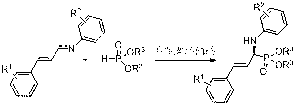Method for preparing optically-active alpha-amino phosphonate derivatives by chiral spiro phosphate catalysis
A technology of amino phosphonate and spirocyclic phosphoric acid, which is applied in the field of chiral preparation of phosphorus-containing compounds, can solve the problems that chiral catalysts cannot be recycled, enantioselectivity is not very high, and reaction substrates are limited, etc. Mild conditions, high purity of optical activity, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]
[0022] The imine (R 1 = H, R 2 =4-OMe; 0.05 mmol), diethyl phosphonite (0.1 mmol) and ( R )-chiral spirocyclic phosphoric acid catalyst [formula (1), 0.05 mmol] was dissolved in xylene (0.8 milliliters), stirred at room temperature for 68 hours, and the reaction was completed. The reaction mixture was purified by silica gel column chromatography to obtain a solid product ( S , E )-α-(4-methoxyphenyl)amino-styrylphosphonic acid diethyl ester, yield 88%, melting point 91-93 °C, chiral purity 87% ee, HPLC analysis: Chiralpak AS -H (hexane / i-PrOH = 85 / 15, 0.6 mL / min), t R (major) 21.3 min, t R (minor) 34.6 min, [α] D 20 = -40.2° (c = 0.70, CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ) δ (ppm) 7.35 (d, J = 7.5 Hz, 2H), 7.32 – 7.22 (m, 3H), 6.80 – 6.64 (m, 5H), 6.22-6.29 (m, 1H), 4.38 (dd, J = 25.4, 6.2 Hz, 1H), 4.21-4.13 (m, 5H), 3.73 (s, 3H), 1.30 (t, J = 7.0 Hz, 6H); 13 C NMR (101 MHz, CDCl 3 ) δ (ppm) 152.79, 140.52 (d, J = 12.9 Hz), 136.29, 132.96 (d, ...
Embodiment 2
[0024]
[0025] The imine (R 1 = H, R 2 =4-OMe; 0.05 mmol), diisopropyl phosphonite (0.1 mmol) and ( R )-chiral spirocyclic phosphoric acid catalyst [formula (1), 0.05 mmol] was dissolved in xylene (0.8 milliliters), stirred at room temperature for 168 hours, the reaction was complete, and the reaction mixture was purified by silica gel column chromatography to obtain a solid product ( S , E )-α-(4-methoxyphenyl)amino-styryl phosphonic acid diisopropyl ester, the yield is 85%, the melting point is 76-78 °C, the chiral purity is 88% ee, HPLC analysis: Chiralpak AD-H (hexane / i-PrOH = 50 / 50, 0.5 mL / min), t R (minor) 19.2 min, t R (major) 33.2 min, [α] D 20 = -12.5° (c = 0.58, CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ) δ (ppm) 7.35 (d, J = 7.5 Hz, 2H), 7.31-7.26 (m, 2H), 7.22 (t, J = 7.0 Hz, 1H), 6.76 (d, J = 8.8 Hz, 2H), 6.65 (d, J = 8.5 Hz, 3H), 6.29-6.24 (m, 1H), 4.80 – 4.71 (m, 2H), 4.31 (d, J = 25.8 Hz, 1H), 4.03 (s, 1H), 3.72 (s, 3H), 1.34 (d, J = 6.1 H...
Embodiment 3
[0027]
[0028] The imine (R 1 =2-NO 2 , R 2 =3-Cl; 0.05 mmol), diethyl phosphonite (0.1 mmol) and ( S )-chiral spirocyclic phosphoric acid catalyst [formula (1), 0.05 mmol] was dissolved in xylene (0.8 milliliters), stirred at room temperature for 88 hours, and the reaction was completed. The reaction mixture was purified by silica gel column chromatography to obtain a liquid product ( R , E )-α-(3-chlorophenyl) amino-(2-nitro) styryl phosphonic acid diethyl ester, yield 92%, chiral purity is 97% ee, HPLC analysis: Chiralpak AD-H ( hexane / i-PrOH = 80 / 20, 1.0 mL / min), t R (minor) 16.9 min, t R (major) 18.7 min, [α] D 20 = + 40.0° (c = 0.35, CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ) δ (ppm) 7.93 (d, J = 7.9 Hz, 1H), 7.58 – 7.52 (m, 2H), 7.42 – 7.36 (m, 1H), 7.24 – 7.17 (m, 3H), 6.77 (t, J = 7.3 Hz, 1H), 6.72 (d, J = 7.7 Hz, 2H), 6.29-6.23 (m, 1H), 4.52 (dd, J = 25.8, 5.9 Hz, 1H), 4.41 – 4.08 (m, 5H), 1.33 (td, J = 7.0, 0.7 Hz, 6H); 13 C NMR (101 MHz, CDC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com