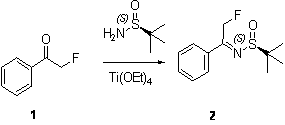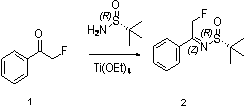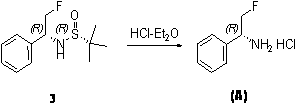Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
59 results about "Prochirality" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step. An achiral species which can be converted to a chiral in two steps is called proprochiral.
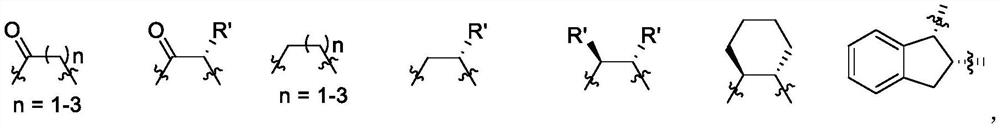
Preparation of chiral amides and amines
ActiveUS20090149549A1Efficient and convenient methodConvenient conversionBiocideOrganic active ingredientsNaphthylamineOxime
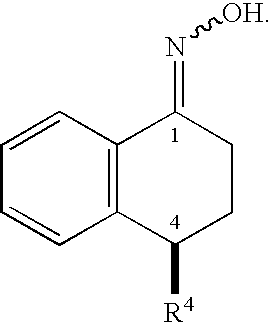
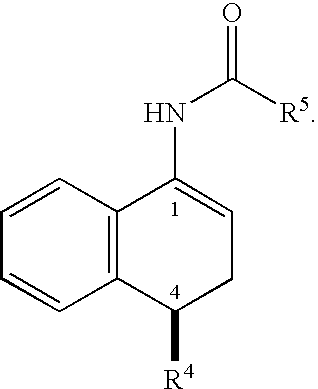
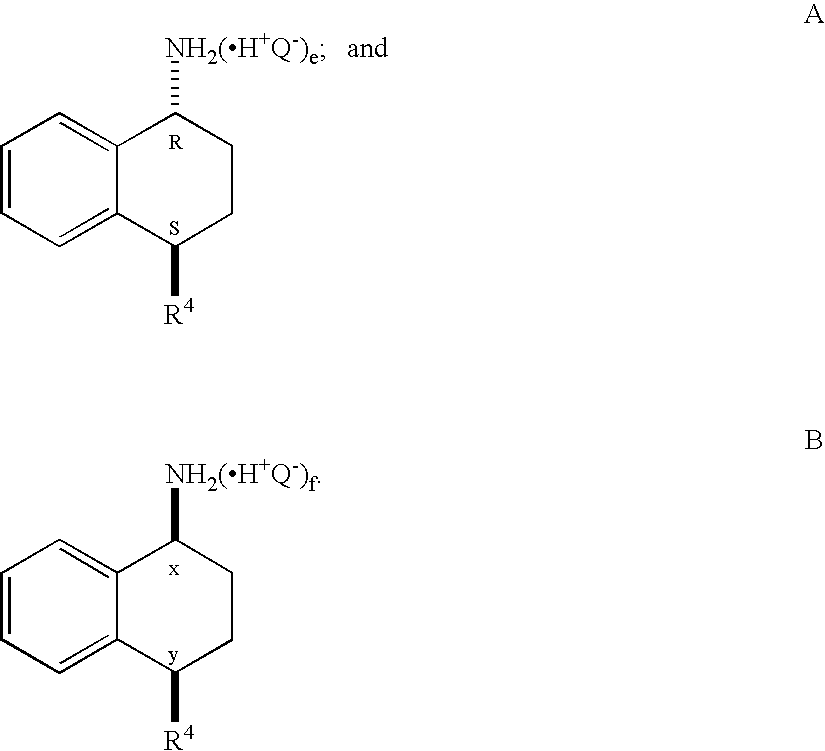
This invention provides a convenient method for converting oximes into enamides. The process does not require the use of metallic reagents. Accordingly, it produces the desired compounds without the concomitant production of a large volume of metallic waste. The enamides are useful precursors to amides and amines. The invention provides a process to convert a prochiral enamide into the corresponding chiral amide. In an exemplary process, a chiral amino center is introduced during hydrogenation through the use of a chiral hydrogenation catalyst. In selected embodiments, the invention provides methods of preparing amides and amines that include the 1,2,3,4-tetrahydro-N-alkyl-1-naphthalenamine or 1,2,3,4-tetrahydro-1-naphthalenamine substructure.
Owner:SUNOVION PHARMA INC
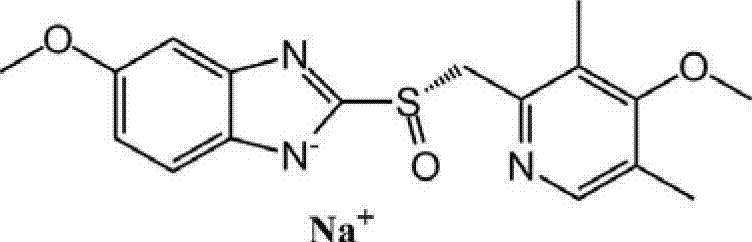
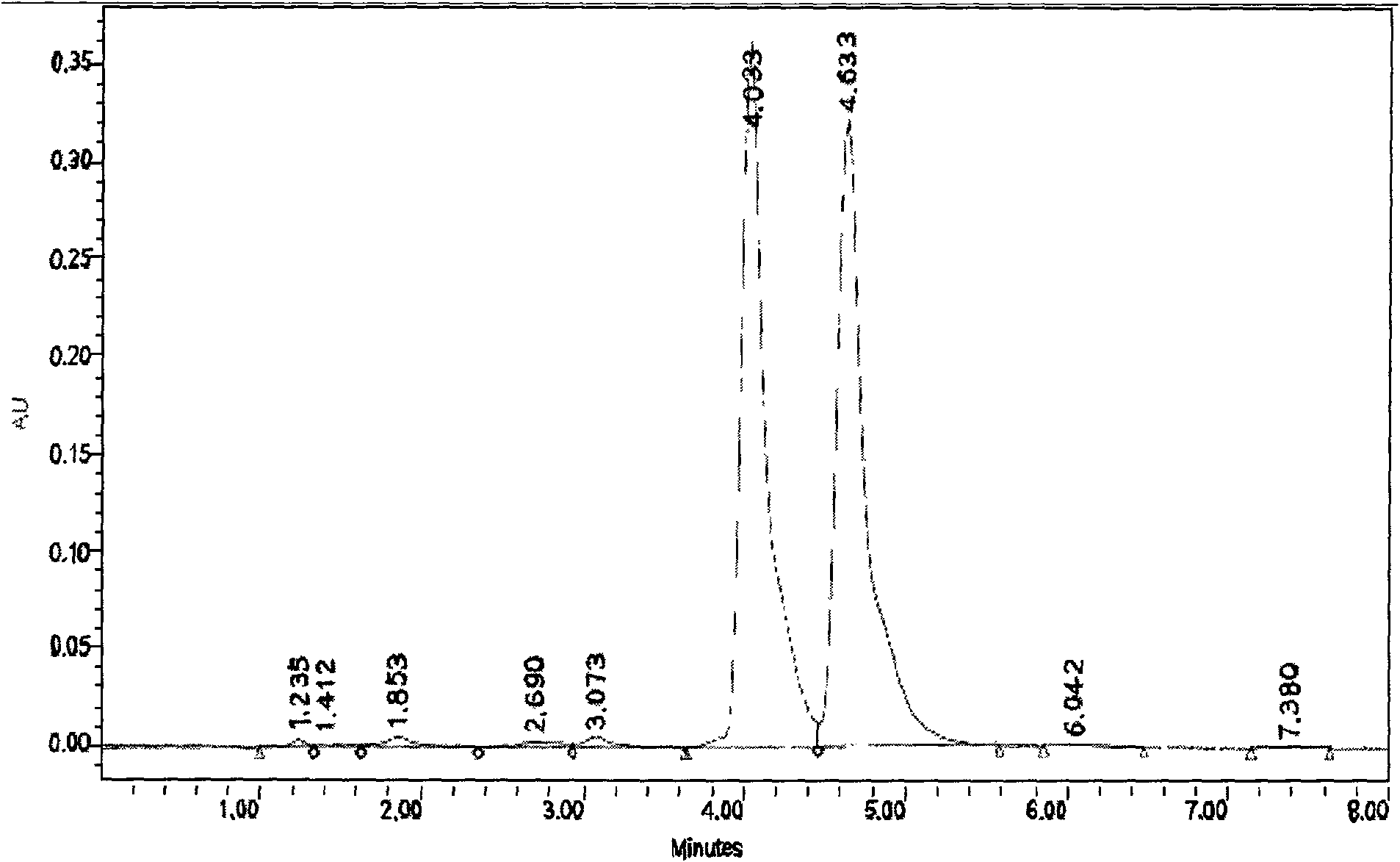
Method for synthesizing esomeprazole sodium
The invention discloses a method for synthesizing esomeprazole sodium. The method comprises the steps as follows: preparing 5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridyl) methylthio-1H-benzimidazole, namely prochirality thioether; preparing crude esomeprazole sodium; refining the crude esomeprazole sodium; adding the prepared prochirality thioether and dried methylbenzene into D-(-) diethyl tartrate and water by stirring, adding titanium isopropylate, and stirring; and adding diisopropylamine at constant temperature, stirring, dropwise adding cumyl hydroperoxide with the mass concentration of 80%, ending the reaction, extracting, salifying, concentrating, washing and carrying out vacuum drying to obtain a crude product, and refining the crude product to obtain the esomeprazole sodium. The method is low in cost, toxicity and pollution, easy to operate, short in reaction time, high in product purity and easy for industrial production.
Owner:KAMP PHARMA
Short-chain dehydrogenase and mutant thereof as well as preparation of gene and application of short-chain dehydrogenase
The invention belongs to the technical field of biology, and relates to wild type short-chain dehydrogenase LfSDR1 and a gene of mutant enzyme of the short-chain dehydrogenase, construction of a recombinant expression vector containing the gene, preparation of recombinant expression transformant, recombinase and a preparation method of the recombinase. Through mutation of the wild type short-chaindehydrogenase LfSDR1, the mutant enzyme having two stereoselectivities (R or S stereoselectivities) is obtained, and catalytic asymmetrical reduction on prochiral ketone is carried out by utilizing the mutant enzyme, such as asymmetrical reduction of catalytic 1-phenyl ethanone compounds. Compared with the wild type short-chain dehydrogenase, the mutant disclosed by the invention has the advantages that the stereoselectivities is improved or reversed, a substrate is catalyzed so as to obtain chiral alcohol having two types (R or S type), such as optical pure (R)- or (S)-2-chloro-1-phenylethanol; moreover, the mutant has a good application value in the field of preparation of chiral alcohol.
Owner:SHENYANG PHARMA UNIVERSITY
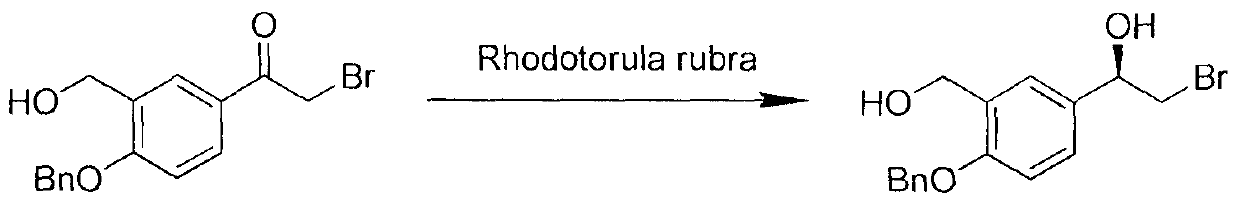
Rhodotorula cell and method for producing optical active alcohols by asymmetric conversion
InactiveCN101565679AImprove conversion rateHigh optical purityFungiMicroorganism based processesAlcoholKetone
The invention discloses a rhodotorula cell and a method for producing optical active alcohols by using the cell. The rhodotorula cell taken as a catalyst can convert substrates of various prochiral ketones under mild reaction condition to obtain corresponding optical active alcohols. The method has high conversion rate of the substrates, high optical purity of products (the enantiomeric excess value is all over 90 percent), and high actual application value and development prospect.
Owner:CHINA PHARM UNIV
Process For The Preparation Of Enantiomerically Enriched Beta Amino Acid Derivatives
ActiveUS20080058522A1Organic compound preparationCarboxylic acid amides optical isomer preparationGreek letter betaMedicinal chemistry
The present invention relates to a process for the efficient preparation of enantiomerically enriched beta amino acid derivatives wherein the amino group is unprotected. The product chiral beta amino acid derivatives are useful in the asymmetric synthesis of biologically active molecules. The process comprises an enantioselective hydrogenation of an amine-unprotected prochiral beta-amino acrylic acid or derivative thereof in the presence of a rhodium metal precursor complexed with a chiral mono- or bisphosphine ligand.
Owner:MERCK SHARP & DOHME LLC +1
Method for synthesizing (R)-salmeterol
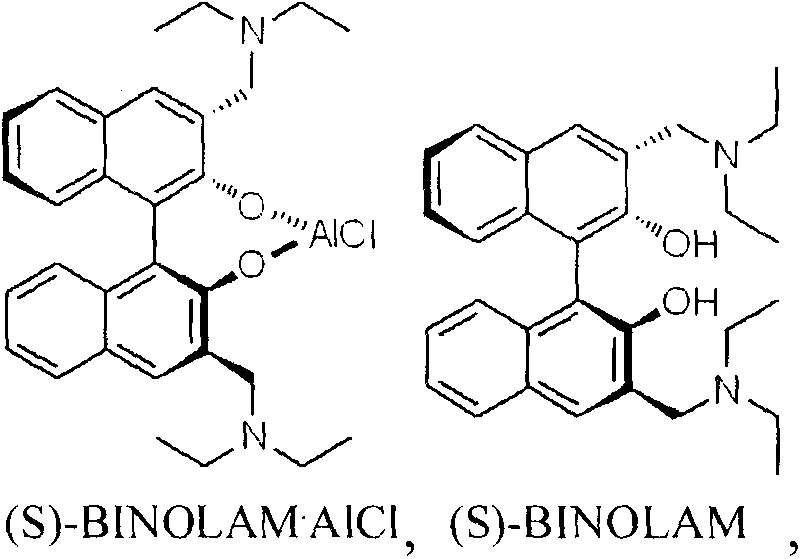
The invention discloses a method for synthesizing (R)-salmeterol. The method is implemented by taking p-hydroxy benzaldehyde as a raw material through the steps of carrying out a methylolation reaction on the p-hydroxy benzaldehyde so as to obtain prochiral aldehyde for protecting double hydroxide radicals in an acetal form; then, through taking (S)-BINOLAM.AlCl as a chiral catalyst, carrying out an asymmetric nucleophilic addition reaction so as to obtain a chiral cyanohydrin intermediate; and reducing the chiral cyanohydrin intermediate so as to obtain primary amine, reacting the primary amine with mesylate [6-(4 phenylbutoxy]hexane, and carrying out hydrolysis on the obtain product so as to remove protecting groups, thereby obtaining a final product (R)-salmeterol. In the invention, a chiral catalyst precursor (S)-BINOLAM can be reused; and the method has relatively high yield and relatively good enantioselectivity, and is short in synthetic route, simple and convenient in operation and low in cost.
