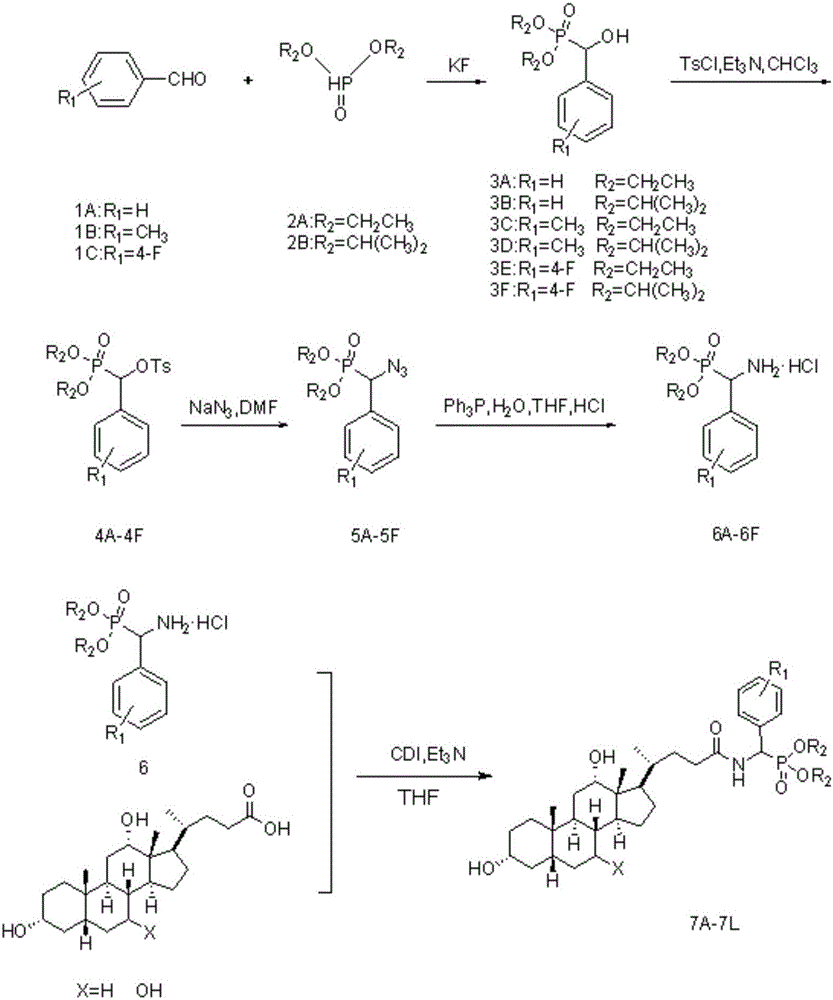
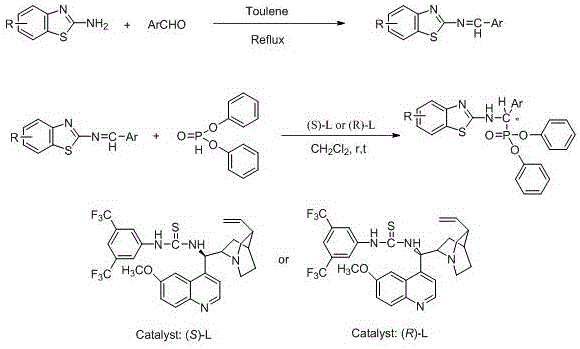
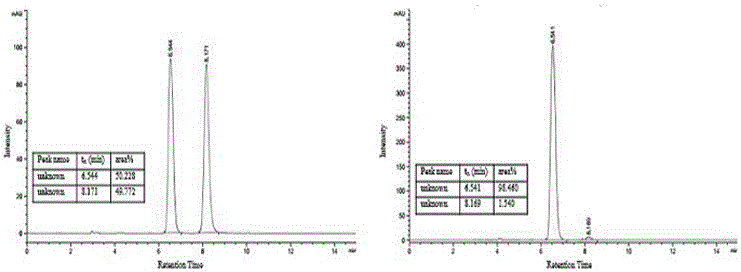
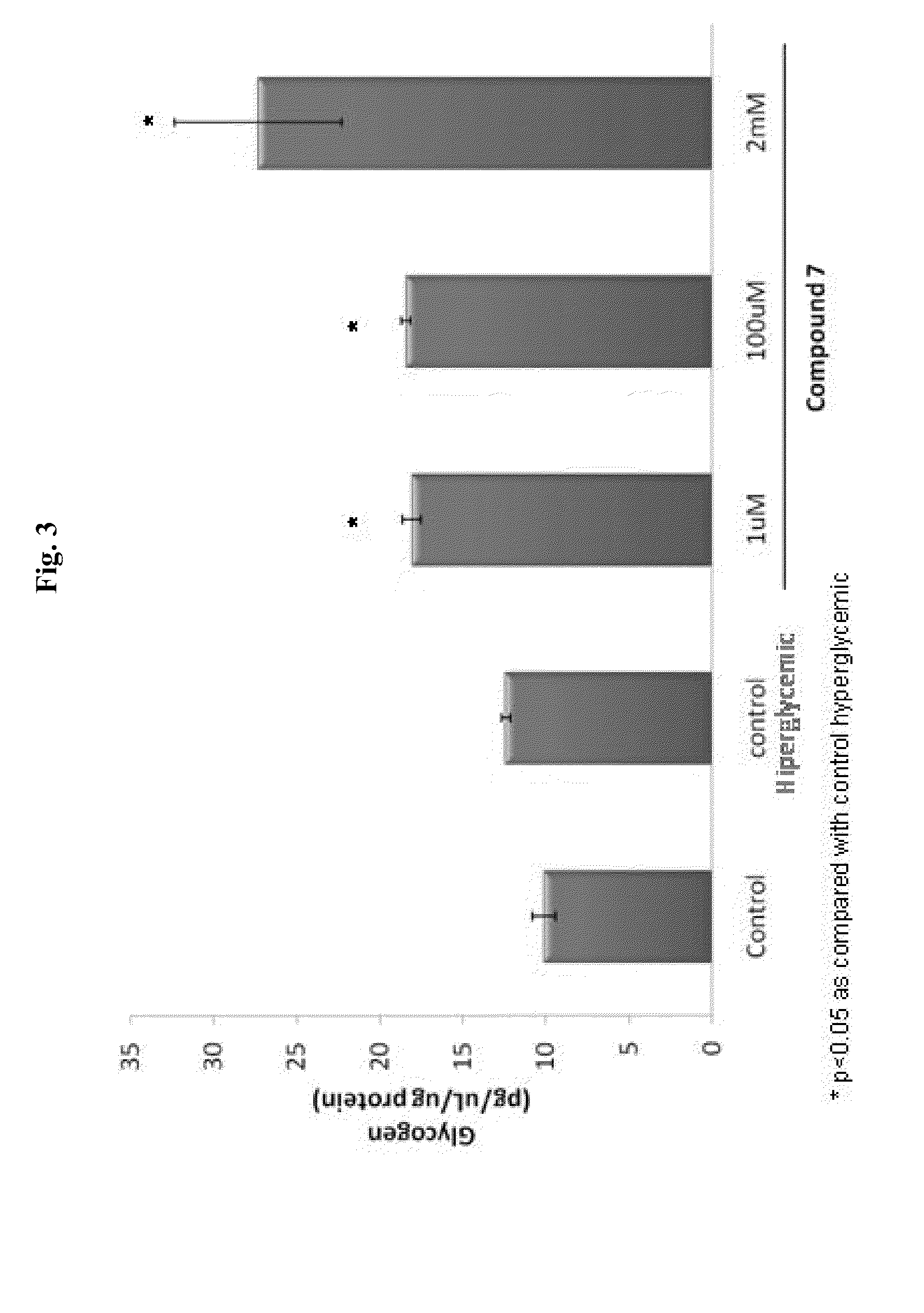
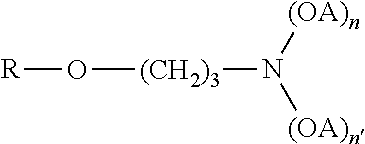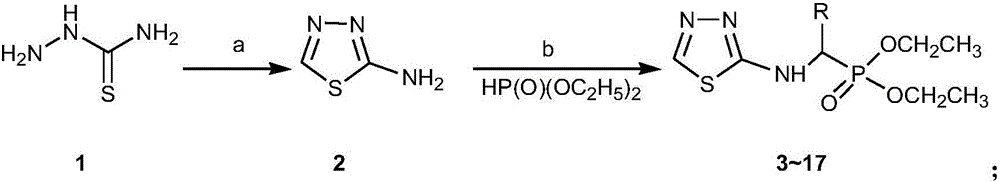Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67 results about "Aminophosphonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phosphonic acid, HPO(OH)2, or its salt, anion, or ester, with an amino group attached.
Herbicidal composition comprising aminophosphate or aminophosphonate potassium salt
The present invention relates to herbicidal compositions comprising aminophosphate or aminophosphonate salts, particularly to herbicidal compositions comprising an aminophosphate or aminophosphonate potassium salt. Preffered compositions of the invention have a high amount and the aminophosphate or aminophosphonate salt. The invention also relates to compositions of matter (or blend) that are especially useful ingredients for preparing the compositions comprising the aminophosphate or aminophosphonate salts.
Owner:RHODIA OPERATIONS SAS
Double-liver-targeting phosphoramidate and phosphonoamidate prodrugs
This application discloses phosphoramidate and phosphonoamidate prodrugs of alcohol-based therapeutic agents, such as nucleosides, nucleotides, acyclonucleosides, C- nucleosides, and C-nucleotides, and use of these prodrugs for treatment of diseases or disorders, including infectious diseases and cancers. This application also discloses a general method for enhancing bioavailability and / or liver-targeting property of alcohol drugs through converting the alcohol drugs to phosphoramidate or phosphonoamidate prodrugs, and methods of preparation of these prodrugs.
