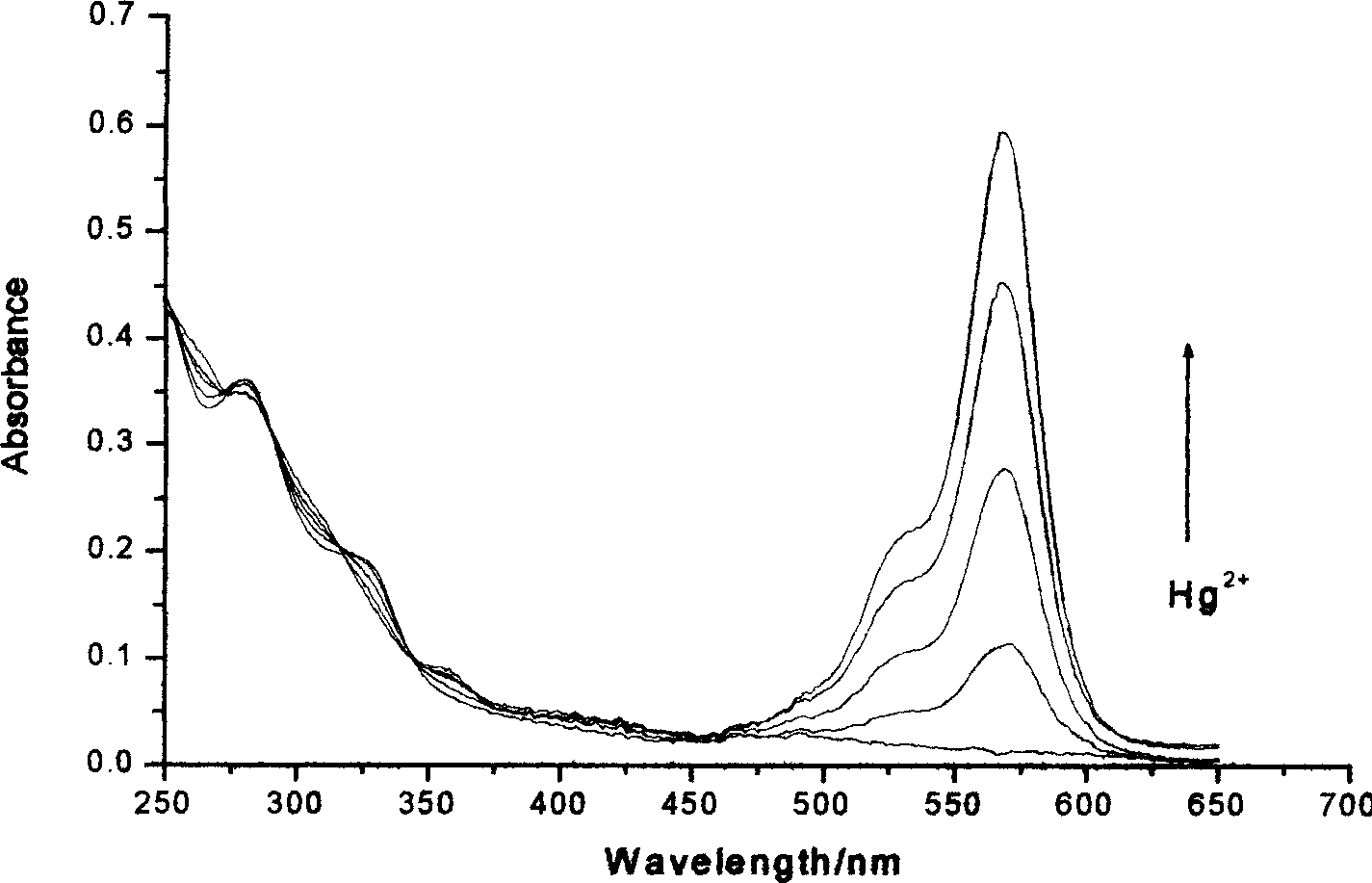
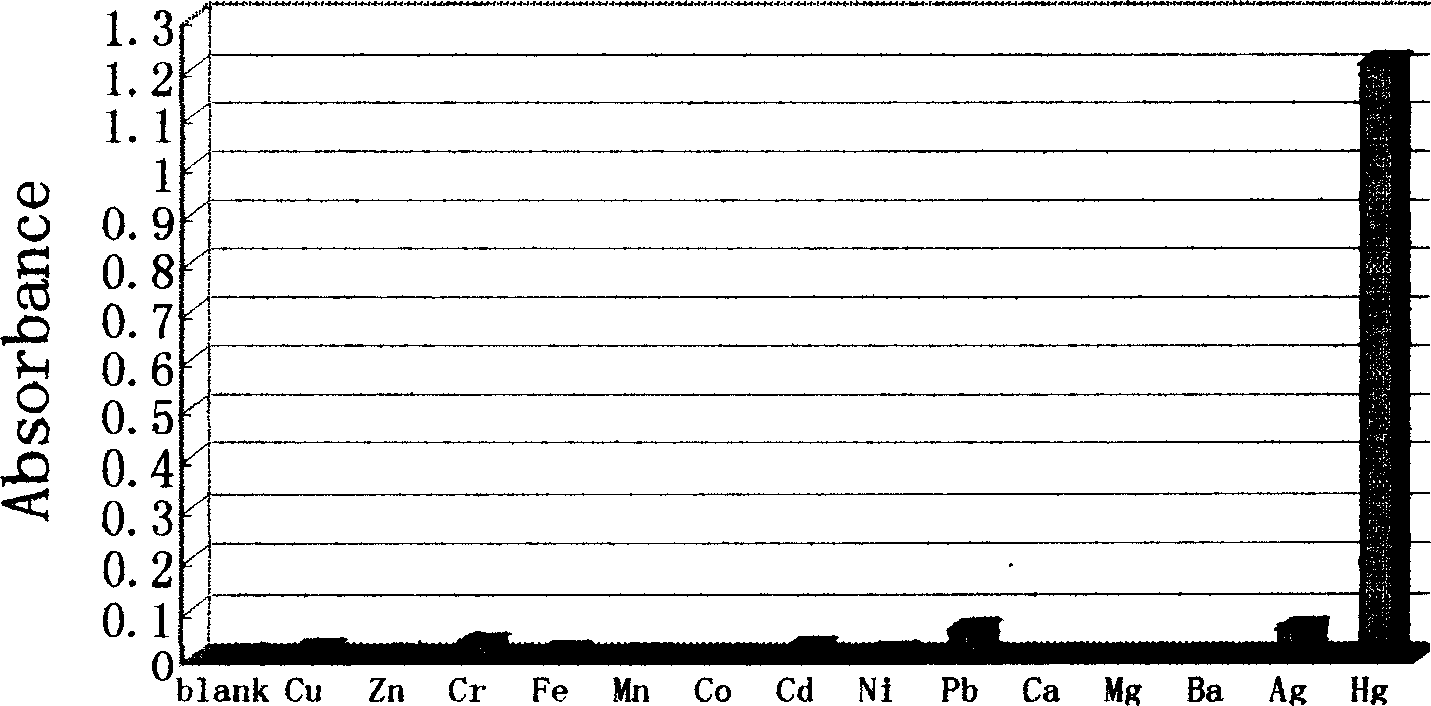
Reagent for detecting mercury ion in water and its preparation method
A technology of chemical reagents and mercury ions, applied in the field of chemical reagents, can solve the problems such as the inability of optical chemical sensors of mercury ions to be practical, and achieve the effects of expanding the scope of use, rapid detection, and high selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of rhodamine B lactam-based phenylthiourea. The structure of rhodamine B lactam phenylthiourea is as follows:
[0031]
[0032] First, synthesize rhodamine B hydrazide, add 1 part of rhodamine B to a round bottom flask, dissolve it with 40 parts of methanol, and then add 15 parts of 80% hydrazine hydrate. Heat to reflux until the purple color of rhodamine B disappears; filter and wash the obtained solid with water to obtain white rhodamine hydrazide, yield: 75%.
[0033] 1 HNMR (CDCl 3 ): δ7.93(m, 1H, ArH), 7.45(m, 2H, ArH), 7.11(m, 1H, ArH), 6.46(d, 2H, xanthene-H), 6.42(d, 2H, xanthene- H), 6.29(dd, 2H, xanthene-H), 3.61(s, 2H, NH 2 ), 3.34(q, 8H, NCH 2 CH 3 ), 1.17(t, 12H, NCH 2 CH 3 ).
[0034] ESI mass spectrometry, C 28 h 32 N 4 o 2 , m / z: 457.3 ([M+H] + ).
[0035] Synthesis of rhodamine B-lactamyl phenylthiourea: In a round bottom flask, add 1 part of rhodamine B hydrazide compound to 40 parts of CH 3 CN dissolves; then ...
Embodiment 2
[0039] Example 2: Preparation of rhodamine B lactam p-methoxyphenylthiourea. The structure of rhodamine B lactam p-methoxyphenylthiourea is as follows:
[0040]
