, wherein at least part of the crystal grain boundaries are exposed on a surface of the lithium-based negative electrode active material, and the area of the exposed surface of the crystal grain boundaries is 0.02 to 0.5 cm2 per square centimeter of surface; 2) a dry polymer electrolyte including a specific ethylene glycol ether, a polymer containing electron-donating oxygen atoms in the skeleton, and a lithium salt; or 3) an amorphous lithium nitride layer formed between the negative electrode and the polymer electrolyte. This reduces the resistance at the interface between the negative electrode and the polymer electrolyte, thereby providing an all solid-state polymer battery with high capacity and excellent cycle characteristics. This also suppresses an increase in internal resistance during storage, thereby providing an all solid-state polymer battery with excellent high-rate discharge characteristics after storage.
Owner:PANASONIC CORP
Hard carbon-metal lithium nitride composite cathode materials and method for preparing same
InactiveCN1877888AReduce the first irreversible capacityImprove the first Coulombic efficiencyElectrode manufacturing processesMixing methodsCapacitanceComposite cathode
The invention discloses a hard carbon-Li metal nitride compound cathode material and making method in the chemical engineering and new source material technological domain, which is characterized by the following: preparing Li-metal nitride based on Li3 powder and metal powder of removing surface oxide; blending hard carbon and Li-metal nitride protected by inert gas atmosphere completely to produce hard carbon-Li metal nitride composite cathode material. The first surplus discharging capacitance can supplement the first irreversible capacitance of hard carbon. The invention possesses little irreversible capacitance, which improves coulomb efficiency and electric chemical activity.
Owner:TSINGHUA UNIV
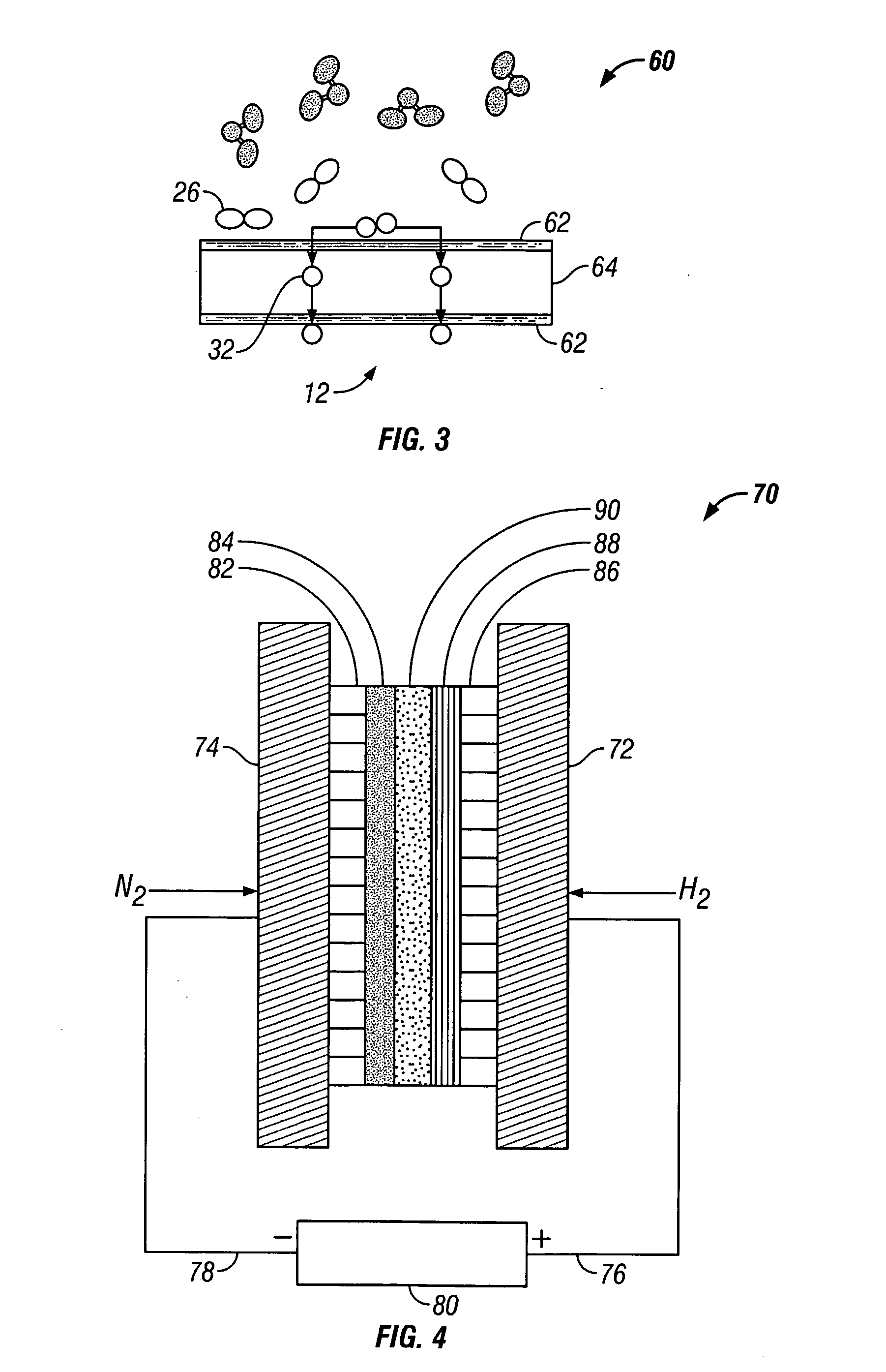
Electrochemical synthesis of ammonia
A method for electrochemical synthesis of ammonia gas comprising providing an electrolyte between an anode and a cathode, providing hydrogen gas to the anode, oxidizing negatively charged nitrogen-containing species present in the electrolyte at the anode to form an adsorbed nitrogen species, and reacting the hydrogen with the adsorbed nitrogen species to form ammonia. Preferably, the hydrogen gas is provided to the anode by passing the hydrogen gas through a porous anode substrate. It is also preferred to produce the negatively charged nitrogen-containing species in the electrolyte by reducing nitrogen gas at the cathode. However, the negatively charged nitrogen-containing species may also be provided by supplying a nitrogen-containing salt, such as lithium nitride, into the molten salt electrolyte mixture in a sufficient amount to provide some or all of the nitrogen consumed in the production of ammonia.
Owner:LYNNTECH
Electrochemical synthesis of ammonia
A method for electrochemical synthesis of ammonia gas comprising providing an electrolyte between an anode and a cathode, providing hydrogen gas to the anode, oxidizing negatively charged nitrogen-containing species present in the electrolyte at the anode to form an adsorbed nitrogen species, and reacting the hydrogen with the adsorbed nitrogen species to form ammonia. Preferably, the hydrogen gas is provided to the anode by passing the hydrogen gas through a porous anode substrate. It is also preferred to produce the negatively charged nitrogen-containing species in the electrolyte by reducing nitrogen gas at the cathode. However, the negatively charged nitrogen-containing species may also be provided by supplying a nitrogen-containing salt, such as lithium nitride, into the molten salt electrolyte mixture in a sufficient amount to provide some or all of the nitrogen consumed in the production of ammonia.
