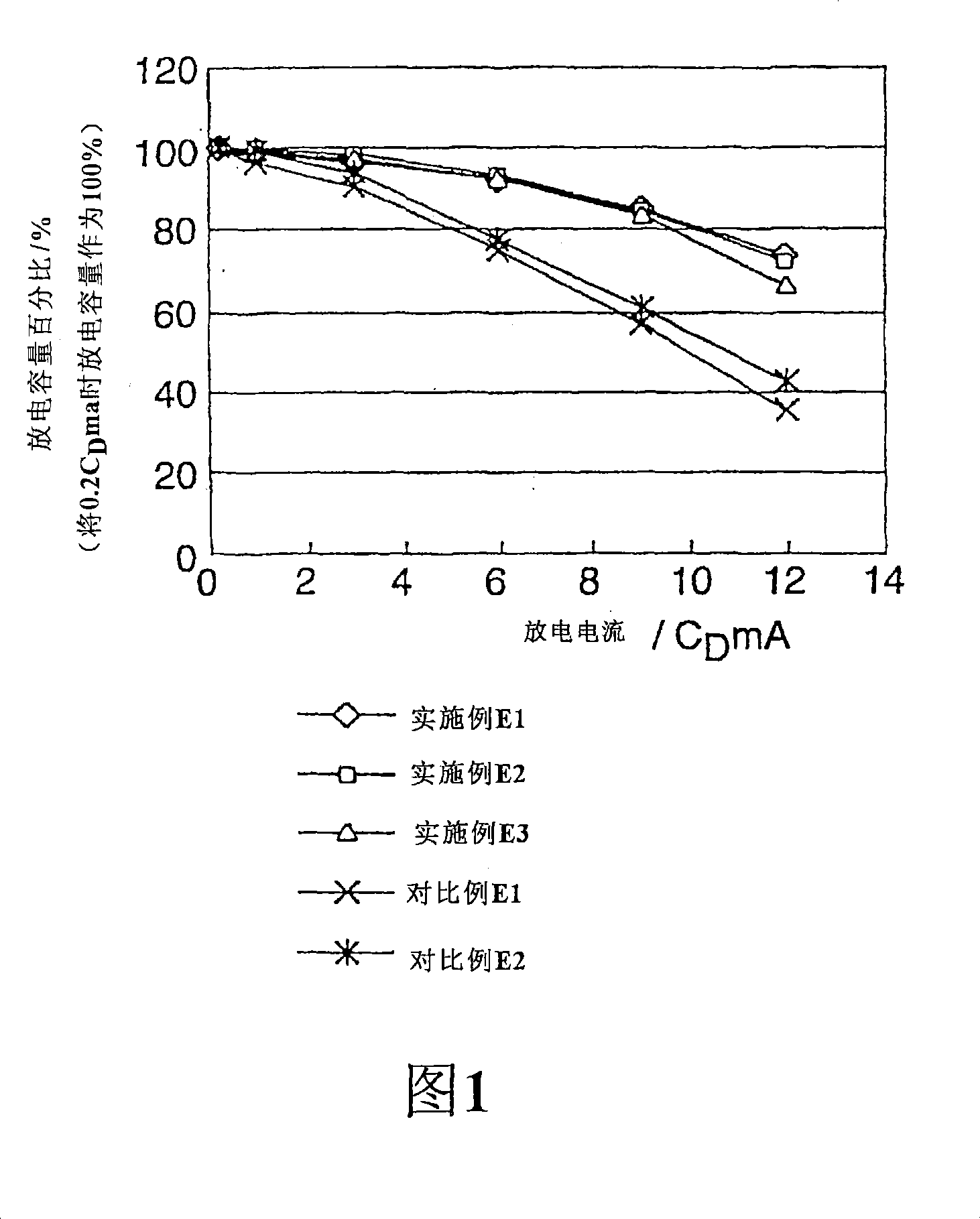
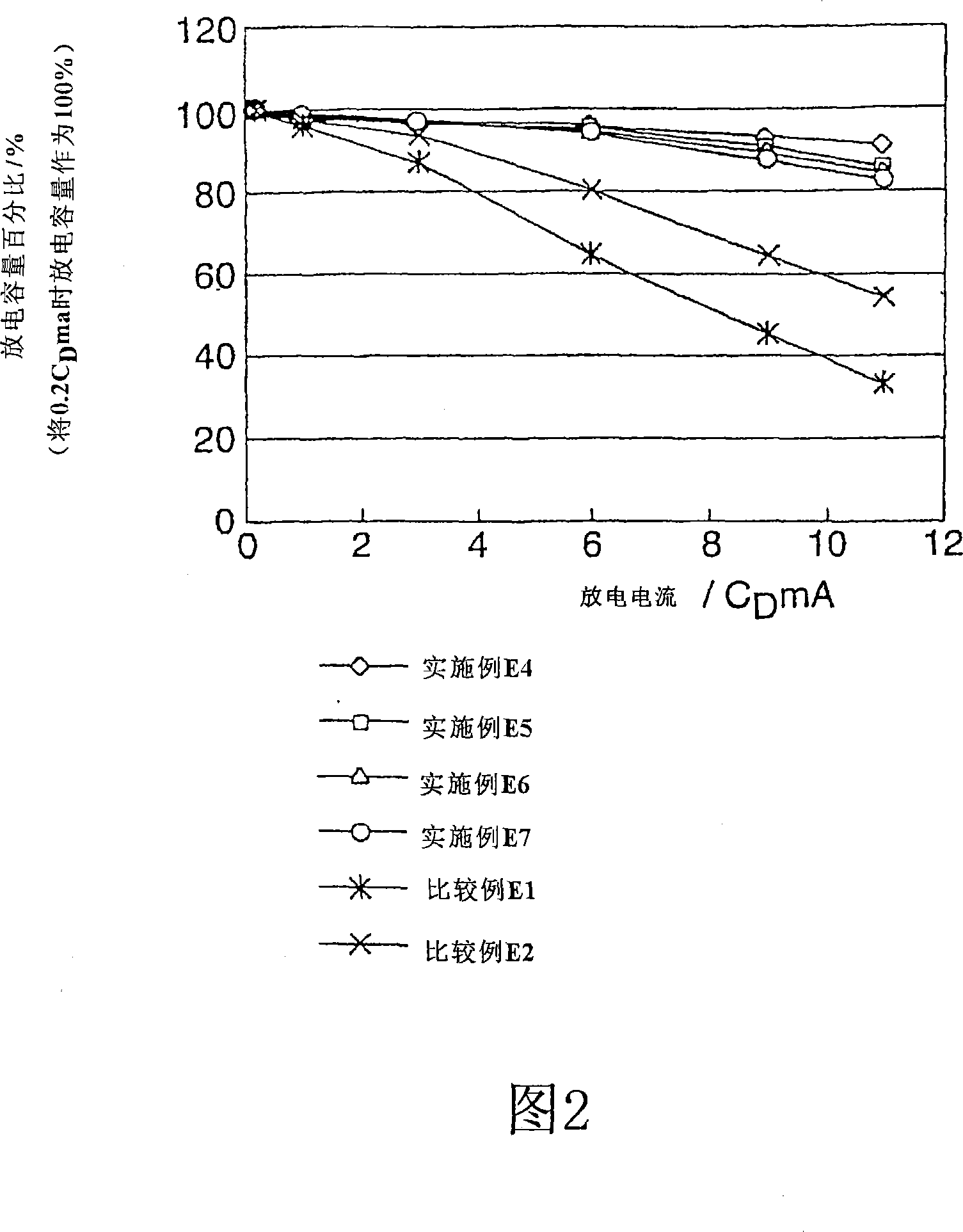
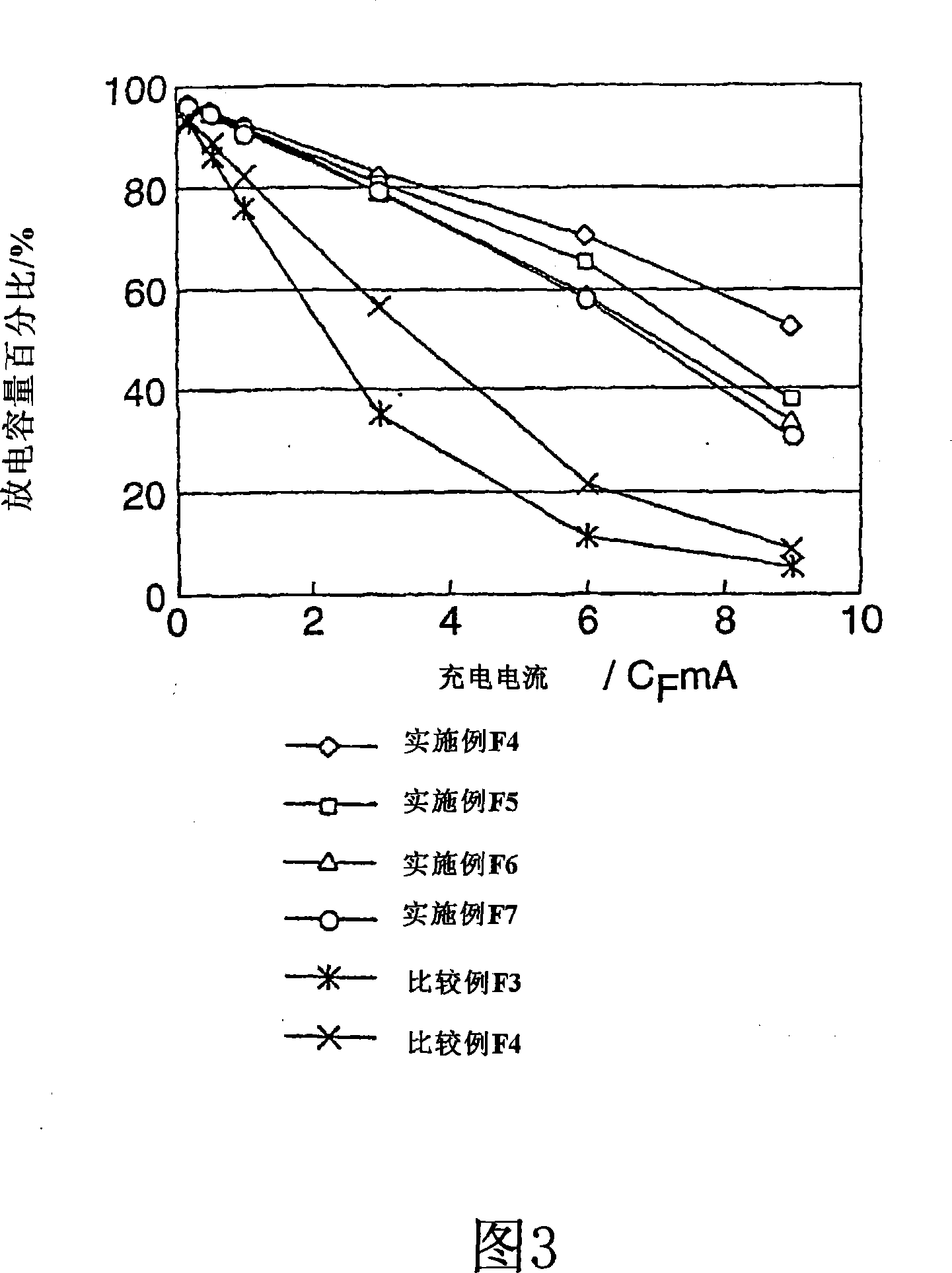
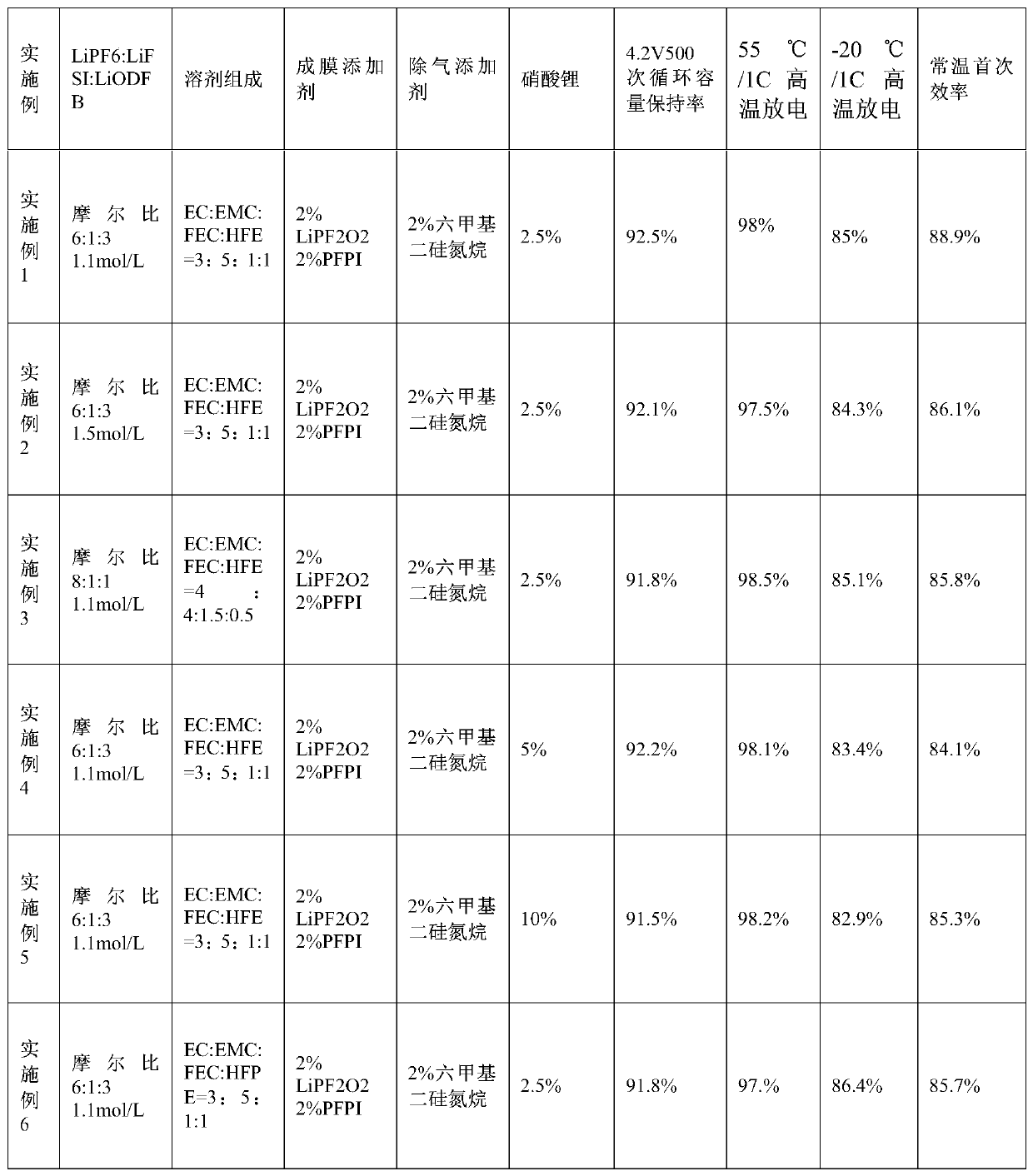
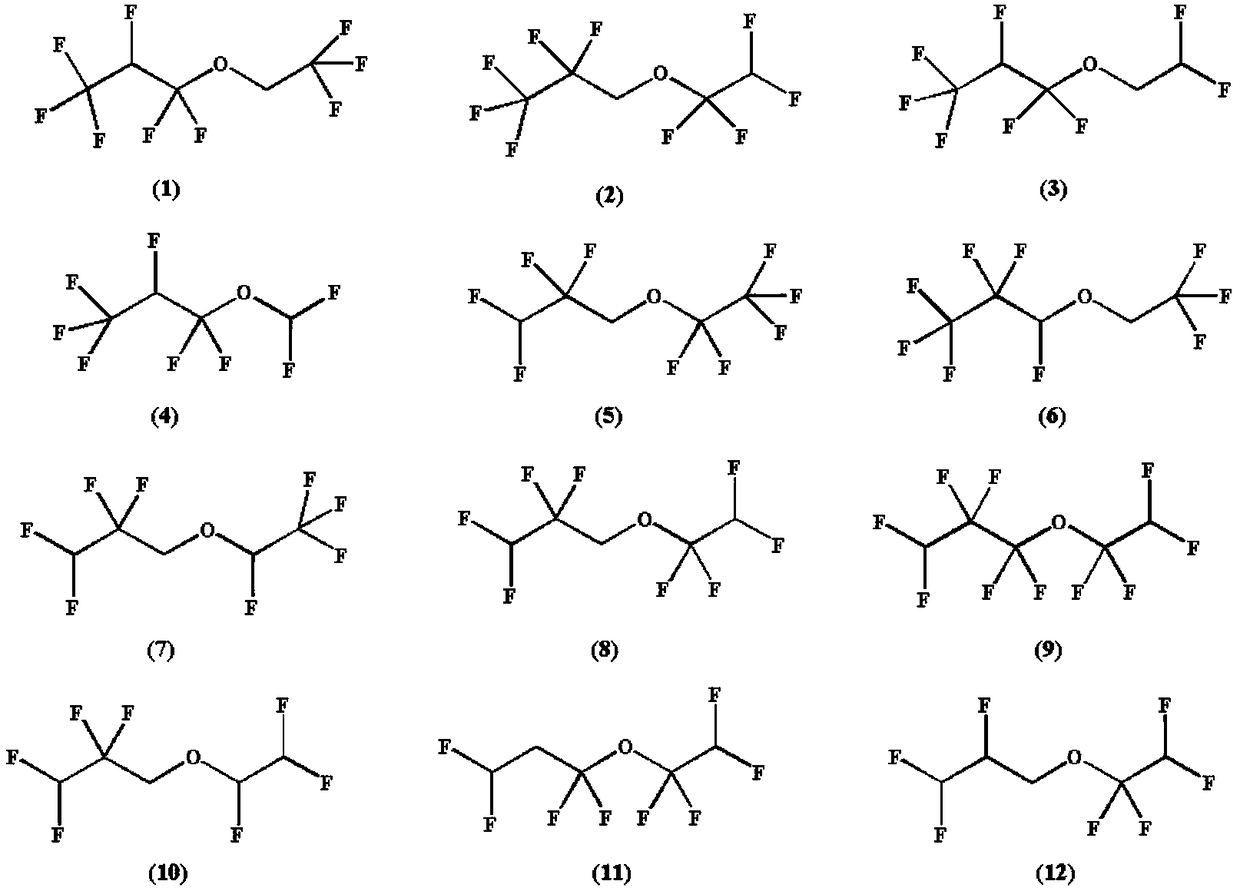
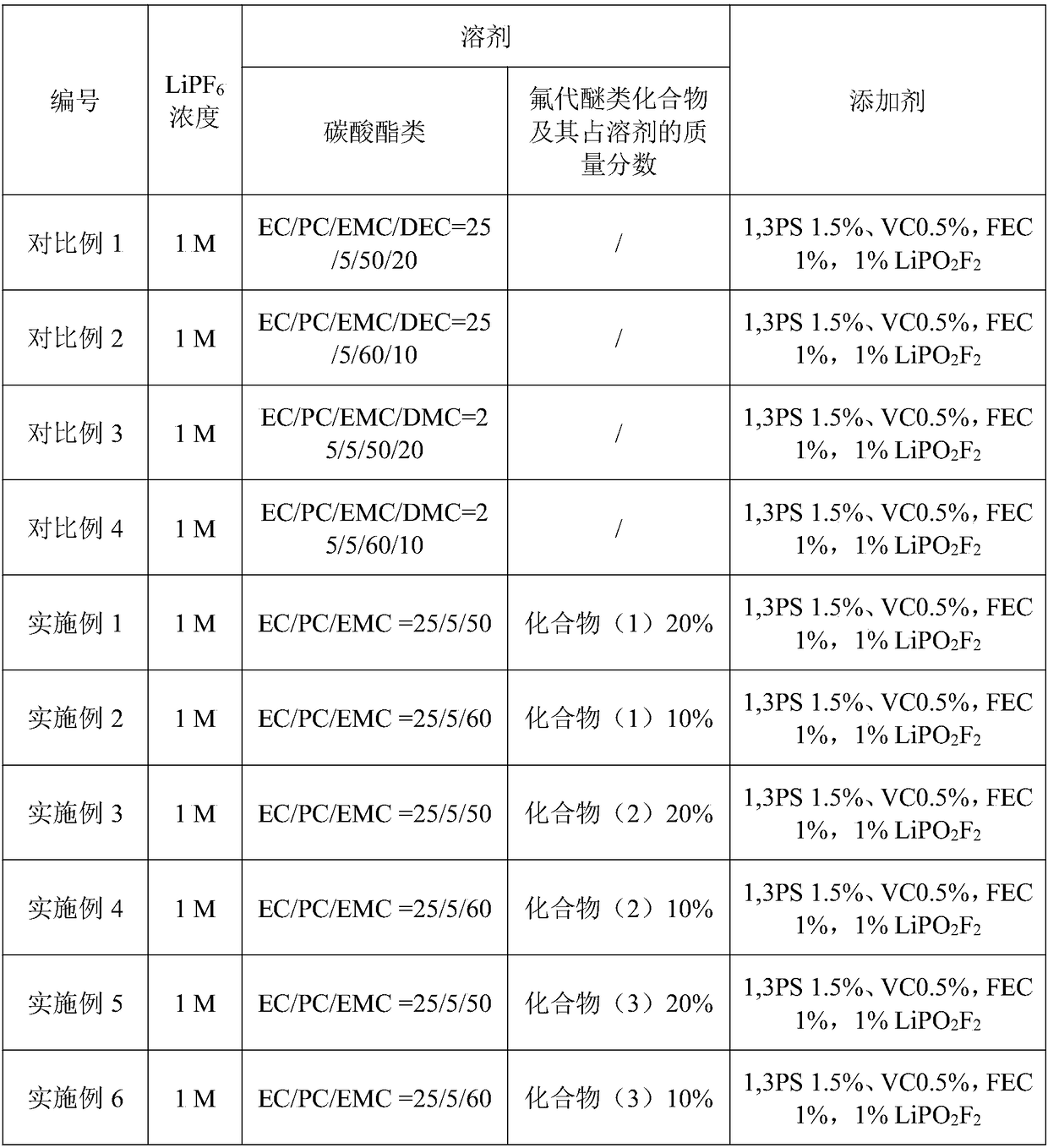
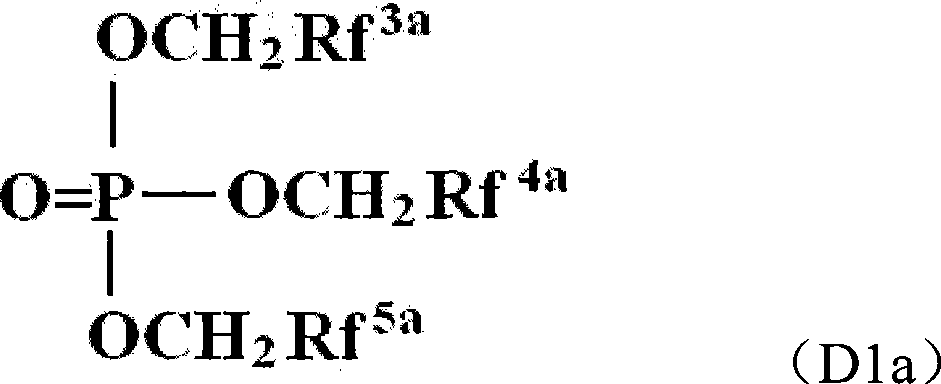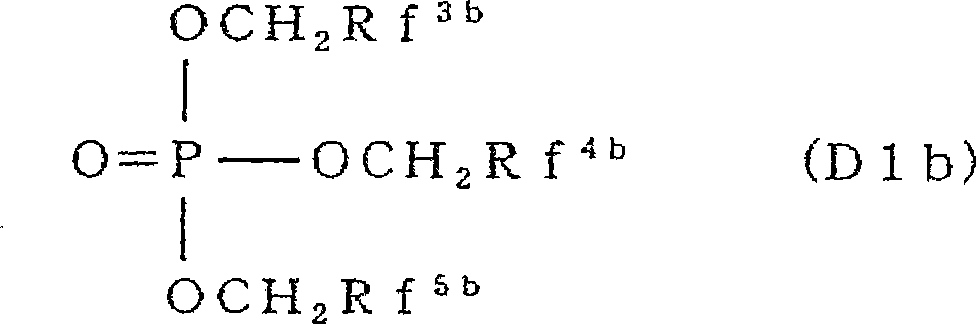Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
128 results about "Fluorinated ether" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
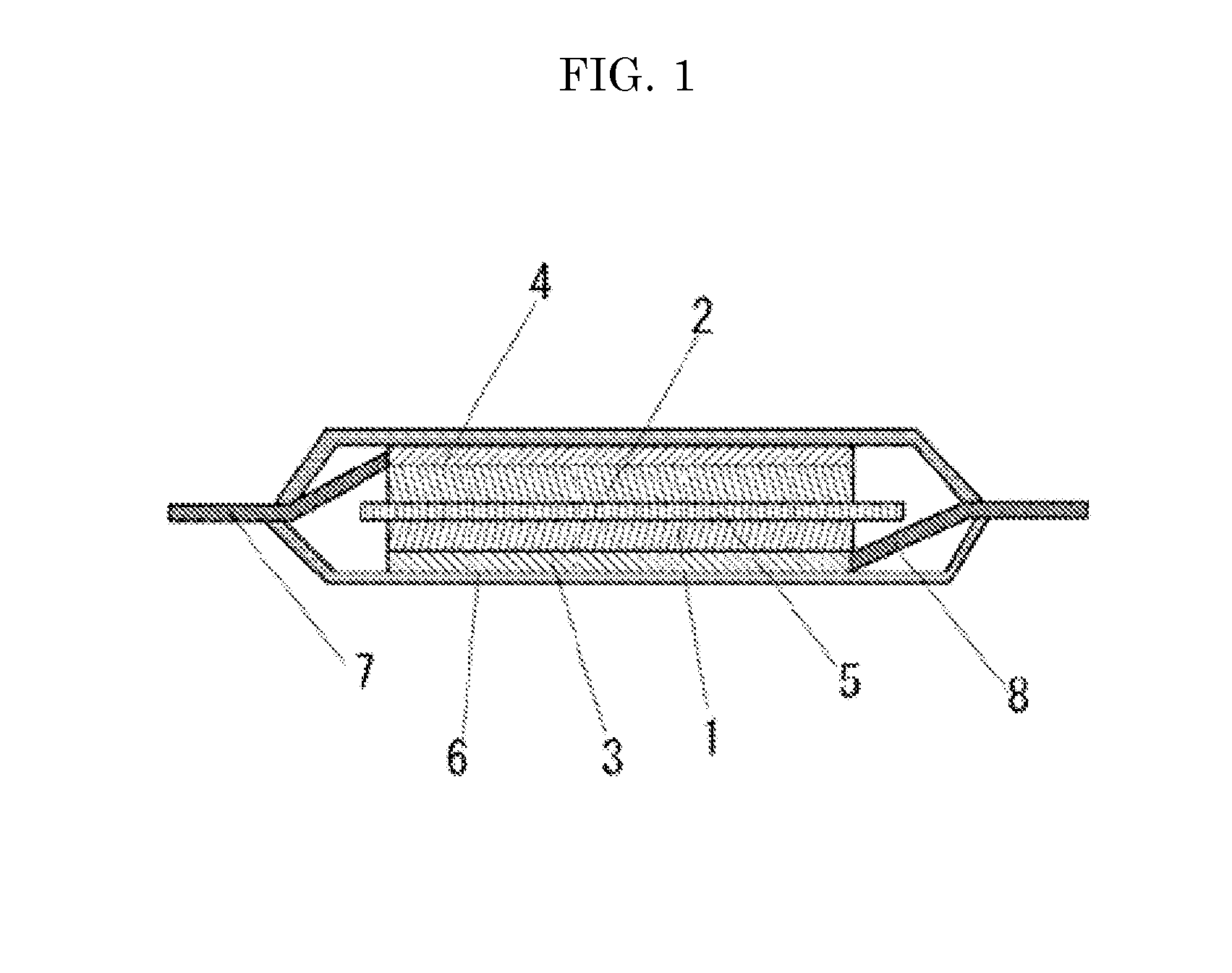
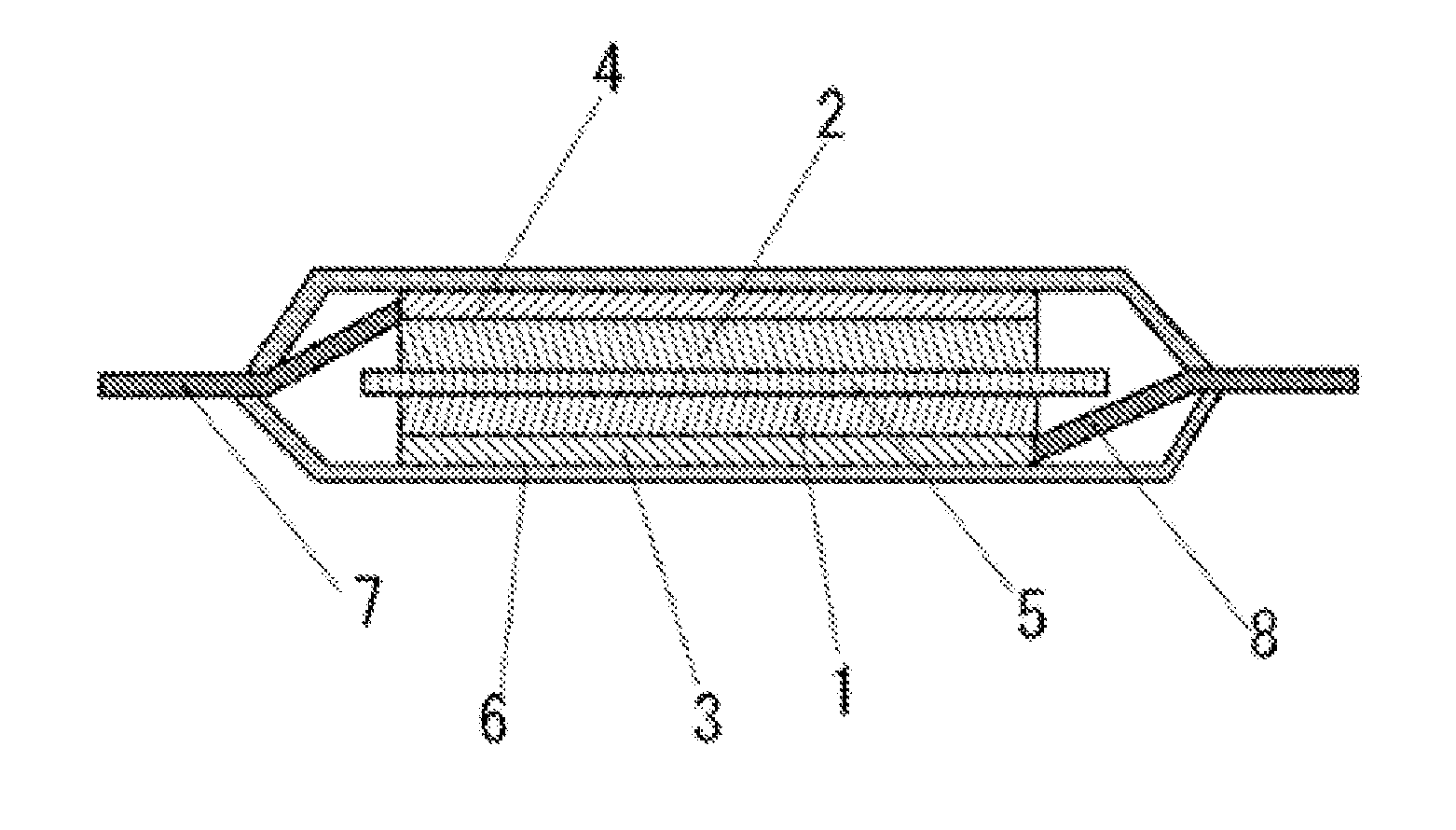