[0041] First, synthesize rhodamine B hydrazide, add 1 part of rhodamine B to a round bottom flask, dissolve it with 40 parts of methanol, and then add 15 parts of 80% hydrazine hydrate. Heat to reflux until the purple color of rhodamine B disappears; filter and wash the obtained solid with water to obtain white rhodamine hydrazide, yield: 65%.
[0042] Synthesis of rhodamine B lactam-based p-methoxyphenylthiourea: In a round-bottomed flask, add 1 part of rhodamine B hydrazide compound to 45 parts of CH 3 CN dissolves; then add 3 parts of p-methoxyphenyl isothiocyanate. After the mixture was heated to reflux for 5 hours, the CH 3 CN, separated by column chromatography, the target product was obtained, yield: 25%.
[0043] ESI mass spectrometry, C 36 h 39 N 5 o 3 S, m / z: 621.9 (M + ).
Embodiment 3
[0044] Example 3: Preparation of rhodamine B lactam p-nitrophenylthiourea. The structure of rhodamine B lactam p-nitrophenylthiourea is as follows:
[0045]
[0046] First, rhodamine B hydrazide was synthesized: 1 part of rhodamine B was added to a round bottom flask, dissolved in 40 parts of ethanol, and then 15 parts of 80% hydrazine hydrate was added. Heat to reflux until the purple color of rhodamine B disappears; filter and wash the obtained solid with water to obtain white rhodamine hydrazide, yield: 65%.
[0047] Synthesis of rhodamine B lactam-based p-nitrophenylthiourea: In a round-bottomed flask, add 1 part of rhodamine B hydrazide compound to 50 parts of CH 3 CN dissolves; then add 3 parts of p-nitrophenyl isothiocyanate. After the mixture was heated to reflux for 6 hours, CH was removed 3 CN, separated by column chromatography to obtain the target product, yield: 30%.
[0048] ESI mass spectrometry, C 35 h 36 N 6 o 4 S, m / z: 636.8 (M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com