Owner:LYNNTECH
Metal lithium negative electrode and preparation method thereof
ActiveCN110660969AEasy to prepareImprove Coulombic efficiencyMaterial nanotechnologyElectrode carriers/collectorsMetallic lithiumLithium oxide
The invention provides a metal lithium negative electrode. The lithium ion battery negative electrode comprises a negative electrode current collector and a negative electrode active material layer deposited in the negative electrode current collector; the negative electrode active material layer is made of metal lithium; the negative electrode current collector comprises a current collector bodyand a gradient conductive ion layer coating the surfaces of pores in the current collector body and the outer surface of the current collector body; the ion and electron transmission of the current collector is ensured; the negative electrode active material layer is deposited on the surface of the gradient conductive ion layer; the current collector body is made of a porous conductive material; the porosity of the porous conductive material is 10%-95%; the gradient conductive ion layer is made of at least one material selected from lithium phosphide, lithium oxide, lithium nitride, lithium sulfide, lithium fluoride, lithium chloride, lithium bromide, lithium iodide and lithium phosphate. The invention further provides a preparation method of the metal lithium negative electrode.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
High-rate graphite negative electrode material, preparation method thereof and lithium ion battery
ActiveCN107946576AImprove structural stabilityHigh affinityCell electrodesSecondary cellsCarbon layerLattice defects
The invention relates to a high-rate graphite negative electrode material, a preparation method thereof and a lithium ion battery. The high-rate graphite negative electrode material comprises a graphite inner core and a hard carbon material layer, wherein the hard carbon material layer wraps a surface of the graphite inner core and is doped with lithium nitride. In the high-rate graphite negativeelectrode material provided by the invention, doping modification on the hard carbon material is performed by the lithium nitride, on one hand, a structure relation between the inner core and a coating layer can be built by lithium hydrophilcity of the inner core and the hard carbon material, and the hydrophilcity and the structural stability of the inner core and the coating layer are improved; and on the other hand, lattice defect can be formed in a carbon layer by lithium nitride doping, the electron mobility is improved, lithium storage combination points are increased, meanwhile, layer distance of a carbon-based material also can be expanded, and the lithium ion migration rate is improved. The graphite negative electrode material has the characteristics of good structural stability, large layer distance, many lithium storage combination points and rapid lithium ion and electron transmission speed, and the rapid charge capability of the lithium ion battery can be improved to a great extent.
Owner:CALB CO LTD +1
Lithium metal protective layer and preparation method thereof and battery with protective layer
InactiveCN111490252AReduced activityThickness is easy to controlCell seperators/membranes/diaphragms/spacersElectrode carriers/collectorsAluminum fluorideSlurry coating
The invention discloses a lithium metal protective layer. The preparation raw materials comprise a metal compound, a conductive agent and a binding agent, wherein the mass ratio of the metal compoundto the conductive agent to the binding agent is (6-8):1:(1-3), the metal compound is one or more of metal nitride, aluminum oxide, aluminum fluoride and Al4Li9, and the metal nitride and lithium metalcan form lithium alloy and lithium nitride through an in-situ electrochemical reaction. The lithium metal protective layer is a porous layer and provides a host material for lithium metal deposition;and in addition, the lithium metal protective layer also generates uniformly dispersed lithium alloy and / or lithium nitride in situ in the battery, so that the lithium affinity sites are enhanced, the lithium ion diffusion capacity is improved, and the generation of lithium dendrites is inhibited. The lithium metal protective layer can be prepared by slurry coating and drying, the preparation method is simple and convenient, the raw material source is cheap, and the thickness of the protective layer is easier to control. The lithium metal battery with the protective layer can inhibit the generation of lithium dendrites, and reduce the activity of lithium metal, and the cycle life of the lithium metal battery can be effectively improved.