Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67 results about "Hafnium tetrachloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
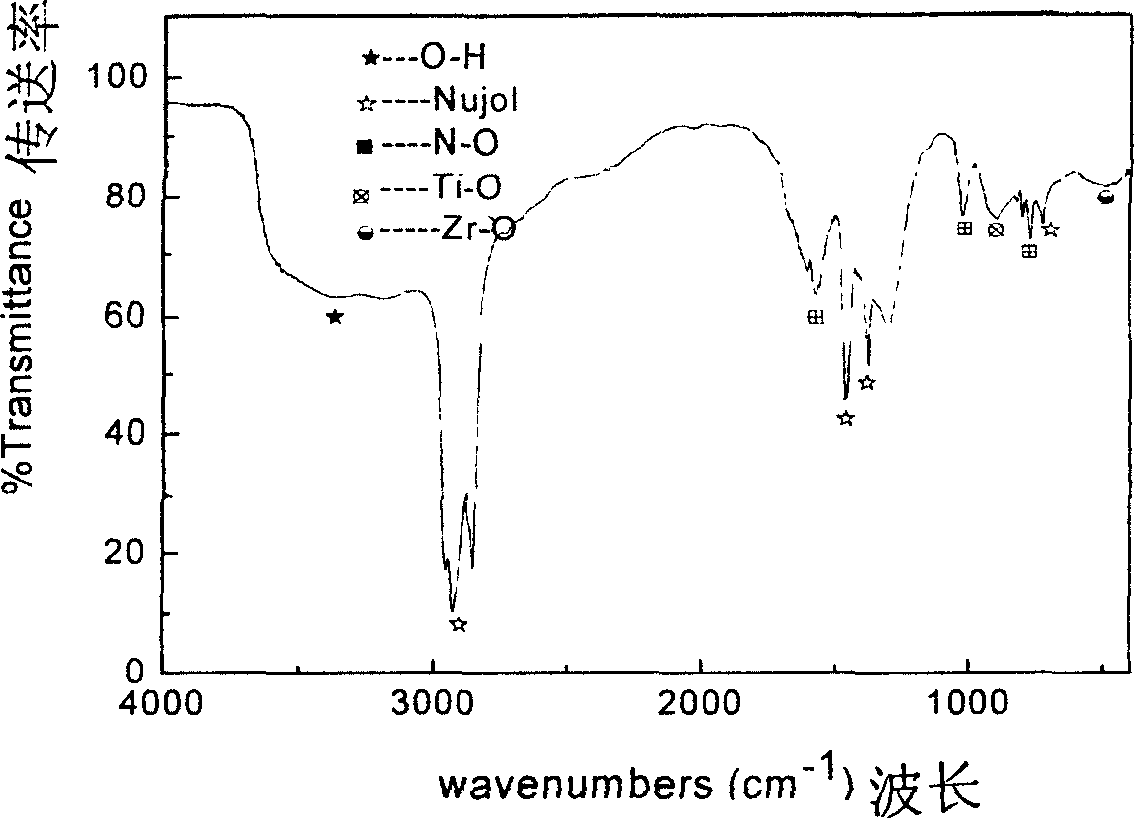
Hafnium(IV) chloride is the inorganic compound with the formula HfCl₄. This colourless solid is the precursor to most hafnium organometallic compounds. It has a variety of highly specialized applications, mainly in materials science and as a catalyst.
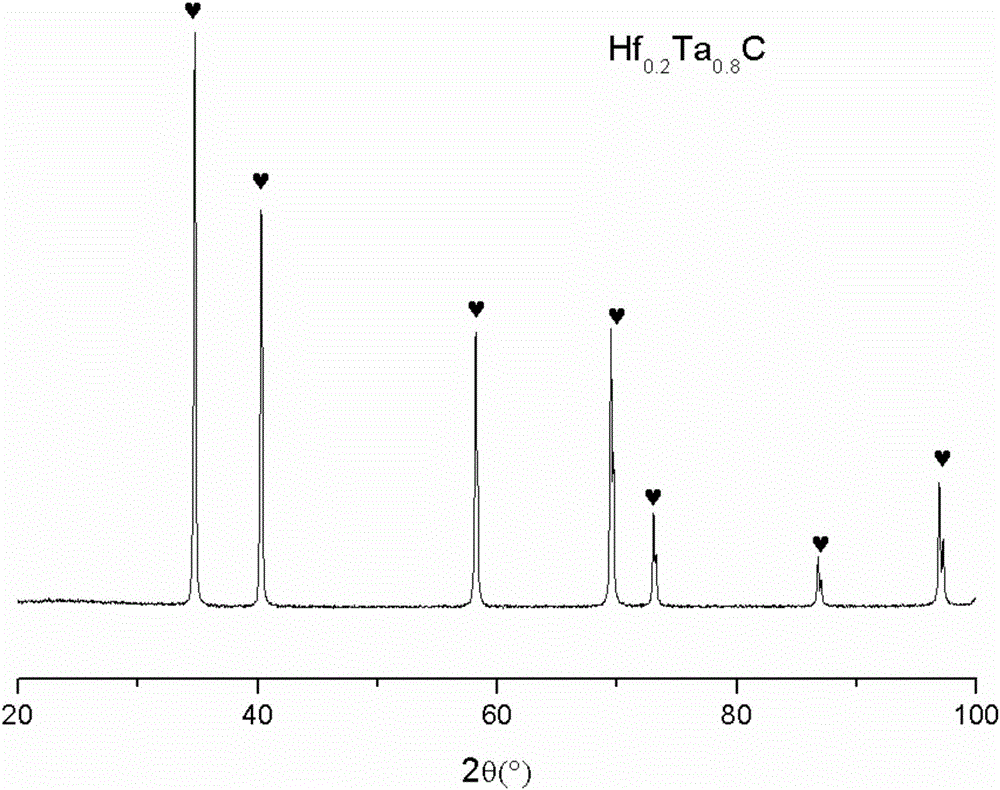
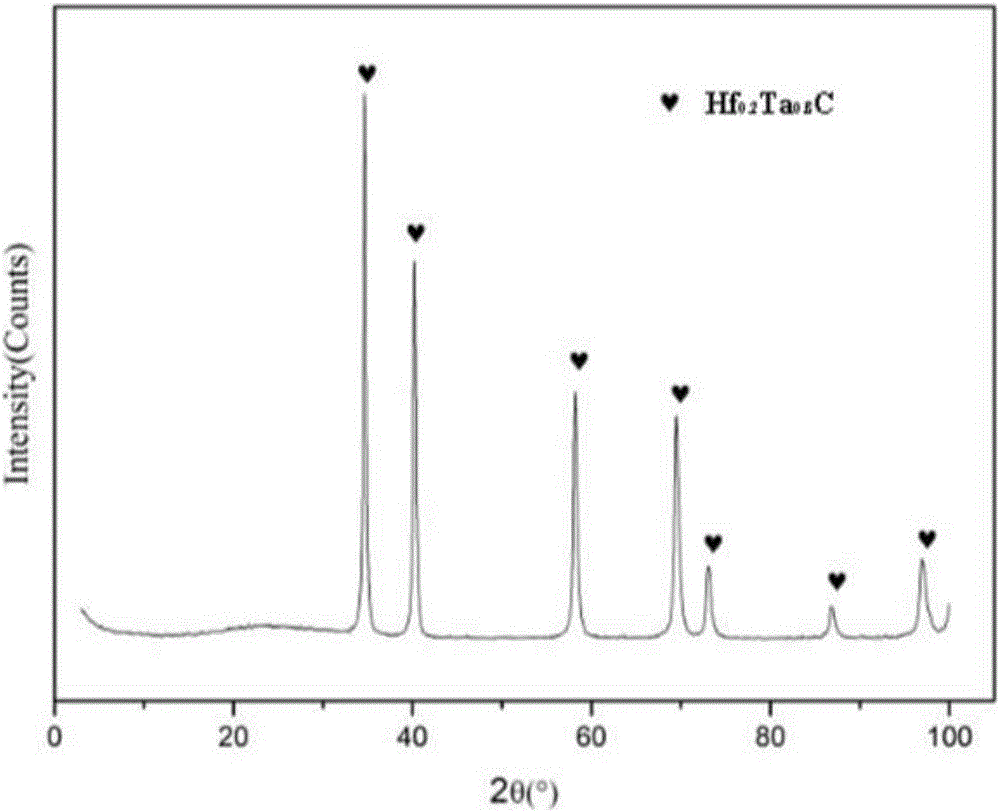
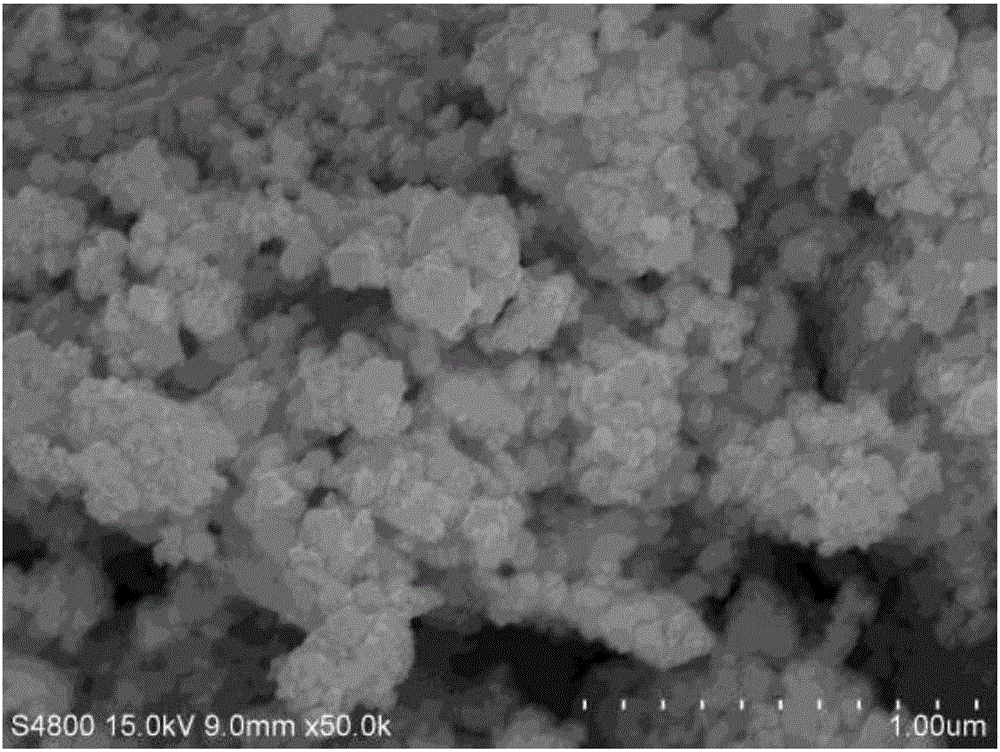
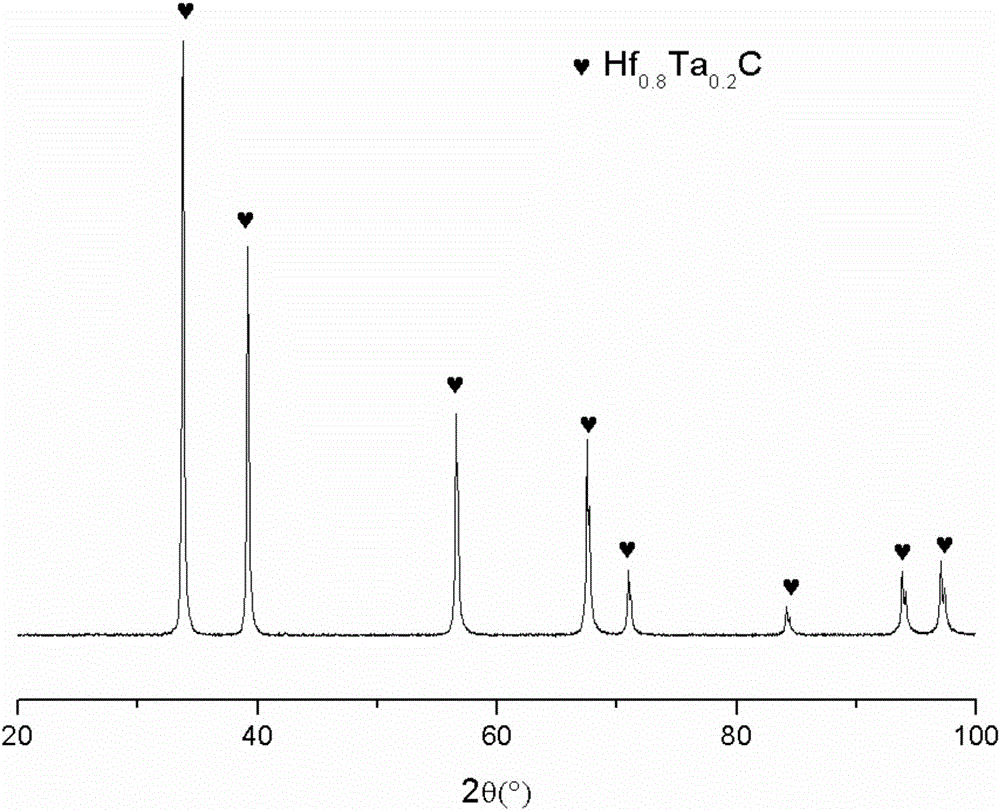
Preparation method of Hf<x>Ta<1-x>C alloy precursor and Hf<x>Ta<1-x>C alloy prepared therefrom
The invention relates to a preparation method of an Hf<x>Ta<1-x>C alloy precursor and Hf<x>Ta<1-x>C alloy prepared therefrom. The method includes the following steps: (1) dispersing hafnium tetrachloride in a solvent and dropwise adding a mixture of monohydric alcohol and triethylamine, after the mixture is all added, performing heating reflux and filtering the solution to obtain a hafnium alkoxide solution; (2) dispersing tantalum pentachloride in a solvent and dropwise adding a mixture of monohydric alcohol and triethylamine, after the mixture is all added, performing heating reflux and filtering the solution to obtain a tantalum alkoxide solution; (3) mixing the hafnium alkoxide solution and the tantalum alkoxide solution, dropwise adding a chelating agent, and then performing heating reflux, dropwise adding a mixture of water and monohydric alcohol, after the mixture is all added, performing reflux and pressure reduced distillation to obtain a hafnium and tantalum polymer precursor; and (4) mixing the hafnium and tantalum polymer precursor with allyl phenolic aldehyde to prepare the Hf-Ta alloy precursor. The Hf<x>Ta<1-x>C alloy precursor has good solubility and long stable storage time and can be used as a fiber-reinforced ceramic based composite material substrate.
