Metal lithium negative electrode and preparation method thereof
A metal lithium and negative electrode technology, applied in the field of dense metal lithium negative electrode and its preparation, can solve the problems of unfavorable uniform deposition of lithium metal, complex preparation process, three-dimensional current collector thickness, etc., and achieve uniform distribution and rapid transmission, preparation method Simple, good cycle stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The present invention also provides a method for preparing a metal lithium negative electrode, comprising the following steps:
[0040] Step S1: providing a current collector body, the current collector body is a porous conductive material, and the porosity of the porous conductive material is 10%-95%.
[0041] In one embodiment, the current collector body is at least one of copper nanowire interweaves, copper foam, and porous copper foil, the pore structure of the current collector body is evenly distributed, and the porosity of the current collector body is further It can be 40%-85%. In other embodiments, the current collector body may also be a porous conductive material of nickel.
[0042] When the current collector body is a copper nanowire or copper nanowire interwoven body, the prefabrication of the copper nanowire current collector body includes the following steps:
[0043] Step S101: provide a precursor solution, the precursor solution includes copper salt, ...
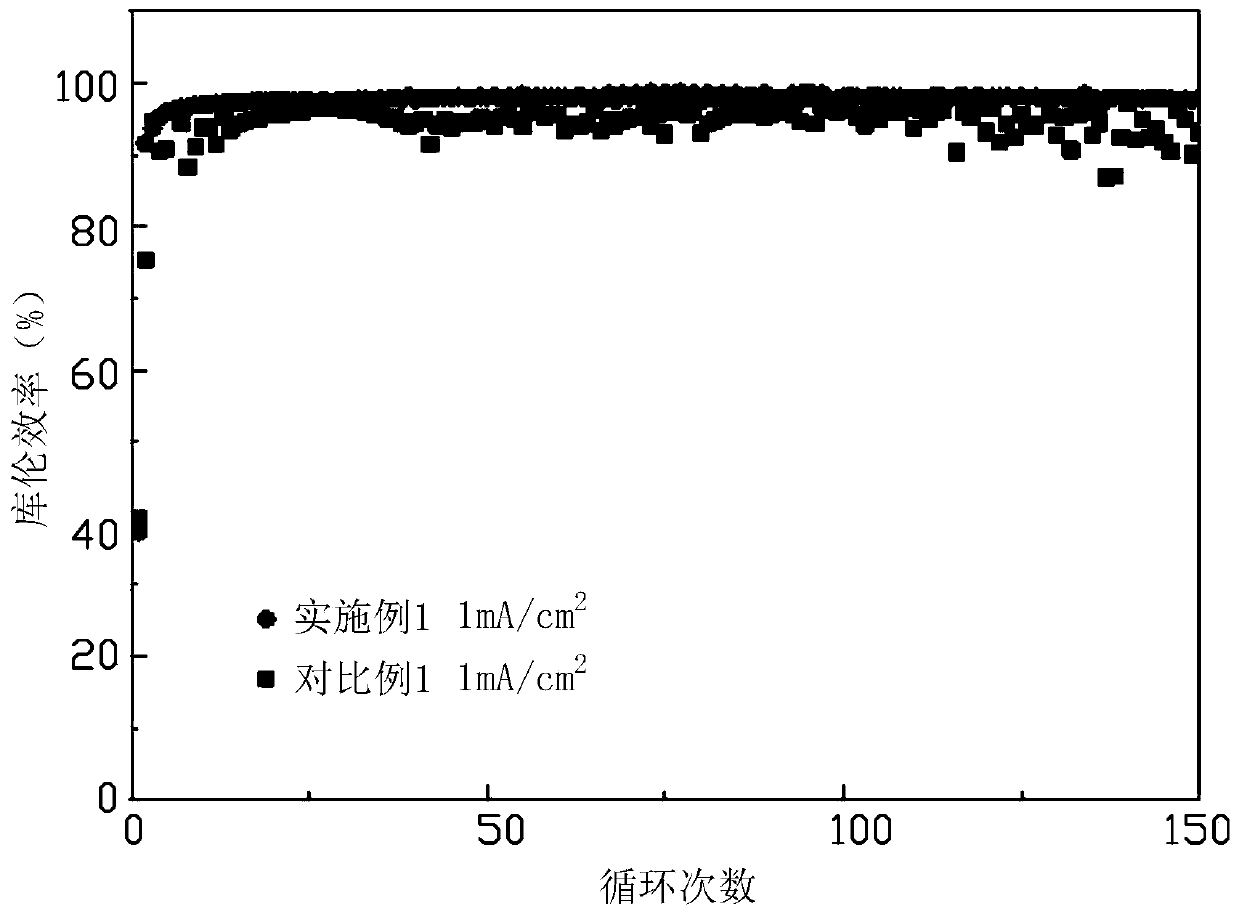
Embodiment 1
[0074] Add copper chloride and tetradecylamine to 250 mL of deionized water to form a first mixed solution, wherein the concentration of copper chloride is 2.8 mg / mL, and the concentration of tetradecylamine is 10.6 mg / mL. Adding 1 g of glucose to the first mixed solution and mixing evenly to obtain a precursor solution, and placing it in a constant temperature oven at 100°C for 8 hours to obtain a copper nanowire dispersion;
[0075] Suction-filtering the copper nanowire dispersion, and washing with isopropanol and ethanol respectively to remove residual surfactants to obtain the copper nanowire current collector body;
[0076]Place the copper nanowire current collector body in a dual-temperature zone tube furnace, heat-treat with argon as a protective gas, place 200mg of sodium hypophosphite in the upstream area of the airflow, and heat it to 300-400°C, and place the copper nanowires in the downstream of the airflow zone, and the temperature was raised to 100° C., and the ...
Embodiment 2
[0089] Add copper chloride and tetradecylamine to 250 mL of deionized water to form a first mixed solution, wherein the concentration of copper chloride is 2.8 mg / mL, and the concentration of tetradecylamine is 10.6 mg / mL. Add 1 g of glucose to the first mixed solution and mix evenly to obtain a precursor solution, and place it in a constant temperature oven at 100°C for 8 hours to obtain a copper nanowire dispersion;
[0090] Suction-filtering the copper nanowire dispersion, and washing with isopropanol and ethanol respectively to remove residual surfactants to obtain the copper nanowire current collector body;
[0091] Place the copper nanowire current collector body in a dual-temperature zone tube furnace, heat-treat with argon as a protective gas, place 200mg of sodium hypophosphite in the upstream area of the airflow, and heat it to 300-400°C, and place the copper nanowires in the downstream of the airflow zone, and the temperature was raised to 100° C., and the reactio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com