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Electrolyte for inhibiting growth of lithium dendrites and lithium battery
PendingCN110416615AImprove deposition and dissolution efficiencyInhibition formationSecondary cells servicing/maintenanceSolid state electrolyteLithium chloride
The invention discloses an electrolyte for inhibiting the growth of lithium dendrites and a lithium battery. The electrolyte comprises an additive, a lithium salt and an organic solvent. The additiveincludes at least one in the group consisting of lithium hexafluorophosphate, lithium perchlorate, lithium bis(trifluoromethane) sulfonate, lithium trifluoromethanesulfonate, lithium fluoroborate, trilithium hexafluoroaluminate, lithium hexafluoroarsenate, lithium fluoride, lithium chloride, lithium bromide, lithium nitrate, lithium polysulfide, lithium nitride, lithium phosphide, lithium oxalateborate, lithium oxide, lithium sulfite, lithium sulfate, lithium acetate, lithium hydroxide and lithium oxalate, and the lithium salt is different from the additive. According to the lithium battery containing an additive, a layer of solid electrolyte membrane can be formed on a surface of the lithium metal negative electrode during charge and discharge, and the polymerization of the electrolyte can be induced to form an oligomer which covers a surface of a lithium negative electrode and a surface of a positive electrode material matched with the lithium negative electrode. The protective layer can effectively inhibit the growth of the lithium dendrites, and therefore, the safety performance of the battery is improved.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method of g-C3N4-suppurted active carbon with photocatalytic function
InactiveCN103495395AOther chemical processesCombustible gas purificationLithium nitrideActivated carbon
The invention discloses a preparation method of active carbon with a photocatalytic function. The preparation method comprises the following steps of taking active carbon, oxazine trichloride (C3N3C13), lithium nitride (Li3N) and the like as main raw materials, and performing processes of heating, pressing and the like in a benzene solvent to prepare the g-C3N4-suppurted active carbon with the photocatalytic function. The g-C3N4-suppurted active carbon can purify pollutants by depending on adsorption performance and catalytic performance under a lighting condition, can purify the pollutants by depending on the adsorption performance of the active carbon under the condition with darkness and insufficient light, and can realize in situ regeneration under illumination, so that the consumption of manpower, material resources and financial resources of a regenerative process is greatly reduced. The successful research of the active carbon plays a role in remission of insufficient supply of active carbon in China.
Owner:FUJIAN AGRI & FORESTRY UNIV
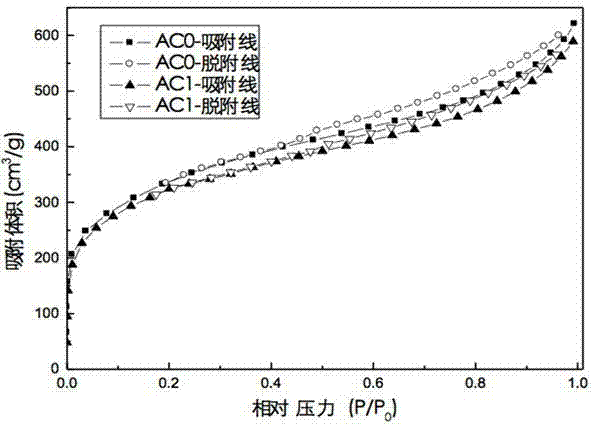
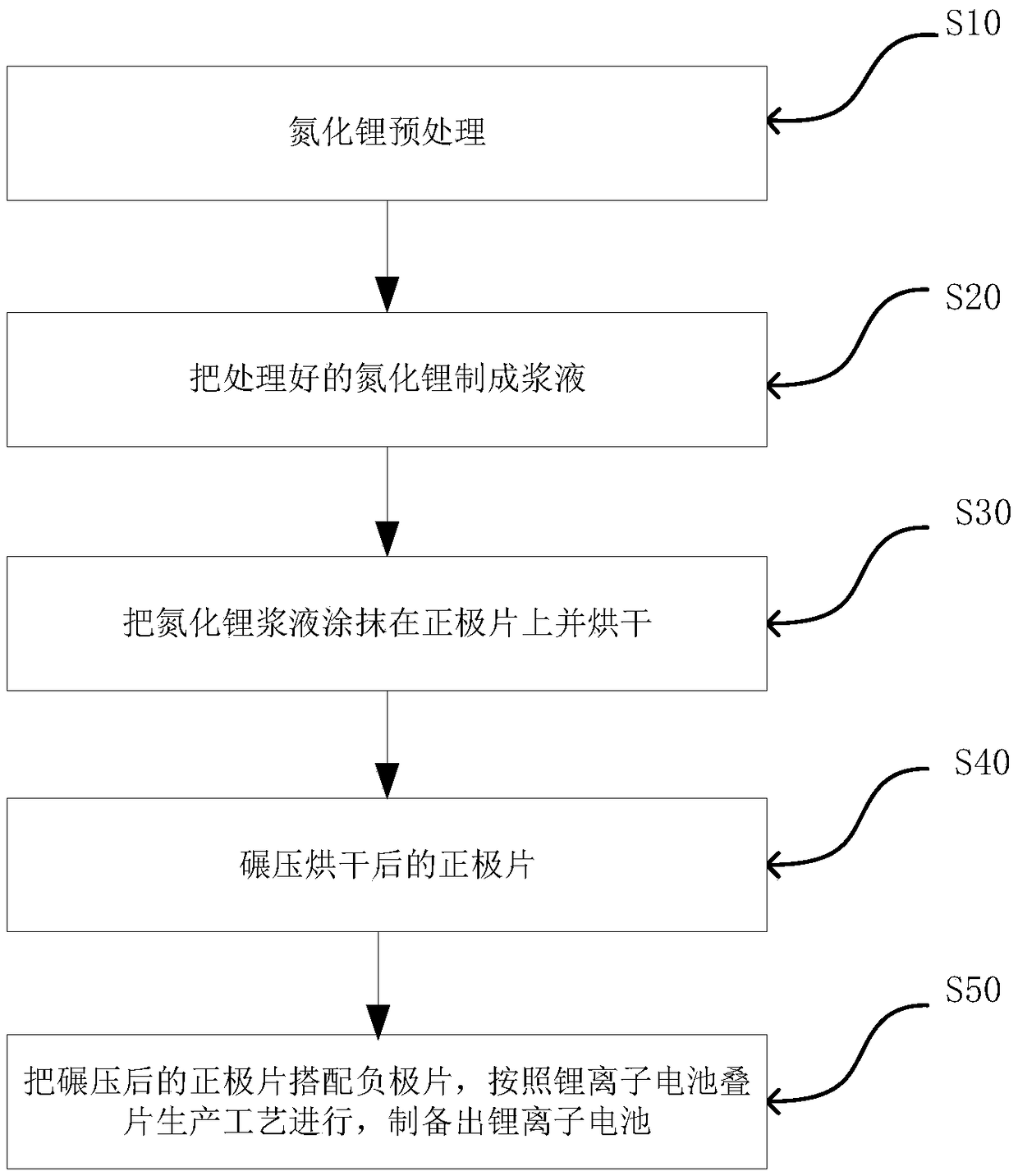
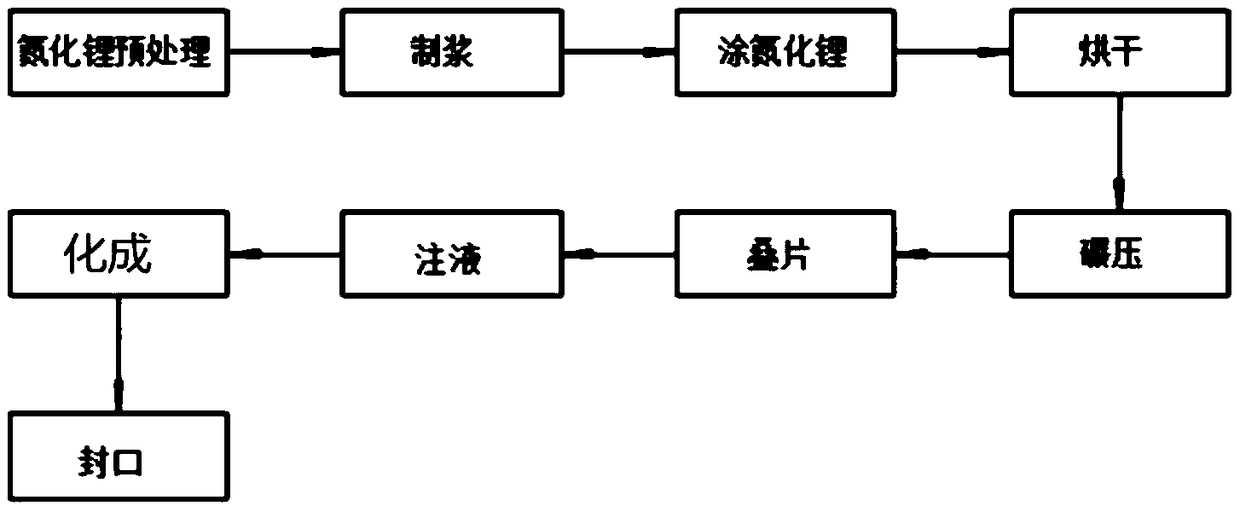
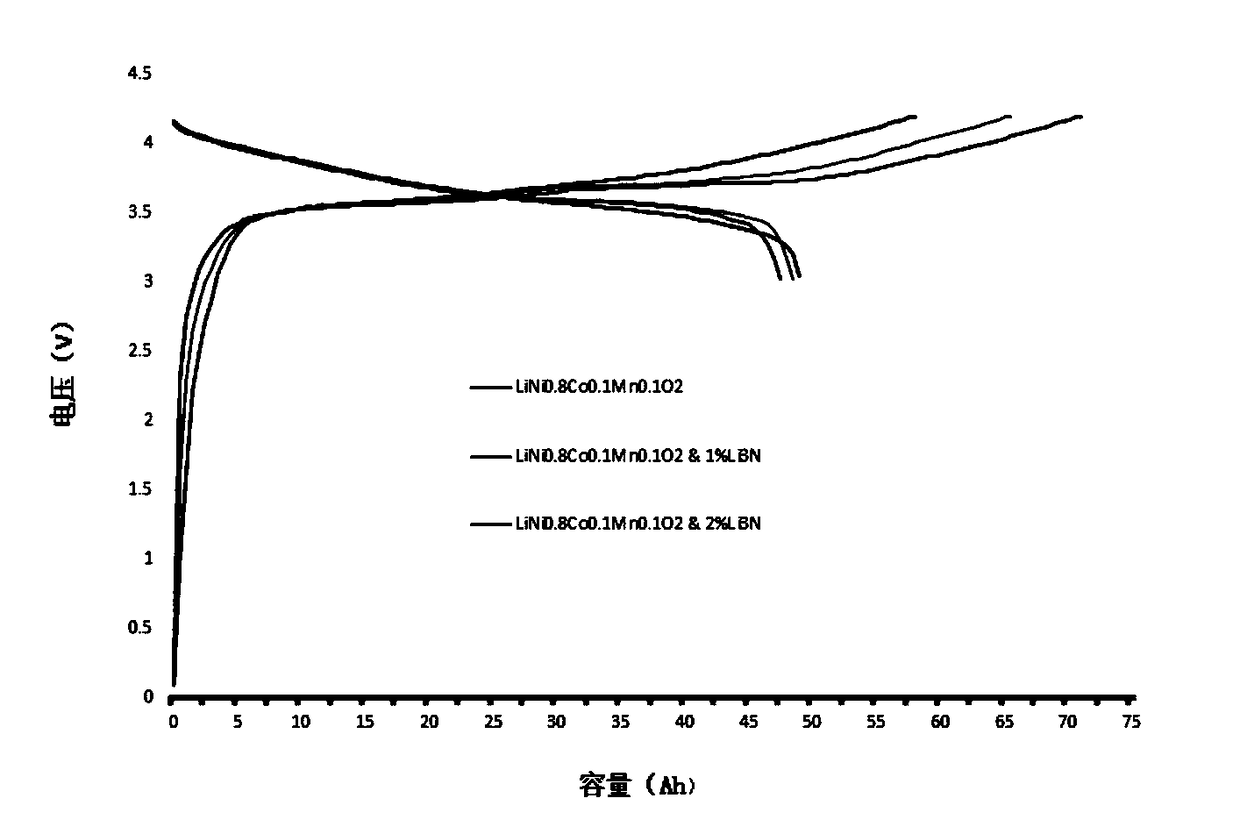
Method for prolonging circulation life of lithium ion battery with lithium nitride
InactiveCN109346679AImprove cycle lifeLittle impact on magnification performanceFinal product manufactureCell electrodesAdhesiveTitanium nitride