Owner:NANJING UNIV OF TECH
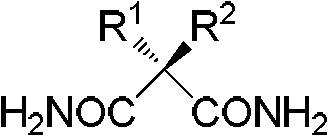
Functionalized unnatural amino acids with quaternary carbon centers and biocatalytic desymmetrization preparation method thereof
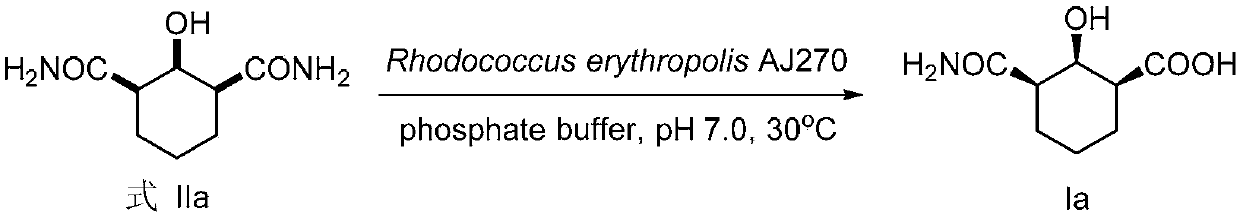
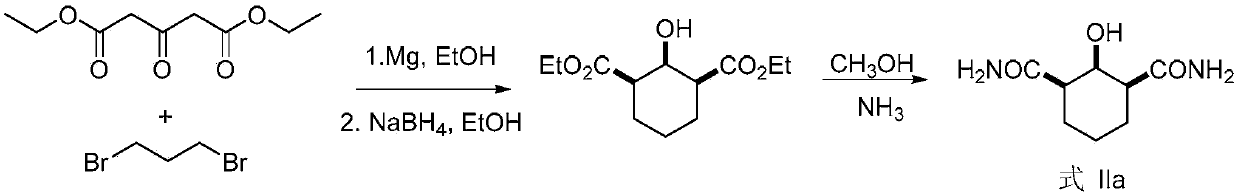
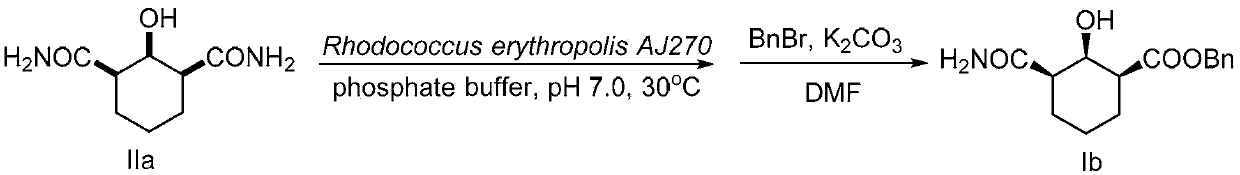
The invention discloses a functionalized unnatural chiral amino acid compounds with quaternary carbon centers and a preparation method thereof. In the invention, a Rhodococcus erythropolis AJ270 microbial system is taken for catalytic hydrolysis of prochiral malonamide compounds with different substituent groups so as to obtain corresponding unnatural amino acids with an amide group. The dosage of the Rhodococcus erythropolis can be adjusted according to the dosage of a substrate. The reaction solvent is a common buffer solution with a pH value of 6.0-8.0, and the reaction is performed at 20-37DEG C for 0.1-120h. The Rhodococcus erythropolis microbial catalytic system has the characteristics of fermentable cultivation and convenient preservation. The method for preparing monoamide carboxylic acid and dicarboxylic acid through bioconversion has the characteristics of simple operation, high efficiency reaction, mild reaction conditions, high enantioselectivity, easy separation of products, and high product purity, and can be used for synthesis of other unnatural chiral amino acids with a quaternary carbon center.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Process for preparation of (2R)-4-oxo-4-[3- (trifluoromethyl)-5,6-dihydro [1,2,4]-triazolo[4,3-a]pyrazin- 7(8H)-yl]-l-(2,4,5-trifluorophenyl)butan-2-amine & new impurities in preparation thereof
The present invention relates to synthesis of β-amino acid derivatives of formula (I) and its salts of formula (Ia) by a novel process. The process comprises the reduction of a protected or unprotected prochiral β-amino acrylic acid or derivative there of, by using borane containing reducing agents at atmospheric pressure. The resulting racemic β-amino compound is resolved to a pure stereoisomer of formula (I), specifically to (2R)-4-oxo-4-[3-Ctrifluoromethyl)-5,6-dihydrol[1,2,4]triazolo[4,3-alpyrazin-7(8H)-yl]-1-(2,4,4-trifluorophenyl)butan-2-amine. In an embodiment the invention disclosed polymorphic forms of formula (I), phosphate salt of formula (I) and also a Dibenzoyl-L-tartaric acid salt of formula (I).
Owner:CADILA HEALTHCARE LTD
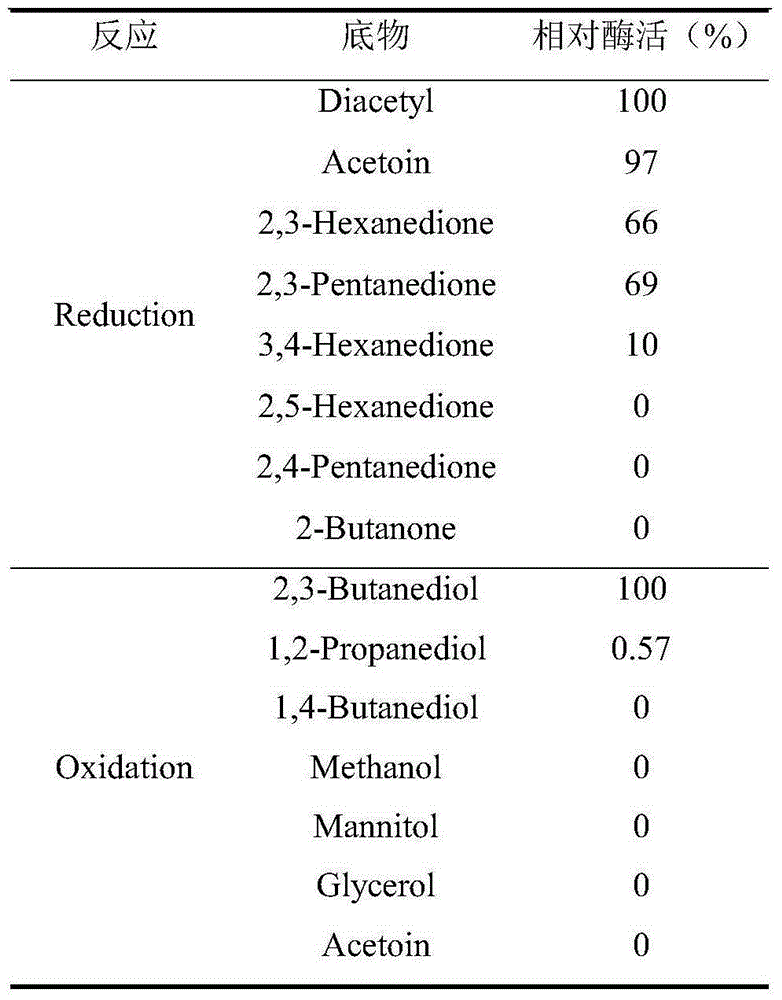
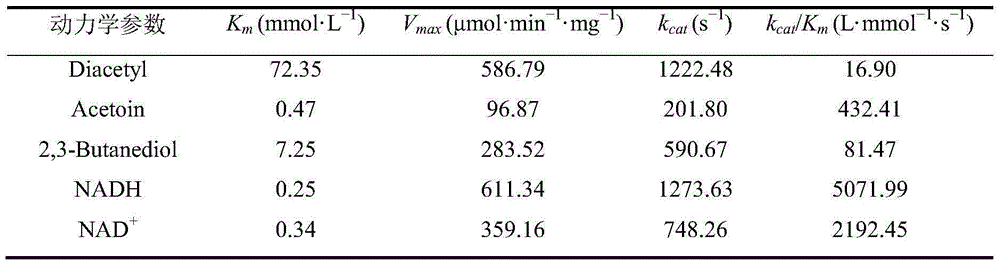
Coding gene of meso-2,3-butanediol dehydrogenase, recombinase and preparation method and application of recombinase
InactiveCN105200068AGood catalyticWide substrate applicabilityBacteriaMicroorganism based processesBacillus licheniformisDiol
The invention discloses a coding gene of meso-2,3-butanediol dehydrogenase, a recombinant expression vector and recombinant expression transformant with the gene, a preparation method of recombinase, and application of the recombinase as a catalyst in an asymmetrically reducing prochiral dicarbonyl compound for preparing optical activity chiral diols. The meso-2,3-butanediol dehydrogenase comes from Bacillus licheniformis CICIMB0109, can be used as a catalyst for efficiently synthesizing chiral diols, is high in catalytic activity and stereoselectivity, mild in suitable reaction condition and friendly to the environment, and has quite good application and development prospects.
Owner:JIANGNAN UNIV
Process for preparation of (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro [1,2,4]-triazolo[4,3-a]pyrazin-7(8H)-yl]-l-(2,4,5-trifluorophenyl)butan-2-amine and new impurities in preparation thereof
The present invention relates to synthesis of β-amino acid derivatives of formula (I) and its salts of formula (Ia) by a novel process. The process comprises the reduction of a protected or unprotected prochiral β-amino acrylic acid or derivative there of, by using borane containing reducing agents at atmospheric pressure. The resulting racemic β-amino compound is resolved to a pure stereoisomer of formula (I), specifically to (2R)-4-oxo-4-[3-Ctrifluoromethyl)-5,6-dihydrol[1,2,4]triazolo[4,3-alpyrazin-7(8H)-yl]-1-(2,4,4-trifluorophenyl)butan-2-amine. In an embodiment the invention disclosed polymorphic forms of formula (I), phosphate salt of formula (I) and also a Dibenzoyl-L-tartaric acid salt of formula (I).
Owner:CADILA HEALTHCARE LTD
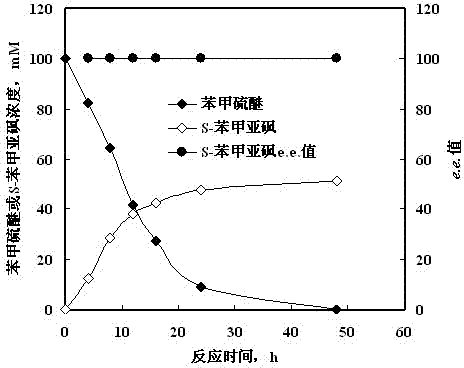
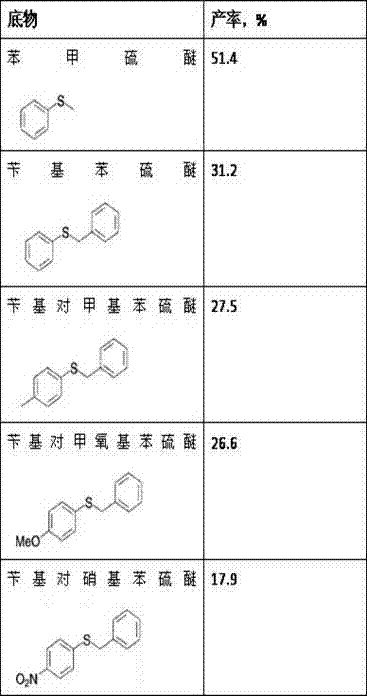
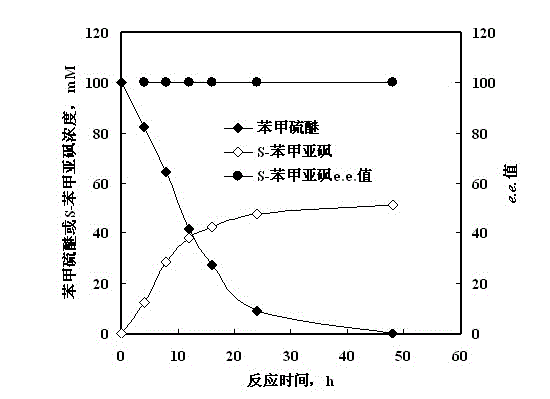
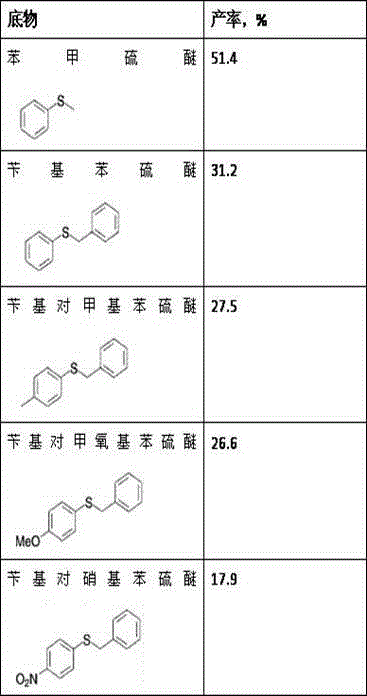
Method for synthesizing chiral sulfoxide from thioether under catalytic action of Rhodococcus
InactiveCN103114109ASimple process routeMild reaction conditionsMicroorganism based processesFermentationPtru catalystCatalytic effect
The invention discloses a method for synthesizing chiral sulfoxide from thioether under the catalytic action of Rhodococcus, belonging to the technical field of microorganisms. By using a resting cell of Rhodococcus sp. CCZU10-1 of which the collection number is CGMCC NO.4911 as a biocatalyst, asymmetric catalytic oxidation is performed on prochiral thioanisole and derivatives thereof, thus synthesizing chiral S-methyl phenyl sulfoxide (e.e.>99.9%) and derivatives thereof. The strain disclosed by the invention and the stereoselective biological oxidation method using the same have the characteristics of favorable catalytic effect, simple operating process route, mild reaction conditions, no pollution and the like.