Owner:HENAN GENUINE BIOTECH CO LTD
Methods of using corrosion-inhibiting cleaning compositions for metal layers and patterns on semiconductor substrates
Provided herein are methods for using corrosion-inhibiting cleaning compositions for semiconductor wafer processing that include an aqueous admixture of at least water, a surfactant and a corrosion-inhibiting compound selected from a group consisting of amino phosphonates, polyamines and polycarboxylic acids. The quantity of the corrosion-inhibiting compound in the admixture is preferably in a range from about 0.0001 wt % to about 0.1 wt % and the quantity of the surfactant is preferably in a range from about 0.001 wt % to about 1.0 wt %. The aqueous admixture may also include sulfuric acid and a fluoride, which act as oxide etchants, and a peroxide, which acts as a metal etchant.
Owner:LEE KWANG WOOK +8
Herbicidal composition comprising an aminophosphate or aminophosphonate salt and a viscosity reducing agent
InactiveUS20110009269A1Good surprisingly compatibilityImprove compatibilityBiocideDead animal preservationCompound (substance)Viscosity
The present invention relates to herbicidal compositions comprising an aminophosphate or aminophosphonate salt, particularly to herbicidal compositions comprising a relatively high amount of aminophosphate or aminophosphonate salt. The compositions comprise an additive compound which is a salt.