The invention discloses a method for prolonging the circulation life of a lithium ion battery with lithium nitride and aims to solve the technical problem that a lithium ion battery is poor in circulation performance. The method comprises the following steps: grinding lithium nitride powder material in a glove tank with protection of argon; mixing NMP (N-Methyl Pyrrolidone) with PVDF (Polyvinylidene Fluoride), carrying out vacuum stirring to obtain an adhesive, and controlling the viscosity of the adhesive within 3000-6000mPa*s; adding the grinded lithium nitride powder, and carrying out vacuum stirring; uniformly coating the surface of an anode piece by the prepared lithium nitride slurry by using a coating machine, drying, and grinding; matching with a corresponding cathode piece, and implementing a conventional process, thereby obtaining a battery. The method is simple in preparation process, device requirements can be met by conventional lithium ion battery production equipment, and the method is applicable to industrial production and application; the lithium nitride is distributed on the surface of the anode piece, and the rate capability of the battery is slightly affected;as the lithium nitride is uniformly distributed on the surface of the anode piece, a dense and uniform SEI membrane can be formed; the circulation life of the lithium ion battery after lithium replenishing of lithium nitride can be prolonged.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
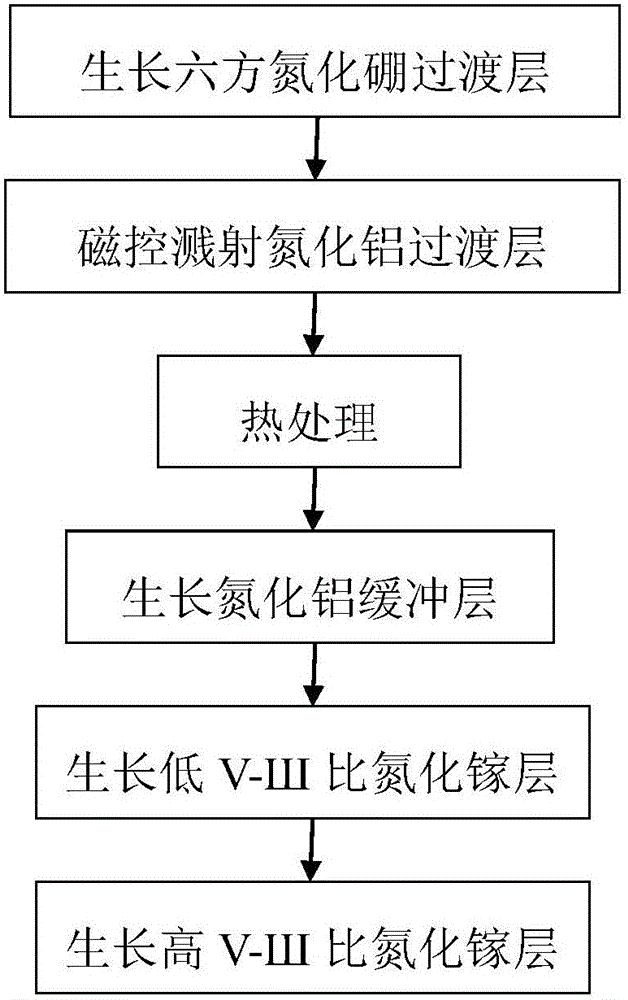
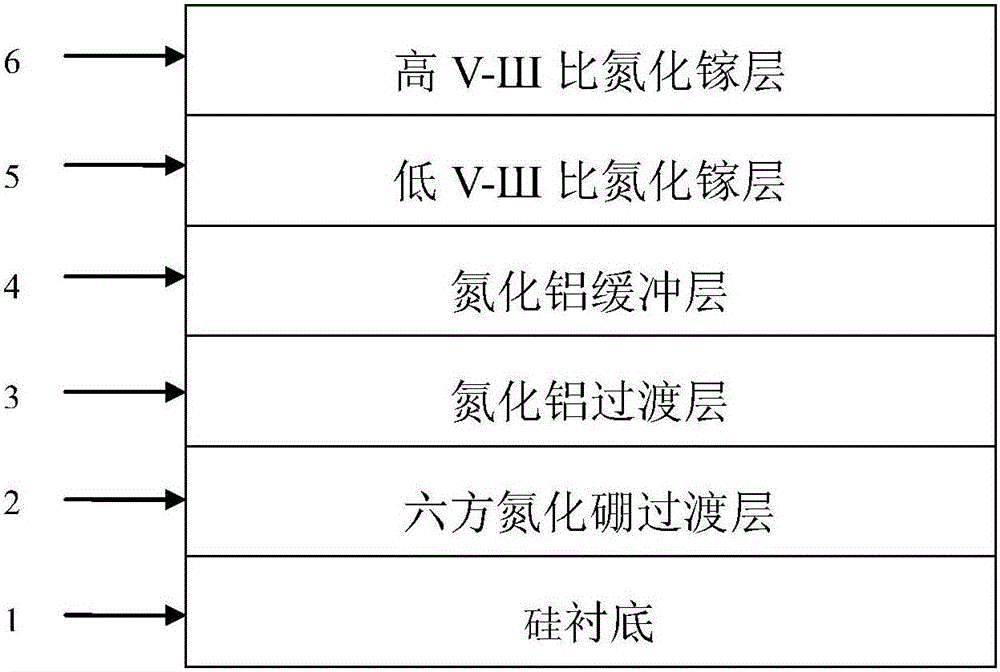
Gallium nitride growing method based on hexagonal boron nitride and magnetron-sputtered aluminum nitride
ActiveCN105861987AOvercoming the problem of only growing on substrates with small lattice mismatchesOvercoming growth problemsVacuum evaporation coatingSputtering coatingSputteringHexagonal boron nitride
The invention discloses a gallium nitride growing method based on hexagonal boron nitride and magnetron-sputtered aluminum nitride. The method is mainly used for improving the quality of gallium nitride material. The method comprises the following growing steps: (1) growing of a boron nitride transition layer; (2) magnetron sputtering of an aluminum nitride transition layer; (3) heat treatment; (4) growing of an aluminum nitride buffer layer; (5) growing of a gallium nitride layer with a low V-SH ratio; and (6) growing of a gallium nitride layer with a high V-SH ratio. A gallium nitride film formed according to the method has the advantages of combining hexagonal boron nitride and magnetron-sputtered aluminum nitride, having high material quality and a large applicable substrate range and being capable of being used for manufacturing high-performance gallium nitride devices.
Owner:XIDIAN UNIV
Preparation method of hydroxyl gallium oxide nanometer crystal
InactiveCN102786078AIncrease productionHigh purityMaterial nanotechnologyGallium/indium/thallium compoundsPrismGallium nitride
The invention relates to a preparation method of hydroxyl gallium oxide nanometer crystal, and belongs to the technical field of preparation of a nanometer material of oxyhydroxide. The preparation method comprises the following steps of: dripping a deionized water solution of CTAB (cetyltrimethyl ammonium bromide) into a benzene solution of gallium chloride under an ultrasonic environment by the adoption of a principle of a liquid-liquid interface reaction, putting the mixed solution into a reaction kettle to carrying out heating reaction, and obtaining a sample of white powders. The hydroxyl gallium oxide is the raw material for compounding gallium nitride and gallium oxide, and the microcosmic morphological feature of the hydroxyl gallium oxide has very important influence on the performances of the prepared gallium nitride and gallium oxide. The hydroxyl gallium oxide nanometer crystal, prepared by the method provided by the invention, is prism-shaped, and has the characteristics of smooth surface, complete shape, uniform size, etc. The preparation method, provided by the invention, conquers the shortcomings that gallium chloride is very easy to absorb water and cause deliquescence, and abandons the complicated step that the pH value must be regulated in the past and does not need the vacuum environment; and has the advantages of simplicity in operation, high yield, little energy consumption, high repeatability and the like.