Owner:临泉县嘉鸿装饰工程有限公司
Non-natural chiral amino acid and biological catalysis desymmetrisation preparation method thereof
InactiveCN102807501AHigh purityEasy to separateOrganic compound preparationDigestive systemCarboxylic acidSolvent
The invention discloses non-natural chiral amino acid compounds and a preparation method thereof. The non-natural chiral amino acid compounds are shown as a formula III. Raw materials for preparing the non-natural chiral amino acid compounds provided by the invention are obtained by using a Rhodococcus erythropolis AJ270 microbial system to catalytically hydrolyze prochiral malonamide compounds with different substituents. The dosage of Rhodococcus erythropolis AJ270 thalluses can be adjusted according to the dosage of substrate. Reaction solvent is commonly used buffer solution with a pH (potential of hydrogen) value being 6.0-8.0, the temperature is 20-37DEG C and reaction time is 0.1-120 hours. The Rhodococcus erythropolis AJ270 microbial catalytic system has the characteristics of fermented cultivation and convenience in storage. The method for preparing chiral monoamine carboxylic acid and dicarboxylic acid through biological conversion has the advantages of simplicity and convenience in operation, high reaction efficiency, moderate reaction conditions, high enantioselectivity, easiness in product separation and high product purity, and can be used for synthesizing non-natural chiral amino acid.
Owner:INST OF CHEM CHINESE ACAD OF SCI
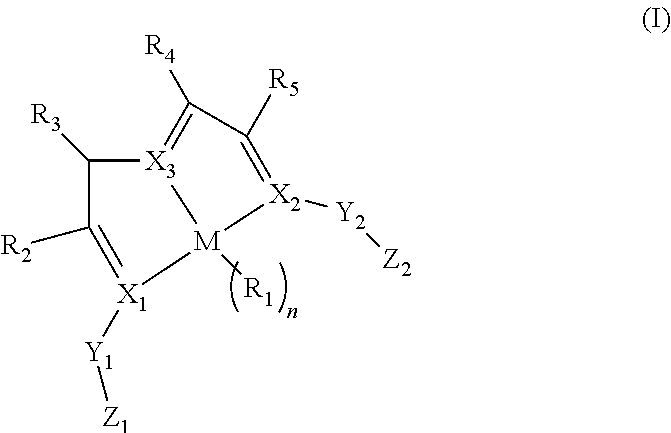
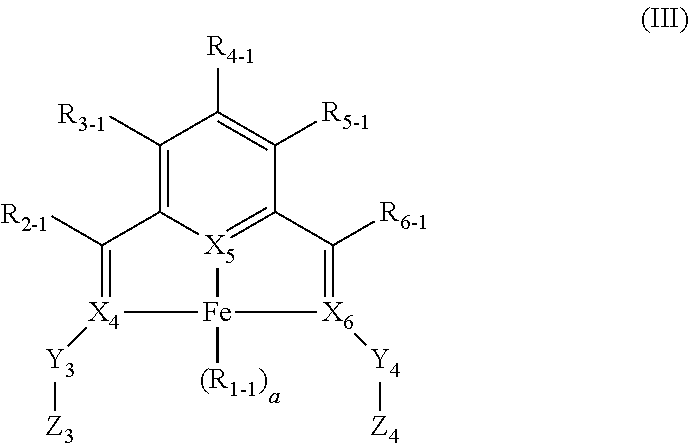
Enantiopure base-metal catalysts for asymmetric catalysis and bis(IMINO)pyridine iron alkyl complexes for catalysis
ActiveUS20130079567A1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCobalt compoundsNickel
Owner:THE TRUSTEES FOR PRINCETON UNIV
Process for the preparation of diisopinocampheylchloroborane
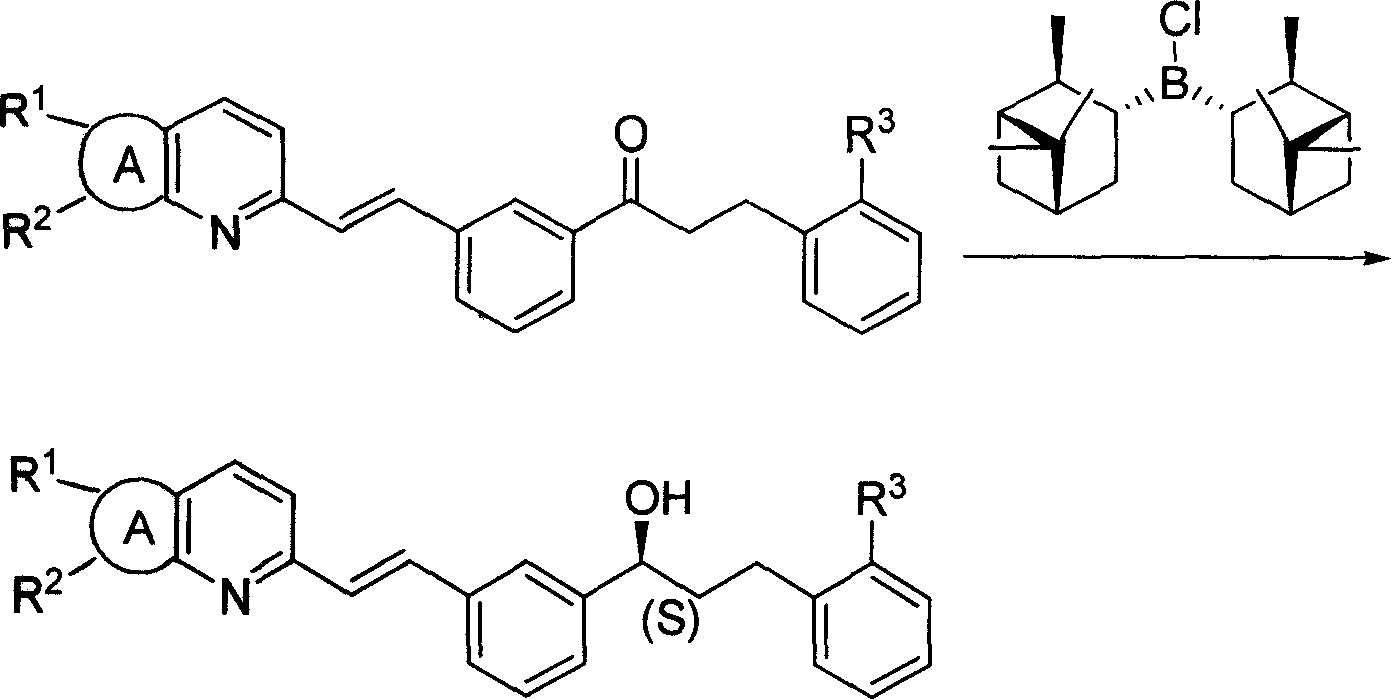
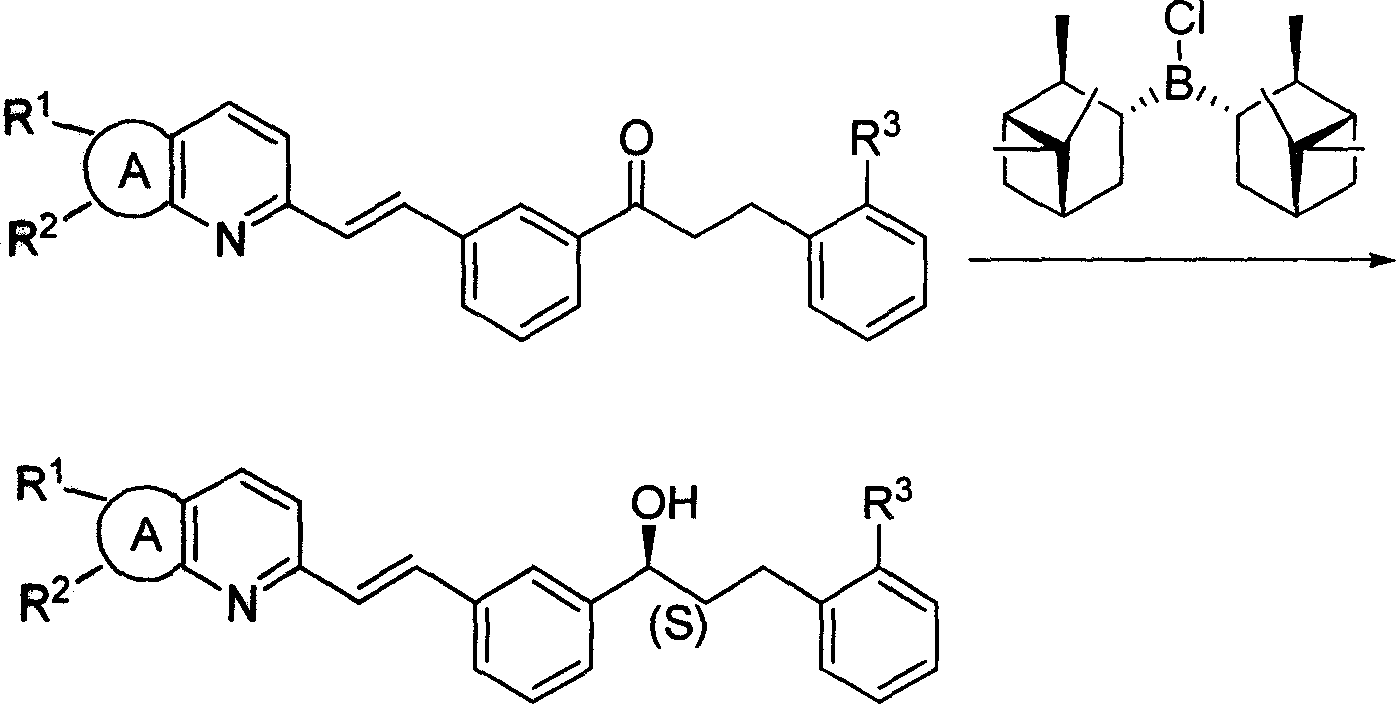
InactiveCN101225089AHigh optical purityReduce manufacturing costOrganic reductionGroup 3/13 element organic compoundsAlcoholKetone
The invention discloses a new low-cost preparation method of B-Chlorodiisopinocampheylborane, which comprises the reaction of sodium borohydride and a-pinene and phosphorus pentachloride. The new low-cost preparation method of B-Chlorodiisopinocampheylborane has the advantages that the mixture with the gotten B-Chlorodiisopinocampheylborane can be directly used for the reduction from prochiral ketones to the corresponding chiral alcohols with high optical purity, and the production cost and hidden danger are greatly reduced.
Owner:SHANGHAI ACEBRIGHT PHARMA GRP +1
Method for synthesizing 3-(Boc-aminomethyl)cyclobutanone
ActiveCN109053496ALow costUnique routeCarbamic acid derivatives preparationOrganic compound preparationSodium methoxideSynthesis methods
The invention relates to a method for synthesizing 3-(Boc-aminomethyl)cyclobutanone. The method for synthesizing 3-(Boc-aminomethyl)cyclobutanone provided by the invention mainly solves the technicalproblems of high raw material cost and relatively difficult post-processing according to the existing synthesis method. The method for synthesizing 3-(Boc-aminomethyl)cyclobutanone provided by the invention comprises the following steps: 3-oxocyclobutanecarboxylic acid and trimethyl orthoformate are reacted in the methanol solution to generate a compound 1; the compound 1 and benzylamine are reacted in the methanol solution under an action of sodium methoxide to generate a compound 2; the compound 2 and sodium bis(2-methoxyethoxy)aluminiumhydride are reacted in tetrahydrofuran solution to generate a compound 3; the compound 3 is subjected to a Pd / C debenzylation and hydrogenation operation in the methanol solution to obtain a compound 4; the compound 4 and Boc2O are reacted in the methanolsolution to obtain a compound 5; the compound 5 is reacted in a 0.05 M hydrochloric acid solution generate a target compound 6. By performing an enzymatic catalyzed deglandulation action of on the corresponding prochiral 3-substituted cyclobutanone, a series of gamma-butyrolactone derivatives, including some spiro derivatives, can be obtained; and all the derivatives are inhibitors having good biological activity.
Owner:GL BIOCHEM SHANGHAI
A class of chiral beta-hydroxyamide compounds, preparation method and applications thereof,
ActiveCN110857276AEasy to storeHigh purityOrganic compound preparationOrganic chemistry methodsCarboxylic acidRhodococcus erythropolis
The invention discloses a chiral beta-hydroxyamide compound and a preparation method thereof, wherein the compound is represented by a formula I. According to the invention, the raw material for preparing a non-natural chiral amino acid compound is obtained by carrying out catalytic hydrolysis on a prochiral diamide compound II with different substituents by using a Rhodococcus erythropolis AJ270microbial system, wherein the use amount of the Rhodococcus erythropolis can be adjusted according to the use amount of a substrate, the reaction solvent is a common buffer solution with the pH valueof 6.0-8.0, the temperature is 20-37 DEG C, the reaction time is 3-120 h, and the Rhodococcus erythropolis microbial catalytic system can be cultured through fermentation and conveniently stored; andwith the application of the biotransformation to prepare chiral monoamide carboxylic acid and dicarboxylic acid, the characteristics of simplicity and convenience in operation, high reaction efficiency, mild reaction conditions, high enantioselectivity, easiness in product separation and high product purity are achieved, and the application prospect is god.