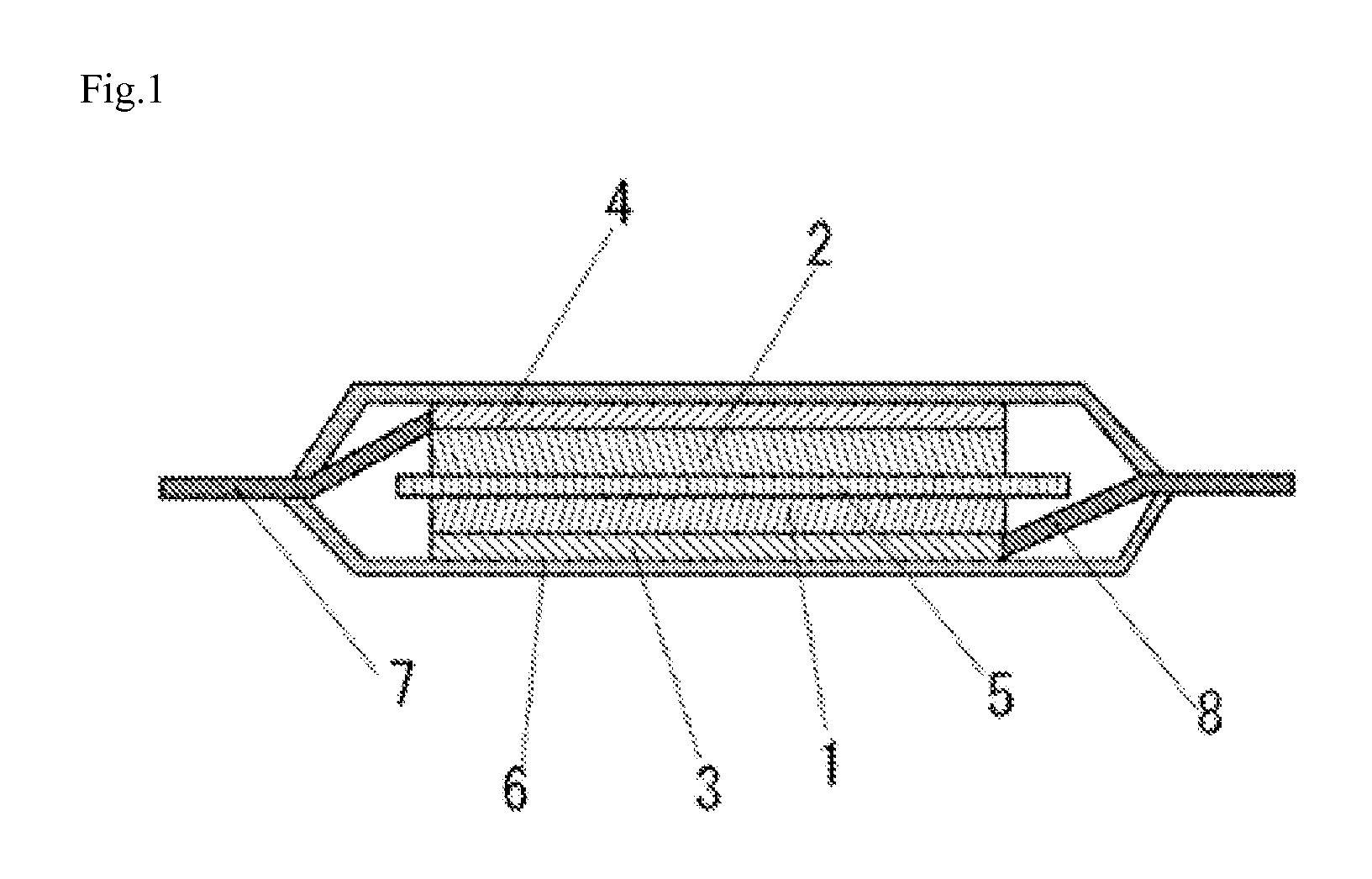
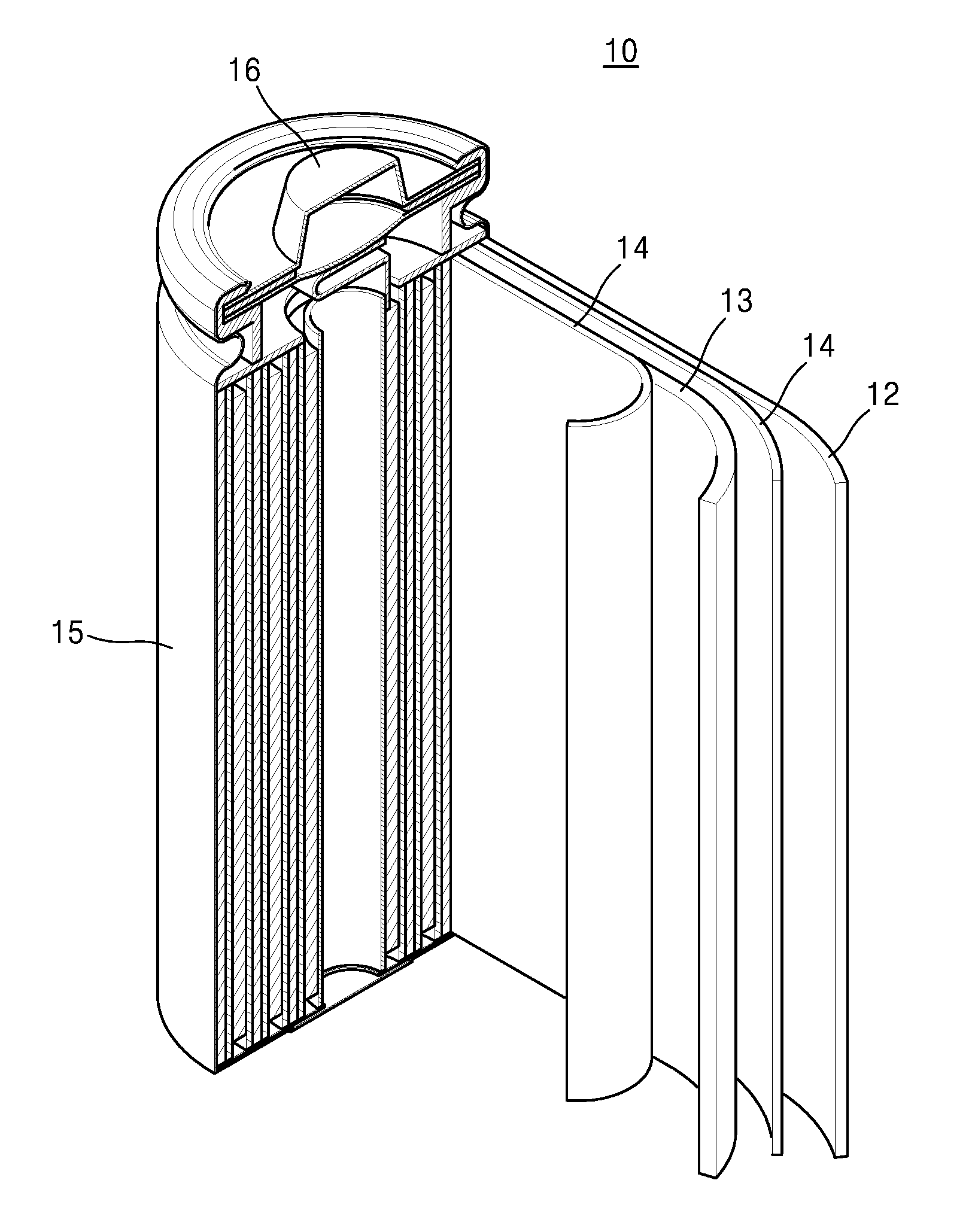
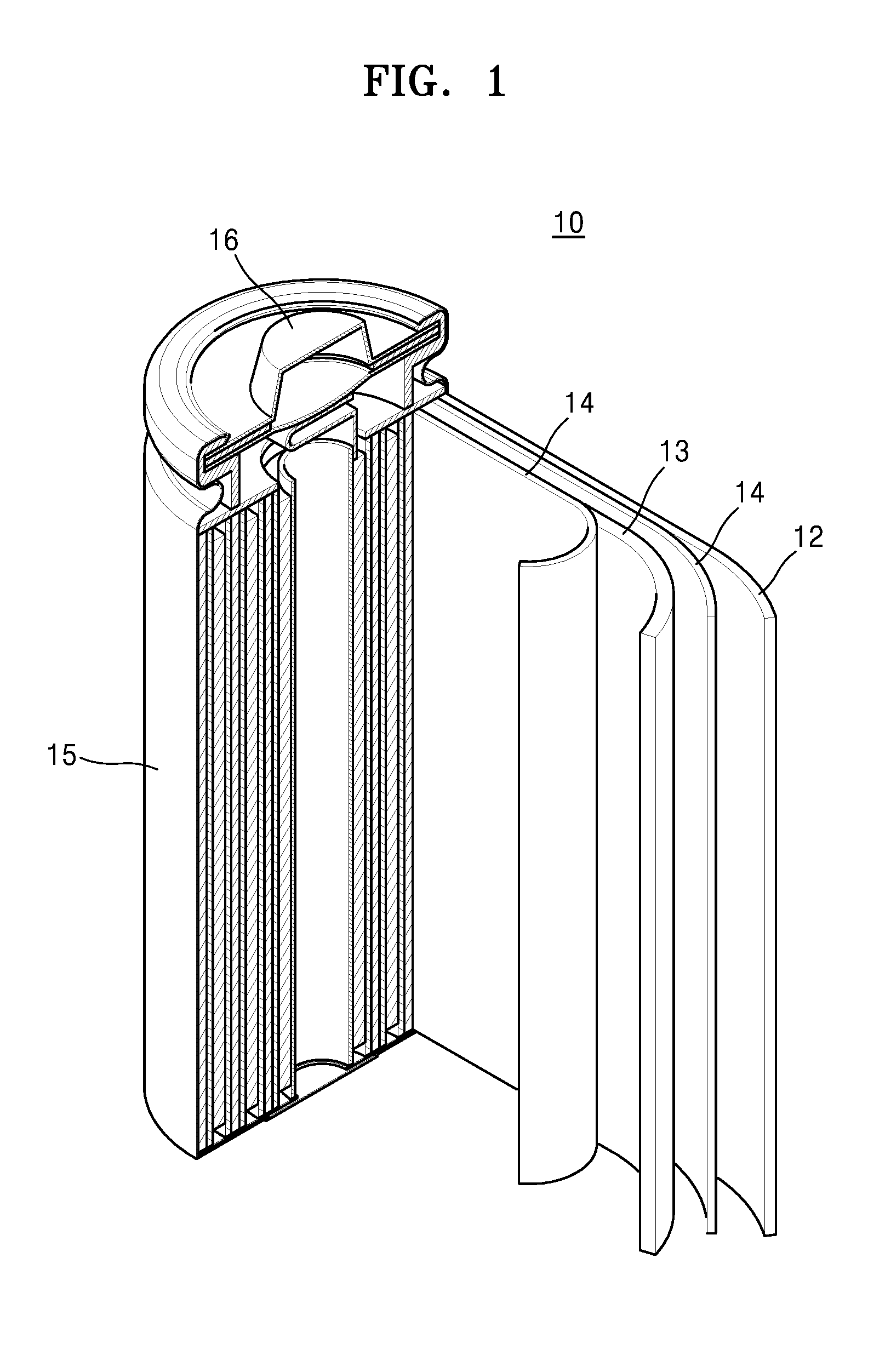
Electrolyte solution for high-capacity lithium-ion battery, preparation method and lithium-ion battery
InactiveCN104900916AAvoid direct contactInhibit cathode decompositionFinal product manufactureElectrolyte accumulators manufactureElectrolytic agentElectrical battery
The invention discloses an electrolyte solution for a high-capacity lithium-ion battery. The electrolyte solution includes non-aqueous solvent, lithium hexafluorophosphate, negative electrode film forming additive, positive electrode surface activity inhibiting additive and transition metal ion complexant; the negative electrode film forming additive includes organic ester negative electrode film forming additive of 1 to 10wt% of the total electrolyte solution and inorganic lithium salt negative electrode film forming additive of 0.5 to 2wt% of the total electrolyte solution; the positive electrode surface activity inhibiting additive includes fluorinated ether additive of 1 to 5wt% of the total electrolyte solution and nitrile additive of 0.1 to 5wt% of the total electrolyte solution; the transition metal ion complexant is of 0.1 to 1.0wt% of the total electrolyte solution. The electrolyte solution is adaptive to the high-capacity lithium-ion battery and is capable of optimizing the circulating performance and high-temperature storage performance of the lithium-ion battery. The invention further provides a preparation method of the electrolyte solution and the high-capacity lithium-ion battery adopting the electrolyte solution.