Owner:RHODIA OPERATIONS SAS
Rosinyl diterpene modified alpha - phosphoramidate, preparation method, and application for anti tumors
InactiveCN101003548AHigh activityIncrease fat solubilityOrganic active ingredientsGroup 5/15 element organic compoundsBenzaldehydeSolvent free
This invention relates to a method for preparing roinyl diterpene modified alpha-aminophosphate, and its anti-tumor application. The method comprises: reacting roinyl amine diterpenoid with substituted benzaldehyde and phosphite at a mol ratio of 1 :( 1-1.05) :( 1-1.1) by solvent synthesis, one-pot synthesis or solvent-free synthesis. The inroduction of roinyl diterpene into aminophosphate can largely improve the liposolubility and bioactivity of the compound. R1 structure comes from natural product rosin, thus has low toxicity.
Owner:JIANGSU QIANGLIN BIO ENERGY
Preparation method of beta-hydroxyphosphonate derivatives
InactiveCN104370960ARich typeRaw materials are easy to getGroup 5/15 element organic compoundsMANGANESE ACETATEEthylic acid
The invention discloses a preparation method of beta-hydroxyphosphonate derivatives. The preparation method comprises that an arylethene derivative, a phosphorus reagent and manganese acetate are dissolved in a solvent and the solution undergoes a reaction at a temperature of 20-60 DEG C to produce the beta-hydroxyphosphonate derivative. Under the action of ammonia water, a beta-aminophosphonate derivative is prepared from the beta-hydroxyphosphonate derivative. The preparation method utilizes the arylethene derivative as an initiator and utilizes multiple easily available raw materials. The beta-hydroxyphosphonate derivatives belong to multiple types and can be directly used and can be used for other further reactions. The preparation method has a short synthesis route, mild reaction conditions, simple reaction and aftertreatment processes and a high yield and is suitable for large-scale production.
Owner:翁后科
A green method for the synthesis of α-aminophosphonates catalyzed by brφnsted acidic ionic liquids
InactiveCN102276649ASimple and fast operationHigh yieldGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen SulfateCatalytic method
The invention relates to a sulfonic acid group functionalized acidic ionic liquid 1-butyl-2-trifluoromethyl-3-sulfonic acid propyl benzimidazole hydrogen sulfate [PSb(2-CF3Bim)][HSO4] A green approach to the catalytic synthesis of α-aminophosphonates. The method has the advantages of simple operation, environmental friendliness, mild reaction conditions, short reaction time, simple post-treatment, high yield and recyclable catalyst.