Owner:INST OF CHEM CHINESE ACAD OF SCI
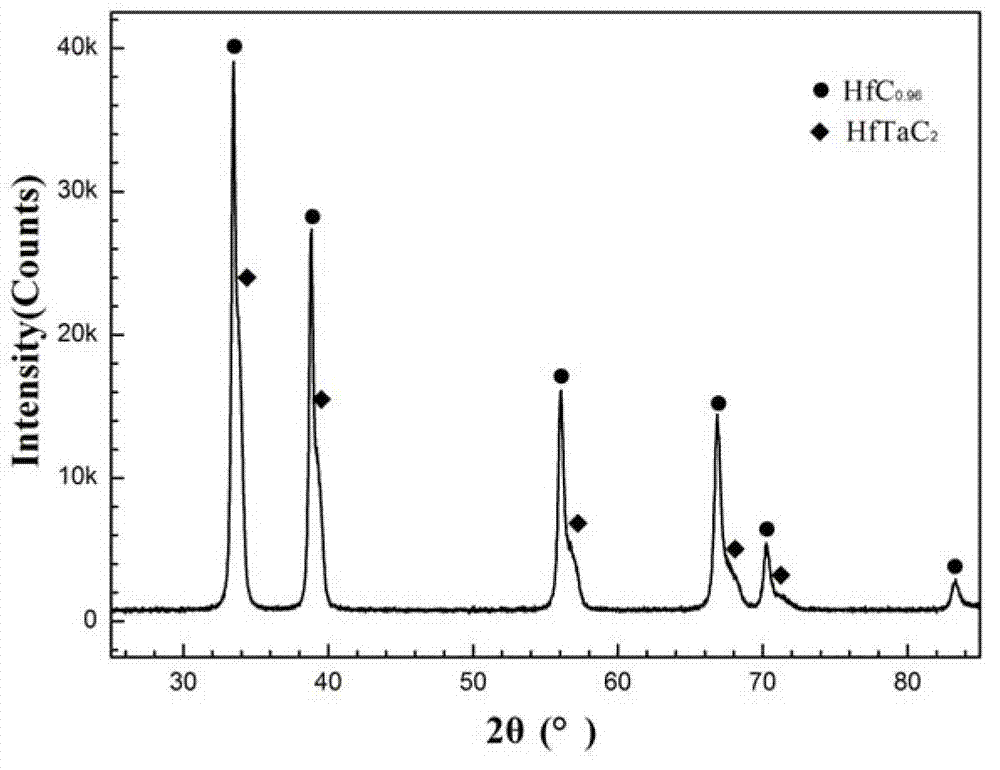
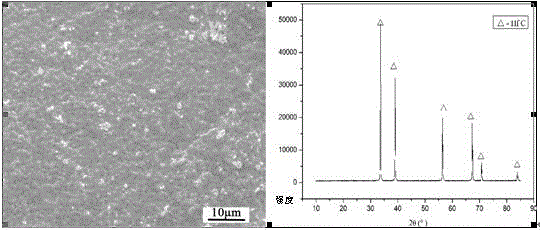
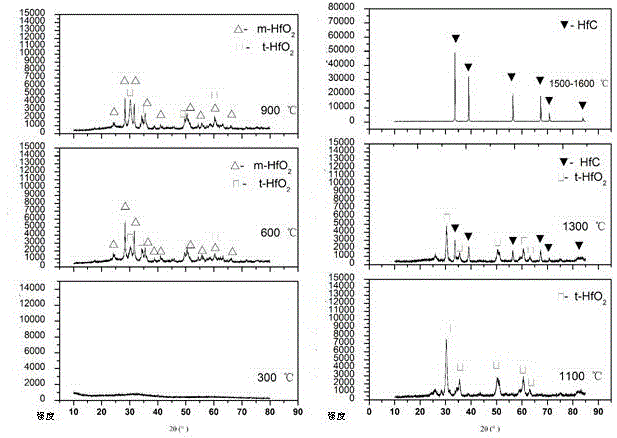
Hf (Ta) C ultra-high-temperature composite coating and preparation method thereof
The invention discloses a Hf (Ta) C ultra-high-temperature composite coating, which consists of HFC with HfTaC2, wherein the molar fraction of HfTaC2 is 6-50 percent, and the HfTaC2 is distributed in the coating uniformly or in a gradient way. The preparation method comprises the steps of: placing a surface-treated substrate material into a low-voltage chemical vapor deposition furnace, and usingmixed powder of hafnium tetrachloride and tantalum pentachloride as a hafnium source and a tantalum source, methane as a carbon source, argon as a diluent gas and hydrogen as a reducing gas; and delivering the mixed powder into a reactor of the deposition furnace, and preparing the Hf (Ta) C ultra-high-temperature composite coating by surface deposition on the substrate material. Limitations of asingle coating in ablation can be overcome, advantages of all phases of the coating can be fully utilized, and requirements for long-time high-temperature protection of the substrate material can be satisfied. The process is simple and convenient to operate, the prepared coating is well combined with the substrate, no interlayer cracks or penetrating cracks can be produced, and the thermal shock resistance and ablation resistance are excellent. The Hf (Ta) C ultra-high-temperature composite coating is suitable for surface coating and high-temperature protection of carbon / carbon composite materials, carbon / ceramic composite materials, graphite, carbide ceramics and other materials.
Owner:CENT SOUTH UNIV
Rare earth hafnate high-entropy ceramic powder through low-temperature synthesis and preparation method
The invention relates to rare earth hafnate high-entropy ceramic powder through low-temperature synthesis and a preparation method, wherein the chemical formula of the rare earth hafnate high-entropyceramic powder is (5RE0.2)2Hf2O7, and rare earth elements RE are La, Ce, Nd, Pr, Sm and Eu. The preparation method specifically comprises the following steps: by taking hafnium tetrachloride (HfCl4) and hydrated rare earth nitrate (RE(NO3)3.xH2O) as raw materials and selecting urea as a combustion agent, mixing the components in a solution, combusting in an air atmosphere at a low temperature to generate fluffy powder, and carrying out high-temperature carbon removal treatment to obtain high-purity (5RE0.2)2Hf2O7 powder. Compared with high-energy ball milling, spray pyrolysis, coprecipitationand other oxide high-entropy ceramic synthesis methods, the method has the advantages of low synthesis temperature, simple operation, high preparation speed and the like.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
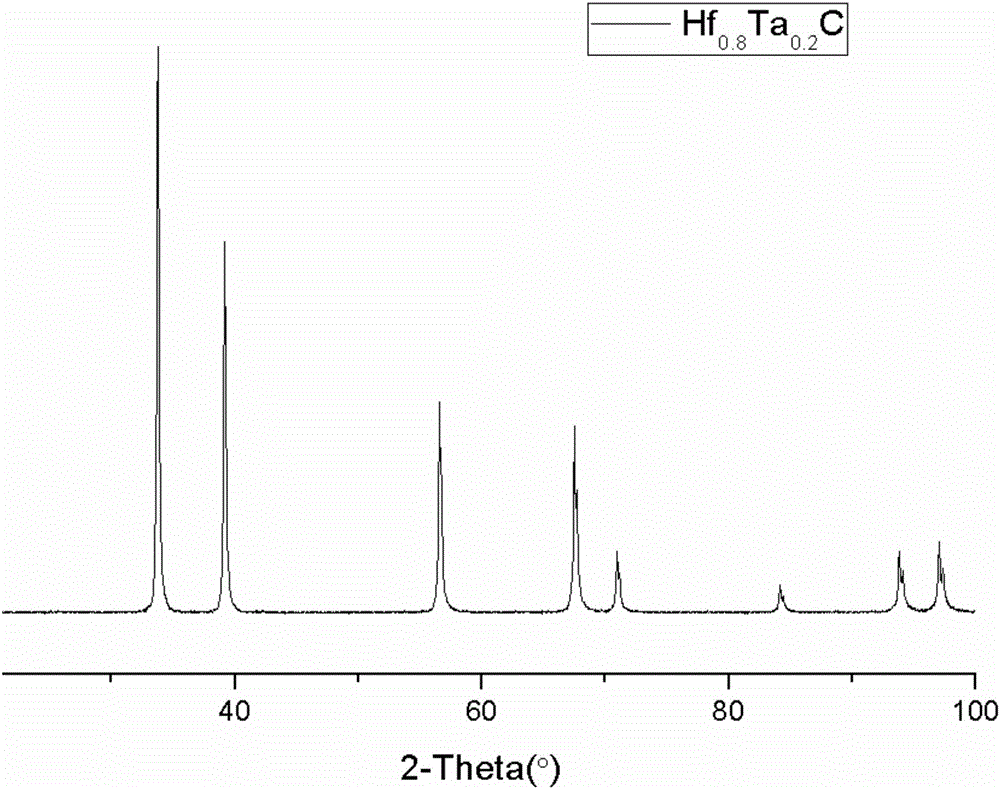
Preparation method of HfxTa1-xC alloy precursor and HfxTa1-xC alloy obtained through method
The invention relates to a preparation method of an HfxTa1-xC alloy precursor and an HfxTa1-xC alloy obtained through the method. The method comprises the steps that 1, hafnium tetrachloride is dispersed in a solvent, monobasic alcohol is dripped into the solvent, triethylamine is dripped into the solvent, after monobasic alcohol and triethylamine are completely dripped, reflux is conducted, filtration is conducted, and a hafnium alkoxide solution is obtained; 2, a chelating agent is dropwise added into the hafnium alkoxide solution, after the chelating agent is completely dripped, reflux is conducted, water and monobasic alcohol are added, after the water and monobasic alcohol are completed dripped, reflux is conducted, and polyhafnoxane is obtained through vacuum distillation; 3, tantalum pentachloride is dispersed in the solvent, monobasic alcohol is dripped into the solvent, triethylamine is dripped into the solvent subsequently, after monobasic alcohol and triethylamine are completely dripped, reflux is conducted, filtration is conducted, and a tantalum alkoxide solution is obtained; 4, a chelating agent is dropwise added into the hafnium alkoxide solution, after the chelating agent is completely dripped, reflux is conducted, water and monobasic alcohol are added, after the water and monobasic alcohol are completed dripped, reflux is conducted, and poly tantalum oxane is obtained through vacuum distillation; 5, polyhafnoxane, poly tantalum oxane and allyl phenol are mixed, and a hafnium tantalum alloy precursor is obtained. The prepared alloy precursor is good in solubility and stable storage life and can serve as a fiber reinforced ceramic-based composite material matrix for use.