Owner:JILIN UNIV
Ammonia synthesis using lithium ion conductive membrane
Ammonia is synthesized using electrochemical and non-electrochemical reactions. The electrochemical reactions occur in an electrolytic cell having a lithium ion conductive membrane that divides the electrochemical cell into an anolyte compartment and a catholyte compartment. The catholyte compartment includes a porous cathode closely associated with the lithium ion conductive membrane. The overall electrochemical reaction is: 6LiOH+N2→Li3N (s)+3H2O+3 / 2O2. The nitrogen may be produced by a nitrogen generator. The non-electrochemical reaction involves reacting lithium nitride with water and / or steam as follows: Li3N (s)+3H2O→3LiOH+NH3 (g). The ammonia is vented and collected. The lithium hydroxide is preferably recycled and introduced into the anolyte compartment. The electrolytic cell is shut down prior to reacting the lithium nitride with water. The cathode is preferably dried prior to start up of the electrolytic cell and electrolyzing Li+ and N2 at the cathode.
Owner:ENLIGHTEN INNOVATIONS INC
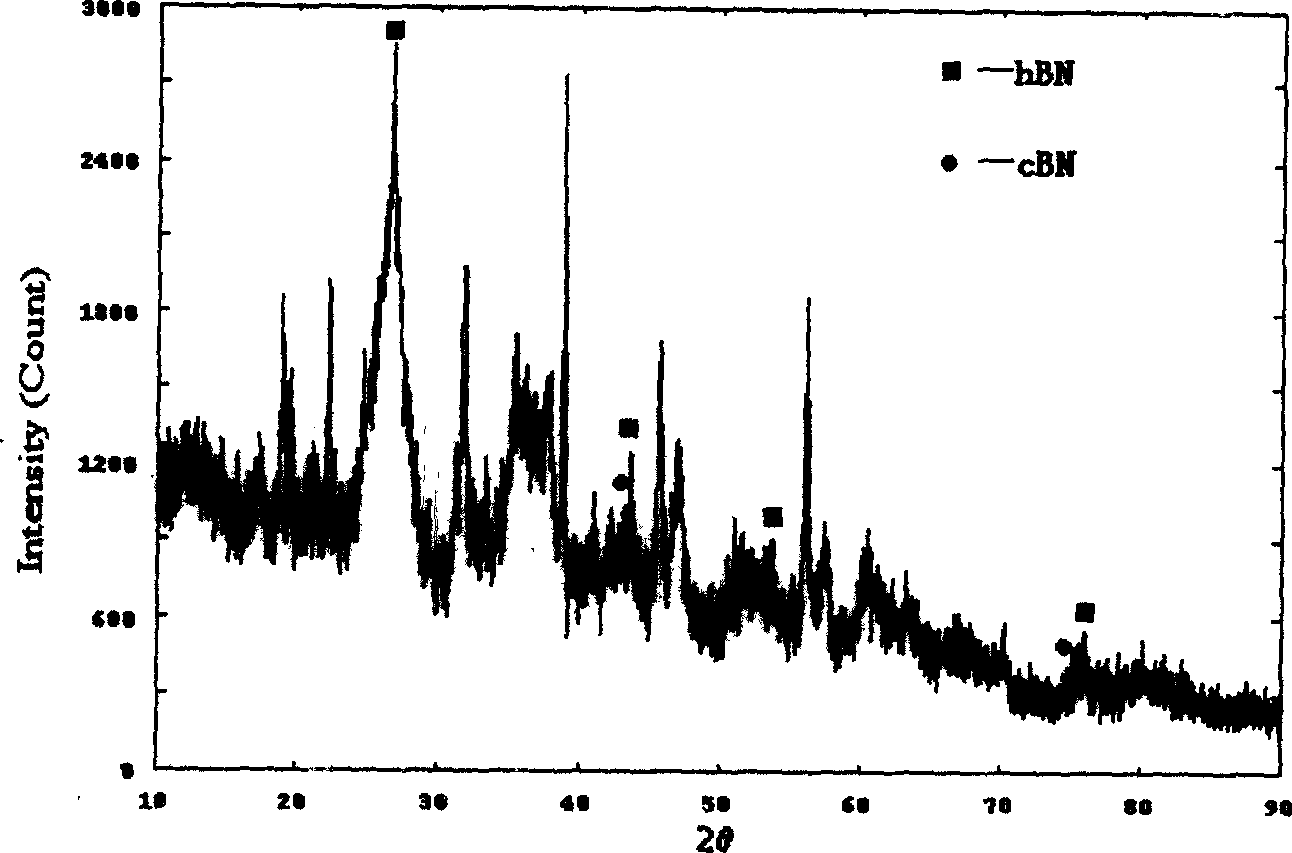
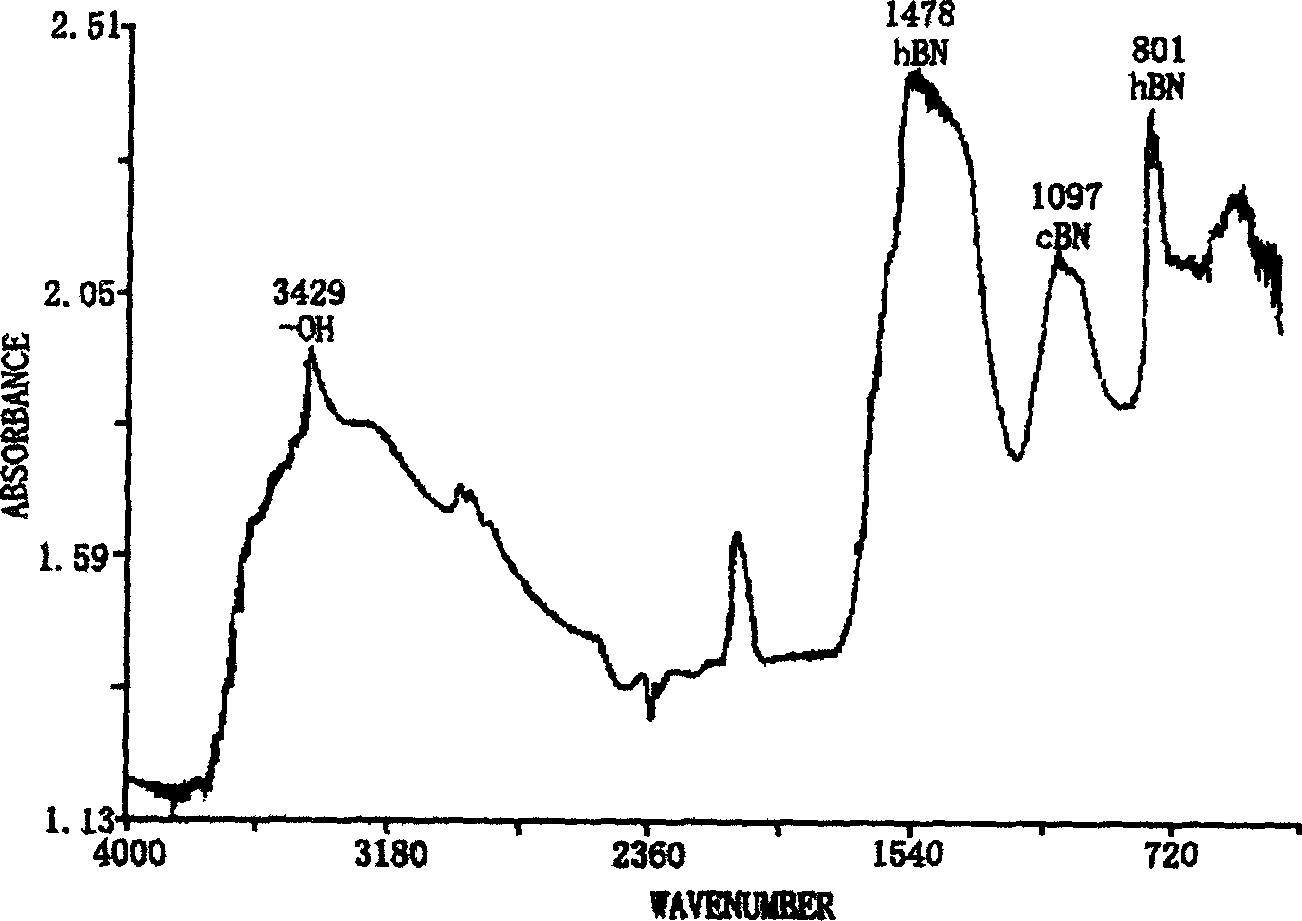
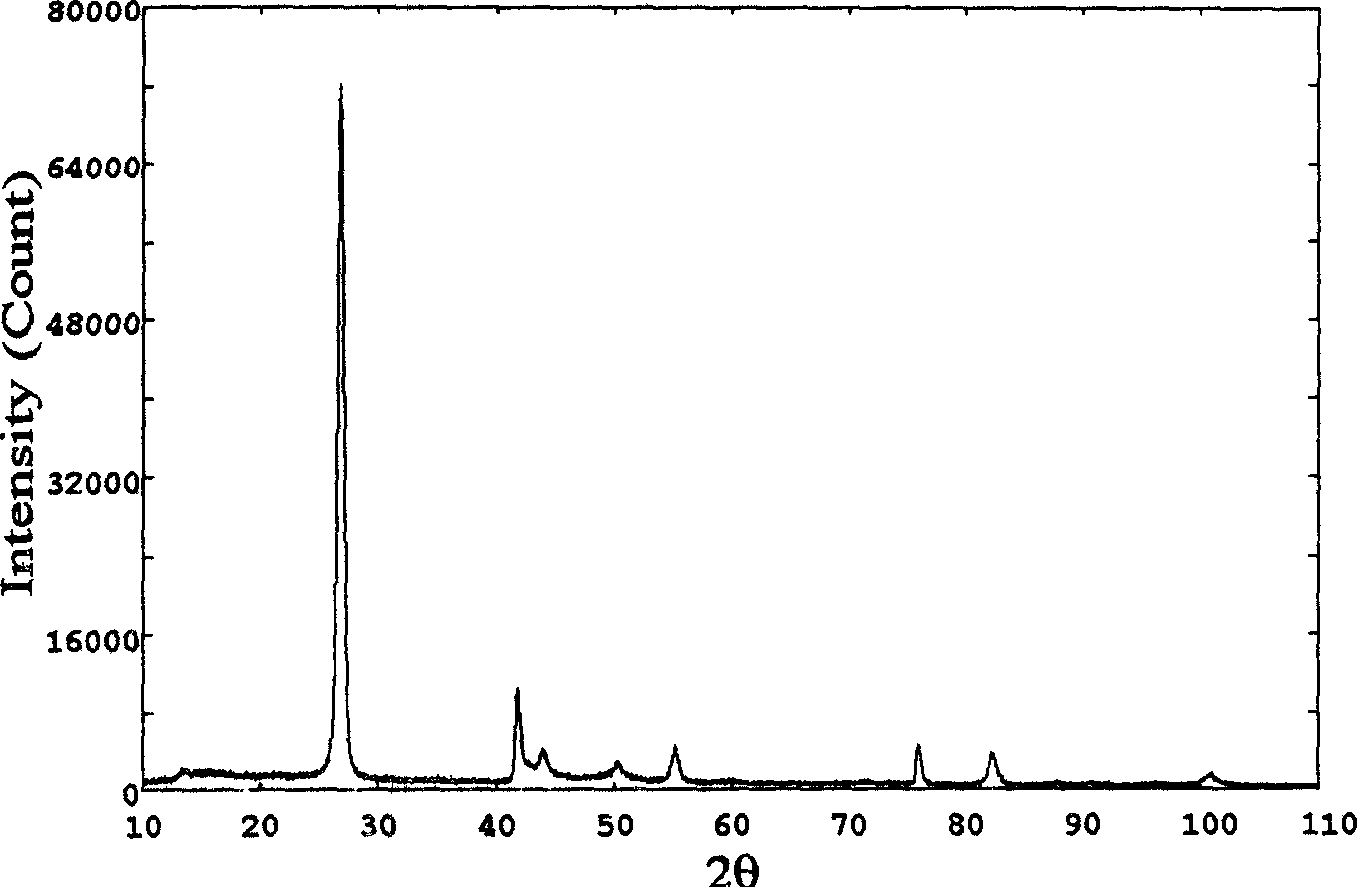
Method for compounding fine-particle cubic boron nitride single crystal
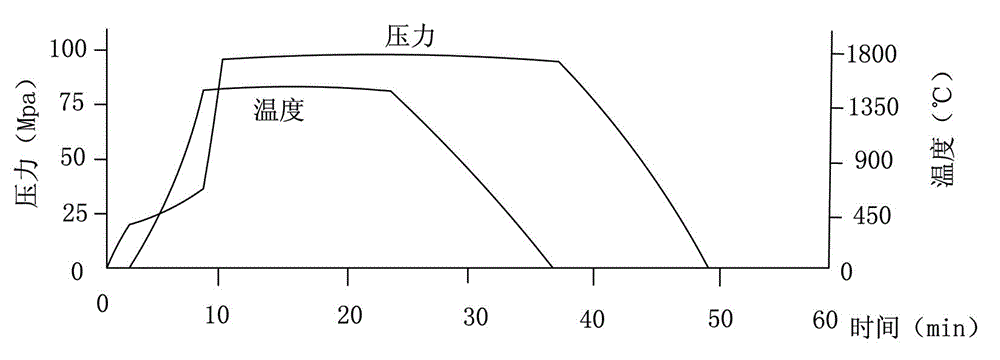
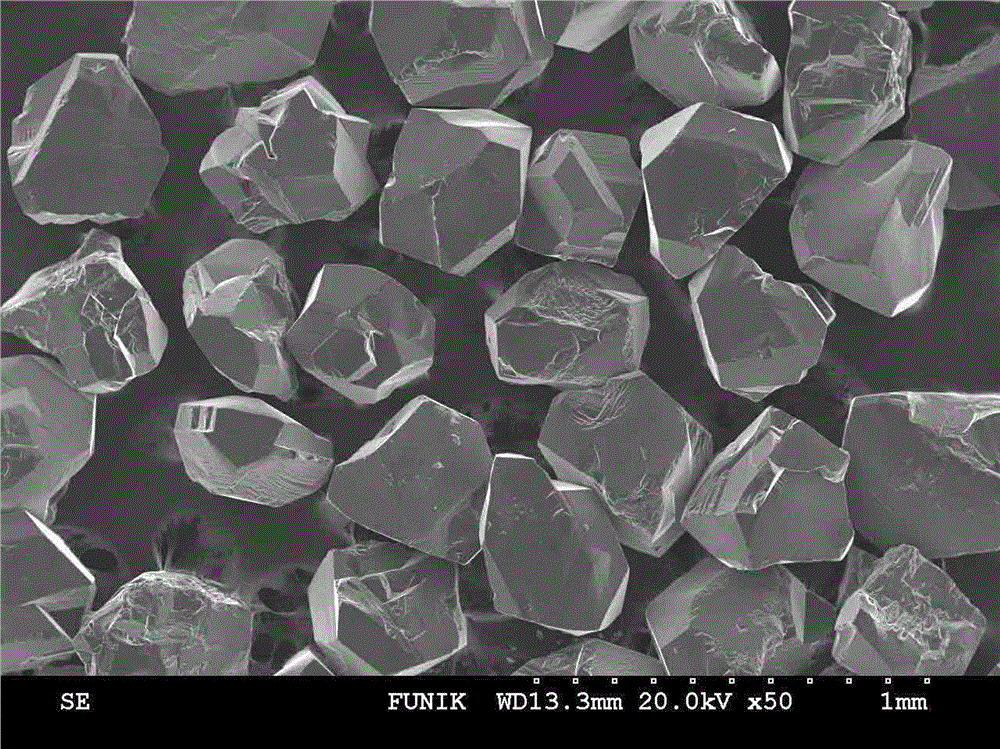
InactiveCN102019153AAvoid wastingThe synthesis process is simplePolycrystalline material growthUltra-high pressure processesMetallurgyHexagonal boron nitride
The invention discloses a method for compounding a fine-particle cubic boron nitride single crystal. In the method, hexagonal boron nitride is used as raw material, lithium nitride is used as catalytic agent and the fine-particle cubic boron nitride product is compounded under the condition of high temperature and high pressure, wherein the weight ratio of the hexagonal boron nitride to the lithium nitride is 1:0.1 to 0.3, the compounding time is 2 to 5 minutes, the compounding temperature is 1300 to 1600 DEG C, and the compounding pressure is 4.5 to 5.5 GPa. After finishing the compounding, the compounded material containing fine-particle cubic boron nitride single crystal is acquired. Compared with the prior art, the invention solves the problems that the traditional compounding method of the fine-particle cubic boron nitride single crystal has many steps, long compounding time and complex post processing and obtains incomplete single crystal shape of majority acquired fine-particle cubic boron nitride. Besides, the method of the invention is short in compounding time, has proper compounding pressure and is beneficial to save instrument loss and industrialized volume production.