Owner:INST OF CHEM CHINESE ACAD OF SCI +1
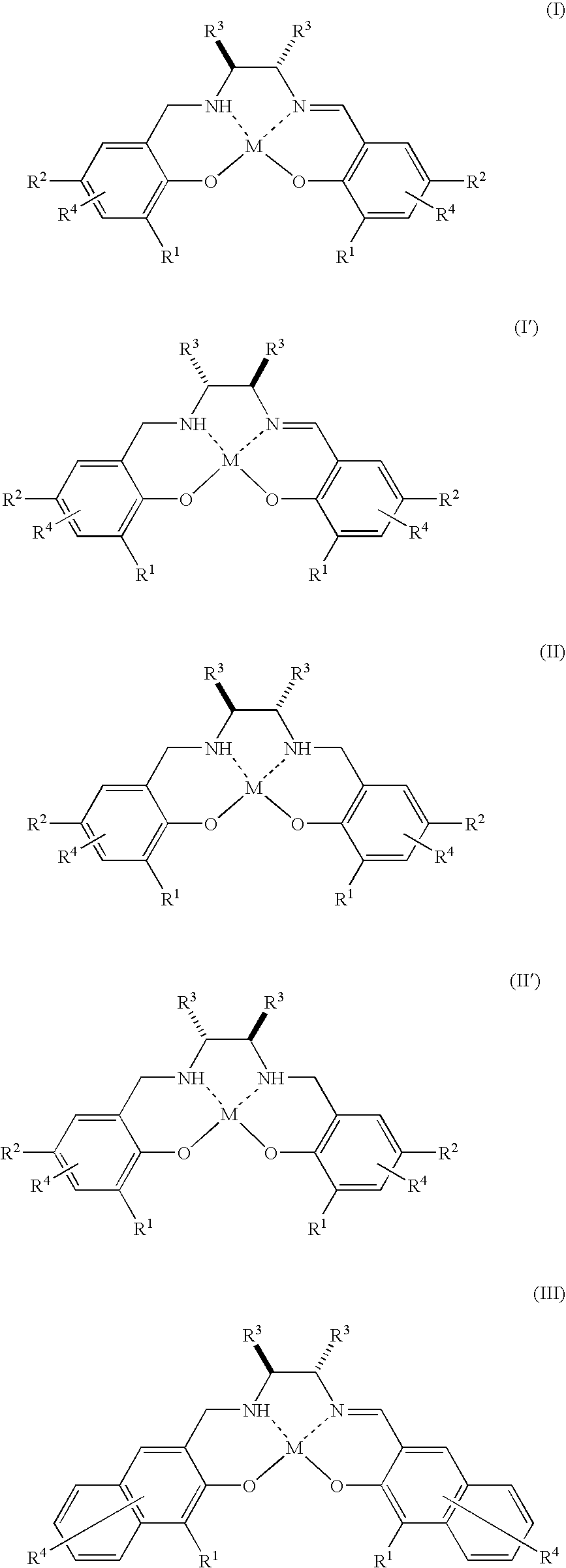
Method for Production of Optically Active Epoxy Compound, and Complex Used Therefor and Process for Producing the Same
InactiveUS20080071099A1Promote epoxidationHigh enantioselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsArylHalogen
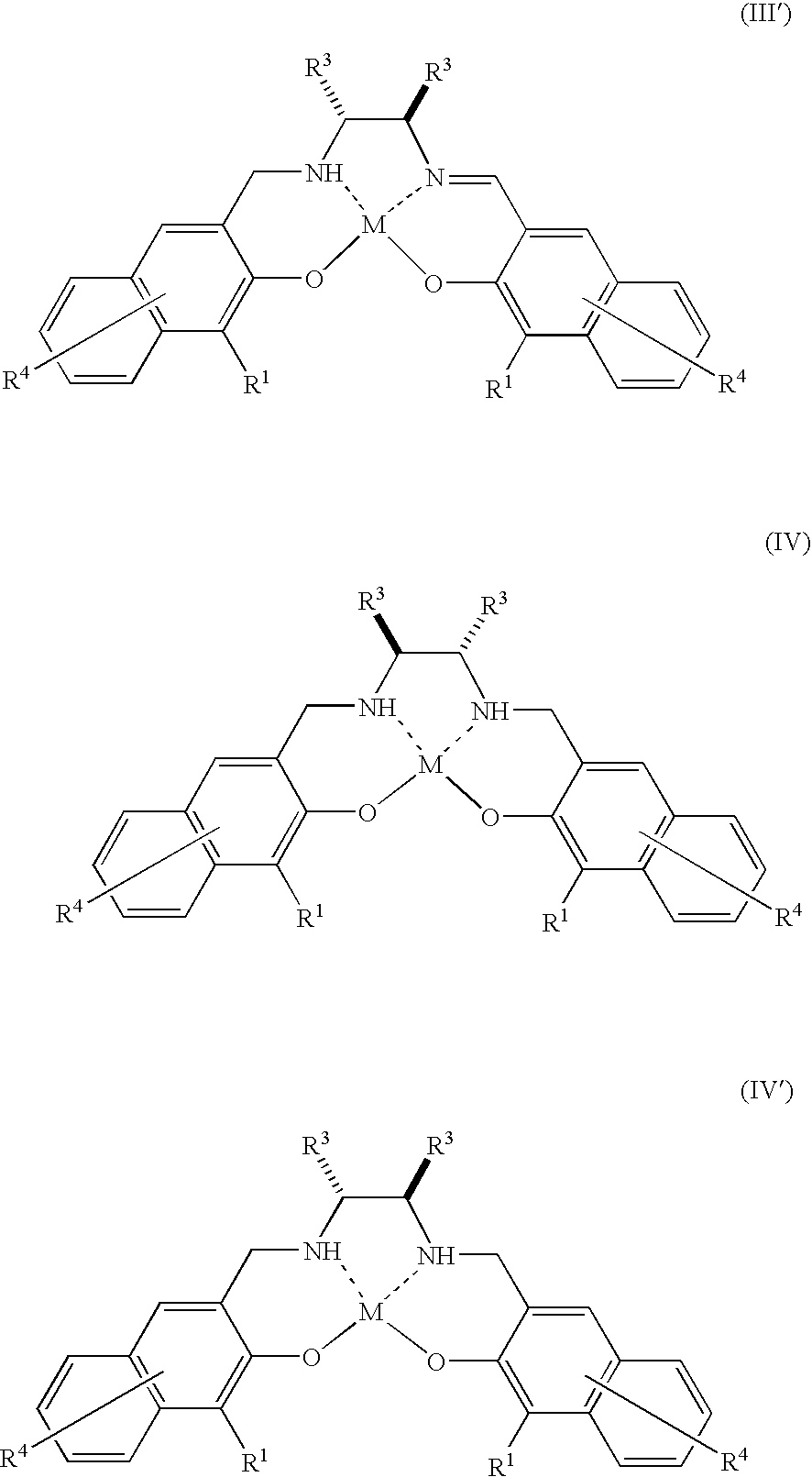
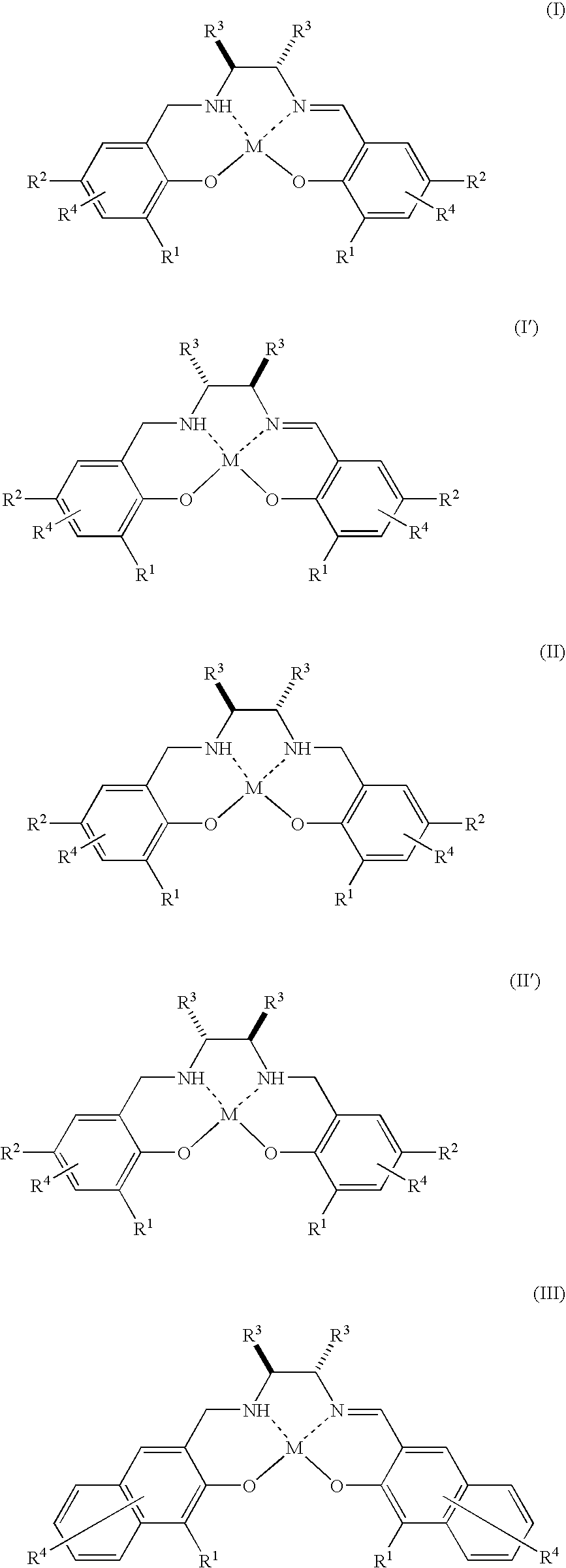
There is provided a method for industrially producing optically active epoxy compounds by asymmetrically epoxidizing prochiral unsaturated compounds with an oxidant using as a catalyst a single substance or a di-μ-oxo dimer derived therefrom represented by any of the following formulae (I), (I′), (II), (II′), (III), (III′), (IV), and (IV′): [wherein R1s are independently an alkyl group or an aryl group; R2s are independently an alkyl group or an aryl group; R3s are independently an alkyl group or an aryl group, provided that two R3s may be bonded with each other to form a ring; R4s are independently a hydrogen atom, a halogen atom, an alkyl group, an alkoxy group, a nitro group, or a cyano group; M is TiY2 (Y is Cl, alkoxide, or a μ-oxo ligand)].
Owner:JAPAN SCI & TECH CORP
Hydrogenation of imines
ActiveUS20110054217A1Improve conversion efficiencyHigh enantiomeric excessOrganic compound preparationOrganic chemistry methodsPtru catalystAsymmetric hydrogenation
The present invention relates to a process for the asymmetric hydrogenation of imines with hydrogen under elevated pressure in the presence of a catalyst system. In particular the present invention relates to the use of the said catalytic system for the enantioselective hydrogenation of prochiral ketimines to asymmetric amines leading to the formation of herbicides.
Owner:UNITED PHOSPHORUS LTD
Reductase, gene and recombinase of reductase and preparation method and application of recombinase
InactiveCN102559623AGood catalyticWide substrate applicabilityBacteriaMicroorganism based processesWild typeCatalytic effect
The invention discloses a reductase, a gene of the reductase, a recombinant expression vector and recombinant expression transformant which contain the gene, a recombinase of the reductase, a preparation method of the recombinase, and an application of the reductase or the recombinase thereof used as a catalyst in the asymmetric reduction of a prochiral carbonyl compound so as to prepare chiral alcohols with optical activities. The reductase or the recombinase thereof can derive from Bacillus sp. of which is the preservation number is CGMCC (China General Microbiological Culture Collection Center) No.2549, can be used as the catalyst in the asymmetric reduction of the prochiral carbonyl compound so as to prepare various chiral alcohols with optical activities, and has high catalytic efficiency and strong stereoselectivity; and the applicable reaction conditions are mild and the reductase or recombinase is environmentally friendly. Compared with the intact cells of the wild-type Bacillus sp. of which preservation number is CGMCC No.2549, the reductase has better catalytic effect and higher substrate applicability; and the reductase has very good industrial application and development prospects.
