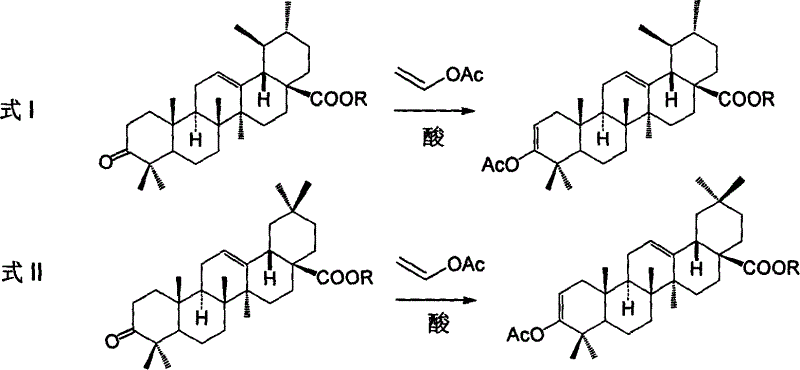
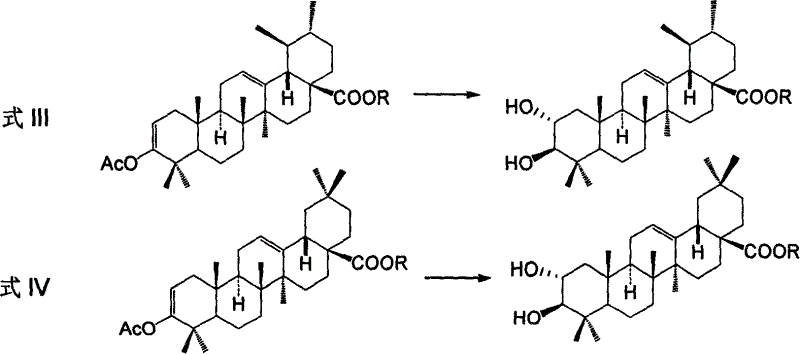
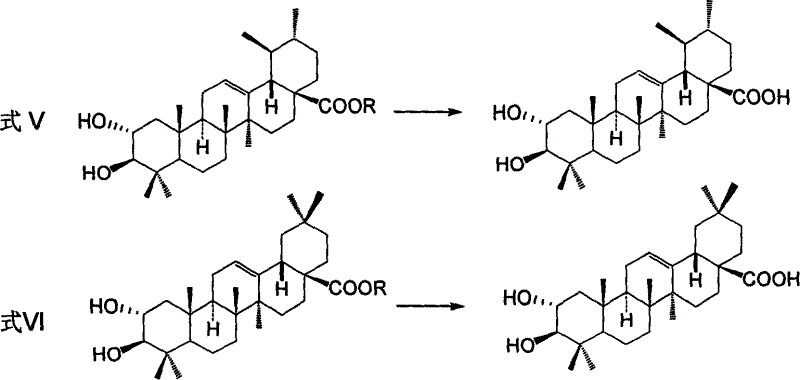
Process for preparing corosolic acid and crataegolic acid
A technology of corosolic acid and maslinic acid, applied in the field of synthesis that can be applied industrially, can solve the problems of high production cost, complex extraction process, conversion of maslinic acid methyl ester, etc., achieve short reaction time, simple reaction conditions, and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Preparation of benzyl ursolic acid (7a, R = benzyl)
[0059] Suspend ursolic acid (10.0g) in 100mL of anhydrous DMF, heat at 100°C to dissolve completely, let stand for a little cooling, then add K 2 CO 3 (6.04g) and benzyl chloride (3.0mL). The mixture was heated at 100°C with stirring until the starting material disappeared (ca. 3 hours). After cooling, filter with suction, and wash the solid with DMF 3 times, 15 mL each time. Pour the mother liquor into 500mL of water, shake while pouring to disperse the precipitated solids, let stand until the solids are completely precipitated, collect the solids by suction filtration, and wash them thoroughly with water. After drying, 11.54 g of white benzyl ursolic acid crude product was obtained (crude yield 96.4%). This crude product was directly used in the next reaction without purification. A pure analytical sample was obtained by column chromatography (eluent, petroleum ether: ethyl acetate = 5:1).
[0060] 1 HNMR (C...
Embodiment 2
[0062] Preparation of methyl ursolic acid (7a, R = methyl)
[0063] Ursolic acid (1.0g) was suspended in 10mL of anhydrous DMF, adding K 2 CO 3 (0.6g) and MeI (0.15mL), stirred at room temperature for 4 hours. After dilution with water, it was extracted with ethyl acetate, and the combined ethyl acetate layer was washed with 1% aqueous hydrochloric acid solution and saturated aqueous sodium chloride solution successively, and dried over anhydrous sodium sulfate. After distilling off the solvent, 1.06 g of a white crude product of methyl ursolic acid was obtained. This crude product was directly used in the next reaction without purification. 0.13 g of the crude product was subjected to silica gel column chromatography (eluent, petroleum ether: ethyl acetate = 6: 1) to obtain 0.12 g of pure methyl ursolic acid.
[0064] 1 HNMR (CDCl 3 , 500MHz): δ0.75, 0.80, 0.93, 1.00, 1.08(each, 3H, s), 0.87(3H, d, J=6.5Hz), 0.95(3H, d, J=6.2Hz), 2.24(1H , d, J=10.5Hz, H-18), 3.22 (1H,...
Embodiment 3
[0066] Preparation of benzyl oleanate (7b, R = benzyl)
[0067] Oleanolic acid (10.0g) was suspended in 100mL of anhydrous DMF, heated at 100°C to dissolve completely, and then added K 2 CO 3 (6.04g) and benzyl chloride (3.0mL). The mixture was heated at 100°C with stirring until the starting material disappeared (ca. 3 hours). After cooling, filter with suction, and wash the solid with DMF 3 times, 15 mL each time. Pour the mother liquor into 500mL of water, shake while pouring to disperse the precipitated solids, let stand until the solids are completely precipitated, collect the solids by suction filtration, and wash them thoroughly with water. After drying, 11.51 g of white crude benzyl oleanate was obtained (crude yield 96%). This crude product was directly used in the next reaction without purification. Pure analytical samples were obtained by recrystallization from ethanol twice.
[0068] 1 HNMR (CDCl 3 , 500MHz): δ0.62, 0.78, 0.88, 0.90, 0.92, 0.98, 1.13(each, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com