Owner:GUANGZHOU TINCI MATERIALS TECH
Lithium sulfur battery
A lithium sulfur battery comprising an electrolyte solvent which comprises at least one fluorosubstituted compound is described. Preferred fluorosubstituted compounds which are predominantly solvents are notably selected from the group consisting of fluorosubstituted carboxylic acid esters, fluorosubstituted carboxylic acid amides, fluorosubstituted fluorinated ethers, fluorosubstituted carbamates, fluorosubstituted cyclic carbonates, fluorosubstituted acyclic carbonates, fluorosubstituted ethers, perfluoroalkyl phosphoranes, fluorosubstituted phosphites, fluorosubstituted phosphates, fluorosubstituted phosphonates, and fluorosubstituted heterocycles. Monofluoroethylene carbonate, cis-difluoroethylene carbonate, trans-difluoroethylene carbonate, 4,4-difluoroethylene carbonate, trifluoroethylene carbonate, tetrafluoroethylene carbonate, 4-fluoro-4-methyl-1,3-dioxolane-2-one, 4-fluoro-4-ethyl-1,3-dioxolane-2-one, 2,2,2-trifluoroethyl-methyl carbonate, 2,2,2-trifluoroethyl-fluoromethyl carbonate are preferred. The solvent may further comprise a non-fluorinated solvent, e.g., ethylene carbonate, a dialkyl carbonate, or propylene carbonate. Use of such fluorinated compound as additive for such batteries and specific electrolyte solutions.
Owner:SOLVAY FLUOR GMBH DE
Fluorinated ketone and fluorinated ethers as working fluids for thermal energy conversion
Polyfluorinated ethers and polyfluorinated ketones and mixtures thereof, preferably polyfluorinated ethers such as methyl(trifluoroethyl)ether (CH3OCH2CF3), methyl(heptafluoropropyl)ether (CH3OCF2CHFCF3), di(trifluoroethyl)ether (CF3CH2OCH2CF3), methyl(hexafluoropropyl)ether (CH3OCF2CF2CHF2), methyl(pentafluoropropyl)ether (CH3OCH2CF2CF3), methyl (perfluorobutyl)ether (C4F9OCH3), ethyl(perfluorobutyl)ether (C4F9OC2H5), and polyfluorinated ketones such as methyl(perfluoromethyl)ketone (CF3COCH3), perfluoromethyl(trifluoroethyl)ketone (CF3CH2COCF3), methyl(perfluooroethyl)ketone (C2F5COCH3), methyl(perfluoropropyl)ketone (F3CF2CF2COCH3), perfluoroethyl(perfluoropropyl)ketone (CF3CF2CF2COC2F5), methyl(octafluorobutyl)ketone (C2F5CFHCF2COCH3), di(perfluoropropyl)ketone (CF3CF2CF2COCF2CF2CF3), and mixtures thereof meet the requirement for not adversely affect atmospheric chemistry and would be a negligible contributor to ozone depletion and to green-house global warming in comparison to the fully halogenated hydrocarbons and are suitable for use as working fluids for u se i n thermal energy conversion systems such as an organic Rankine cycle system.
Owner:HONEYWELL INT INC
Lithium secondary cell
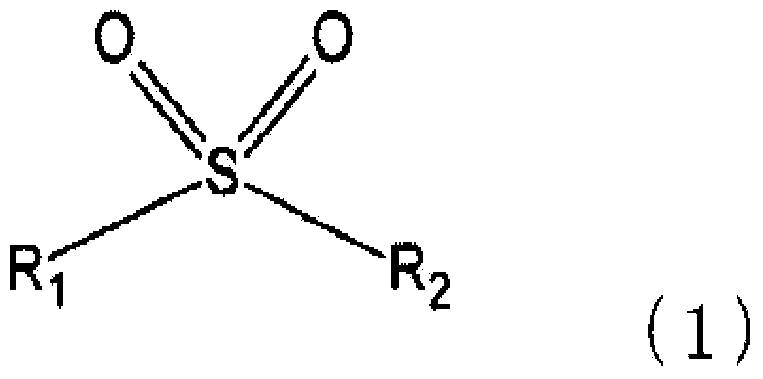
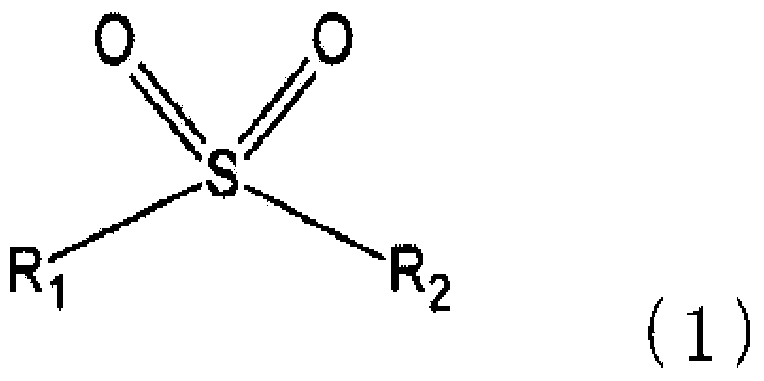
The present invention pertains to a secondary cell having a positive electrode capable of occluding and releasing lithium, and a liquid electrolyte containing a non-aqueous electrolytic solvent, the secondary cell being characterized in that: the positive electrode has a positive electrode active material operating at 4.5 V or higher relative to lithium; and the non-aqueous electrolytic solvent contains a sulfone compound represented by a predetermined formula and a fluorinated ether compound represented by a predetermined formula.
Owner:NEC CORP
Electrolyte solution for lithium-sulfur battery
ActiveCN107681197AAvoid negative effectsIncrease capacityLi-accumulatorsSecondary cells servicing/maintenanceSolubilityNon solvent
The invention discloses an electrolyte solution for a lithium-sulfur battery. The electrolyte solution comprises an electrolyte lithium salt, an ionic liquid, a non-solvent liquid and an additive, wherein the viscosity of the non-solvent liquid is lower than that of the used ionic liquid; the solubility of the lithium salt and polysulfide lithium formed in charge and discharge processes in the non-solvent liquid is much lower than the corresponding solubility in the ionic liquid; the additive is another lithium salt with a film-forming function, which is different from the electrolyte lithiumsalt; and fluorinated ether can be selected as the non-solvent liquid. The electrolyte solution mainly aims at developing a complementary synergistic effect of the ionic liquid and the non-solvent liquid; and through assist of the film-forming lithium salt, on one hand, the viscosity of an ionic liquid-based electrolyte solution is reduced and the ionic conductivity of the electrolyte solution isimproved, and on the other hand, the capacity of the electrolyte solution for inhibiting dissolving and shuttling of the polysulfide lithium is strengthened. By adopting the electrolyte solution disclosed by the invention, various negative effects, caused by the polysulfide lithium, to the lithium-sulfur battery are greatly avoided, and the properties, such as the capacity, the cycle performance and the rate capability of the battery are improved as a whole.
Owner:XIAN UNIV OF SCI & TECH
Fluorinated ether as electrolyte co-solvent for lithium metal based anode
ActiveUS20180062206A1Improve cycling efficiency and durabilityIncrease capacityFinal product manufactureElectrode carriers/collectorsLithium metalEther
The performance and durability of an electrochemical cell using a lithium metal based anode and a compatible lithium-accepting cathode are improved by the use of a suitable lithium electrolyte salt and a new liquid co-solvent mixture for the electrolyte. The co-solvent mixture comprises a non-aqueous ionic liquid, conductive of lithium ions, and a liquid fluorinated organic ether.
Owner:GM GLOBAL TECH OPERATIONS LLC
Non-aqueous electrolytic solutions and electrochemical cells comprising the same
ActiveUS20140038059A1Imparting non-flammabilityReduce concentrationFinal product manufactureElectrode carriers/collectorsLithiumAlkylphosphate
Owner:SHENZHEN CAPCHEM TECH
Electrolyte solution
Owner:DAIKIN IND LTD
Non-aqueous electrolyte solution for secondary batteries, and lithium ion secondary battery
InactiveUS20150171476A1Reduced responseAvoid stabilityCell electrodesOrganic electrolyte cellsCarboxylic acidSolvent
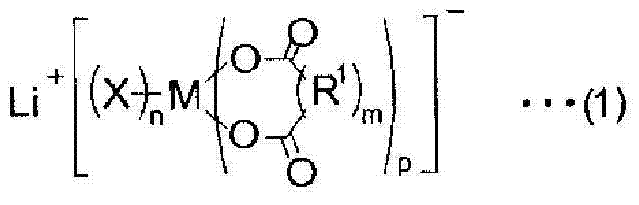
To provide a non-aqueous electrolyte solution for secondary batteries, which has a low reactivity with a positive electrode and a negative electrode and which is excellent in stability to prevent thermal runaway of a secondary battery and excellent also in battery properties such as cycle properties and rate properties, and a lithium ion secondary battery employing such a non-aqueous electrolyte solution.A non-aqueous electrolyte solution for secondary batteries, comprising a lithium salt composed of a specific complex such as lithium difluoro(oxalato)borate, a fluorinated solvent (A) containing at least one member selected from the group consisting of a fluorinated ether compound, a fluorinated chain carboxylic acid ester compound and a fluorinated chain carbonate compound, and a cyclic carboxylic acid ester compound (B); and a lithium ion secondary battery employing such a non-aqueous electrolyte solution.