Owner:EAST CHINA UNIV OF SCI & TECH
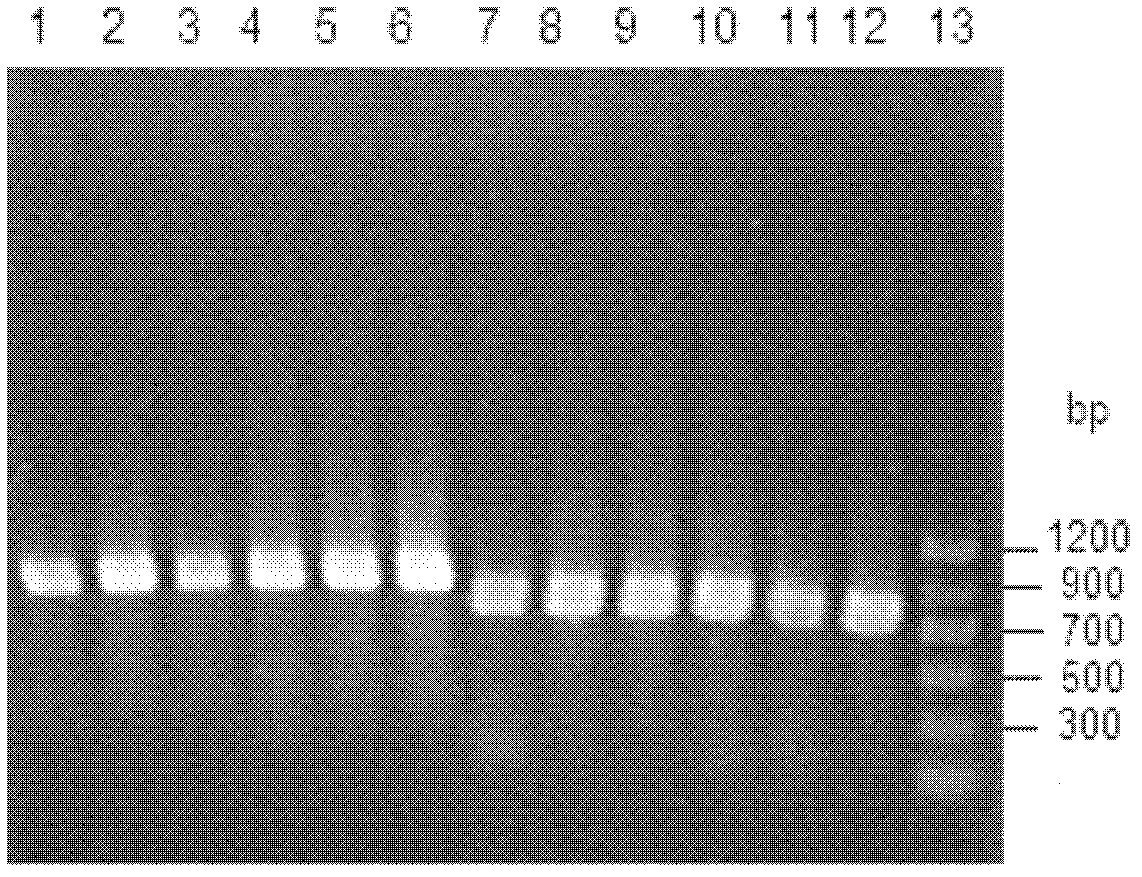
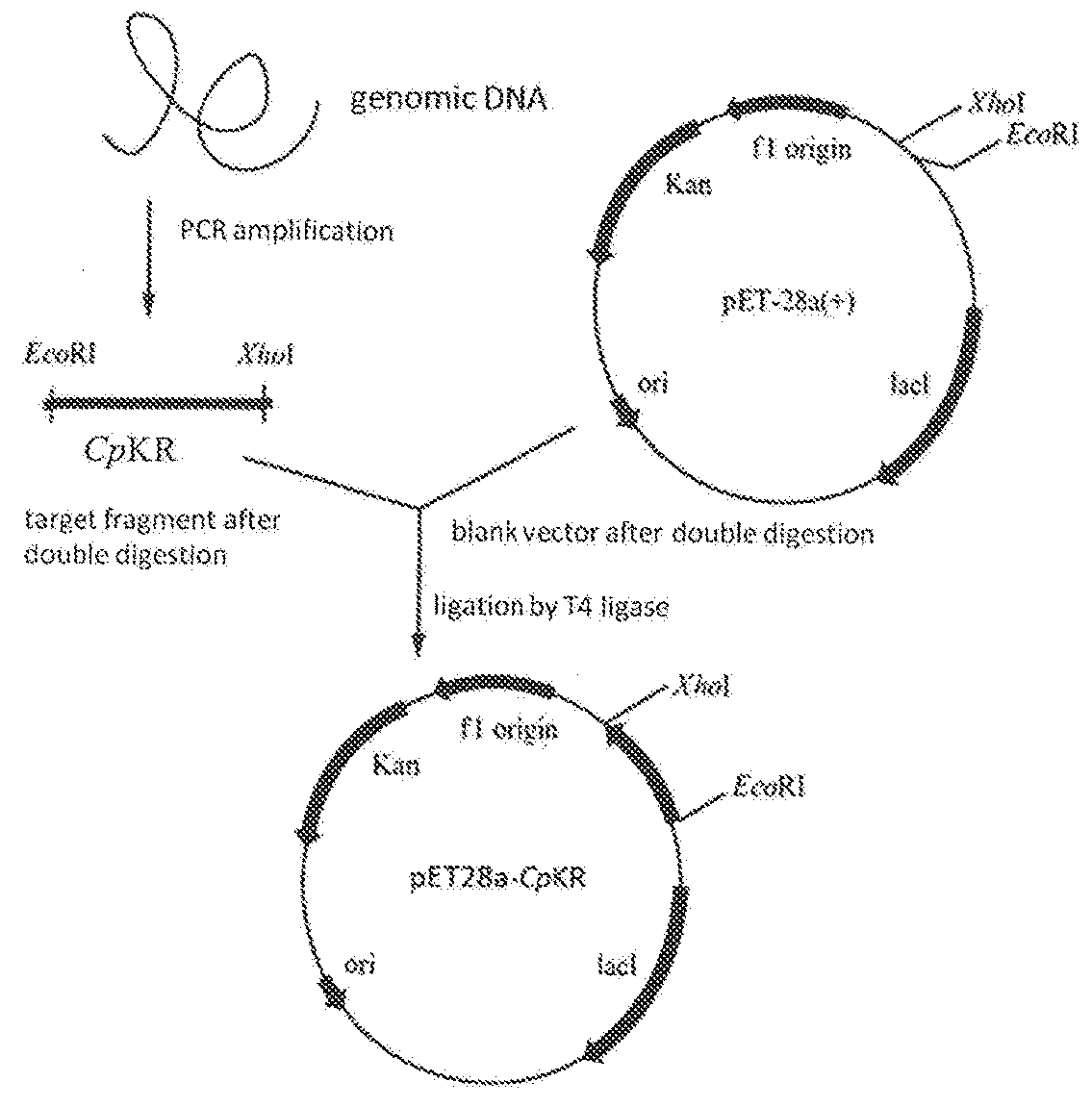
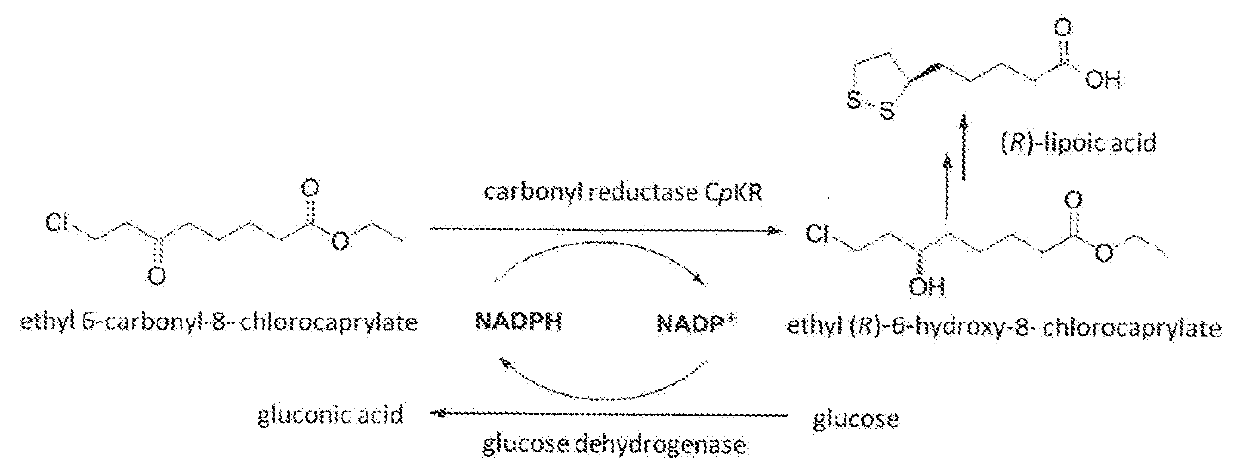
Candida carbonyl reductase and method for preparing (r)-lipoic acid precursor
ActiveUS20180265875A1High yieldHigh optical purityFungiOxidoreductasesPtru catalystCarbonyl Reductase
Disclosed herein is Candida parapsilosis CGMCC 9630, the carbonyl reductase expressed by said strain and the encoding gene and amino acid sequence thereof, the recombinant expression vector and recombinant expression transformant containing said gene sequence, and use of whole cells of Candida parapsilosis, carbonyl reductase or corresponding recombinant transformant thereof as catalyst in catalyzing asymmetric reduction of prochiral carbonyl compounds, particularly reduction of 6-carbonyl-8-halogenocaprylate to prepare the synthetic precursor of (R)-α-lipoic acid, (R)-6-hydroxy-8-halogenocaprylate. In comparison to other methods of asymmetric reduction for preparing (R)-6-hydroxy-8-halogenocaprylate, the disclosure has advantages of high substrate concentration, mild reaction conditions, environmental friendship, high yield, and high optical purity of the product, and thus has good prospect in industrial production of (R)-α-α-lipoic acid.
Owner:EAST CHINA UNIV OF SCI & TECH
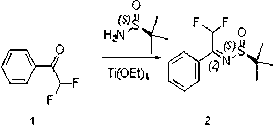
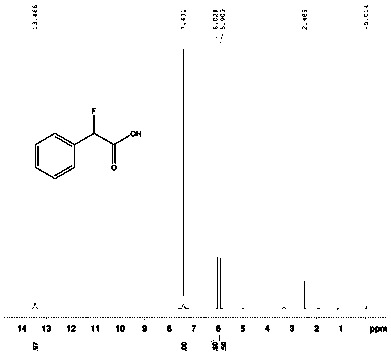
Preparation method of chiral 2-fluoromethyl phenyl ethylamine
ActiveCN102976954ASimple and efficient synthesisEfficient synthesisOrganic compound preparationAmino compound preparationEthyl groupLewis acid catalysis
The invention relates to a preparation method of chiral 2-fluoromethyl phenyl ethylamine, and mainly solves the technical problems of complex and high cost in a conventional synthesis method of the chiral 2-fluoromethyl phenyl ethylamine compound. The preparation method of the chiral 2-fluoromethyl phenyl ethylamine comprises the steps of a first step reaction for obtaining chiral (2-fluoromethyl-1-phenyl-ethyl)-tert-butyl sulfoximine (2) in a solvent via lewis acid catalytic condensation reaction and the effect with chiral tert-butanesulfinyl amide by using 2-fluoro-1-acetophenone (1) as a raw material; a second step reaction for obtaining chiral (2-fluoromethyl-1-phenyl-ethyl)-tert-butyl sulfinamide (3) by reacting the compound (2) with a reducing agent; and a third step reaction for obtaining a target product of chiral 2-fluoromethyl phenyl ethylamine by hydrolyzing the compound (3) in a mixed condition of the solvent and hydrochloric acid.