Owner:XINJIANG UNIVERSITY
Corrosion resistant wire-grid polarizer and method of fabrication
A polarizing device (10), such as a wire-grid polarizer, includes a substantial mono-layer (24) of a corrosion inhibitor without adversely affecting the optical properties of the polarizing device. The polarizing device can include an optical element (14) and a nano-structure (16), such as an array of a plurality of spaced-apart, elongated elements (32) disposed on a substrate (28). The corrosion inhibitor is chemically bonded to surfaces of the elements to form the mono-layer. The mono-layer can have a thickness less than 100 Angstroms. A method of forming the substantial mono-layer can include treating the polarizing device with an amino phosphonate, such as a nitrilotris (methylene) triphosphonic acid.
Owner:MOXTEK INC
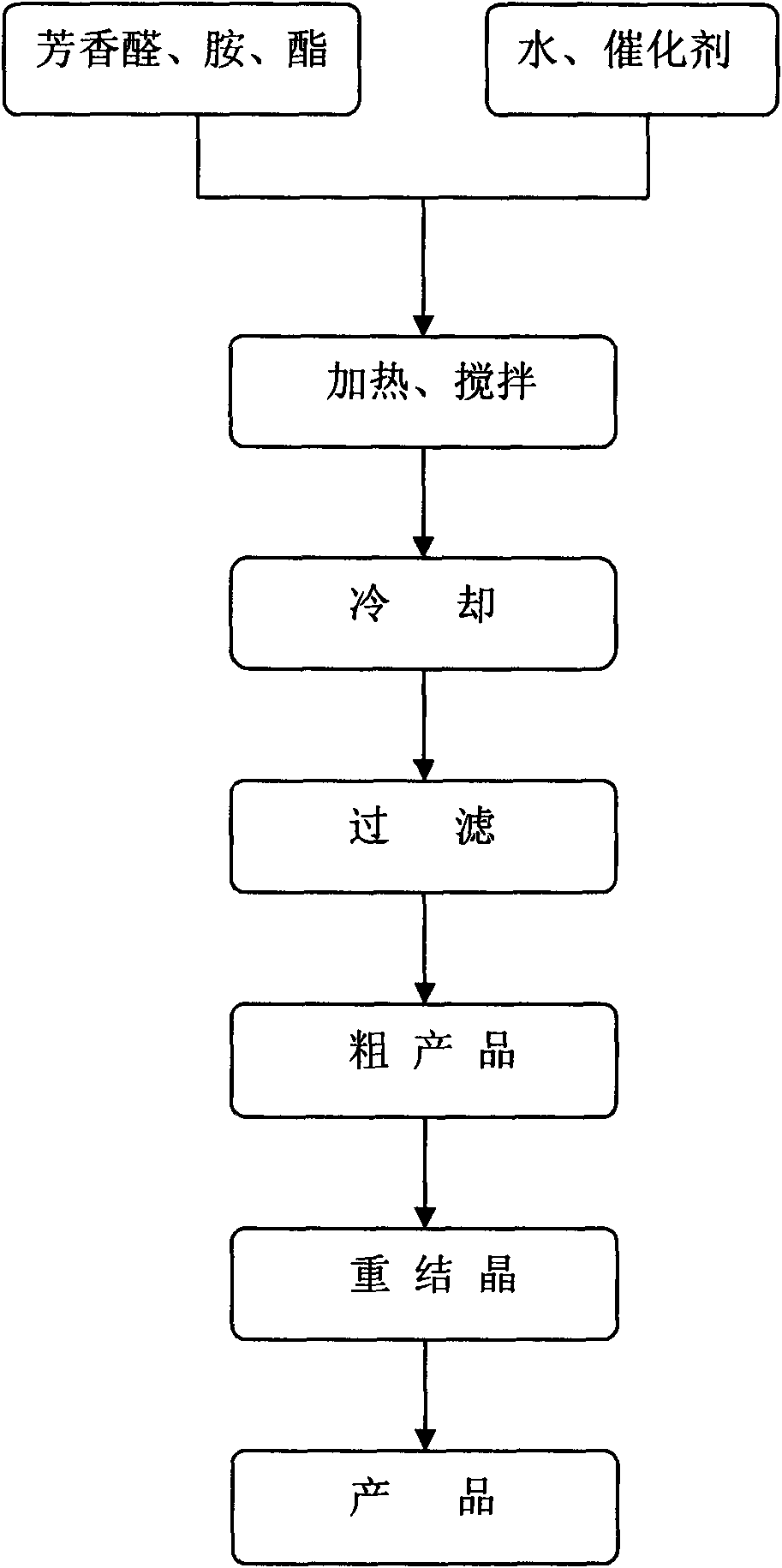
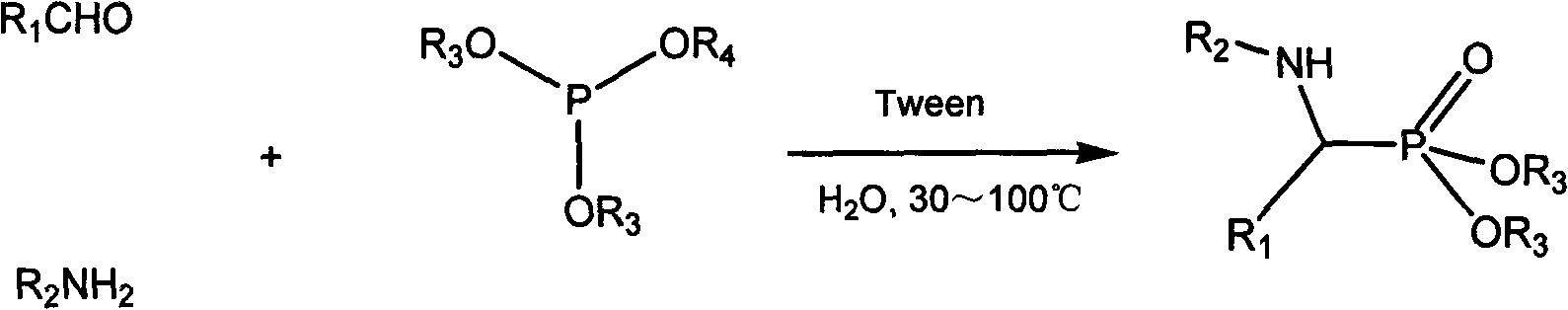
Method for synthesizing alpha-aminophosphonate through water phase cleaning
InactiveCN102321120AAvoid pollutionWide variety of sourcesGroup 5/15 element organic compoundsOrganic solventSurface-active agents
The invention discloses a method for synthesizing alpha-aminophosphonate through water phase cleaning. Aromatic aldehyde, amine and phosphite ester are used as raw materials, non-ionic surface active agents are used as catalysts, water replaces organic solvents to be used as reaction media, the heating and stirring reaction is carried out at ordinary pressure, and the synthesis of the target compound is realized. Compared with the prior art, the method has the advantages that (1) Tween-20 and Tween-60 non-ionic surface active agents are adopted, raw material resources are wide, the preparation is convenient, and safety and reliability are realized; (2) the catalytic activity is high, the use consumption is little, the stability on the water is realized, the catalysts are not inactivated, and the cyclic use can be realized; and (3) a water phase reaction method is adopted, the environment pollution caused by the organic solvent use is avoided, in addition, the post treatment is convenient, the reaction is safe and stable, the industrial amplification is easy, and energy saving and emission reduction effects are obvious. The method belongs to an efficient and environment-friendly method for synthesizing alpha-aminophosphonate compounds, and the large-scale industrial production is favorably realized.