Owner:ASAHI GLASS CO LTD
Non-aqueous electrolyte and non-aqueous electrolyte secondary battery
ActiveUS20100124708A1Improve wettabilityReducing gas generationOrganic chemistryElectrolytic capacitorsHigh temperature storagePolyolefin
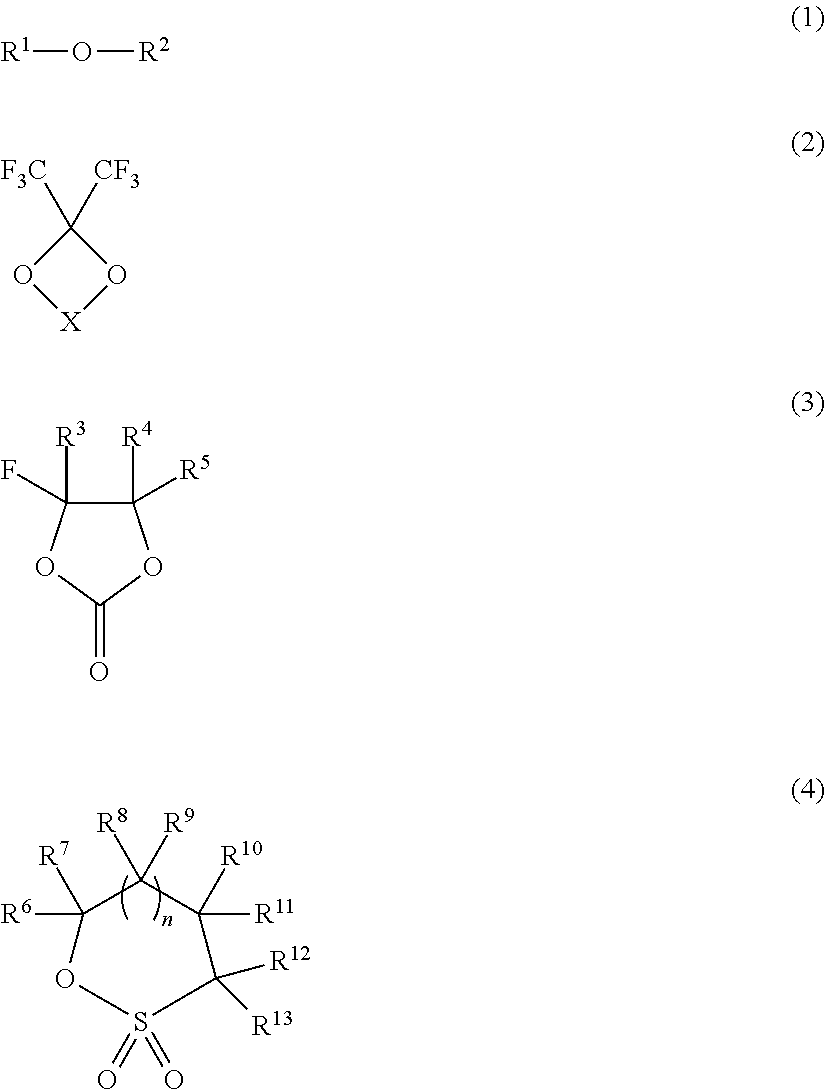
A non-aqueous electrolyte is provided that includes a non-aqueous solvent and an electrolyte salt, wherein the non-aqueous solvent contains a fluorinated ether (1) represented by the following Formula: HCF2CF2CF2CH2—O—CF2CF2H (1). This non-aqueous electrolyte has good wettability to a polyolefin separator, can provide a battery with excellent load characteristics for a long period, does not easily decompose in the battery under high-temperature storage, and causes little gas generation due to decomposition. Furthermore, a non-aqueous electrolyte secondary battery is provided that includes a positive electrode, a negative electrode, a separator, and the above-described non-aqueous electrolyte.
Owner:PANASONIC CORP
Lithium secondary battery
ActiveUS20150140443A1Capacity deteriorationSuppresses gas evolutionFinal product manufactureElectrode carriers/collectorsLithiumSolvent
The present invention relates to a secondary battery cg a positive electrode capable of absorbing and releasing lithium, and a electrolyte solution containing a non-aqueous electrolytic solvent, wherein the positive electrode has a positive electrode active material which operates at 4.5 V or more relative to lithium, and wherein the non-aqueous electrolytic solvent contains a sulfone compound represented by a predetermined formula and a fluorinated ether compound represented by a predetermined formula.
Owner:NEC CORP
Electrochemical device
InactiveCN101490893AImprove featuresExcellent battery characteristicsSecondary cellsSolubilityHeat resistance
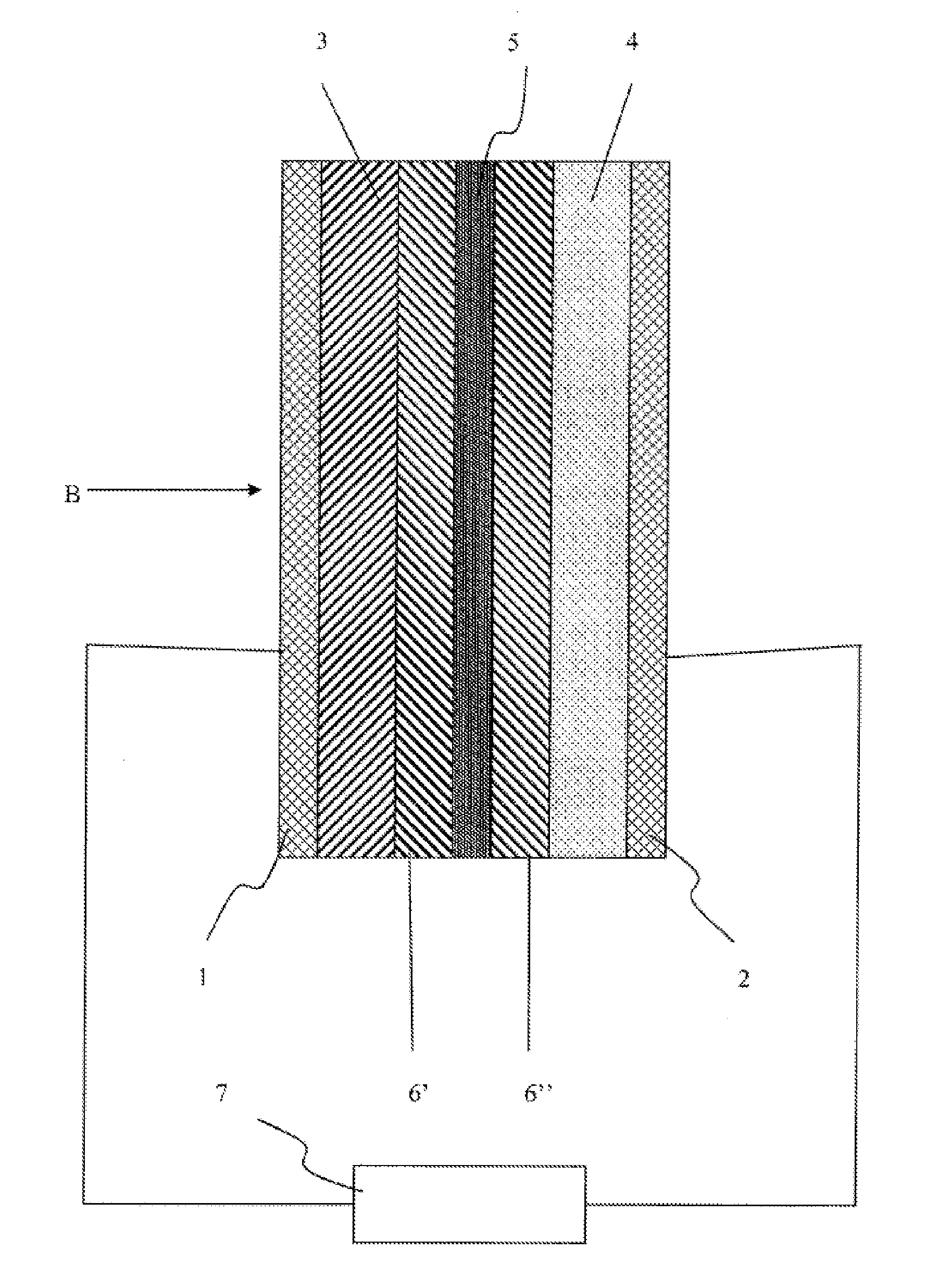
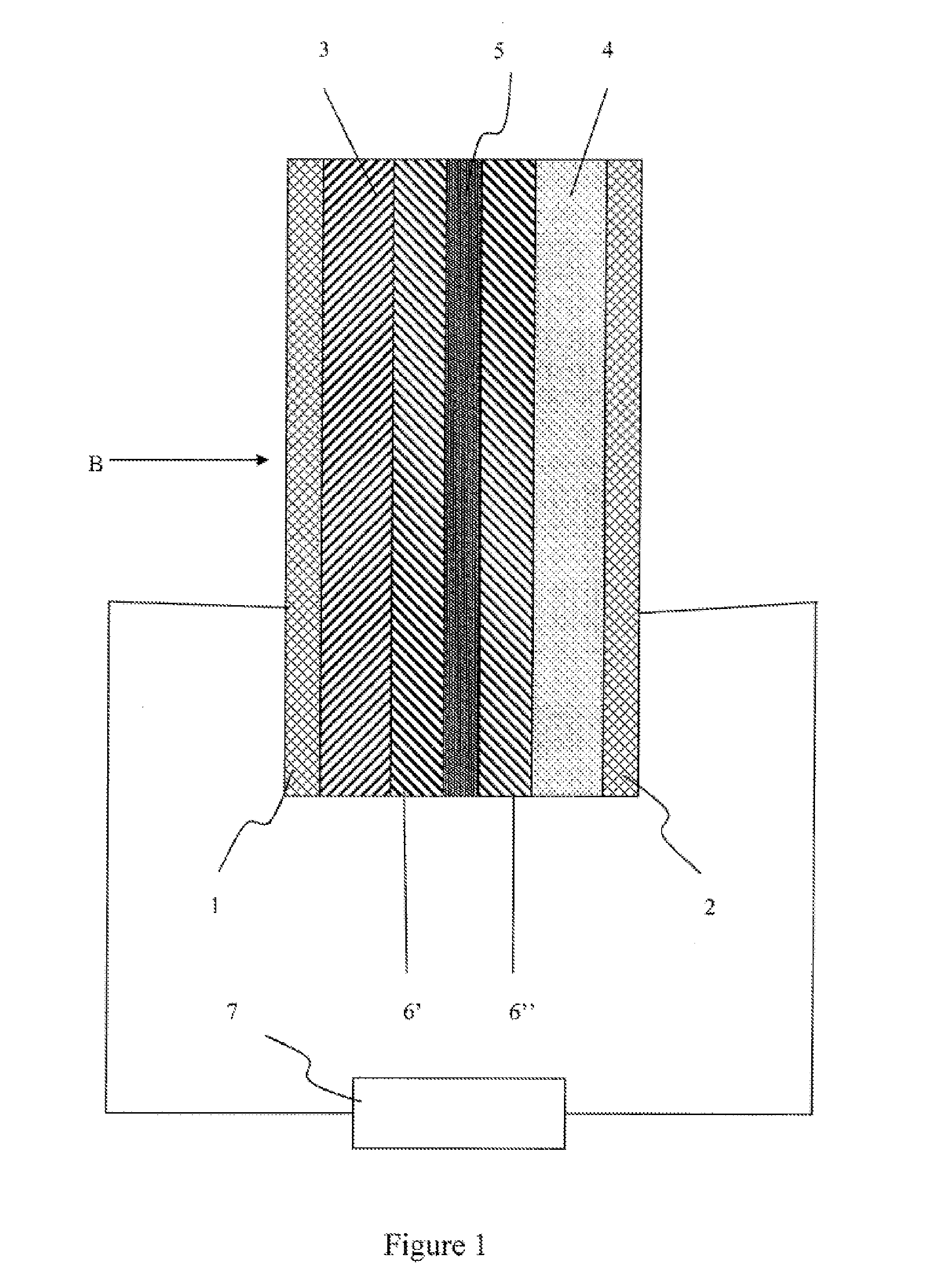
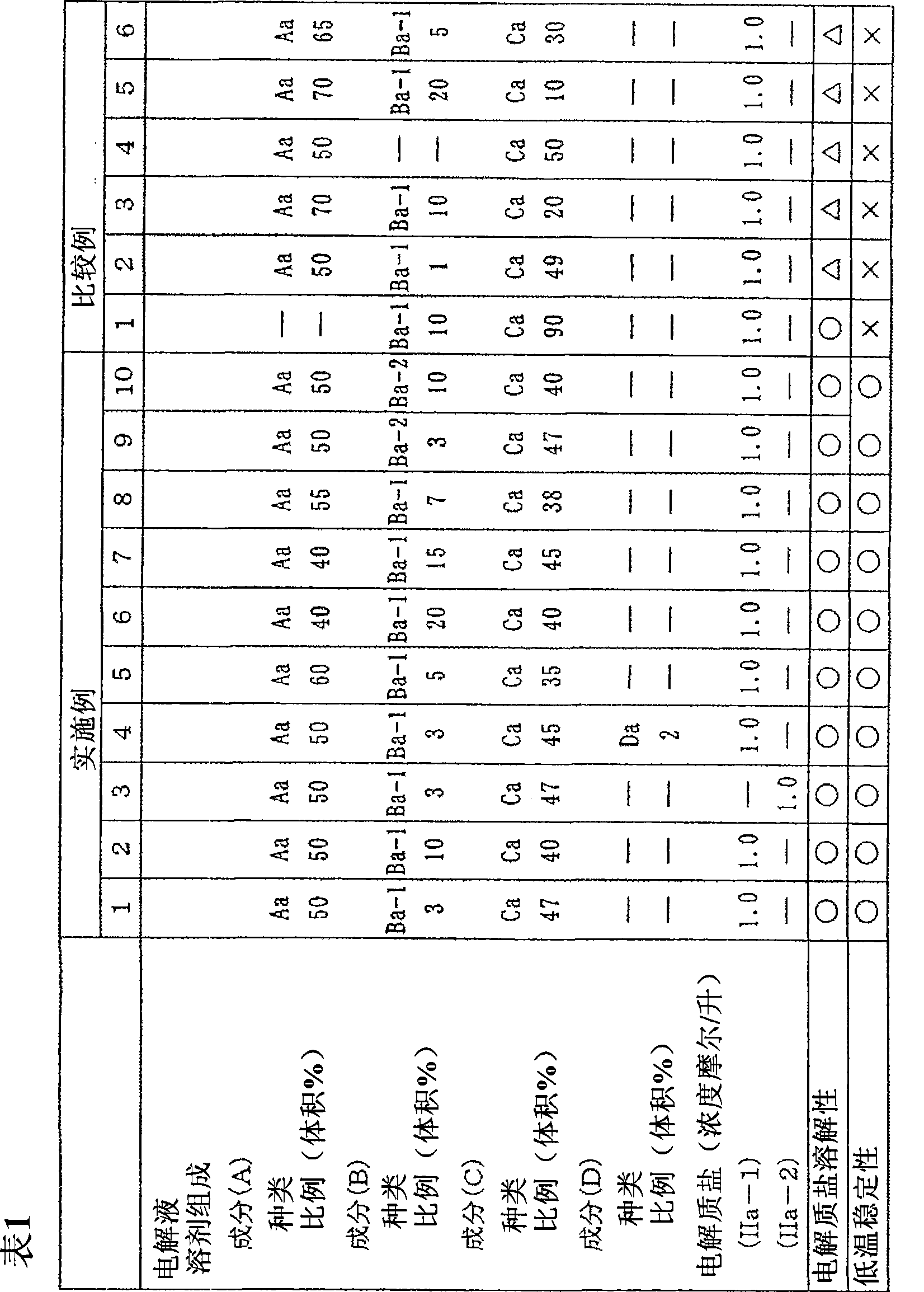
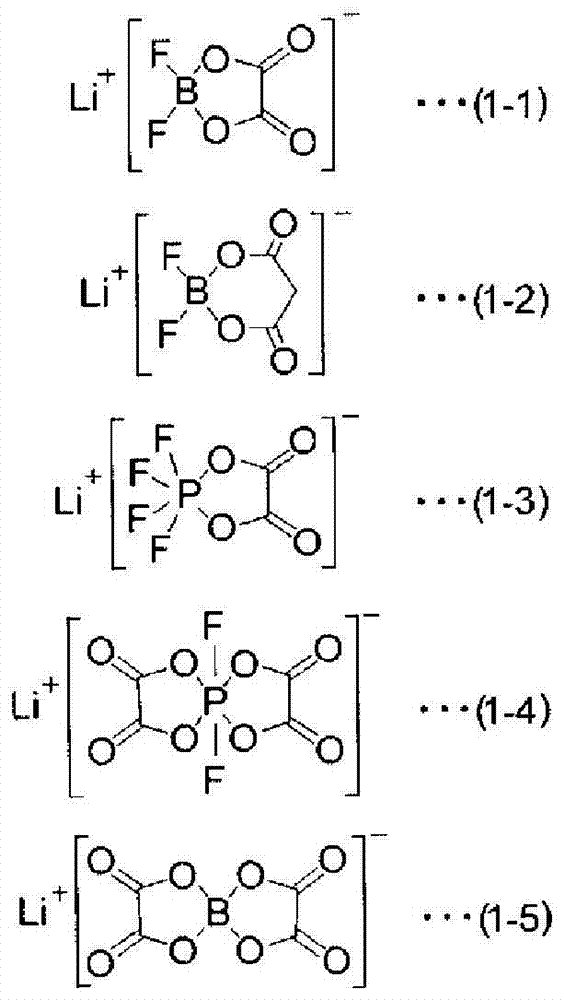