Owner:自贡中天胜新材料科技有限公司
Synthetic method of tetra(dimethylamino)zirconium
InactiveCN103910640ALow toxicityEasy to operateOrganic compound preparationAmino compound preparationArgon atmosphereDistillation
The invention relates to a synthetic method of tetra(dimethylamino)zirconium, which comprises the following steps: 1)under argon atmosphere, adding dimethylamine and hexane in a three-necked bottle according to a proportion that 100-300 milliliters n-hexane is added in 100g dimethylamine, uniformly stirring, and placing the reaction bottle between the temperature of -20--60 DEG C, adding a n-butyllithium solution drop by drop in the reaction bottle, stirring after dropping, and reacting for 10 hours; 2)adding zirconium tetrachloride in the above reaction system, keeping the temperature of the reaction system between -20-0 DEG C, after adding zirconium tetrachloride, stirring the reaction system under the protection of inert gas to react for 24-30 hours; and 3)after the reaction is finished, removing a solvent of the reaction under one atmospheric pressure, after solvent n-hexane is completely removed, performing underpressure distillation, and collecting the fraction at 110-112 DEG C / 4mmHg which is the tetra(dimethylamino)zirconium compound. According to the invention, the reaction employs the raw material dimethylamino lithium and hafnium tetrachloride which are simple and easy to obtain, the operation is simple, and the cost is reduced.
Owner:NANJING UNIV
Preparation method and application of metal organic framework material
ActiveCN107353412ALarge adsorption capacityGood chemical stabilityProductsOther chemical processesAcetic acidN dimethylformamide
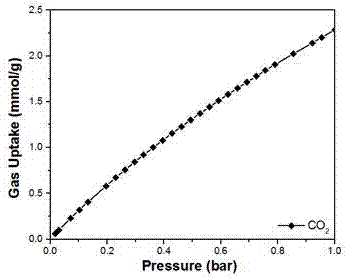
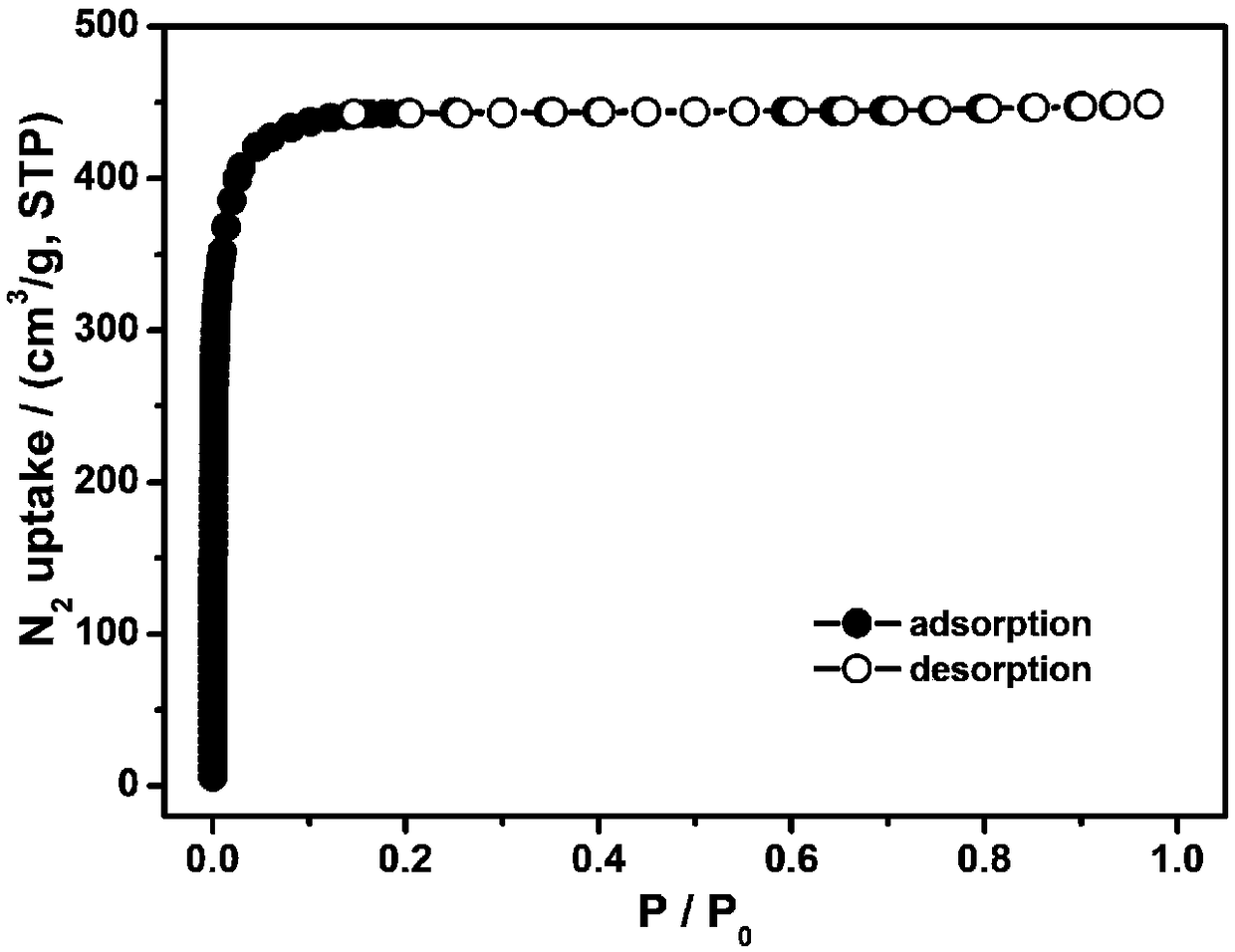
The invention relates to a preparation method and application of a metal organic framework material, and belongs to the technical field of crystalline materials. Under the condition that the temperature is not higher than 40 DEG C, zirconium tetrachloride, hafnium tetrachloride and 2-amino terephthalic acid are dissolved in a mixed organic phase to obtain a mixed solution A, wherein the mixed organic phase is an organic mixture of N,N-dimethylformamide and acetic acid; at the temperature of 100-120 DEG C, the mixed solution A reacts for 12-24 h under the stirring condition, the product is filtered, washed with N,N-dimethyl formamide and subjected to vacuum drying, and then the metal organic framework material is obtained. The metal organic framework material prepared through the method can be used for adsorbing carbon dioxide (CO2) and has the advantages of being high in adsorption rate and capable of being repeatedly used.
Owner:KUNMING UNIV OF SCI & TECH
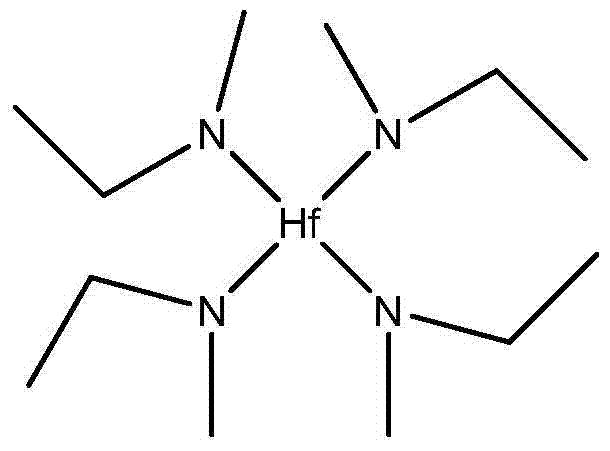
Tetrakis(ethylmethylamino)hafnium synthesis method
InactiveCN103601750AEasy to operateSimple and fast operationGroup 4/14 element organic compoundsAlkaneN-Butyllithium
The invention relates to a tetrakis(ethylmethylamino)hafnium synthesis method, which comprises the following synthesis steps: (1) under an inert atmosphere, adding methylethylamine and an alkane solvent to a three-necked bottle, carrying out mechanical stirring, placing the reaction bottle into an environment with a temperature of -10 to -80 DEG C, adding a n-hexane solution of n-butyl lithium to the reaction bottle in a dropwise manner, and carrying out a stirring reaction after completing the addition; (2) adding hafnium tetrachloride to the reaction system, maintaining the temperature of the reaction system to not more than 60 DEG C, carrying out a stirring reaction on the reaction system under the protection of the inert gas after completing the addition; and (3) after completing the reaction, removing the reacting solvent under an atmospheric pressure, carrying out vacuum distillation after completely removing the solvent, and collecting the 110-115 DEG C / 4-5 mmHg fraction, wherein the fraction is the tetrakis(ethylmethylamino)hafnium compound. According to the present invention, the reaction adopts the simple and easily available materials such as methylethylamine, butyl lithium and hafnium tetrachloride as the raw materials, the operations are simple, and the cost is reduced.
Owner:NANJING UNIV
Catalyst for conversion of ethyl levulinate into gamma-valerolactone and preparation method of catalyst
ActiveCN107398301AReduced activityHigh activityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsMetal-organic frameworkGamma-Valerolactone
The invention provides a catalyst for conversion of ethyl levulinate into gamma-valerolactone and a preparation method of the catalyst. The catalyst comprises two parts, namely, sulfonic acid functionalized hafnium-based-metal organic framework material SO3H-MOF (Hf) and noble metal Ru nano-particles, wherein the noble metal Ru accounts for 0.5%-5% of the total weight of the catalyst, and the sulfonic acid group SO3H accounts for 0-20% of the total weight of the catalyst. The preparation method comprises the steps as follows: adding hafnium tetrachloride, terephthalic acid and monosodium 2-sulfoterephthalate to a mixed solution of dimethylformamide and acetic acid, and performing crystallizing, washing and drying to obtain the sulfonic acid functionalized hafnium-based-metal organic framework material SO3H-MOF (Hf); then, adding a RuCl3 solution dropwise to obtain a catalyst precursor, performing reduction on the catalyst precursor with sodium borohydride, and performing aftertreatment on the catalyst precursor with hydrochloric acid to obtain the catalyst. The catalyst has better catalytic activity, selectivity and reusability when used for conversion of ethyl levulinate into gamma-valerolactone.