Owner:SHANGHAI STA PHARMA R&D CO LTD
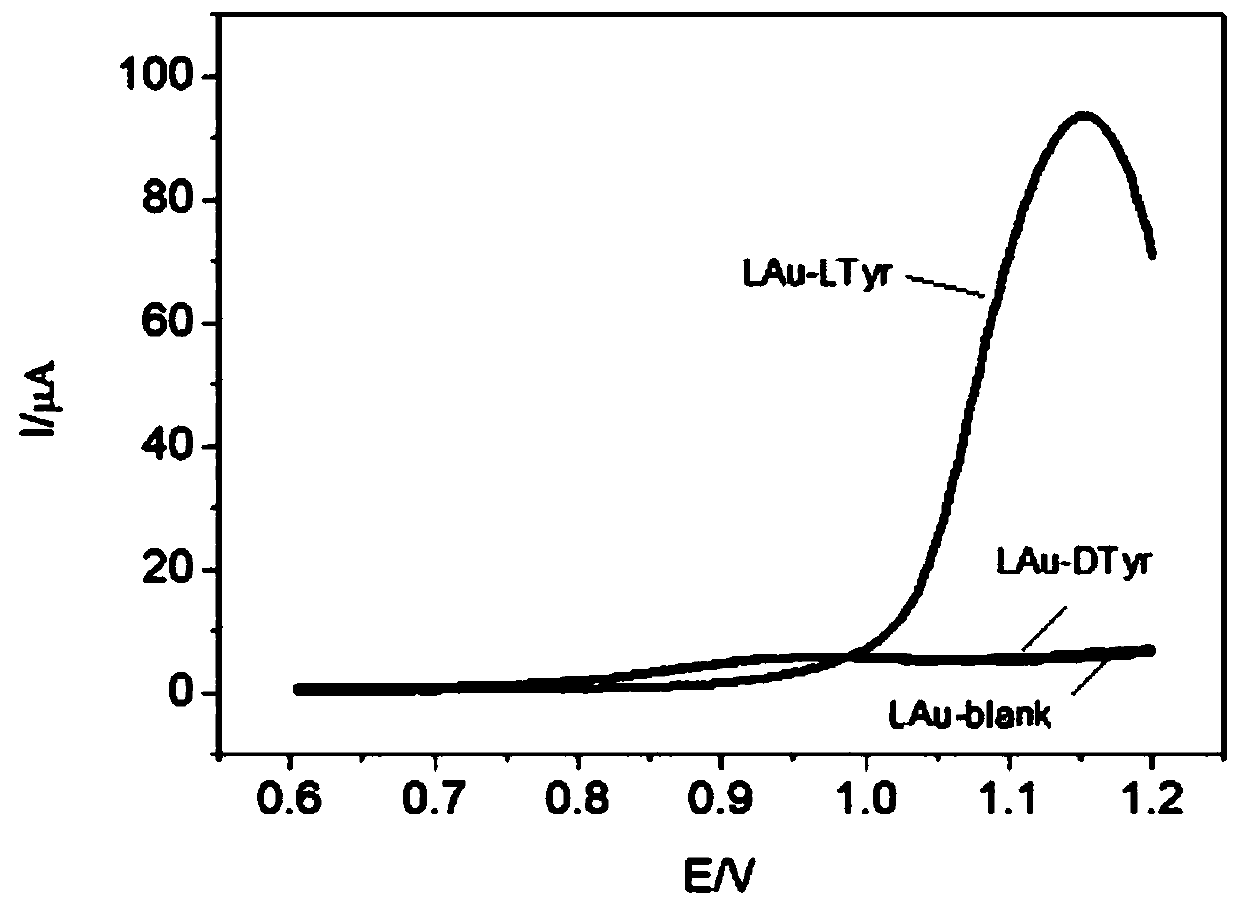
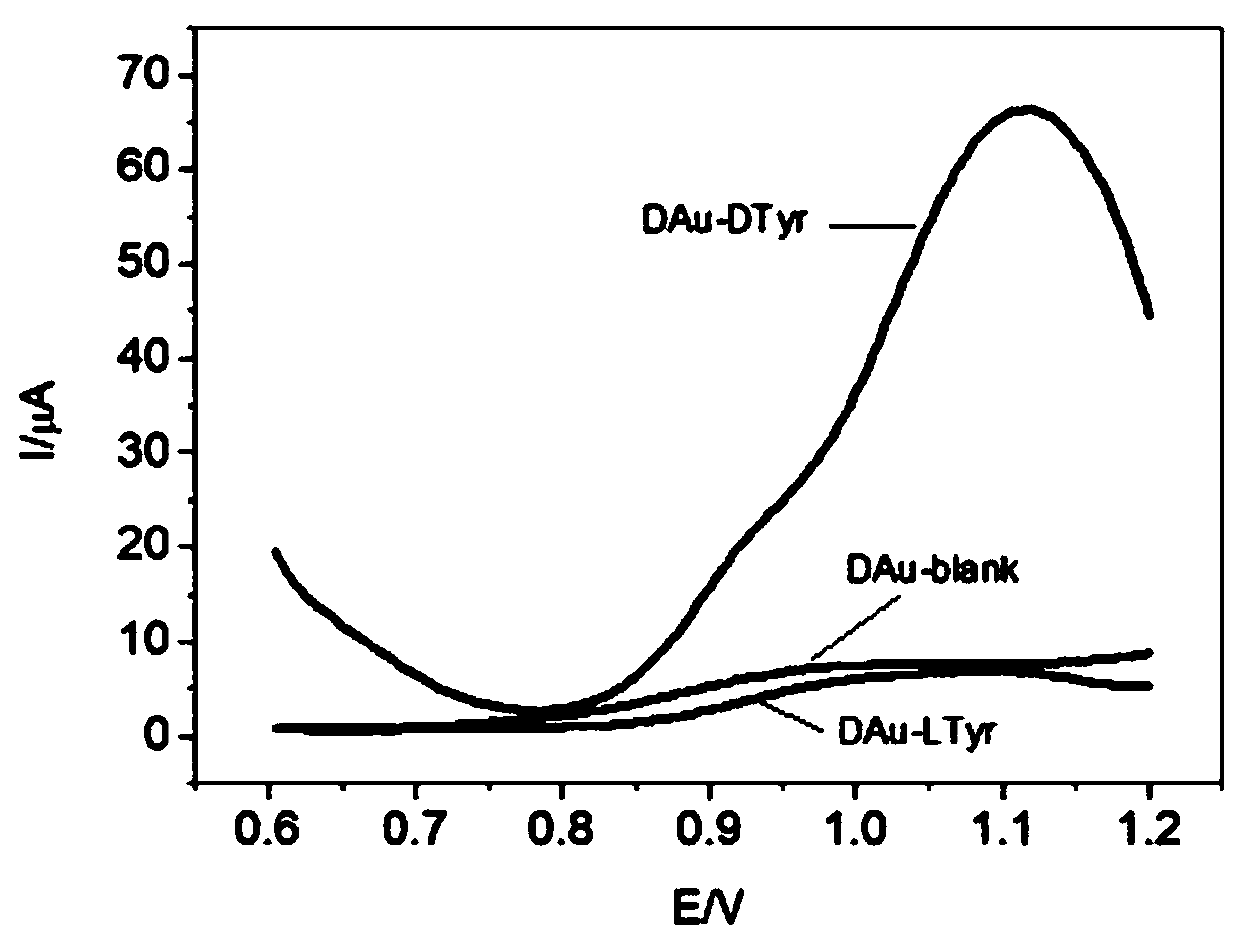
Chiral electrochemical sensor and application thereof
ActiveCN111474225AStrong chiral recognition abilityTo achieve the purpose of simultaneous detectionMaterial electrochemical variablesBULK ACTIVE INGREDIENTElectrochemistry
The invention relates to a chiral electrochemical sensor based on intrinsic chiral nanocrystals and application thereof, belongs to the technical field of electrochemical sensors, and solves the technical problems in the existing chiral electrochemical sensor that the enantiomer distinguishing capacity is poor, complete distinguishing is not achieved, in-situ simultaneous distinguishing cannot beachieved, and the service life is extremely short. The chiral electrochemical sensor comprises an electrode and intrinsic chiral nanocrystals fixed on the surface of the electrode. The intrinsic chiral nanocrystal is proposed for the first time to be used for constructing the chiral electrochemical sensor, the chiral interface modified by the chiral nanocrystal material has an intrinsic chiral recognition capability, and the difference is generated between the chiral interface modified by the chiral nanocrystal material and a non-covalent bond acting force between electrode chiral interfaces.Targeted chiral molecular isomers can be truly distinguished by the non-covalent bond acting force and the molecular chiral group associated party. The electrochemical chiral sensor can be used for identifying amino acids in foods, active ingredients in chiral medicines and chiral isomers in human bodies.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
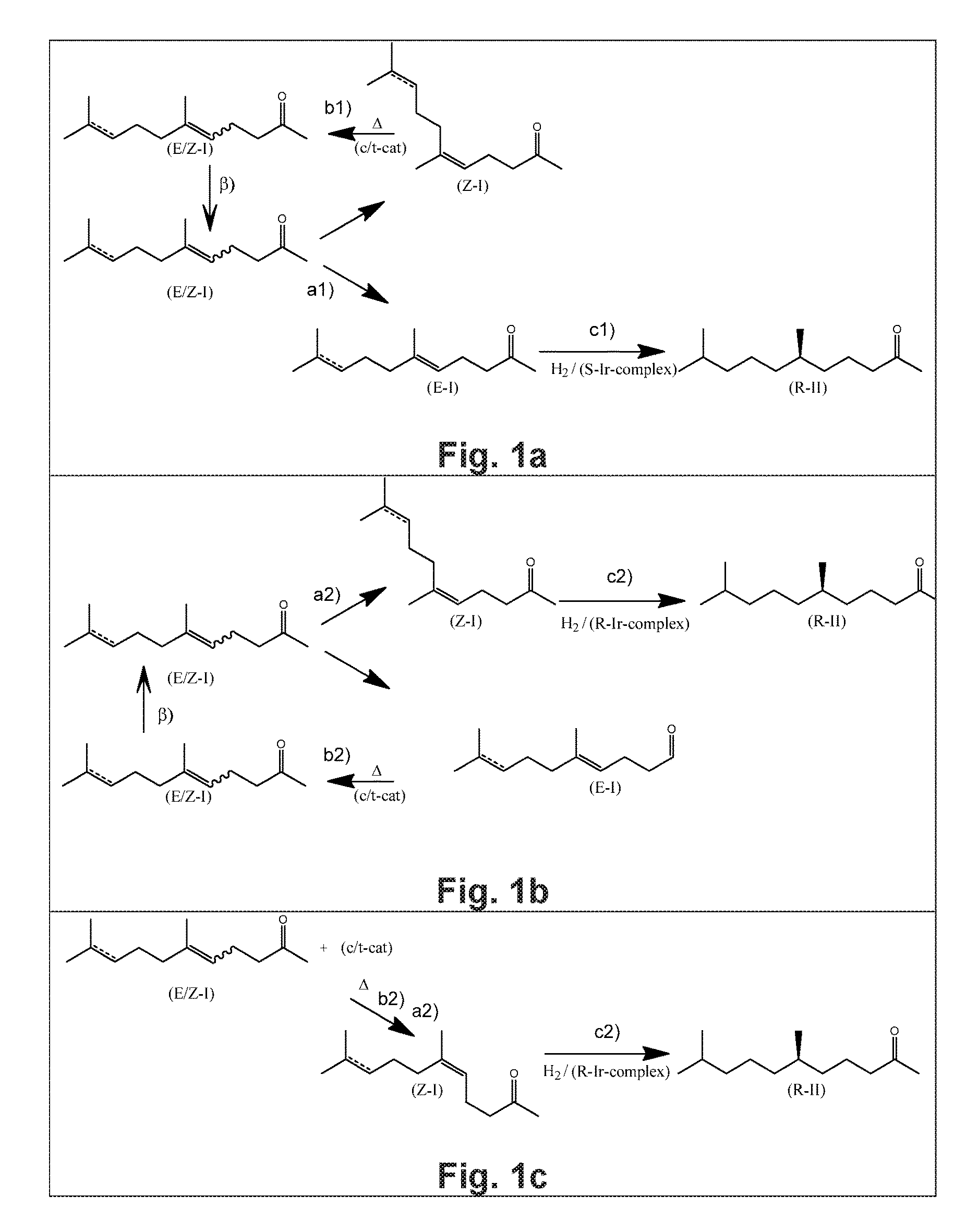
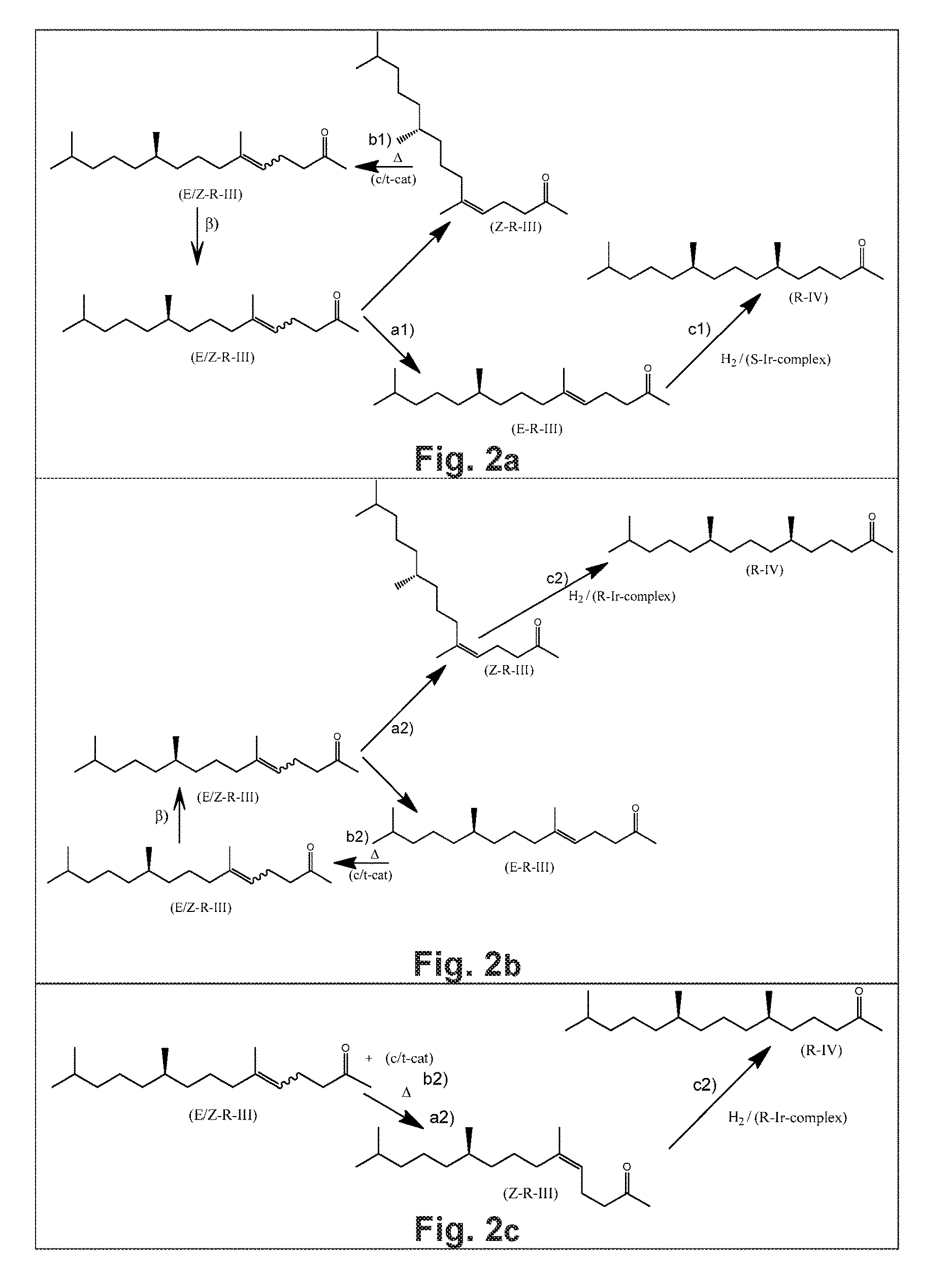
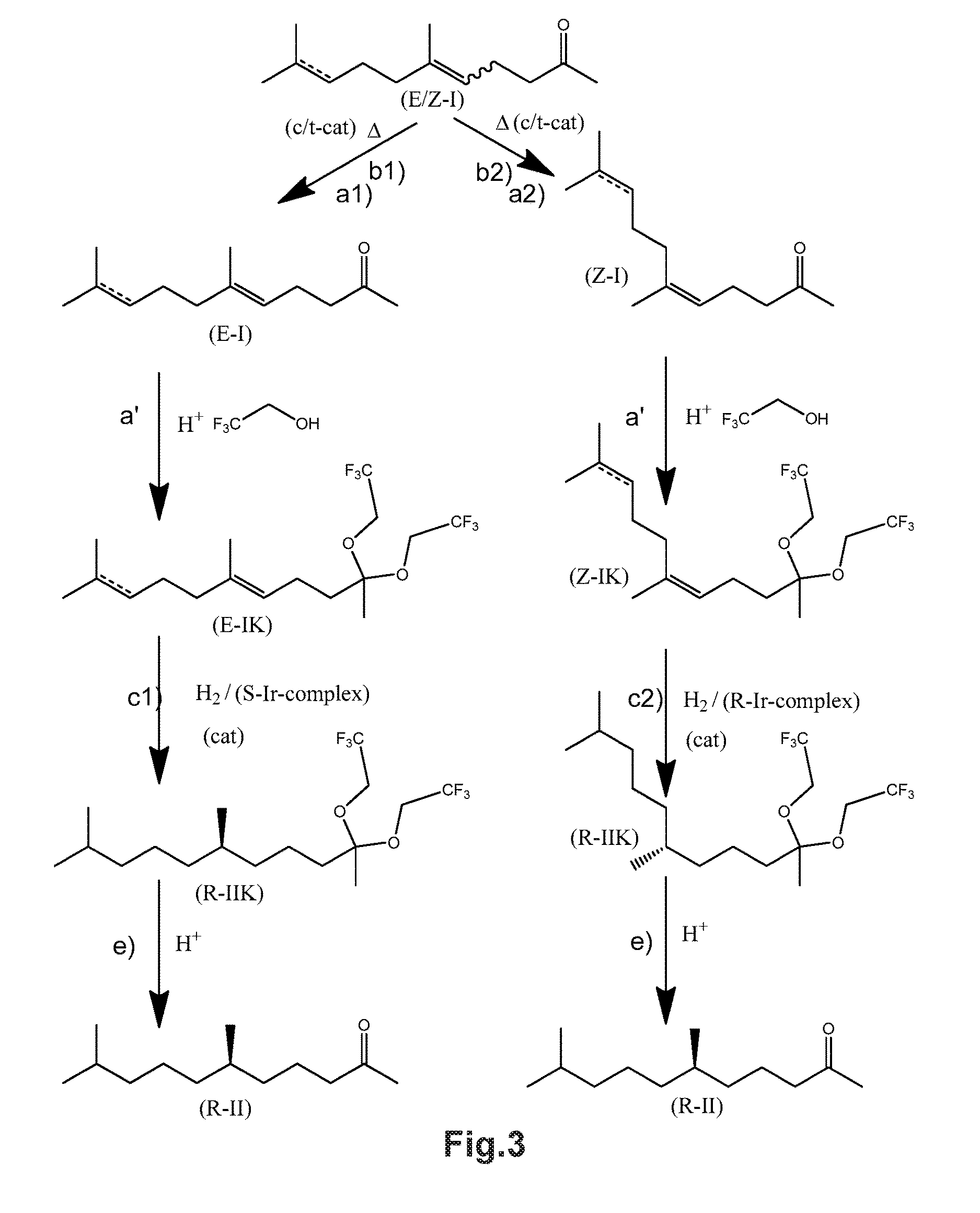
Using mixtures of E/Z isomers to obtain quantitatively specific products by combining asymmetric hydrogenation and isomerization
ActiveUS9573872B2Organic compound preparationOrganic chemistry methodsIsomerizationChemical compound
The present invention relates to a process of manufacturing compound having stereogenic centers from a mixture of E / Z isomers of unsaturated compounds having prochiral double bonds. The hydrogenation product has a specific desired configuration at the stereogenic centers. The process involves an asymmetric hydrogenation and an isomerization step. The process is very advantageous in that it forms the desired chiral product from a mixture of stereoisomers of the starting product in an efficient way.