Owner:YANCHENG TEACHERS UNIV
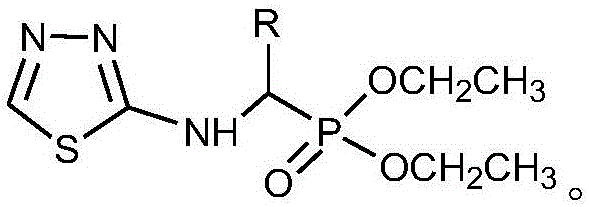
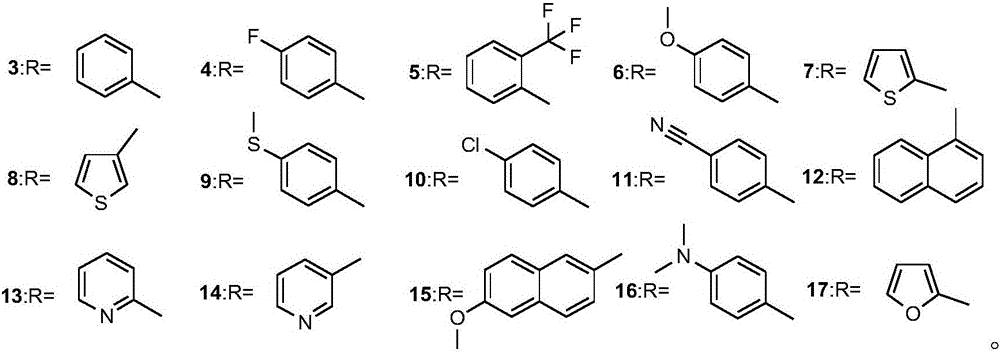
Alpha-amino phosphonate compound with 2-amino-1, 3, 4-thiadiazole structure and preparation method and application of alpha-amino phosphonate compound
InactiveCN106117268AWide range of usesImprove the bactericidal effectBiocideGroup 5/15 element organic compoundsEconomic benefitsMythimna separata
The invention discloses an alpha-amino phosphonate compound with a 2-amino-1, 3, 4-thiadiazole structure and a preparation method and application of alpha-amino phosphonate compound. The alpha-amino phosphonate compound has the sterilizing effect of alpha-amino phosphonate compounds and has a pesticide effect by introducing the 2-amino-1, 3, 4-thiadiazole group, and the alpha-amino phosphonate compound is wide in application range and good in sterilizing and pesticide effect by combining the alpha-amino phosphonate compound with the 2-amino-1, 3, 4-thiadiazole group. The preparation method is few in step, simple to operate, high in yield and productivity, capable of saving a large amount of time and cost in actual production and good in economic benefit. The 2-amino-1, 3, 4-thiadiazole has an inhibition effect on the growth and proliferation of alternaria solani and on the growth of mythimna separata and can be used as the pesticide intermediate to apply to the preparation of different agricultural fungicide.
Owner:GUANGXI TEACHERS EDUCATION UNIV
Rhein aminophosphonate derivatives, and synthetic method and applications thereof
InactiveCN103524555ANovel structureImprove anti-tumor activityOrganic active ingredientsGroup 5/15 element organic compoundsAcetic acidOrganic layer
The invention discloses a series of rhein aminophosphonate derivatives, and a synthetic method and applications thereof. The synthetic method of the rhein aminophosphonate derivatives comprises: taking rhein and an alpha-aminophosphonate as raw materials, dissolving in a polar solvent, in the presence of a catalyst HOBT and a condensing agent EDAC, reacting completely; and adding trichloromethane into the reaction liquid, washing with water, collecting the organic layer, applying to silica gel for column chromatography, eluting with a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:4-100 to obtain the corresponding derivative. The rhein aminophosphonate derivatives have the structural general formula (I) as shown in the description, wherein R is p-bromophenyl, o-bromophenyl, m-bromophenyl, p-fluorophenyl, o-fluorophenyl, p-chlorophenyl, m-chlorophenyl, o-chlorophenyl, m-methoxyphenyl, o-methoxyphenyl, phenyl, naphthyl, p-methoxyphenyl, m-methylphenyl, p-methylphenyl, m-fluorophenyl or anthryl.
Owner:GUANGXI NORMAL UNIV
Herbicidal composition comprising an aminophosphate or aminophosphonate salt and a viscosity reducing agent
InactiveCN101932236ALow viscosityEasy to pourBiocideDead animal preservationChemical compositionViscosity
The present invention relates to herbicidal compositions comprising an aminophosphate or aminophosphonate salt, particularly to herbicidal compositions comprising a relatively high amount of aminophosphate or aminophosphonate salt. The compositions comprise an additive compound which is a salt.
Owner:RHODIA OPERATIONS SAS
Efficient and novel method for preparing aminophosphonate through catalytic synthesis of hafnium tetrachloride
InactiveCN105646576AHigh catalytic activityReduce dosageGroup 5/15 element organic compoundsPhosphorous acidCatalytic effect
The invention discloses an efficient and novel method for preparing aminophosphonate through catalytic synthesis of hafnium tetrachloride. With hafnium tetrachloride as a catalytic reagent, aryl / alkyl aldehyde, aryl / alkyl amine and phosphorous acid diester / triester as raw materials, and ethyl alcohol as a solvent, a one-pot reaction is performed for 0.5-2.0 h at 60 DEG C to generate corresponding aminophosphonate, wherein the dosage of the hafnium tetrachloride catalytic reagent is 2 mol% that of aldehyde, and the concentration of aldehyde in an ethanol solution and the concentration of amine in the ethanol solution are both 1.0 mol / L. Hafnium tetrachloride used in the method is high in catalytic activity, small in dosage, capable of achieving the optimum catalytic effect at the dosage of 2 mol%, universally applicable to various aryl aldehyde / amine substrates and alkyl aldehyde / amine substrates and high in product yield. According to the method, reaction conditions are mild, heating is performed just at 60 DEG C, the ethyl alcohol solvent does not need drying, a reaction system does not need gas protection, the reaction speed is high, aftertreatment and purification are easy, and it is only needed to concentrate the reaction system and directly perform conventional silica-gel column chromatography.