Owner:ZHEJIANG NORMAL UNIVERSITY
Hydrothermal method for preparing HfO2 nano-particles
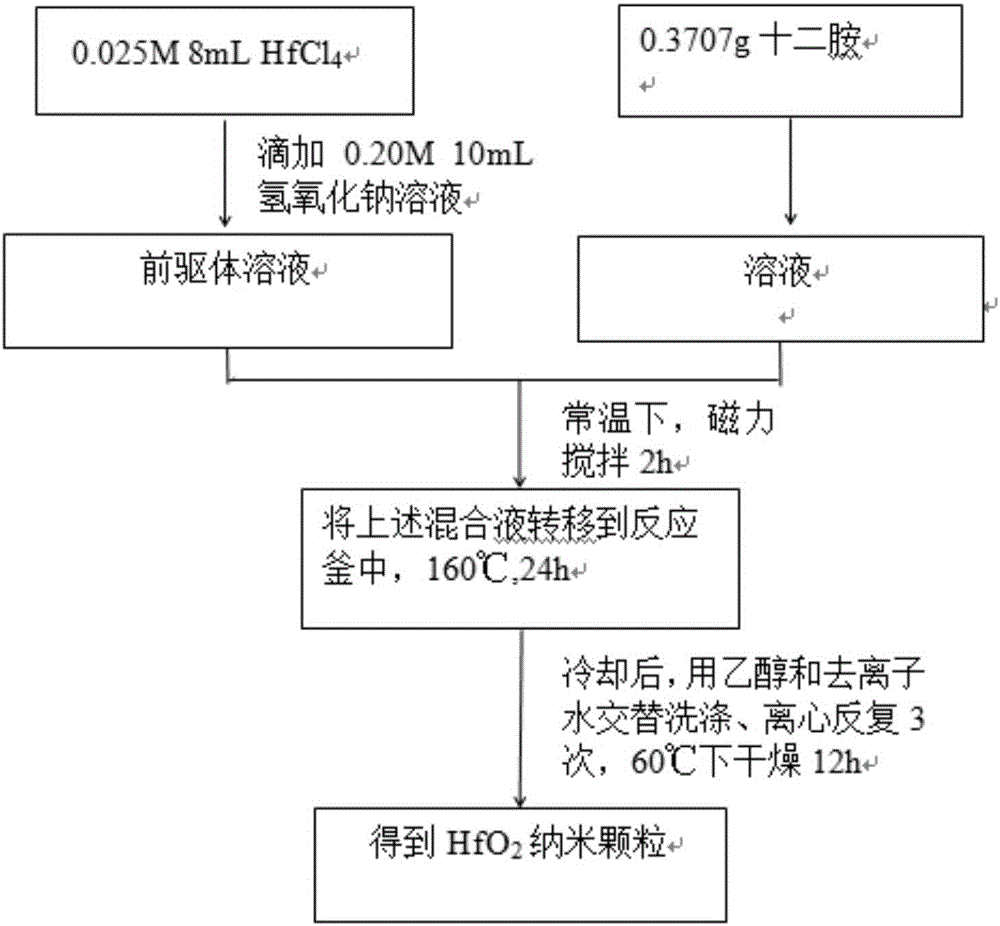
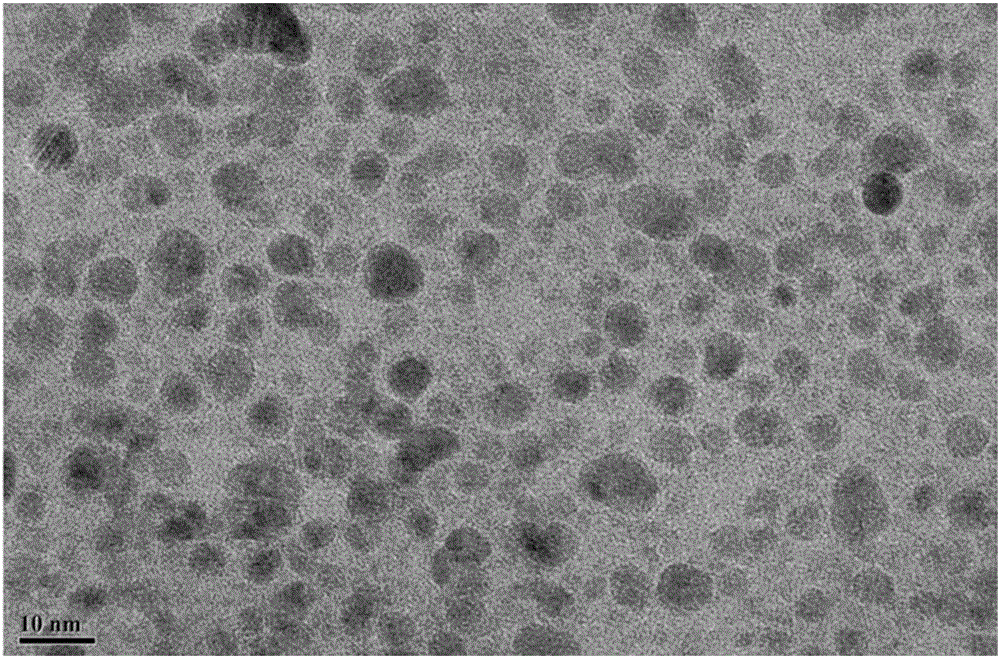
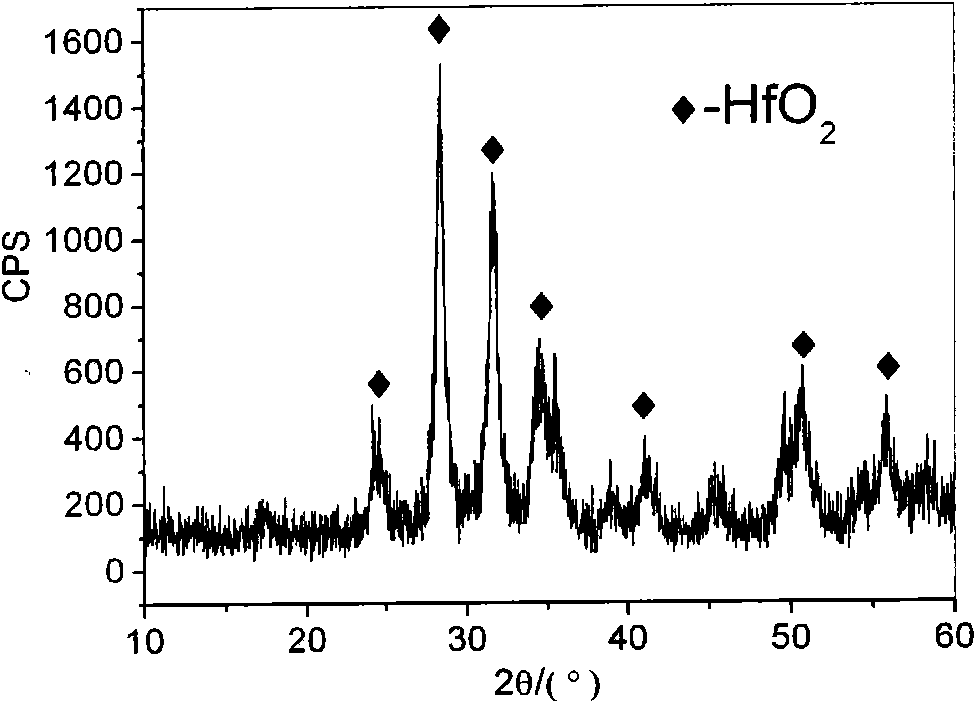
The invention relates to a hydrothermal method for preparing HfO2 nano-particles. According to the invention, hafnium tetrachloride is dissolved in water, such that a hafnium oxychloride solution is obtained; a sodium hydroxide solution is dropped into the hafnium oxychloride solution, such that a precursor solution is obtained; dodecylamine is dissolved in a solvent, such that a dodecylamine solution is obtained; the dodecylamine solution is mixed with the above precursor solution, and the mixture is subjected to normal-temperature magnetic stirring, such that a mixed solution is carried out; a hydrothermal reaction is carried out; and separation, washing and drying are carried out, such that the nano-particles are obtained. The method provided by the invention is simple and convenient, and has good repeatability low cost. The method can be used in mass production. The prepared HfO2 nano-particles have uniform particle size distribution, and have a good application prospect.
Owner:DONGHUA UNIV +1
Preparation method of hafnium oxide powder with nano-porous structure
The invention provides a preparation method of hafnium oxide powder with a nano-porous structure, which belongs to the field of nano-powder materials. The preparation method is characterized by adopting the solid phase method, taking hafnium tetrachloride and benzoic acid as raw materials, mixing the two according to the molar ratio of 1:2-4, carrying out uniform ball milling, then calcining for 2h at the temperature of 500-800 DEG C and finally obtaining the hafnium oxide powder with the nano-porous structure, wherein the pore size is 50-100nm, and the particle size of the hafnium oxide is 20-100nm. The preparation method has the advantages of simple process, safe operation and high efficiency, and the synthesized nano-hafnium oxide powder has larger specific surface area.
Owner:SHANDONG UNIV OF TECH
Method for separating zirconium and hafnium tetrachlorides with the aid of a melted solvent
InactiveUS6929786B2Improve productivityEfficient separationUsing liquid separation agentVapor condensationMolten stateMetal chloride
A method of separating zirconium and hafnium tetrachlorides using a solvent comprising firstly an alkaline metallic solvent comprising a salt made up of an alkali metal chloride and an acidic metal chloride A, for example a chloroaluminate or an alkaline chloroferrate, and secondly an acidic metal or metalloid chloride B of acidity that is less than that of the acidic metal chloride A. The acidic metal or metalloid chloride B may be selected from chlorides of Mg, Zn, and Cu. The method may be a continuous separation method by selective absorption of the tetrachloride vapors by the solvent in the substantially or totally molten state.
Owner:CO EUROPENNE DU ZIRCONIUM CEZUS
Production of hafnium nitride thin-membrane materials from ion beam epitaxial growth apparatus
InactiveCN1778985AHigh purityLow costVacuum evaporation coatingSputtering coatingIsotopeNitrogen gas
A method of producing hafnium nitride(HfN) thin-film material by ion beam epitaxy growing equipment. The process includes selecting hafnium tetrachloride (ZrCl4) powder andnitrogen(N2) as the materials of producing low-energy isotope Hf+ ion beam and N+ ion beam ,choosing twoion beams epitaxy growing equipment with mass separation function and Energetic-Ions depositing function, accurate controlling dosage and mixture ratio ,ion energy and growth temperature of the ion beams; obtaining hafnium nitride thin-film with high quality growth and low temperature extension in ultrahigh vacuum chamber. Ití»s a practical and economical method for producing hafnium nitride thin-film material in semiconductor domain owing to its convenience in growth controlling and optimizing.