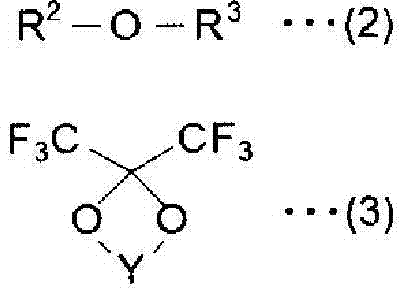
The invention provides an electrochemical device having an electrolyte solution comprising: (I) a solvent for dissolving an electrolyte salt, which comprises (A) a fluorinated ether represented by the formula (1), (B) a cyclic carbonate and (C) a linear carbonate compatible with both the fluronated ether (A) and the cyclic carbonate (B); and (II) the electrolyte salt, wherein the solvent (I) contains the fluorinated ether (A) in an amount of 30 to 60% by volume, the cyclic carbonate (B) in an amount of 3 to 40% by volume and the linear carbonate (C) in an amount of 10 to 67% by volume relative to the total volume of the solvent (I). Rf-O-Rf (1) wherein Rf and Rf2 independently represent a fluorinated alkyl group having 3 to 6 carbon atoms. The electrochemical device hardly causes phase-separation even at lower temperatures, and has excellent flame retardancy and heat resistance, can highly dissolve an electrolyte salt, has a high discharge capacity, and is excellent in charge / discharge cycle property.
Owner:DAIKIN IND LTD
Secondary battery
ActiveUS20130280600A1Positive electrodesNon-aqueous electrolyte accumulator electrodesElectrolytic agentElectrical battery
The object of an exemplary embodiment of the invention is to provide a secondary battery which contains a positive electrode active material operating at a potential of 4.5 V or higher and which has good cycle property at a high temperature. An exemplary embodiment of the invention is a secondary battery, comprising a positive electrode that can absorb and desorb lithium and an electrolyte liquid; wherein the positive electrode comprises a positive electrode active material that operates at a potential of 4.5 V or higher with respect to lithium; and wherein the electrolyte liquid comprises a fluorinated ether represented by a prescribed formula and a cyclic-type sulfonate represented by a prescribed formula.