Owner:JIANGXI SCI & TECH NORMAL UNIV
Method for synthesizing chiral aminophosphonates through organocatalytic Friedel-Crafts reactions
InactiveCN108117569AHigh reactivityHigh enantioselectivityGroup 5/15 element organic compoundsAsymmetric synthesesPhosphoric acidOrganocatalysis
The invention provides a method for synthesizing chiral aminophosphonates through organocatalytic Friedel-Crafts reactions. An organic catalyst used in the method is chiral phosphoric acid. Corresponding chiral aminophosphonates with quaternary carbon centers can be prepared by catalyzing asymmetric Friedel-Crafts reactions of a series of imidophosphonates with indole, and enantiomeric excess is up to 98%. The method provided by the invention is simple, practical and feasible; the catalyst is commercially available; and reaction conditions are mild. In addition, since the chiral aminophosphonates is synthesized through the asymmetric Friedel-Crafts reactions, high enantioselectivity and good yield are realized, and the reactions have the advantages of atomic economy, environmental friendliness and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing phosphonic acid modified graphene or phosphonate modified graphene
ActiveCN108570072AImprove stabilityHigh yieldOther chemical processesGroup 5/15 element organic compoundsSolventCvd graphene
The invention discloses a method for synthesizing phosphonic acid modified graphene or phosphonate modified graphene and belongs to the field of inorganic novel materials. The method comprises the following steps: firstly, dissolving amino phosphonate in a solvent and controlling the temperature; adding sodium nitrite and acid and uniformly stirring; then adding the graphene and reacting; filtering and washing to obtain the phosphonate modified graphene; mixing the phosphonate modified graphene, sodium hydroxide and water, and heating to react; adjusting the acidity and filtering to obtain thephosphonic acid modified graphene. The method disclosed by the invention has the advantages of simple technological flow, mild conditions, high safety and low cost, and the stability of a product isimproved; the possibility of industrialized production of synthesizing a lot of the phosphonic acid modified graphene or the phosphonate modified graphene is realized.
Owner:XINXIANG UNIV
N-substituted benzothiazolyl-1-substituted phenyl-0,0-dialkyl-alpha-amino phosphonate ester derivatives preparation and application
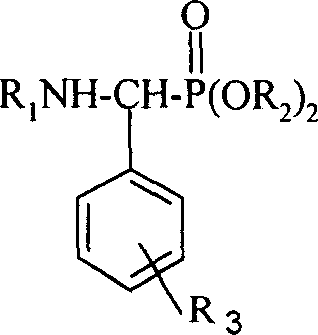
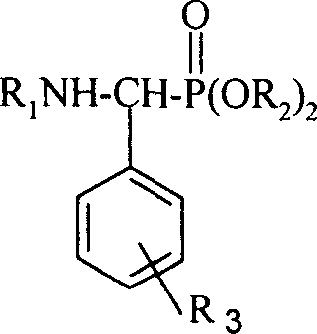
The present invention discloses a medicine with actions of resisting plant virus and resisting tumor-N-substituted benzothiazolyl-1-substituted phenyl-0.0-dialkyl-alpha- aminophosphonates derivative, its preparation method and biological activity. Said invention also provides its structure formula, and also provides its extensive application for inhibiting tobacco mosaic virus (TMV) activity and inhibiting activity of human body prostatic cancer cell PC3 and others.
Owner:GAUNGXI TIANYUAN BIOCHEM
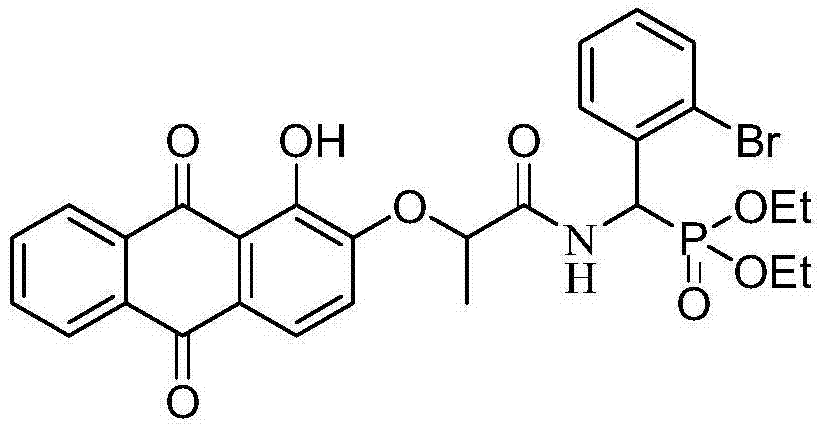
Alizarin aminophosphonate derivatives and their synthesis method and use
InactiveCN104744510ANovel structureImprove anti-tumor activityOrganic active ingredientsGroup 5/15 element organic compounds9,10-DihydroanthracenePropanoic acid
The invention discloses alizarin aminophosphonate derivatives and their synthesis method and use. The synthesis method comprises that alizarin and 2-bromopropionic acid as raw materials are dissolved in a polar solvent, the solution undergoes a reaction in the presence of potassium hydroxide as an acid binding agent to produce 2-(1-hydroxy-9, 10-dioxo-9,10-dihydroanthracene-2-yloxy)propionic acid, the 2-(1-hydroxy-9,10-dioxo-9,10-dihydroanthracene-2-yloxy)propionic acid and alpha-aminophosphonate as raw materials are dissolved in a polar solvent, the solution undergoes a full reaction in the presence of 1-hydroxybenzotriazole as a catalyst and 1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride as a condensing agent, trichloromethane is added into the reaction produce solution, a water washing process is carried out, and an organic layer is collected and then is subjected to chromatography purification by a silicagel column. The alizarin aminophosphonate derivatives have a general structural formula (I).