Owner:INST OF SEMICONDUCTORS - CHINESE ACAD OF SCI
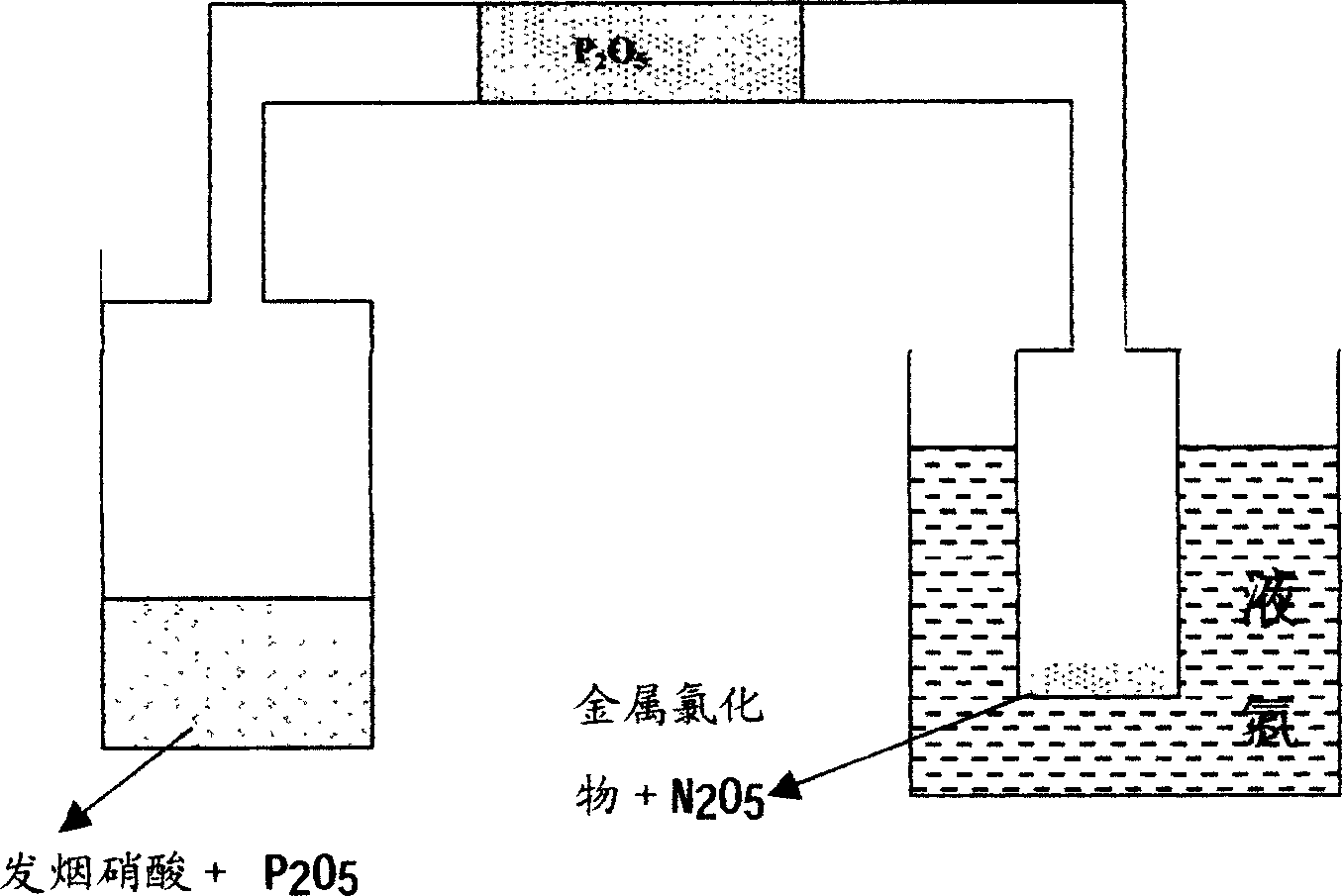
Method of synthesis of hafnium nitrate for HfO2 thin film deposition via ALCVD process
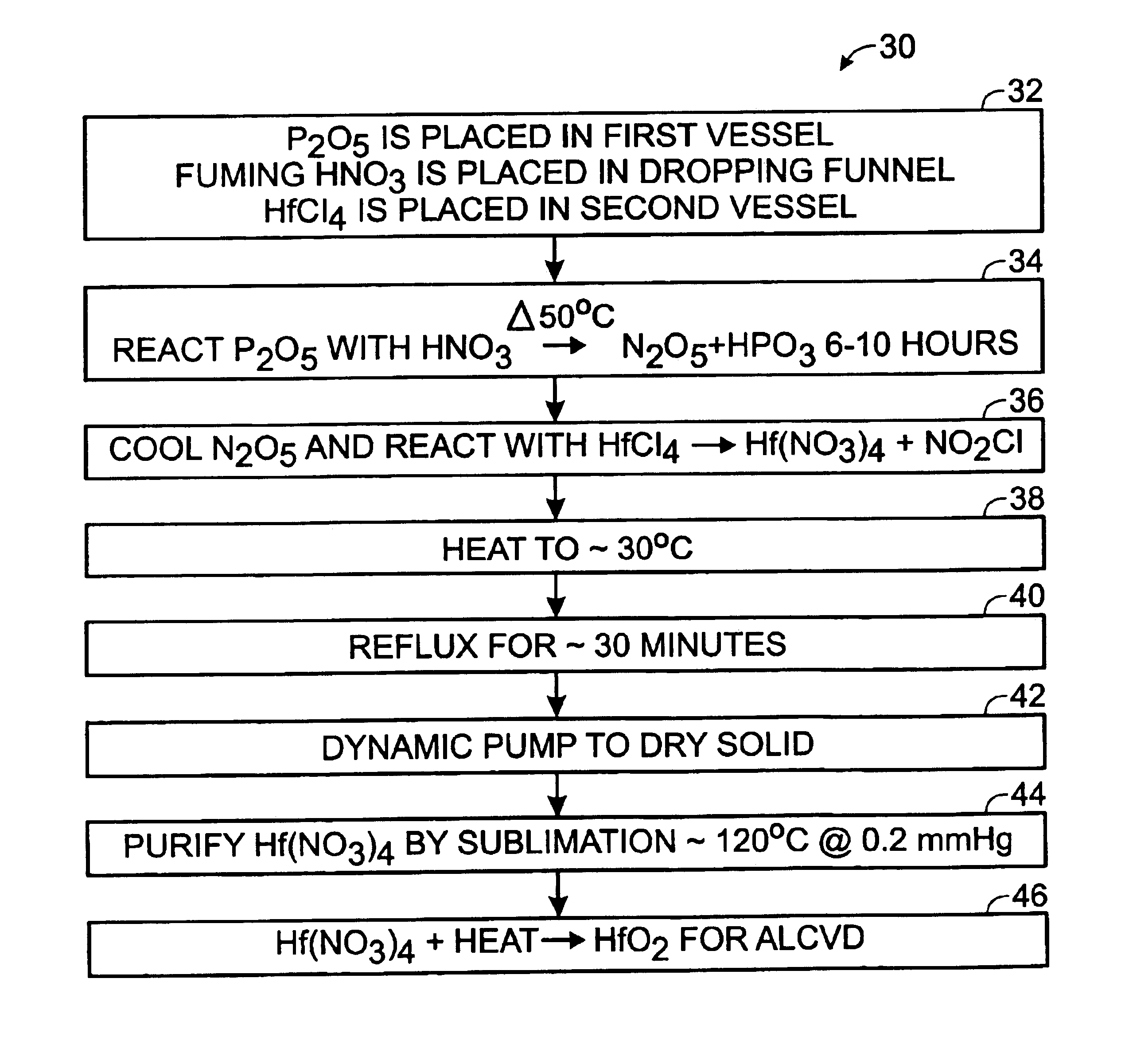
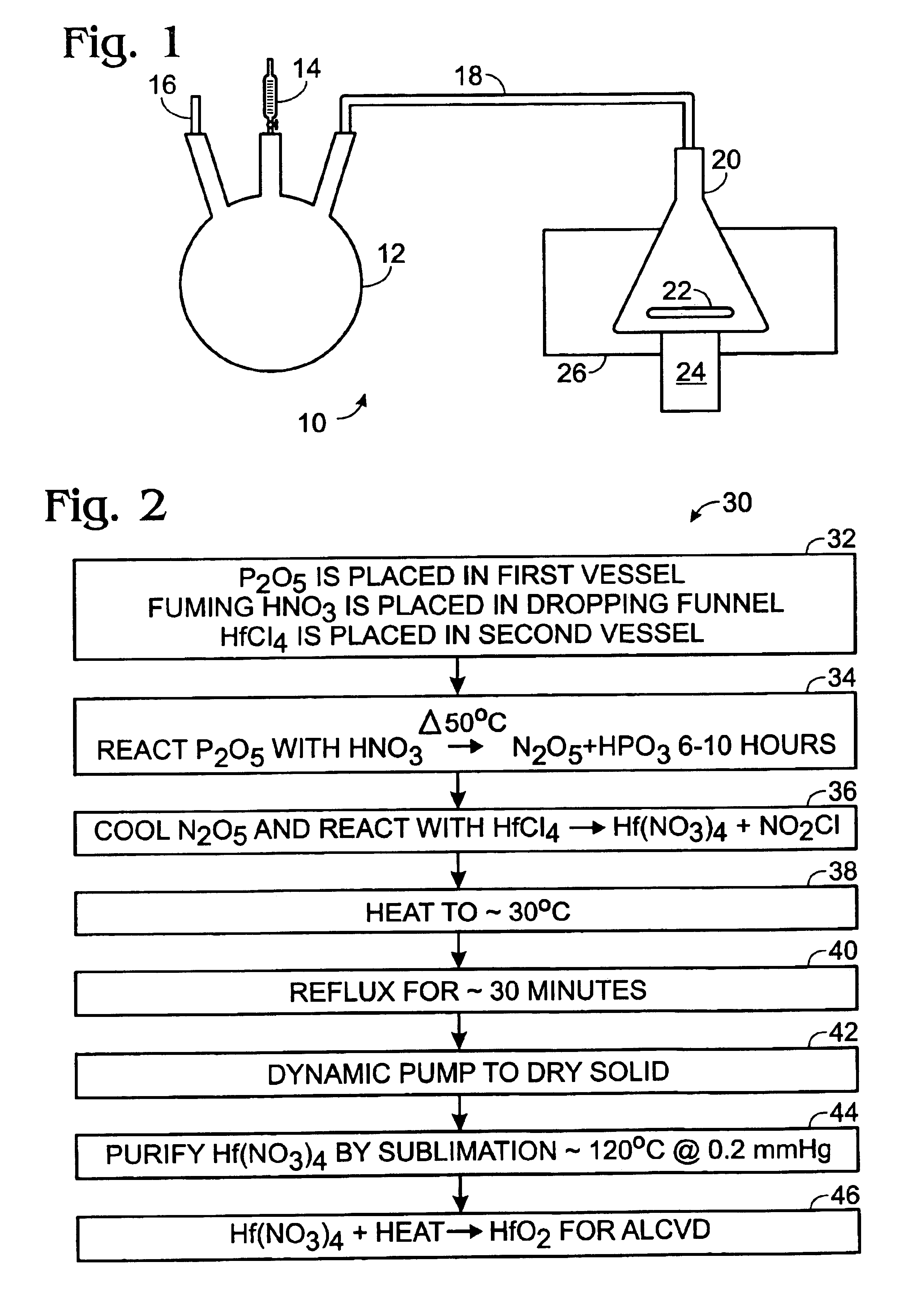
A method of preparing a hafnium nitrate thin film includes placing phosphorus pentoxide in a first vessel; connecting the first vessel to a second vessel containing hafnium tetrachloride; cooling the second vessel with liquid nitrogen; dropping fuming nitric acid into the first vessel producing N2O5 gas; allowing the N2O5 gas to enter the second vessel; heating the first vessel until the reaction is substantially complete; disconnecting the two vessels; removing the second vessel from the liquid nitrogen bath; heating the second vessel; refluxing the contents of the second vessel; drying the compound in the second vessel by dynamic pumping; purifying the compound in the second vessel by sublimation to form Hf(NO3)4, and heating the Hf(NO3)4 to produce HfO2 for use in an ALCVD process.
Owner:SHARP LAB OF AMERICA INC
Efficient and novel method for preparing aminophosphonate through catalytic synthesis of hafnium tetrachloride
InactiveCN105646576AHigh catalytic activityReduce dosageGroup 5/15 element organic compoundsPhosphorous acidCatalytic effect
The invention discloses an efficient and novel method for preparing aminophosphonate through catalytic synthesis of hafnium tetrachloride. With hafnium tetrachloride as a catalytic reagent, aryl / alkyl aldehyde, aryl / alkyl amine and phosphorous acid diester / triester as raw materials, and ethyl alcohol as a solvent, a one-pot reaction is performed for 0.5-2.0 h at 60 DEG C to generate corresponding aminophosphonate, wherein the dosage of the hafnium tetrachloride catalytic reagent is 2 mol% that of aldehyde, and the concentration of aldehyde in an ethanol solution and the concentration of amine in the ethanol solution are both 1.0 mol / L. Hafnium tetrachloride used in the method is high in catalytic activity, small in dosage, capable of achieving the optimum catalytic effect at the dosage of 2 mol%, universally applicable to various aryl aldehyde / amine substrates and alkyl aldehyde / amine substrates and high in product yield. According to the method, reaction conditions are mild, heating is performed just at 60 DEG C, the ethyl alcohol solvent does not need drying, a reaction system does not need gas protection, the reaction speed is high, aftertreatment and purification are easy, and it is only needed to concentrate the reaction system and directly perform conventional silica-gel column chromatography.
Owner:JIANGXI SCI & TECH NORMAL UNIV
Visible-light responsive hafnium-based metal-organic framework material and preparation method thereof
ActiveCN108997591AImprove thermal stabilityGood chemical stabilityOrganic-compounds/hydrides/coordination-complexes catalystsAbsorption capacityMetal-organic framework
The invention discloses a visible-light responsive hafnium-based metal-organic framework material and a preparation method thereof, and belongs to the intersection field of inorganic chemistry, organic chemistry and material chemistry. According to the invention, hafnium tetrachloride is used as a metal salt, trans ethylene bisaminobenzoic acid is used as an organic ligand, and the novel hafnium-based metal-organic framework crystal material is prepared according to a solvothermal method; a metal-organic framework compound prepared according to the method provided by the invention is relatively high in thermal stability and chemical stability, and large in specific surface area and visible light absorption capacity. The preparation technology provided by the invention is simply and convenient to operate, and has important significance for the preparation of porous visible-light responsive hybrid materials and the application thereof in the field of photocatalysis.