Owner:DSM IP ASSETS BV
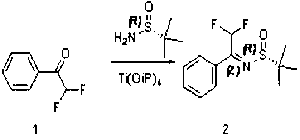
Preparation method of chiral alpha-difluoromethyl phenyl ethylamine
InactiveCN102976953ASimple and efficient synthesisEfficient synthesisOrganic compound preparationAmino compound preparationProchiralityEthylamines
The invention relates to a preparation method of chiral alpha-difluoromethyl phenyl ethylamine, and mainly solves the technical problems of complex and high cost in a conventional synthesis method of the chiral alpha-difluoromethyl phenyl ethylamine compound. The preparation method of the chiral alpha-difluoromethyl phenyl ethylamine comprises the steps of a first step reaction for obtaining chiral (2,2-difluoromethyl-1-phenyl-ethyl)-tert-butyl sulfoximine (2) in a solvent via lewis acid catalytic condensation reaction and the effect with chiral tert-butanesulfinyl amide by using 2,2-difluoro-1-acetophenone (1) as a raw material; a second step reaction for obtaining chiral (2,2-difluoromethyl-1-phenyl-ethyl)-tert-butyl sulfinamide (3) by reacting the compound (2) with a reducing agent; and a third step reaction for obtaining a target product of chiral alpha-difluoromethyl phenyl ethylamine (A) by hydrolyzing the compound (3) in a mixed condition of the solvent and hydrochloric acid.
Owner:上海药明康德新药开发有限公司 +1
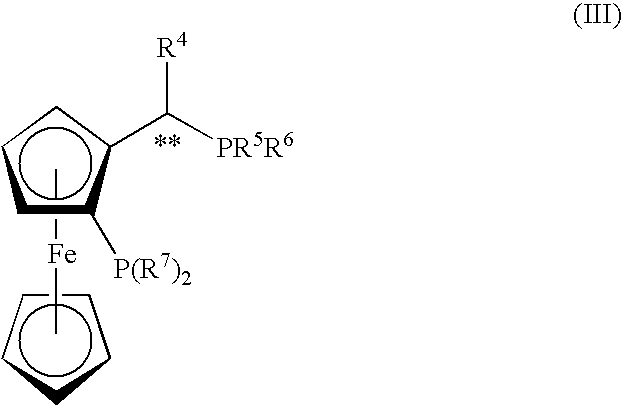
Chiral ferrocene PNNO tetradentate ligand and application thereof in asymmetric hydrogenation reaction
ActiveCN114315917AEasy to retouchHigh catalytic activityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsKeto acidPhosphine
The invention discloses a chiral ferrocene PNNO tetradentate ligand and application of the chiral ferrocene PNNO tetradentate ligand in asymmetric hydrogenation reaction, and belongs to the field of fine chemical engineering. The ligand disclosed by the invention is a novel chiral tetradentate phosphine ligand, and the ligand has the advantages of simplicity in synthesis, easiness in modification, good stability and the like. Metal complexes of the ligands show very excellent catalytic activity and extremely high enantioselectivity in asymmetric hydrogenation reaction of ketone, and alpha, beta, beta, beta-tetracarboxylic acid can be efficiently converted into alpha, beta, beta-tetracarboxylic acid, alpha, beta-tetracarboxylic acid and alpha, beta-tetracarboxylic acid. Prochiral ketones such as alpha, beta-unsaturated ketone, simple aryl alkyl ketone, alpha-hydroxy aryl alkyl ketone, alpha-amino aryl alkyl ketone, alpha-chloro aryl alkyl ketone, beta-keto ester and aryl (hetero) aryl ketone are reduced into corresponding chiral alcohols, and the method has important industrial application value.
Owner:WUHAN UNIV
Method for synthesizing chiral sulfoxide from thioether under catalytic action of Rhodococcus
InactiveCN103114109BSimple process routeMild reaction conditionsMicroorganism based processesFermentationPtru catalystCatalytic effect
The invention discloses a method for synthesizing chiral sulfoxide from thioether catalyzed by Rhodococcus, belonging to the technical field of microbes. Using the resting cells of a strain of Rhodococcus sp.CCZU10-1 with the preservation number CGMCCNo.4911 as a biocatalyst, the chiral S -Benzyl sulfoxide (e.e. >99.9%) and its derivatives. The bacterial strain and the stereoselective biological oxidation method of the present invention not only have good catalytic effect, but also have the characteristics of simple operation process route, mild reaction conditions, no pollution and the like.
Owner:临泉县嘉鸿装饰工程有限公司
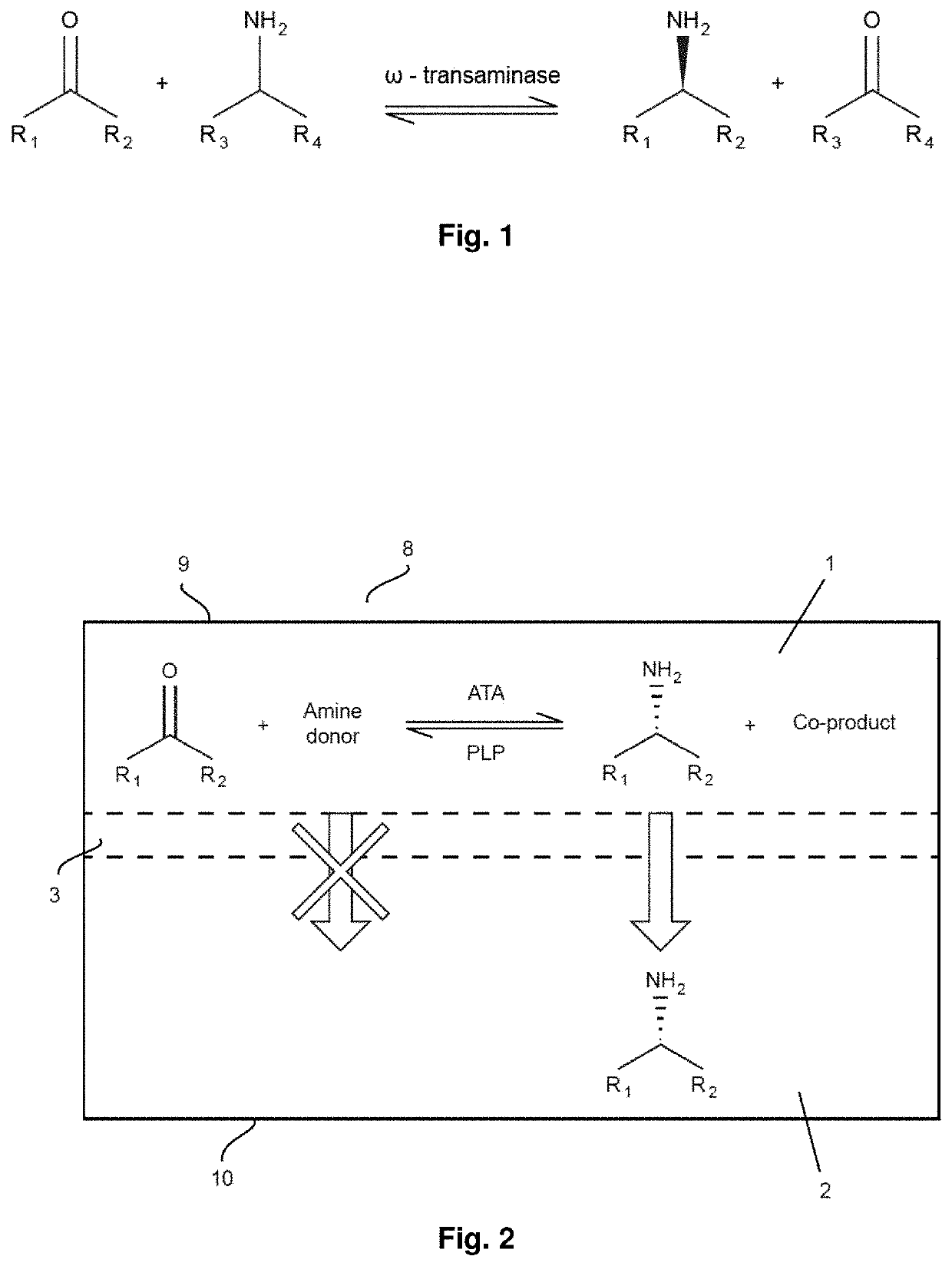
Method for producing chiral amines
ActiveUS20210062233A1Avoiding amino donor lossLong-term and scalable applicationTransferasesFermentationCombinatorial chemistryHigh molecular mass
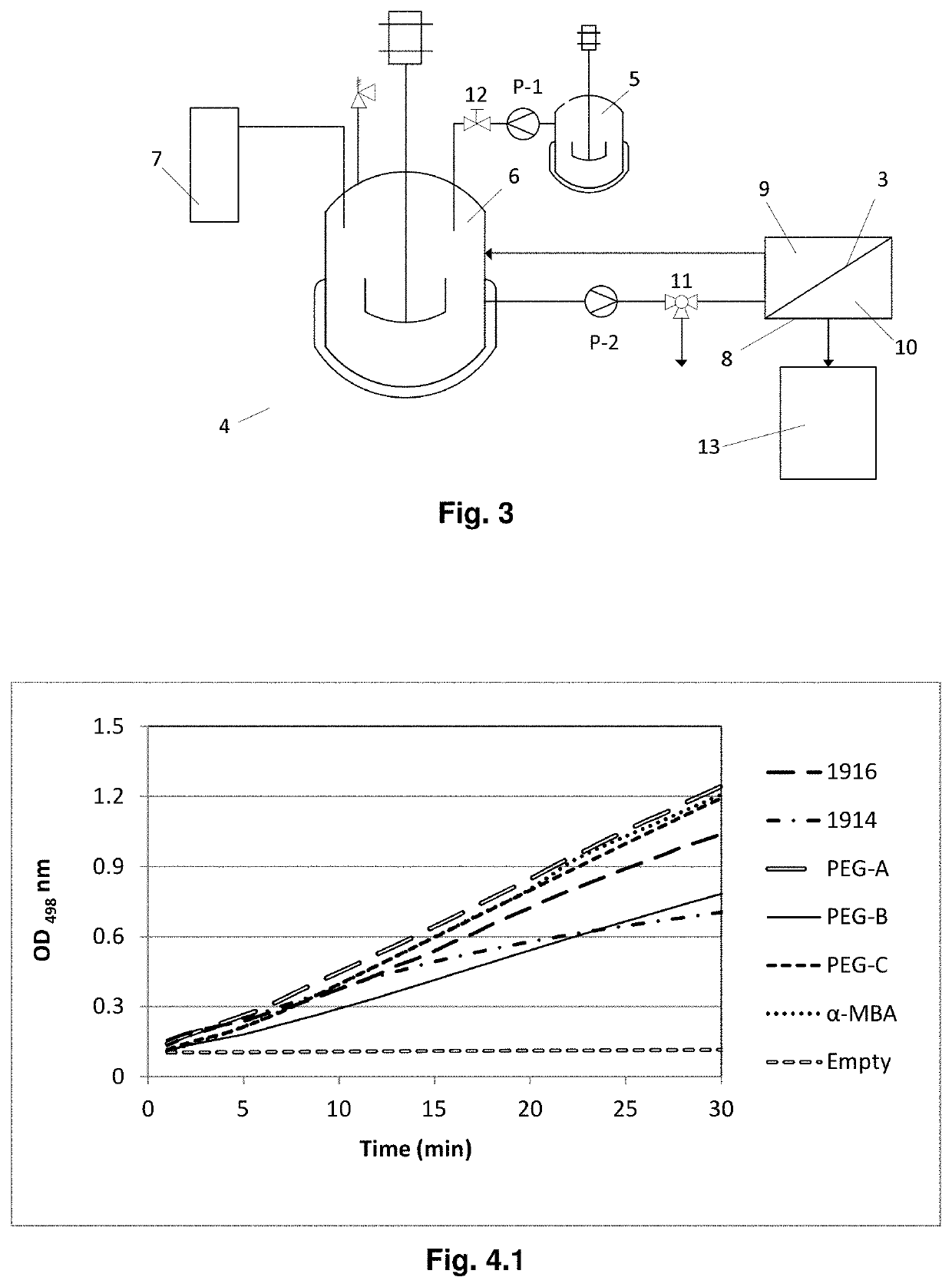
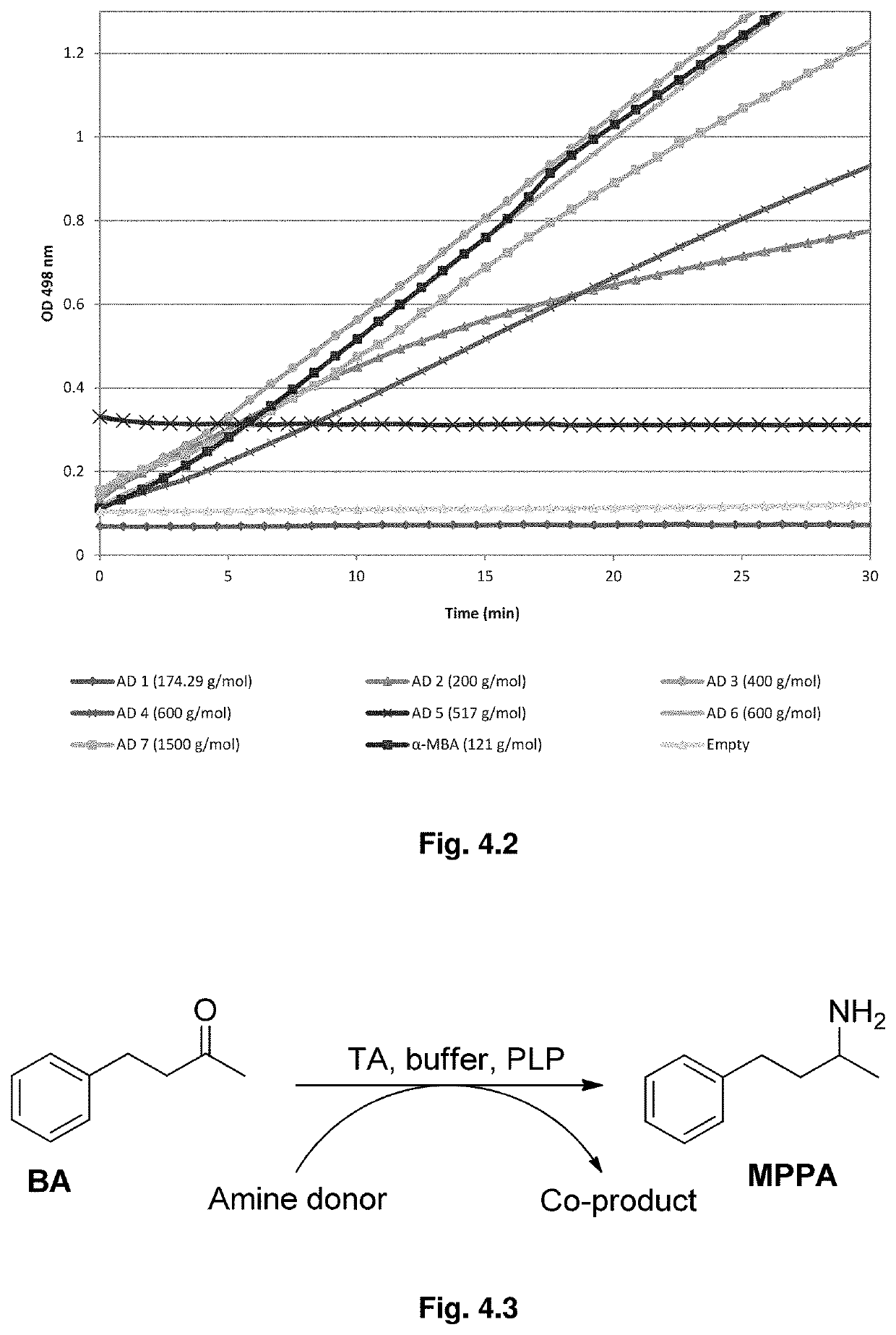
A method for producing a chiral amine may include performing a transamination reaction of a prochiral amino acceptor and an amino donor in a first solution in the presence of a transaminase, thereby forming a chiral amine and a co-product in the first solution. The amino donor is a high molecular weight (HMW) amino donor. In some examples, the molecular weight of the HMW amino donor is at least 150 g / mol. In some examples, the amino donor is affixed on a support, the total molecular weight of the amino donor and the support being at least 150 g / mol.