Owner:GUANGXI NORMAL UNIV
Method for synthesizing chiral gamma-aminophosphonate
ActiveCN110066294AHigh yieldHigh enantioselectivityGroup 5/15 element organic compoundsOrganic chemistry methodsOrganocatalysisEnantio selectivity
Provided is a method for synthesizing chiral gamma-aminophosphonates. Starting from azadiene and phosphite, reacting is conducted under the action of an inorganic base and an organic catalyst to obtain the chiral gamma-aminophosphonate containing various substituents. The method has simple and practical operation, high yield and high enantioselectivity.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Cholic acid-alpha-amino phosphonate derivative and synthesis method thereof
InactiveCN105732758AGood antitumor activityGood biocompatibilitySteroidsCholic acidSynthesis methods
The invention belongs to the field of medicine chemistry and particularly relates to a cholic acid-alpha-amino phosphonate derivative and a preparation method thereof.Cholic acid and phosphate ester serve as raw materials, and the cholic acid-alpha-amino phosphonate derivative with the following general structural formula is synthesized.This kind of compositions has good anti-tumor activity, the human hepatocellular carcinoma cell (HepG2) growth inhibition rate of some compositions is as high as 77.44%, and the advantages of the compositions are better than those of the control drug Amonafide.Please see the general structural formula in the description.
Owner:HENAN ACADEMY OF SCI CHEM RES INST CO LTD
Polyaminomethylenephos phonate derivatives
InactiveUS20050171376A1Convenience to mergeReduce heat exchangeGroup 5/15 element organic compoundsScale removal and water softeningPrecipitationAminophosphonate
A new class of polyaminophosphonate derivatives usable as inhibitors of precipitation and dispersants in aqueous systems. The products in object have complete tolerance of calcium and can be employed in severe conditions of use in the water treatment and detergent fields.
Owner:GIOVANNI BOZZETTO
Chiral alpha-amino phosphonate ester compounds having anti-virus activity and containing benzothiazole heterocycle, preparation and applications thereof
ActiveCN104987350ARaw materials are easy to getMild reaction conditionsGroup 5/15 element organic compoundsDisinfectantsTobacco mosaic virusThiourea
The present invention discloses a class of chiral alpha-amino phosphonate ester compounds having anti-virus activity and containing benzothiazole heterocycle, preparation and applications thereof, wherein the structure of the compound is represented by the following general formula (I). According to the present invention, thiourea or thiourea quinidine is adopted as a chiral catalyst, a 4A molecular sieve is adopted as a cocatalyst, and dichloromethane is adopted as solvent to rapidly synthesize the high yield and high optical activity chiral alpha-amino phosphonate ester compound containing benzothiazole heterocycle at a room temperature; and especially treatment, protection and activity passivation of the compound R-4h on cucumber mosaic virus are superior to the commercial agent ningnanmycin, treatment, protection and activity passivation of the compound R-4q on tobacco mosaic virus are superior to the commercial agent ningnanmycin, and the compounds R-4h and R-4q provide good inhibition effects on tobacco mosaic virus (TMV), cucumber mosaic virus (CMV), Southern rice black-streaked dwarf virus (SRBSDV) and the like, have good universality, and can be used for anti-plant virus chiral pesticide preparation. The formula (1) is defined in the specification.
Owner:GUIZHOU UNIV
Chirality oliopeptide phosphonate thiourea derivatives and application and preparation method thereof
The invention provides chirality oliopeptide phosphonate thiourea derivatives and application and a preparation method thereof. A series of chirality oliopeptide phosphonate thiourea derivatives of novel structures are designed and synthesized. Each derivative comprises an amino acid skeleton, a thiourea-based functional group and an amino phosphonate component, and the molecular structure is provided with multiple chirality centers. Meanwhile, the compounds have a good inhibiting effect on plant viruses, and are suitable for preparing plant virus resistant agents and particularly suitable for new pesticide creation and research for tobacco mosaic viruses (TMV). The preparation method is good in reliability, good symmetry selectivity and high yield are achieved, and the material basis is provided for follow-up research. The material source is simple, and the material is easy to obtain, low in cost and good in use effect.
Owner:GUIZHOU MEDICAL UNIV +1
Herbicidal composition comprising an aminophosphate or aminophosphonate salt and an n-alkyl-pyrrolidone solvent
InactiveUS20120040832A1Reduce the environmentImprove securityBiocideDead animal preservationOrganic solventAdditive ingredient
The present invention relates to herbicidal compositions comprising an aminophosphate or aminophosphonate salt, for example to herbicidal compositions comprising an aminophosphate or aminophosphonate potassium salt. Preferred compositions of the invention have a high amount and the aminophosphate or aminophosphonate salt. The invention also relates to compositions of matter (or blend) that are especially useful ingredients for preparing the compositions comprising the aminophosphate or aminophosphonate salt. In particular, these ingredients are a surfactant such as an amine oxide surfactant, and an organic solvent comprising an N-alkylpyrrolidone wherein the alkyl group comprises at least 2 carbon atoms.
Owner:RHODIA OPERATIONS SAS
Beta-Hydroxy-Gamma-Aminophosphonates and Methods for the Preparation and Use Thereof
InactiveUS20100087400A1Inhibit carnitine acyltransferasesBiocideNervous disorderDiseaseInsulin dependent diabetes
The present invention provides β-hydroxy-γ-aminophosphonates, β-amino-γ-aminophosphonates, and analogs thereof that inhibit carnitine acyltransferases. The invention also provides compositions comprising these β-hydroxy-γ-aminophosphonates, β-amino-γ-aminophosphonates, and analogs, and methods of the use of such compounds and compositions in the treatment, amelioration or prevention of pathological conditions, diseases or disorders that are linked with fatty acid metabolism, such as non-insulin dependent diabetes or obesity. The invention also provides processes for the preparation of such compounds and compositions.