Owner:NORTHEAST FORESTRY UNIVERSITY
Industrialized production method for tetra(methylethylamino)hafnium
InactiveCN106916178AEasy to getLow priceGroup 4/14 organic compounds without C-metal linkagesAlkanePurification methods
The invention discloses a synthesis and industrialized purification method for tetra(methylethylamino)hafnium. The method comprises the following steps: adding an n-butyl lithium.alkane CnH(2n+2) (n is not smaller than 6) solution into an inert atmosphere shielded reactor, dropwise adding N-methylethylamine into the reactor under the condition of stirring, carrying out a reaction, keeping the temperature of a reaction system to 0 DEG C to -30 DEG C, and carrying out a reaction for 8 to 12 hours while keeping the temperature to 0 DEG C to -30 DEG C after dropwise adding is completed; then, adding hafnium tetrachloride in a staged manner, carrying out a reaction for 8 to 12 hours while keeping the temperature to 0 DEG C to -30 DEG C, and maintaining a reflux reaction for 4 to 8 hours; and carrying out cooling, then, removing a byproduct, i.e., lithium chloride through a solid-liquid separator, transferring a solution to a distiller, carrying out depressurizing firstly until the pressure intensity is 10mmHg to 50mmHg to distill off an alkane CnH(2n+2) (n is not smaller than 6) solvent, and then, carrying out depressurizing until the pressure intensity is 1mmHg to 2mmHg, so as to distill off the product, i.e., tetra(methylethylamino)hafnium (TEMAH). According to the method disclosed by the invention, selected reagents are moderately-priced and readily available, and the reaction process is moderate and is free of potential safety hazards; due to high-boiling-point alkane, the reaction yield is increased; by arranging a buffer tank, the production efficiency is increased; and the reaction is free of wastes, so that the method is pollution-free to environments.
Owner:苏州复纳电子科技有限公司
Preparation method and application of hafnium carbide precursor impregnation liquid
InactiveCN105601278ASolve application problemsImprove high temperature ablation resistanceCarbon compositesDistillation
The invention relates to a preparation method and an application of a hafnium carbide precursor impregnation liquid. The preparation method is characterized in that hafnium tetrachloride and ROH undergo a nucleophilic substitution reaction under the protection of nitrogen, and R is an ethyl group, a propyl group, a cyclohexyl group or a benzyl group; and the preparation method comprises the following steps: adding hafnium tetrachloride to toluene or xylene, cooling a reaction system to 0DEG C or below, adding the ROH to the reaction system, reacting, and rising the temperature of the reaction system to room temperature; carrying out pumping filtration on a precipitate in the reaction system to obtain a solution A, removing impurities in the solution A to obtain a solution B, and extracting an organic phase in the solution B to obtain a solution C; and adding a drying agent to the solution C, carrying out pumping filtration, and carrying out reduced pressure distillation to obtain the hafnium carbide precursor impregnation liquid. Uniform introduction of a HfC ceramic phase prepared in the invention to a carbon / carbon composite material matrix effectively solves the problem of application of the carbon / carbon composite material at a high temperature, and introduction of the hafnium carbide ceramic phase greatly improves the high temperature ablation resistance of the C / C composite material and widens the application range of the C / C composite material.
Owner:CENT SOUTH UNIV
Synthesis method of hafnium tetraethoxide
ActiveCN102040478ALow toxicityEasy to operatePreparation of metal alcoholatesAlcohol ethylPhysical chemistry
The invention relates to a synthesis method of hafnium tetraethoxide, which comprises the following steps: (1) in a nitrogen atmosphere, adding 5.8g of diced sodium and 100mL of ethanol into a 500mL three-necked bottle, mechanically stirring until the sodium completely reacts, and cooling to room temperature; adding 16g of hafnium tetrachloride into the reaction system in batches while keeping the temperature of the reaction system not higher than 60 DEG C; after adding the hafnium tetrachloride, keeping the temperature of the reaction system at 40-65 DEG C, reacting for 2-8 hours under the condition of mechanical stirring, and evaporating out the residual ethanol to obtain a dry solid; and adding 150mL of phenylmethane to the solid, stirring, filtering, concentrating the filtrate to precipitate white solids, and drying the white solids in vacuum to obtain white crystals. The yield of the method is 80-85%.
Owner:马鞍山南大高新技术研究院有限公司
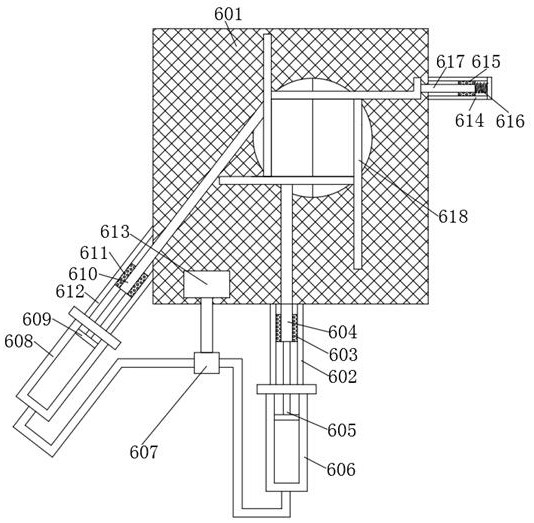
Reducing furnace for preparing sponge hafnium
Owner:南通晶朋新材料科技有限公司
Hafnium tetrachloride preparation method
The invention discloses a hafnium tetrachloride preparation method which includes the processing steps that hafnium oxide and carbon powder are smashed, the smashed hafnium oxide and the smashed carbon powder are evenly mixed into a mixture, microwave drying and dehydration are carried out on the mixture, the dried mixture is added into a chlorination furnace, chlorine is added for chlorination, after an reaction, hafnium tetrachloride gas generated by a fluidized bed passes a condenser, then solid hafnium tetrachloride is obtained, and tail gas is exhausted after leaching in a channel. The method is short in process procedure, the metal recycle rate is high, the yield of the single furnace is high, occupied area is small, equipment is small in number, concentrated and easy to manage, environmental protection is facilitated, no chlorine leaks due to new process material matching, materials are conveyed in a closed condition, powder emission is reduced, and resources are saved.
Owner:CHINA NUCLEAR JINGHUAN ZIRCONIUM IND
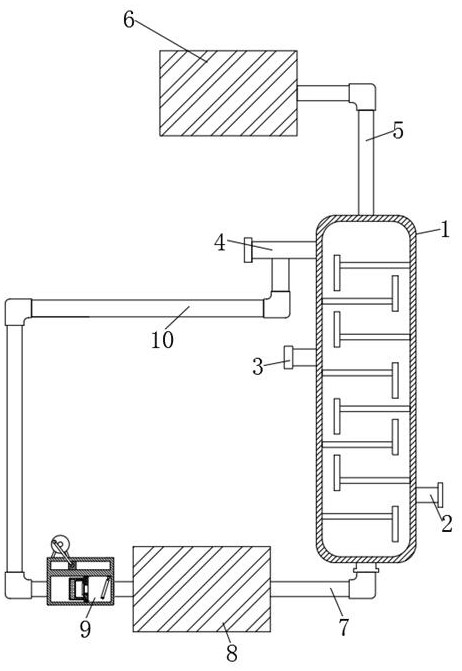
Method for recovering hafnium tetrachloride in crude zirconium tetrachloride purification process
The invention provides a method for recovering hafnium tetrachloride in a crude zirconium tetrachloride purification process. The invention provides a preparation device for the recovery method. The device comprises a rectifying tower; a steam inlet is formed in the lower right side of the rectifying tower; a fourth rail plate is fixedly connected to the rear side of a material containing plate; fourth moving plates are slidably connected to the inner side of the fourth rail plate; first connecting rods are rotatably connected to the front sides of the fourth moving plates; and first fixing shafts are rotatably connected to the lower sides of the first connecting rods. According to the method for recovering hafnium tetrachloride in the crude zirconium tetrachloride purification process ofthe invention, the first connecting rod on the left side is driven to rotate through engagement of the first connecting rods, so that the fourth moving plates are opened outwards, then hafnium tetrachloride on the material containing plate is pushed into a round hole in the material containing plate, and therefore collection is conducted. With such a structure adopted, hafnium tetrachloride is easy to collect, the recovery efficiency of the hafnium tetrachloride is high, the hafnium tetrachloride can be collected regularly, and the recovery rate of the hafnium tetrachloride can be calculated.
Owner:宝钛华神钛业有限公司
Method for synthesizing hafnocene dichloride
The invention relates to a method for synthesizing hafnocene dichloride, which comprises the following process steps: depolymerizing cyclopentadiene: depolymerizing the cyclopentadiene under inert atmosphere, and collecting cyclopentadiene monomers obtained by distillation for standby; heating a solvent of diglycol ether and sodium wires under the inert atmosphere, then dripping the cyclopentadiene monomers, carrying out reaction after completing the dripping, and obtaining diglycol ether solution of cyclopentadienyl sodium; dripping the well prepared cyclopentadienyl sodium solution into n-hexane suspension of hafnium tetrachloride solids, and carrying out reaction at the temperature of 15-35 DEG C; firstly removing the n-hexane under one atmospheric pressure, further removing the diglycol ether, obtaining dark gray solids, carrying out extraction on the solids by using a Soxhlet extractor, filtrating extraction solution, depressurizing, removing the solvent by evaporation, cooling, then precipitating the solids, and obtaining a target product. The method firstly improves the dispersion of sodium, thereby improving the yield of the produced cyclopentadienyl sodium; and then the reaction is carried out with the solid hafnium tetrachloride in the suspension in the solvent, thereby obtaining the target product with higher efficiency.
Owner:NANJING UNIV
Lomposite metallic inorganic source of zirconium hafnium and titanium composited anhydrous nitrate and its synthesis method
InactiveCN1693533APrevent volatilizationAvoid problems caused by thermal decomposition performance differencesZirconium compoundsTitanium compoundsMetal chlorideNitrate
A metallic composite inorganic source with the formula: (ZrxTiy)(NO3)4, (HfxTiy)(NO3)4, where x+y=1, x=0.1-0.9 and y=0.1-0.9, is prepared from TiCl4 and anhydrous ZrCl4 or HfCl4 through reaction between said metal chlorides and N2O4 in the cold trap of liquid N2, removing residual NOx to obtain white powder, sublimating at 100-120 deg.C to generate white crystals on the container's wall at -20-0 deg.C, and removing residual Nox.
Owner:NANJING UNIV
Catalyst for converting ethyl levulinate into gamma-valerolactone and preparation method thereof
ActiveCN107398301BReduced activityHigh activityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsMetal-organic frameworkGamma-Valerolactone
The invention provides a catalyst for conversion of ethyl levulinate into gamma-valerolactone and a preparation method of the catalyst. The catalyst comprises two parts, namely, sulfonic acid functionalized hafnium-based-metal organic framework material SO3H-MOF (Hf) and noble metal Ru nano-particles, wherein the noble metal Ru accounts for 0.5%-5% of the total weight of the catalyst, and the sulfonic acid group SO3H accounts for 0-20% of the total weight of the catalyst. The preparation method comprises the steps as follows: adding hafnium tetrachloride, terephthalic acid and monosodium 2-sulfoterephthalate to a mixed solution of dimethylformamide and acetic acid, and performing crystallizing, washing and drying to obtain the sulfonic acid functionalized hafnium-based-metal organic framework material SO3H-MOF (Hf); then, adding a RuCl3 solution dropwise to obtain a catalyst precursor, performing reduction on the catalyst precursor with sodium borohydride, and performing aftertreatment on the catalyst precursor with hydrochloric acid to obtain the catalyst. The catalyst has better catalytic activity, selectivity and reusability when used for conversion of ethyl levulinate into gamma-valerolactone.
