Bridged diamidino group-IV metal catalyst and method for preparing same
A metal catalyst and a bridging technology, which are applied in the field of bridged bisamidine group IV metal catalysts and their preparation, can solve the problems of complex synthesis methods, harsh synthesis conditions, easy deactivation and the like, and achieve simple preparation methods and easy-to-obtain raw materials. , the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] (1) Preparation and characterization of ligand lithium salt
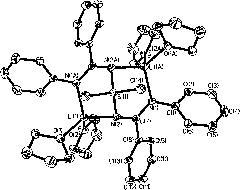
[0060] Under the protection of nitrogen and ice water bath, dimethylsilyl bridged diphenylamine (6.0g, 24.8mmol) was dissolved in ether (200cm 3 ), under stirring, slowly add LiBu n n-hexane solution (2.8mol dm -3 , 17.7cm 3 , 49.6mmol), after the reaction mixture returned to room temperature, continued to stir for 2 hours, cooled the reaction solution to 0°C, and added PhCN (5.06cm 3 , 49.6mmol), after the reaction mixture returned to room temperature, continue to stir for 5 hours, and recrystallize the white solid obtained after the reaction with tetrahydrofuran to obtain a yellow crystal bridged bis-amidine ligand lithium salt 1a, Yield: 17.2g (93%), mp 86-88°C. 1 H NMR (300MHz, C 6 D. 6 ): δ7.46-6.67 (m, 20H; phenyls), 3.52 (t, J HH =6.0Hz, 16H; OCH 2 of THF), 1.35 (p, J HH =3.3, 2.7Hz, 16H; 3, 4-2CH 2 of THF), 0.24 (s, 3H; SiMe 2 ); 13 CNMR (75MHz, C 6 D. 6 ): δ175.7 (N-C-N), 154.0, 143.8...
Embodiment 2
[0068] Preparation and characterization of catalyst 3b
[0069] (1) The preparation of ligand lithium salt is the same as in Example 1.
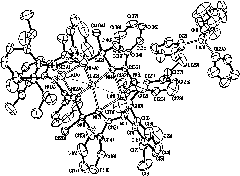
[0070] (2) Vacuumize the Schlenk bottle and pass N 2 After replacing three times, add 1b (1.43g, 2.42mmol) and 20mL of tetrahydrofuran, and slowly add ZrCl 4 (0.56g, 2.42mmol), the reaction mixture was gradually returned to room temperature, and stirring was continued for 12 hours. The reaction solvent and volatile matter were drained under vacuum, and the residue was dissolved in dichloromethane (25 mL), extracted and filtered, concentrated under reduced pressure, and left for a period of time to grow pale yellow crystal compound 3b. Yield: 1.02g (63%).M.p.: 227~229℃. 1 H NMR (300MHz, CDCl 3 ): δ7.33~6.83(m, 16H; phenyls), 3.75(s, 4H; OCH 2 of THF), 2.30(s, 12H; Me on phenyls), 1.54(s, 4H; 3,4-2CH 2 of THF), 0.68 (s, 6H; SiMe 2 ); 13 C NMR (75MHz, CDCl 3 ): δ176.6 (N-C-N), 147.0, 137.7, 135.4, 133.3, 131.3, 130.6, 130.1, 126.7 (p...
Embodiment 3
[0072] Preparation and characterization of catalyst 3c
[0073] (1) The preparation of ligand lithium salt is the same as in Example 1.
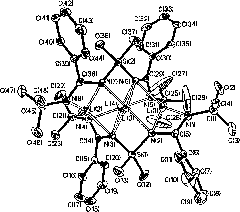
[0074] (2) Vacuumize the Schlenk bottle and pass N 2 After replacing three times, add 1c (1.31g, 0.85mmol) and 20mL of tetrahydrofuran, and slowly add ZrCl 4 (0.40g, 1.70mmol), the reaction mixture was gradually returned to room temperature, and stirring was continued for 12 hours. The reaction solvent and volatile matter were drained under vacuum, and the residue was dissolved in dichloromethane (25 mL), extracted and filtered, concentrated under reduced pressure, and a colorless crystal compound 3c was grown after standing for a period of time. Yield: 0.95g (66%).M.p.: 256~257℃. 1 H NMR (300MHz, CDCl 3 ): δ7.30~7.00(m, 16H; phenyls), 3.82(t, 4H; OCH 2 of THF), 3.66 (m, 4H; CH (CH 3 ) 2 ), 1.58 (p, 4H; 3, 4-2CH 2 of THF), 1.08, 0.70(d, s, 24H; CH(CH 3 ) 2 ), 0.75(s, 6H; SiMe 2 ); 13 C NMR (75MHz, CDCl 3 ): δ175.0 (N-C-N), 146...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| The molecular weight distribution | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com