Owner:NUCITEC DE C V
Biphenyl alpha-aminophosphonate compound as well as preparation method and application thereof
InactiveCN104804040AHas inhibitory effectRaw materials are easy to getGroup 5/15 element organic compoundsDisinfectantsCucumber mosaic virusStructural formula
The invention discloses a biphenyl alpha-aminophosphonate compound as well as a preparation method and an application thereof. The structural formula of the compound is shown in the specification. The compound has very good antiviral activity on plant viruses and can be applied to preparation of anti-plant-virus pesticides, especially anti-CMV (cucumber mosaic virus) pesticides. The preparation method of the biphenyl alpha-aminophosphonate compound has the advantages that raw materials are easy to obtain, reaction conditions are mild, a reaction process is simple to operate, a reagent is cheap and the like.
Owner:张国平
Prodrugs of fluorinated acyclic nucleoside phosphonates
InactiveCN108137630AOrganic active ingredientsGroup 5/15 element organic compoundsSide chainHepatitis B virus
The present invention relates to novel phosphonoamidate prodrugs of acyclic nucleoside phosphonates with a 3-fluoro-2-(phosphonomethoxy)propyl side chain. The invention also relates to the use of these novel phosphonate-modified nucleosides to treat or prevent viral infections and their use to manufacture a medicine to treat or prevent viral infections, particularly infections with viruses such asthe hepatitis B virus, the human immunodeficiency virus, the human cytomegalovirus and the varicella zoster virus.
Owner:KATHOLIEKE UNIV LEUVEN
Method for preparing optically-active alpha-amino phosphonate derivatives by chiral spiro phosphate catalysis
InactiveCN103073583AMild reaction conditionsSimple processOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsPtru catalystOrganic synthesis
The invention discloses a method for preparing optically-active alpha-amino phosphonate derivatives by chiral spiro phosphate catalysis. Chiral spiro phosphate serves as a catalyst, imine and dialkyl phosphate are stirred in an organic solvent at room temperature, and high-purity optically-active alpha-amino phosphonate derivatives are prepared by a simple post-treatment purification process. The method is mild in reaction condition, simple in process and convenient in operation. The catalyst is high in stability and can be reused. The prepared optically-active alpha-amino phosphonate derivatives are high in potential biological activity and can be used as organic synthesis intermediates.
Owner:ZHEJIANG UNIV
Β-hydroxy-γ-aminophosphonates and methods for the preparation and use thereof
The present invention provides β-hydroxy-γ-aminophosphonates, β-amino-γ-aminophosphonates, and analogs thereof that inhibit carnitine acyltransferases. The invention also provides compositions comprising these β-hydroxy-γ-aminophosphonates, β-amino-γ-aminophosphonates, and analogs, and methods of the use of such compounds and compositions in the treatment, amelioration or prevention of pathological conditions, diseases or disorders that are linked with fatty acid metabolism, such as non-insulin dependent diabetes or obesity. The invention also provides processes for the preparation of such compounds and compositions.
Owner:NUCITEC DE C V
Aminophosphonate compound, and application thereof in extraction of lithium in alkaline solution containing lithium ions
ActiveCN113801159AImprove extraction efficiencyEasy to separateGroup 5/15 element organic compoundsProcess efficiency improvementArylPhysical chemistry
The invention provides an aminophosphonate compound, and application thereof in extraction of lithium in an alkaline solution containing lithium ions, and relates to the technical field of metal ion extraction. The structure of the aminophosphonate compound is as shown in formula (I). In the formula (I), R1 is a straight chain of C1-C20 straight-chain or branched-chain alkyl, C1-C20straight-chain or branched-chain alkoxy, or C6-C10 aryl; R2 and R3 are respectively and independently hydrogen, C1-C10 straight-chain or branched-chain alkyl, C1-C10 alkoxy, or C6-C10 aryl; R < 3 > is hydrogen, C1-C10 straight-chain or branched-chain alkyl, C1-C10 alkoxy, or C6-C10 aryl; R4 and R are respectively and independently C1-C10 straight-chain or branched-chain, saturated or unsaturated, substituted or unsubstituted alkyl groups, or C6-C10 aryl; and n is an integer of 1-4. The aminophosphonate compound can be widely applied to extraction of lithium ions in the alkaline solution containing the lithium ions.
Owner:ZHENGZHOU UNIV
Furyl alpha-aminophosphonate chitosan derivative and its preparation method
ActiveCN102516413AHigh antibacterial activityImprove solubilityBiocideFungicidesAntibacterial activityMicrowave power
Belonging to ocean chemical engineering technologies, the invention specifically relates to a furyl alpha-aminophosphonate chitosan derivative and its preparation method. The general formula of the derivative is shown as formula I, wherein, R is an alkyl group and n=4-4000. The preparation method comprises: taking chitosan as a matrix, which is mixed with dialkyl phosphite and furfural, and conducting reaction at a temperature of 0-120DEG C and under microwave power of 10-1000W for 1-60min, immersing the reactant with a solvent, then conducting pumping filtration, washing the filter cake with the solvent, and carrying out drying so as to obtain the furyl alpha-amidophosphonate chitosan derivative. Specifically, the chitosan, the dialkyl phosphite and the furfural are in a molar ratio of 1:1-3:1-3. In the invention, furyl alpha-aminophosphonate is introduced into a chitosan structure, and the two generate synergistic effects, thus significantly improving the antibacterial activity of chitosan.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com