Owner:ZHEJIANG NORMAL UNIVERSITY
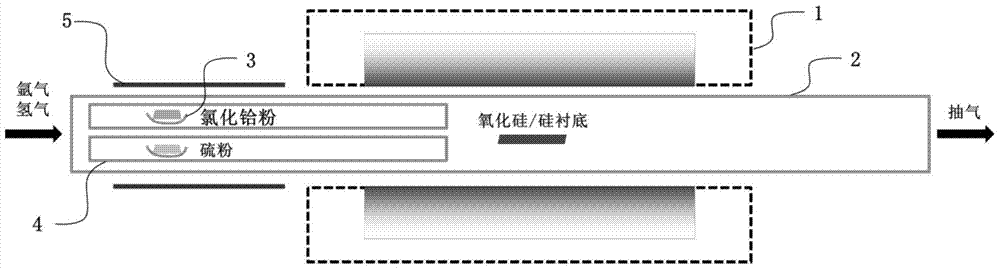
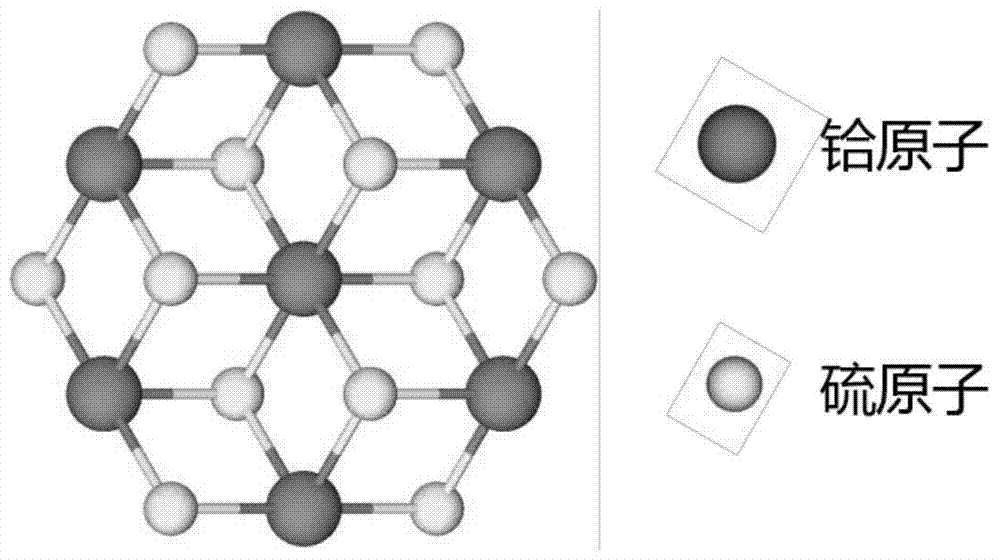
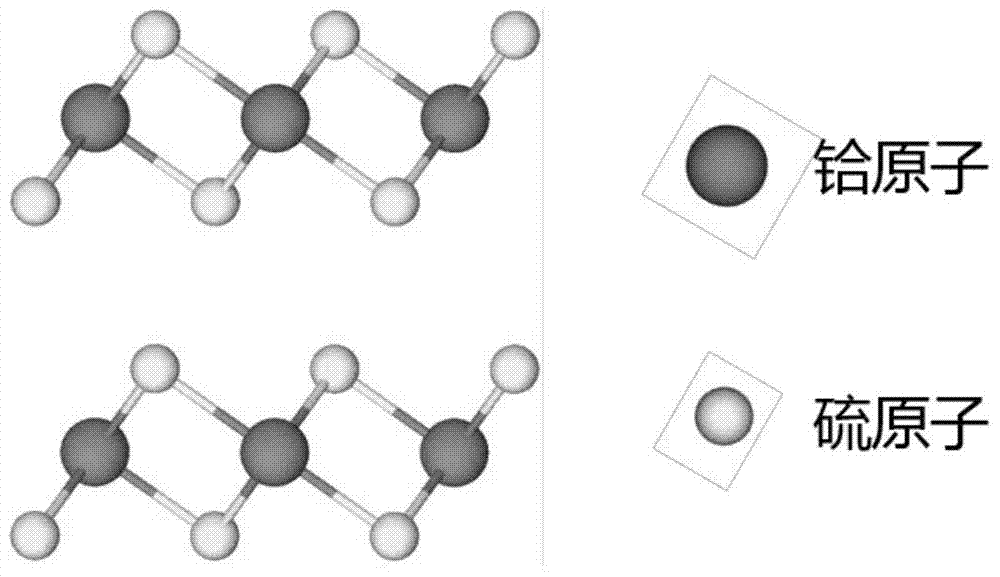
Preparation method of vertically aligned hafnium disulfide nanosheets by chemical vapor deposition
The invention discloses a method for preparing vertically arranged hafnium disulfide nanosheets by chemical vapor deposition, which comprises the following steps: (1) cleaning the substrate; (2) putting the substrate, sulfur powder and hafnium tetrachloride powder into a vacuum tube (3) Evacuate the vacuum tube furnace to a high vacuum, and feed in argon and hydrogen; (4) Heat up, keep warm, and cool down the tube furnace in sequence; (5) Take out the substrate, and the substrate is vertically arranged hafnium disulfide nanosheets; the vertically arranged hafnium disulfide nanosheets prepared by the present invention have good uniformity, high quality, and large area, and are useful for the basic research of hafnium disulfide nanomaterials and the potential application of related two-dimensional nano-optoelectronic devices A reliable method for sample preparation is provided, and the vertically arranged nanostructures have large specific surface area and high surface activity, and have broad application prospects as catalysts.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Production of hafnium nitride thin-membrane materials from ion beam epitaxial growth apparatus
InactiveCN100424221CLow costSave energyVacuum evaporation coatingSemiconductor/solid-state device manufacturingIsotopeNitrogen gas
A method of producing hafnium nitride(HfN) thin-film material by ion beam epitaxy growing equipment. The process includes selecting hafnium tetrachloride (ZrCl4) powder andnitrogen(N2) as the materials of producing low-energy isotope Hf+ ion beam and N+ ion beam ,choosing twoion beams epitaxy growing equipment with mass separation function and Energetic-Ions depositing function, accurate controlling dosage and mixture ratio ,ion energy and growth temperature of the ion beams; obtaining hafnium nitride thin-film with high quality growth and low temperature extension in ultrahigh vacuum chamber. It's a practical and economical method for producing hafnium nitride thin-film material in semiconductor domain owing to its convenience in growth controlling and optimizing.
Owner:INST OF SEMICONDUCTORS - CHINESE ACAD OF SCI
Method for synthesizing hafnium tetra-tert-butoxide
InactiveCN102351891AEasy to operateLow costGroup 4/14 element organic compoundsPotassium tert-butoxideTert-butoxy
The invention relates to a method for synthesizing hafnium tetra-tert-butoxide, which comprises the following steps of: adding potassium tert-butoxide and n-hexane into a three-necked bottle in nitrogen atmosphere, and uniformly stirring; adding hafnium tetrachloride into a reaction system in a molar ratio of the hafnium tetrachloride to the potassium tert-butoxide of 1:4.4-1:4.8, and keeping the temperature of the reaction system to be between 20 and 60 DEG C; keeping the temperature of the reaction system to be between 40 and 65 DEG C after the hafnium tetrachloride is completely added, and reacting for 6 to 10 hours with stirring under the protection of inert gas; removing a reacted solvent under one atmospheric pressure, distilling under reduced pressure after the solvent n-hexane is completely removed, and collecting 90 to 92 DEG C / 5mmHg fractions, namely a hafnium tetra-tert-butoxide compound. Simple and readily available raw materials, namely the potassium tert-butoxide and the hafnium tetrachloride are reacted, the method is easy to operate, and cost is reduced.
Owner:NANJING UNIV
Preparation method of high-purity metal hafnium
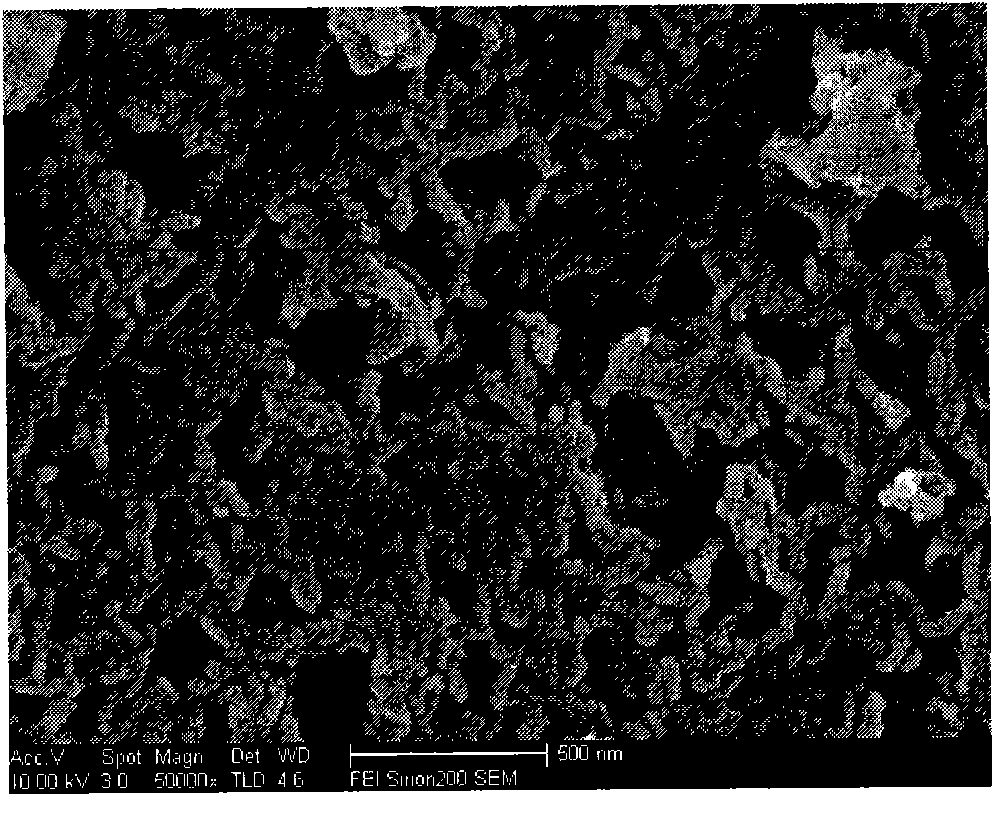
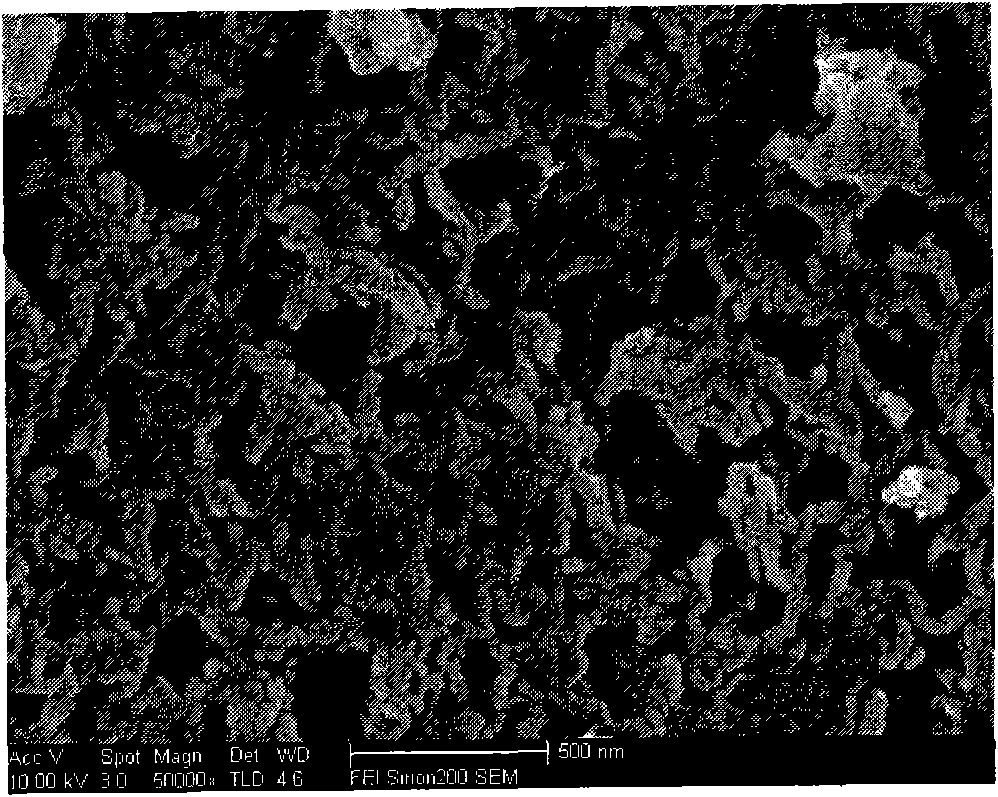
The invention discloses a preparation method of high-purity metal hafnium. The preparation method comprises the steps that tetrachloride hafnium solid is dissolved into deionized water, an oxygen hafnium chloride solution is obtained, a sodium hydroxide solution is slowly dropwise added into the oxygen hafnium chloride solution at the room temperature, and mixed liquid is obtained; the mixed liquid is poured into a high-pressure water thermal reaction kettle, and the reaction kettle is placed into a drying oven; suspension liquid of the reaction kettle is poured into a flask, solid obtained through centrifugation is alternately cleaned by ethyl alcohol and the deionized water, centrifugation is carried out repeatedly for three times, a product finally obtained is dried at 40-60 DEG C for 12-24 h, and hafnium oxide is obtained; and an excessive amount of Ca powder and the hafnium oxide are mixed, and are subjected to reduction in a sealed reactor, and the high-purity metal hafnium is obtained. According to the preparation method, the hafnium oxide prepared by a hydrothermal method is uniform in size, the metal hafnium obtained through reduction of the Ca powder is high in purity, and the metal hafnium is uniform in size.