Owner:VLAAMSE INSTELLING VOOR TECHNOLOGISCH ONDERZOEK NV VITO
Bio-catalytic method for synthesizing optical activity 2R-fluorocarboxylic acid and 2R-hydroxy carboxylic acid
InactiveCN109468347ASimple process routeMild reaction conditionsFermentationFluoroacetic acidCatalytic method
The invention discloses a bio-catalytic method for synthesizing optical activity 2R-fluorocarboxylic acid and 2R-hydroxy carboxylic acid. The method comprises the following steps: with alpha-fluoroacetic acid or alpha-fluoroacetic acid and derivatives thereof as a substrate, and fluoroacetic acid dehalogenase as a biocatalyst, catalyzing the substrate to carry out a defluorinated hydrolysis reaction, and finally producing and separating to obtain chirally pure 2R-fluorocarboxylic acid and 2R-hydroxy carboxylic acid and derivatives thereof. The stereoselectivity is high, the step of catalytically preparing the 2R-fluorocarboxylic acid and 2R-hydroxy carboxylic acid is simple, the preparation is realized in one step, and the problems existing extensively in synthesis of the conventional chiral compounds such as low optical purity, low yield, environmental pollution and the like can be solved. In addition, the method disclosed by the invention has the advantages that the reaction system is enlarged to g-grade experiments, and the product with high yield and ee value can be obtained. Therefore, the method disclosed by the invention is applied to industrial production, and is low in cost, and the energy consumption is low.
Owner:HUNAN NORMAL UNIVERSITY
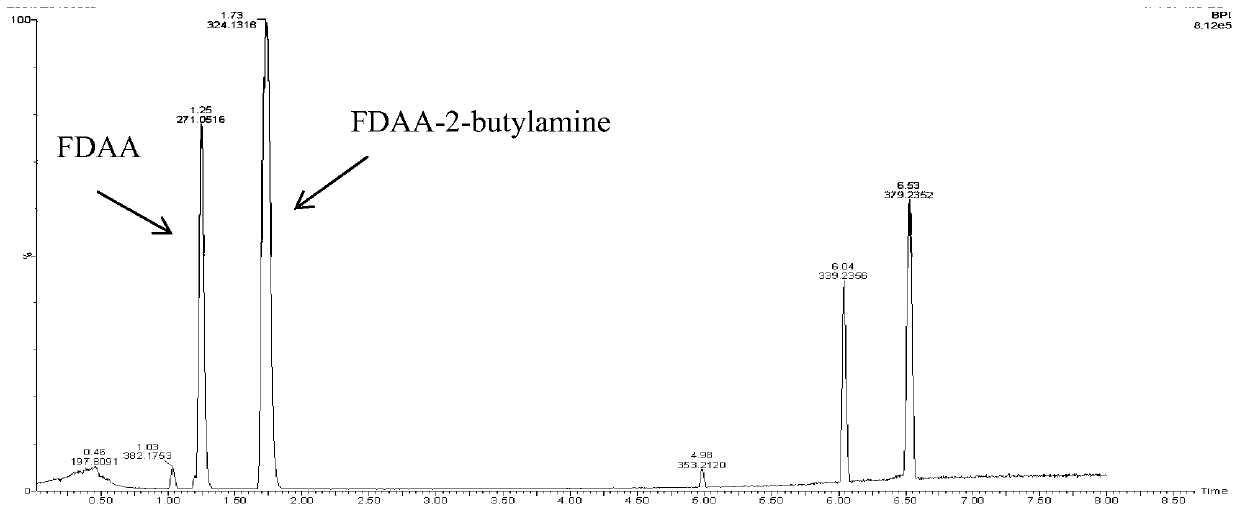
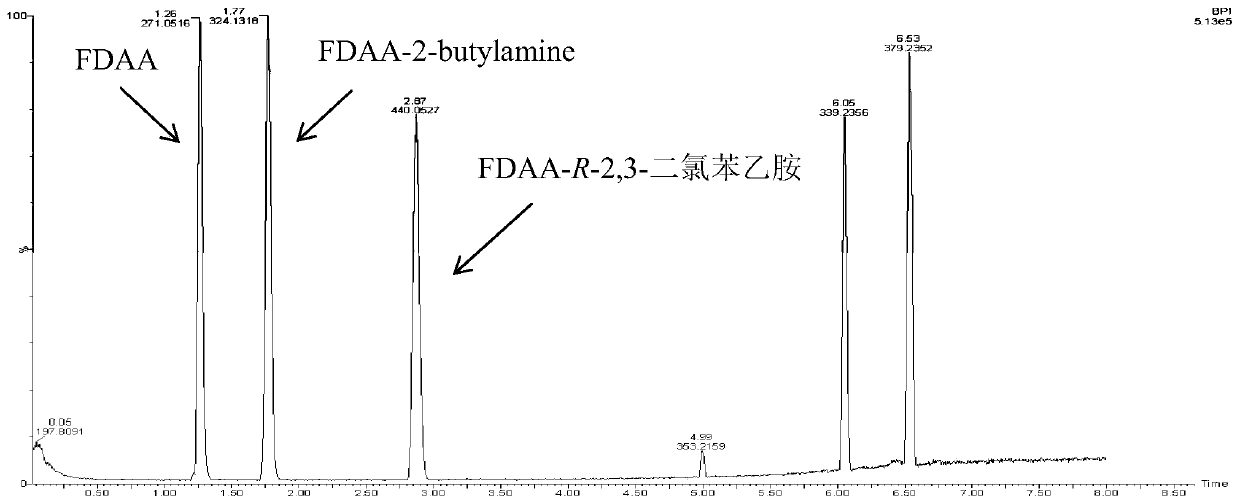
New application of (R)-omega-transaminase mutant
InactiveCN110964705AAddressing Purity IssuesSolve yieldTransferasesFermentationPtru catalystAcetophenone
The invention discloses an application of a (R)-omega-transaminase mutant. The amino acid sequence of the (R)-omega-transaminase mutant is shown as SEQ ID NO.1. 2,3-dichloroacetophenone or 2,5-dichloroacetophenone is taken as a substrate. The (R)-omega-transaminase mutant is taken as a catalyst. Catalytic reaction is carried out in the presence of an amino donor, and (R)-2,3-dichlorophenylethylamine or (R)-2,5-dichlorophenylethylamine is synthesized. According to the method, (R)-omega-transaminase is used as a biocatalyst for catalytic generation of a substrate 2,3-dichloroacetophenone or 2,5-dichloroacetophenone, and the product can be used as a drug intermediate for synthesis of related drugs. The method is high in stereoselectivity, simple in preparation process and convenient to extract. The problems of low optical purity, low yield, environmental pollution and the like widely existing in current chiral compound synthesis are solved.
Owner:ZHEJIANG UNIVERSITY OF SCIENCE AND TECHNOLOGY
Alcohol dehydrogenase mutant and use thereof
ActiveUS20210363500A1Improve thermal stabilityImprove conversion efficiencyOxidoreductasesFermentationChlorobenzeneAlcohol ethyl
The invention discloses an alcohol dehydrogenase mutant and use thereof. The alcohol dehydrogenase mutant of the present invention has high thermal stability and enables high catalytic efficiency and high conversion rate (i.e. space time yield) in the asymmetric reduction of prochiral diaryl ketones to produce chiral diaryl alcohols. Therefore, the alcohol dehydrogenase mutant of the present invention has extremely high prospect of application in the production of chiral diaryl alcohols, such as (S)-(4-chlorophenyl)-(pyridin-2-yl)-methanol, (R)-(4-chlorophenyl)-(pyridin-2-yl)-methanol.
Owner:JIANGNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







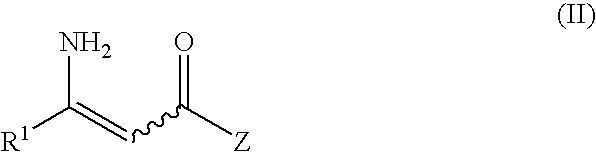



![Process for preparation of (2R)-4-oxo-4-[3- (trifluoromethyl)-5,6-dihydro [1,2,4]-triazolo[4,3-a]pyrazin- 7(8H)-yl]-l-(2,4,5-trifluorophenyl)butan-2-amine & new impurities in preparation thereof Process for preparation of (2R)-4-oxo-4-[3- (trifluoromethyl)-5,6-dihydro [1,2,4]-triazolo[4,3-a]pyrazin- 7(8H)-yl]-l-(2,4,5-trifluorophenyl)butan-2-amine & new impurities in preparation thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4330bf79-53dd-4f2f-9502-c92e47207d80/US20110213149A1-20110901-D00000.png)
![Process for preparation of (2R)-4-oxo-4-[3- (trifluoromethyl)-5,6-dihydro [1,2,4]-triazolo[4,3-a]pyrazin- 7(8H)-yl]-l-(2,4,5-trifluorophenyl)butan-2-amine & new impurities in preparation thereof Process for preparation of (2R)-4-oxo-4-[3- (trifluoromethyl)-5,6-dihydro [1,2,4]-triazolo[4,3-a]pyrazin- 7(8H)-yl]-l-(2,4,5-trifluorophenyl)butan-2-amine & new impurities in preparation thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4330bf79-53dd-4f2f-9502-c92e47207d80/US20110213149A1-20110901-D00001.png)
![Process for preparation of (2R)-4-oxo-4-[3- (trifluoromethyl)-5,6-dihydro [1,2,4]-triazolo[4,3-a]pyrazin- 7(8H)-yl]-l-(2,4,5-trifluorophenyl)butan-2-amine & new impurities in preparation thereof Process for preparation of (2R)-4-oxo-4-[3- (trifluoromethyl)-5,6-dihydro [1,2,4]-triazolo[4,3-a]pyrazin- 7(8H)-yl]-l-(2,4,5-trifluorophenyl)butan-2-amine & new impurities in preparation thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4330bf79-53dd-4f2f-9502-c92e47207d80/US20110213149A1-20110901-D00002.png)
![Process for preparation of (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro [1,2,4]-triazolo[4,3-a]pyrazin-7(8H)-yl]-l-(2,4,5-trifluorophenyl)butan-2-amine and new impurities in preparation thereof Process for preparation of (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro [1,2,4]-triazolo[4,3-a]pyrazin-7(8H)-yl]-l-(2,4,5-trifluorophenyl)butan-2-amine and new impurities in preparation thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/87dd183f-d910-4682-a9ec-c2ffba9ed4f7/US08476437-20130702-D00001.png)
![Process for preparation of (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro [1,2,4]-triazolo[4,3-a]pyrazin-7(8H)-yl]-l-(2,4,5-trifluorophenyl)butan-2-amine and new impurities in preparation thereof Process for preparation of (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro [1,2,4]-triazolo[4,3-a]pyrazin-7(8H)-yl]-l-(2,4,5-trifluorophenyl)butan-2-amine and new impurities in preparation thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/87dd183f-d910-4682-a9ec-c2ffba9ed4f7/US08476437-20130702-D00002.png)
![Process for preparation of (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro [1,2,4]-triazolo[4,3-a]pyrazin-7(8H)-yl]-l-(2,4,5-trifluorophenyl)butan-2-amine and new impurities in preparation thereof Process for preparation of (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro [1,2,4]-triazolo[4,3-a]pyrazin-7(8H)-yl]-l-(2,4,5-trifluorophenyl)butan-2-amine and new impurities in preparation thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/87dd183f-d910-4682-a9ec-c2ffba9ed4f7/US08476437-20130702-D00003.png)