Owner:柳州市大荣非金属材料有限公司
Manufacturing method for cubic boron nitride crystal
ActiveCN104607110AHigh strengthImprove thermal stabilityUltra-high pressure processesHexagonal boron nitrideTitanium nitride
The invention provides a manufacturing method for a cubic boron nitride crystal. The manufacturing method comprises the following steps: uniformly mixing hexagonal boron nitride powder, catalyst powder and additives, and pressing the mixture into a synthesis stick, wherein the catalyst powder comprises lithium nitride and lithium hydride, and the additives comprise ammonium fluoride, titanium nitride and aluminium nitride; carrying out high-pressure high-temperature synthesis on the synthesis stick to obtain a cubic boron nitride crystal crude product; crushing the cubic boron nitride crystal crude product to obtain a massive cubic boron nitride crystal crude product; sequentially soaking the massive cubic boron nitride crystal crude product by water and acid-base to obtain a massive cubic boron nitride crystal finished product. The cubic boron nitride crystal obtained by the manufacturing method disclosed by the invention is an equiareal golden crystal, has the characteristics of high strength, high thermal stability, high wear resistance, high crack resistant capability, high thermal stability and the like, is suitable for long-life electroplated tools and ceramic bond systems, as well as is applied to processing hardened carbon tool steel, hard alloys, alloy steel, and nickel-based and cobalt-based high-temperature alloy materials.
Owner:FUNIK ULTRAHARD MATERIAL
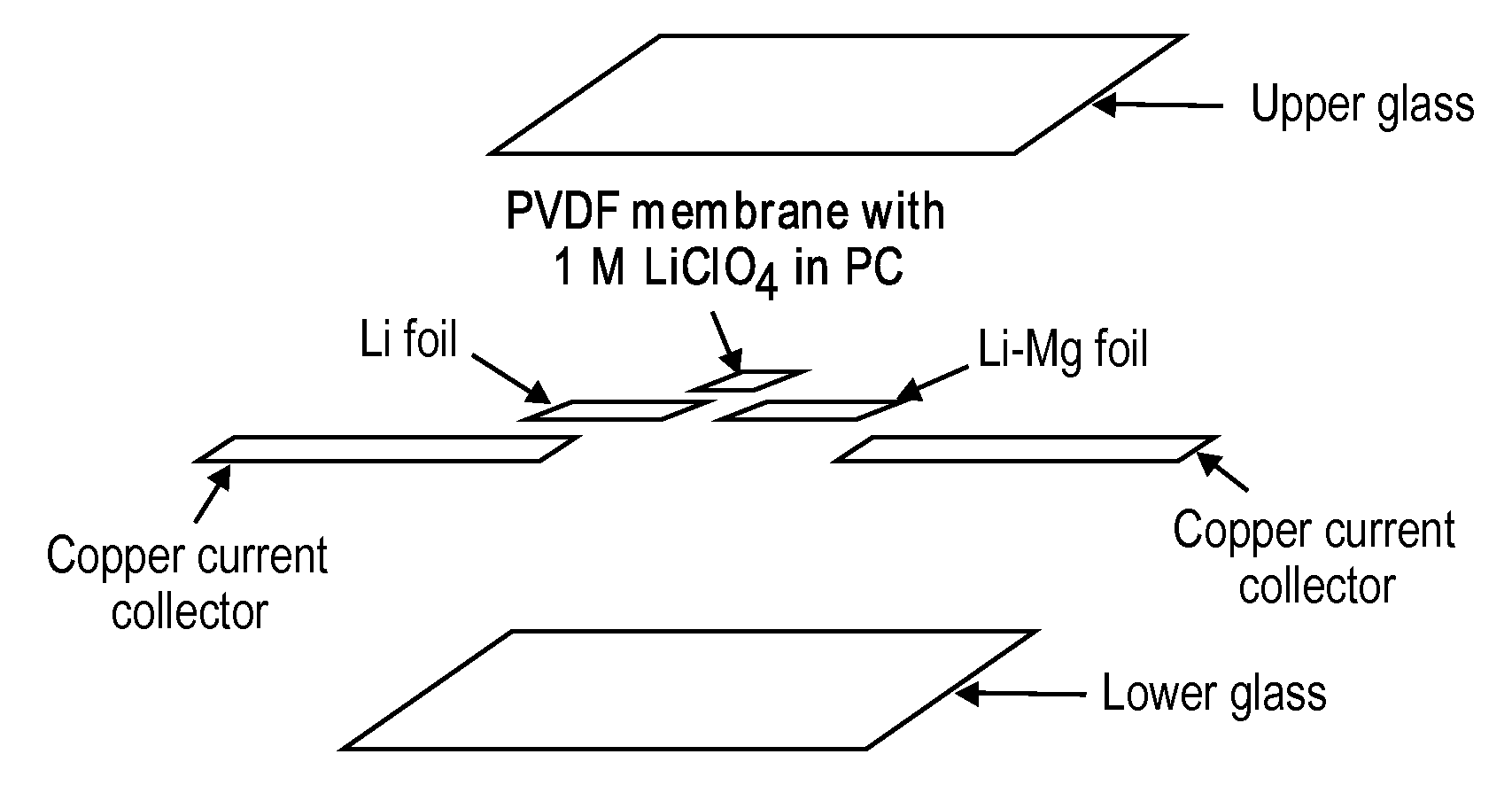
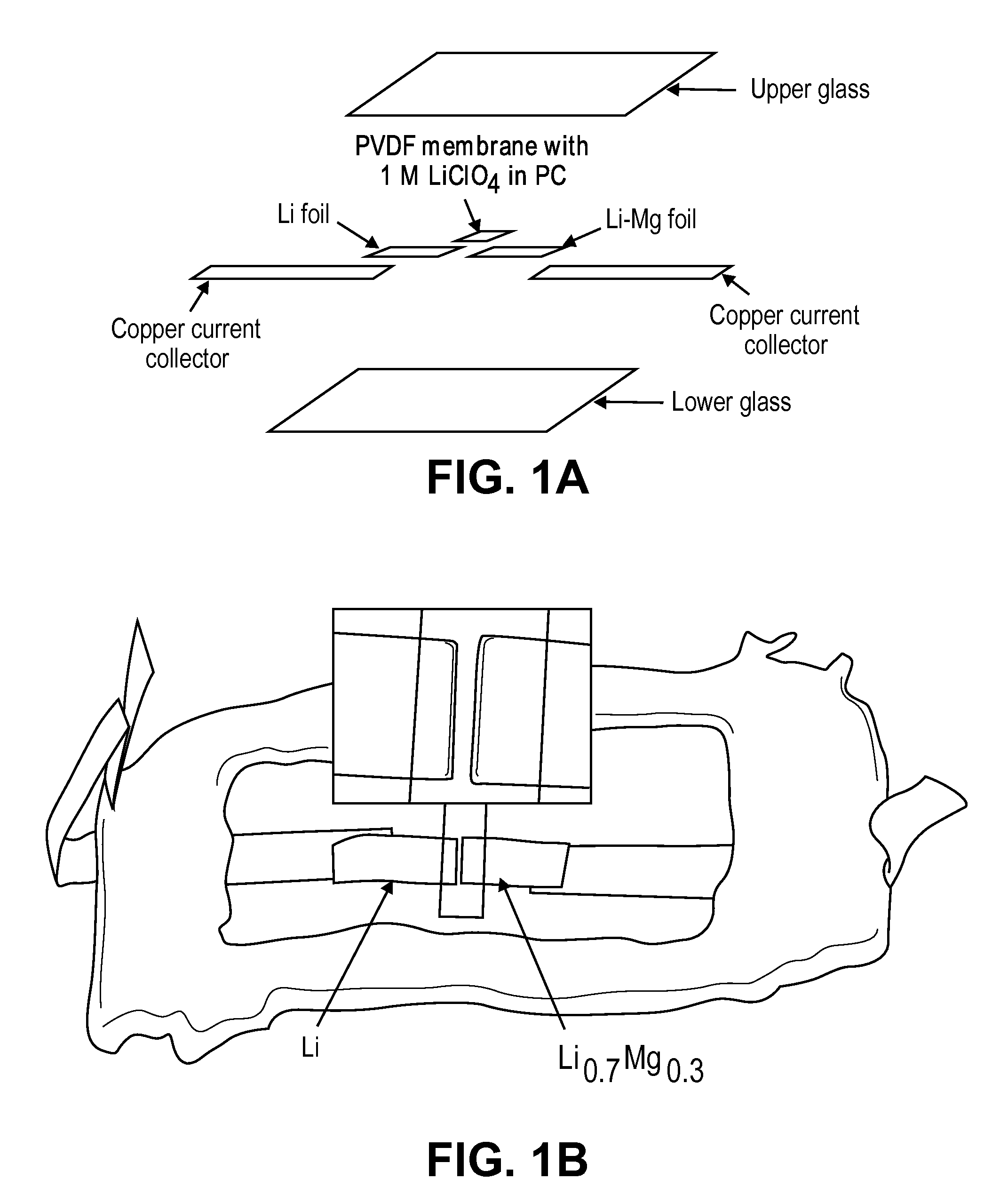
Solid solution lithium alloy cermet anodes
ActiveUS20100181528A1Easy to produceMinimal post reaction manipulationNitrogen-metal/silicon/boron binary compoundsCell temperature controlChemical reactionCeramic composite
A metal-ceramic composite (“cermet”) has been produced by a chemical reaction between a lithium compound and another metal. The cermet has advantageous physical properties, high surface area relative to lithium metal or its alloys, and is easily formed into a desired shape. An example is the formation of a lithium-magnesium nitride ceiniet by reaction of lithium nitride with magnesium. The reaction results in magnesium nitride grains coated with a layer of lithium. The nitride is inert when used in a battery. It supports the metal in a high surface area form, while stabilizing the electrode with respect to dendrite formation. By using an excess of magnesium metal in the reaction process, a cermet of magnesium nitride is produced, coated with a lithium-magnesium alloy of any desired composition. This alloy inhibits dendrite formation by causing lithium deposited on its surface to diffuse under a chemical potential into the bulk of the alloy.
Owner:RGT UNIV OF CALIFORNIA
Protective layers in lithium-ion electrochemical cells and associated electrodes and methods
ActiveCN107078277ASolid electrolytesFinal product manufactureLithium oxideMetal–air electrochemical cell
Protective layers in lithium-ion electrochemical cells, and associated electrodes and methods, are generally described. The protective layers may comprise lithium-ion-conductive inorganic ceramic materials, such as lithium oxide, lithium nitride, and / or lithium oxysulfide. The resulting lithium-ion electrochemical cells may exhibit enhanced performance, including reduced capacity fade rates and reduced self-discharge rates.
Owner:SION POWER CORP
Ion mobility spectrometer with device for generating ammonia gas
ActiveUS20140319332A1Particle separator tubesMaterial analysis by electric/magnetic meansDopantAlkaline earth metal