Owner:南通晶朋新材料科技有限公司
Preparation method for synthesizing lanthanum hafnate powder by using sol-gel process
InactiveCN111943669AChemical composition is easy to controlCalcination temperature is lowPhysical chemistryGlycol synthesis
The invention relates to a preparation method for synthesizing lanthanum hafnate powder by using a sol-gel process. The method comprises the following steps: dissolving hafnium tetrachloride (HfCl4) and lanthanum nitrate hydrate (La(NO3)3.xH2O) in deionized water, conducting sufficient stirring, adding a certain amount of citric acid powder, and performing stirring at 50-80 DEG C for 1-3 hours toform sol; weighing a certain amount of ethylene glycol, dissolving the ethylene glycol in the solution obtained in the step 1, and carrying out stirring for 2-4 hours at a temperature of 70-90 DEG C to form gel; and fully drying the gel obtained in the step 2, and calcining the gel at a temperature of 800-100 DEG C for 8-10 hours to obtain the lanthanum hafnate (La2Hf2O7) powder. The preparation method disclosed by the invention has the advantages of controllable chemical components of the synthetic powder, low calcining temperature, good uniformity, high purity and the like.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Novel method for preparing hafnium acetylacetonate
InactiveCN101417938BReduce usageHigh yieldPreparation of aldehyde/ketone chelatesBenzenePhysical chemistry
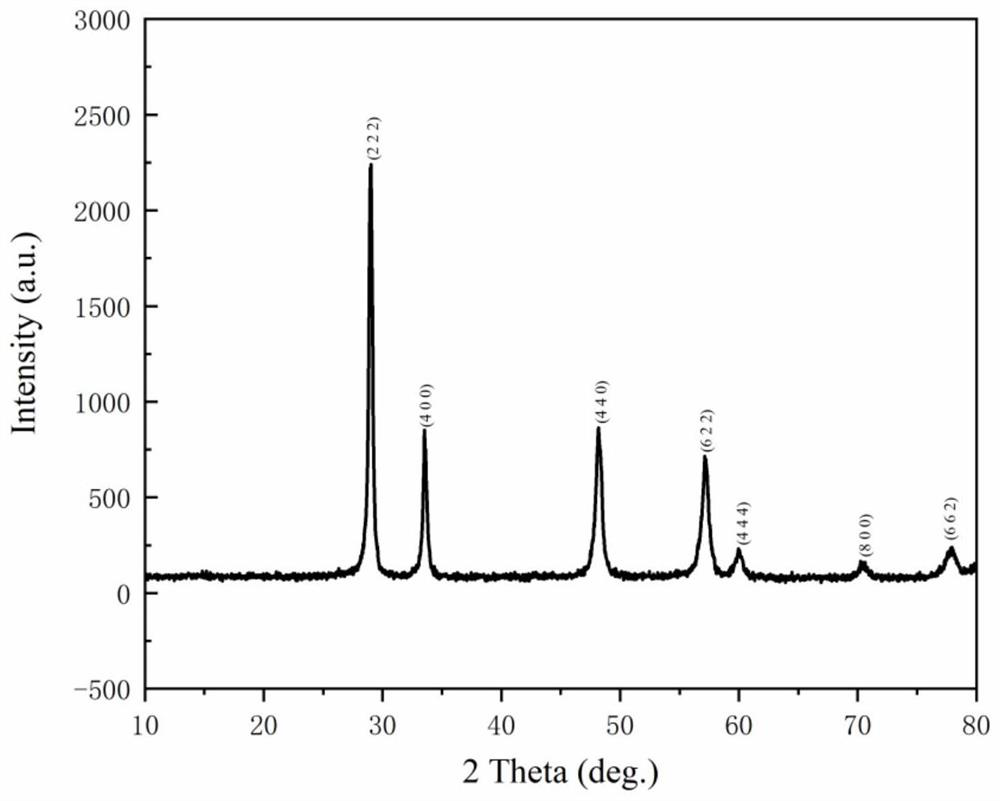
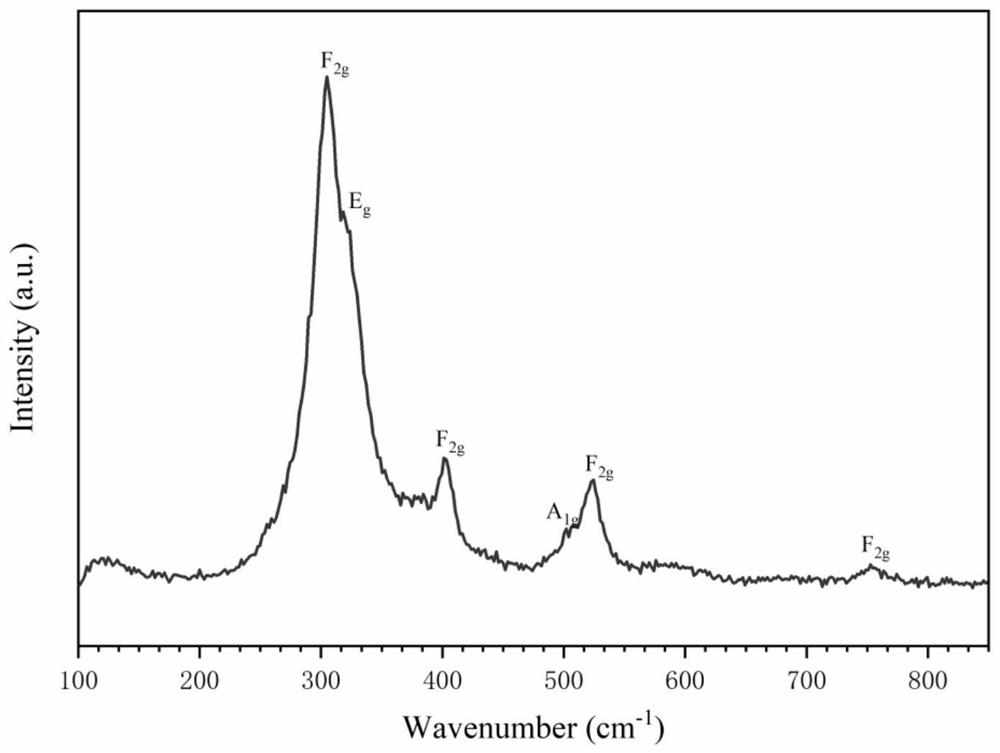
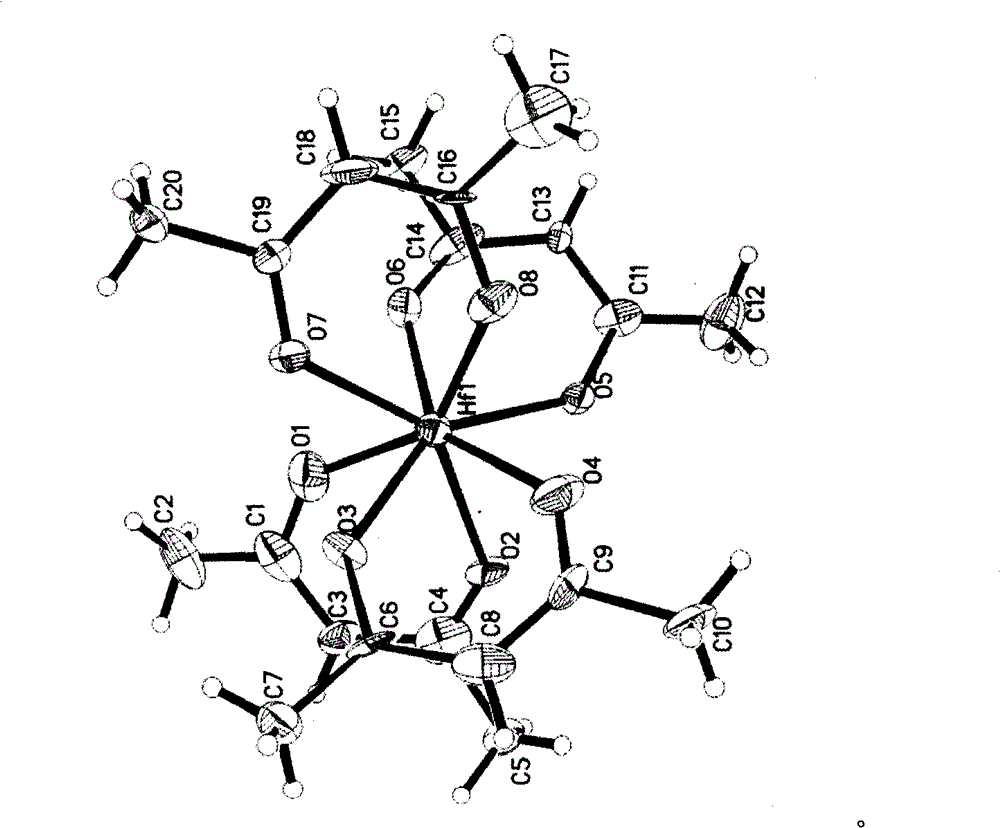
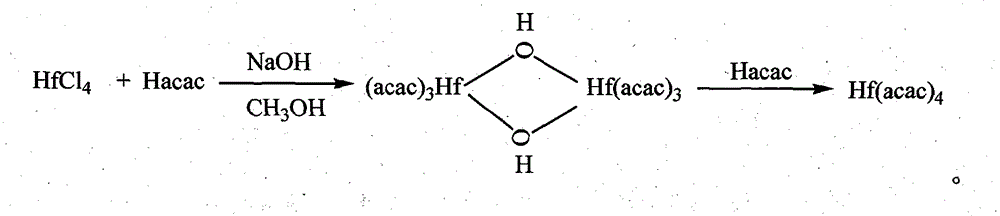
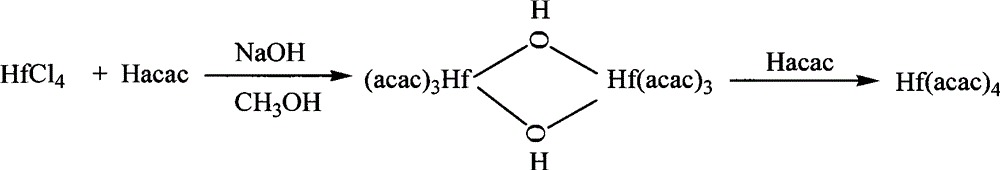
The invention discloses a new technology to prepare (Hf(acac)4), and the technological process is that: anhydrous HfCl4 is dissolved in methanol, and Hacac is added; NaOH is used for adjusting the pH value of the solution; a new intermediate complex Hf2(OH)2(acac)6 is generated after reaction; then Hacac is added for reflux reaction, and the intermediate complex is transferred to the target compound Hf(acac)4. The technology has the advantages that, synthesis technology and purification process are integrated; productivity and purity are high; no special regional sublimation device is required during the purification process; the use of extremely toxic substance benzene is avoided, and scale production can be realized.
Owner:KUNMING INST OF PRECIOUS METALS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com