The present invention relates to ion mobility spectrometry, in particular to methods and devices for generating and delivering of ammonia gas as dopant into the ionization region of an ion mobility spectrometer. It provides an ion mobility spectrometer (IMS) with an ion source and device for generating ammonia gas, wherein the device comprises a dopant reservoir filled with alkali metal nitride or alkaline earth metal nitride, preferably lithium nitride and / or magnesium nitride, said reservoir being fluidly coupled to the ion source and to a water reservoir.
Owner:BRUKER OPTICS GMBH & CO KG
Organic electroluminescent device and production method thereof
InactiveCN104638162AImprove efficiencyImprove luminous efficiencySolid-state devicesSemiconductor/solid-state device manufacturingSilver iodideElectron
The invention discloses an organic electroluminescent device comprising a conductive anode, a hole injection layer, a hole transport layer, a luminescent layer, an electron transport layer, an electron injection layer and a cathode. The electron injection layer comprises a first doped layer, a second doped layer, a third doped layer, a fourth doped layer and a fifth doped layer stacked in order. The first doped layer, the second doped layer, the third doped layer, the fourth doped layer and the fifth doped layer are made of mixture made by doping a doped material to a base material; the doped material comprises a silver compound and an alkali metal compound; the silver compound is silver iodide, silver sulfide, silver chloride, silver fluoride or silver bromide; the alkali metal compound is lithium fluoride, lithium azide, lithium nitride, cesium fluoride, cesium azide or cesium nitride. The invention further provides a production method of the organic electroluminescent device.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Method for synthesizing boron nitride from aether boron trifluoride and lithium nitride
A process for preparing boron nitride from boron trifluoride ether and lithium nitride by solvent heat synthesis method includes adding benzene as solvent in reactor, adding lithium nitride, stirring, adding boron trifluoride ether, stirring, closing reactor, heating to 250-500 deg.C, holding the temp, cooling, dissolving in ionized water, centrifugal separation of supernatant, repeating the dissolving and separation steps, immersing in solution of hydrochloric acid, water washing, centrifugal separation of deposit, and drying.
Owner:UNIV OF SCI & TECH BEIJING
All solid polymer battery
InactiveCN101595592ASuppresses increase in interface resistanceLower resistanceSolid electrolyte cellsActive material electrodesHigh rateInternal resistance
This invention provides an all solid polymer battery comprising 1) a lithium negative electrode active material containing crystal grains and crystal grain boundaries, at least a part of the crystal grain boundaries being exposed on the lithium negative electrode active material, the exposed area of the crystal grain boundaries being 0.02 to 0.5 cm per cm of the surface, 2) a dry polymer electrolyte containing a specific ethylene glycol ether, a polymer containing an electron donating oxygen atom in its skeleton and a lithium salt, or 3) a noncrystalline lithium nitride layer provided between a negative electrode and a polymer electrolyte. This constitution can provide an all solid polymer battery which can realize reduced resistance of the interface of the negative electrode and the polymer electrolyte and has a high capacitance and excellent cycle properties. Further, the all solid polymer battery can realize the suppression of an increase in internal resistance during storage and is excellent in high rate discharge properties after storage.
Owner:PANASONIC CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com