Owner:NEC CORP
Electrolyte for lithium secondary battery and lithium secondary battery employing the same
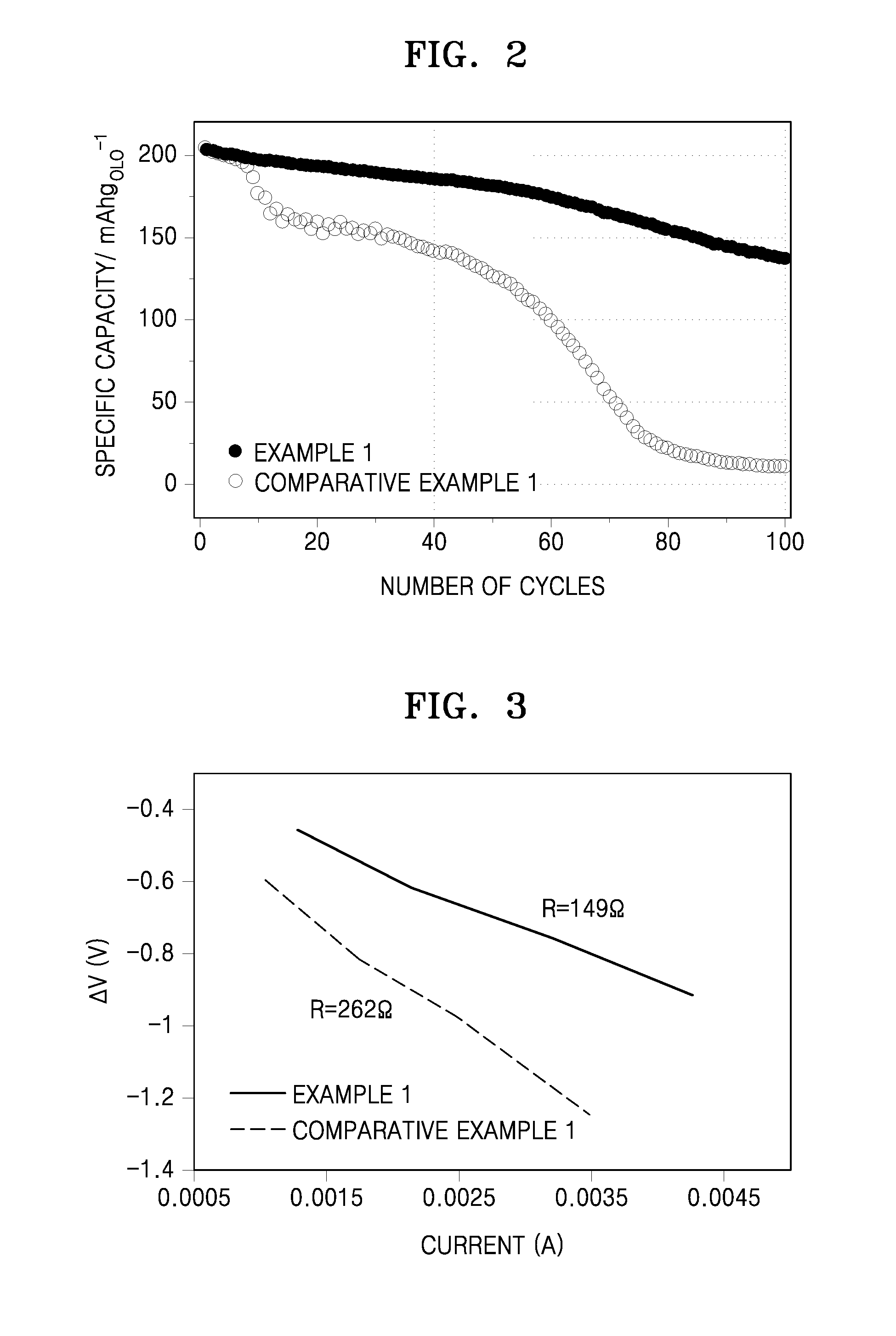
An electrolyte for a lithium secondary battery, the electrolyte including: a lithium salt, an organic solvent, and an organic fluorinated ether compound represented by Formula 1:R1—O—CF2—CHF—R2 Formula 1wherein, in Formula 1, R1 is a C3-C10 alkyl group or a C3-C10 cycloalkyl group, and R2 is fluorine, a C1-C10 alkyl group, a C3-C10 cycloalkyl group, a C1-C10 fluorinated alkyl group, or a C3-C10 fluorinated cycloalkyl group.
Owner:SAMSUNG ELECTRONICS CO LTD +1
Nonaqueous electrolyte solution for secondary batteries and lithium ion secondary battery
InactiveCN104737356AImprove stabilityReduced responseCell electrodesElectrolytesAqueous electrolyteCarboxylic acid
To provide a non-aqueous electrolyte solution for secondary batteries, which has a low reactivity with a positive electrode and a negative electrode and which is excellent in stability to prevent thermal runaway of a secondary battery and excellent also in battery properties such as cycle properties and rate properties, and a lithium ion secondary battery employing such a non-aqueous electrolyte solution. A non-aqueous electrolyte solution for secondary batteries, comprising a lithium salt composed of a specific complex such as lithium difluoro(oxalato)borate, a fluorinated solvent (A) containing at least one member selected from the group consisting of a fluorinated ether compound, a fluorinated chain carboxylic acid ester compound and a fluorinated chain carbonate compound, and a cyclic carboxylic acid ester compound (B); and a lithium ion secondary battery employing such a non-aqueous electrolyte solution.
Owner:ASAHI GLASS CO LTD
Nonaqueous electrolytic solution for electrochemical energy devices
InactiveCN1993848AHybrid capacitor electrolytesProtecting/adjusting hybrid/EDL capacitorElectrolysisBoiling point
A non-aqueous mixed solvent for use in a non-aqueous electrolytic solution for electrochemical energy devices which can enhance the high current charging and discharging capacity and low-temperature charging and discharging capacity of an electrochemical energy device, and prevent the device from damage at high temperatures. The solvent comprises an aprotic solvent and at least one fluorinated ether having a boiling point of 80 DEG C or more and being represented by R1-O-Rf1 (formula 1), R2-O-(Rf2-O)n-R3 (formula 2) or Rfh1-O-A-O-Rfh2 (formula 3). Also electrolytic solutions comprising such solvents and electrochemical energy devices containing such electrolytic solutions.
Owner:3M INNOVATIVE PROPERTIES CO
Silicon-based negative electrode electrolyte and lithium ion power battery
InactiveCN110336078AHas antioxidant capacityImprove electrochemical performanceSecondary cellsOrganic electrolytesDifluorophosphateHigh temperature storage
The invention discloses a silicon-based negative electrode electrolyte and a lithium ion power battery. The silicon-based negative electrode electrolyte comprises lithium salt, a fluorinated ether-containing organic solvent, a film-forming additive, a degassing additive and lithium nitrate, and is characterized in that the film-forming additive is lithium difluorophosphate and pentafluorophenyl isocyanate (PFPI). The lithium salt used in the electrolyte of the invention is mainly composed of high-conductivity lithium bisfluorosulfonimide, lithium hexafluorophosphate and lithium oxalyldifluoroborate; the three kinds of lithium salt are mixed to work under high voltage and temperature; the lithium oxalyldifluoroborate forms SEI films at both positive and negative electrodes, the battery still has good electrochemical performance even without adding other film-forming additives, and due to the existence of fluorinated ether and adiponitrile, the electrolyte has a certain antioxidant capacity; and due to the existence of the degassing additive, the gas production amount during cycle and high temperature storage processes of the battery can be reduced.
Owner:深圳市天劲新能源研究院
Lithium ion secondary battery
InactiveUS20130065136A1Good rate characteristicsExcellent in characteristic high-temperature cycleCell electrodesOrganic electrolyte cellsLithiumVolume concentration
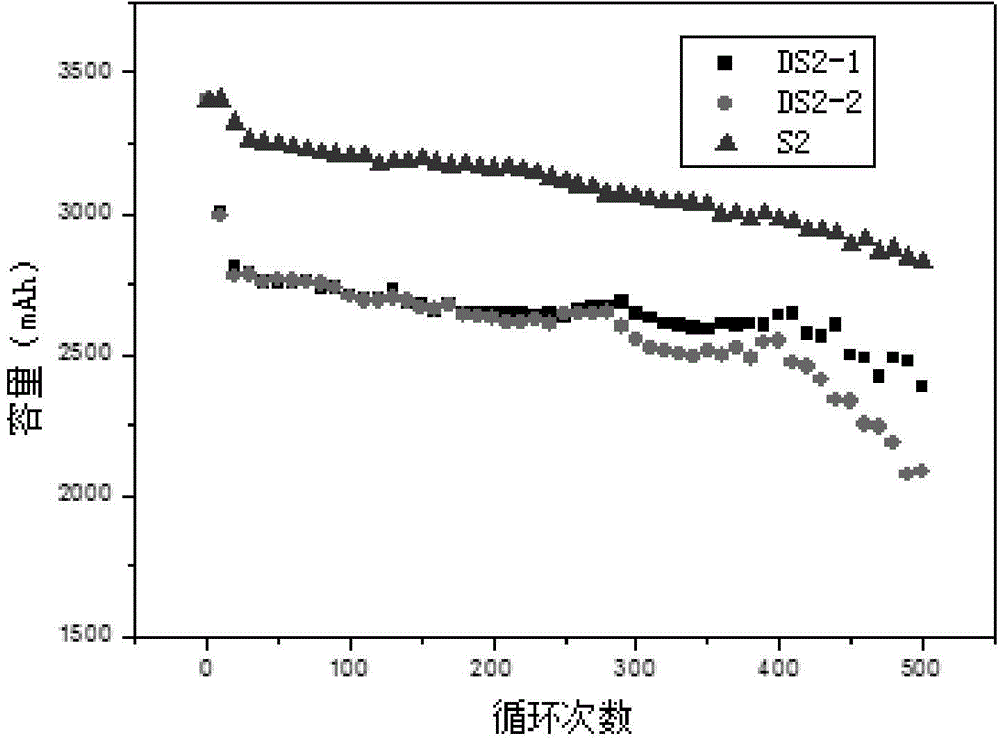
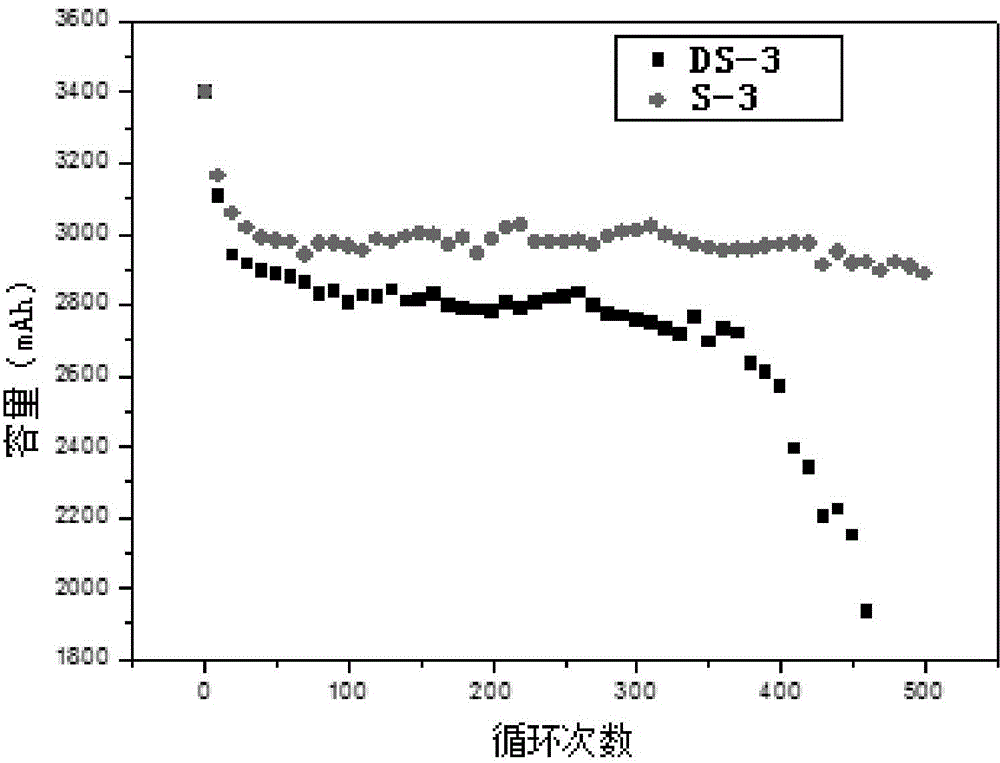
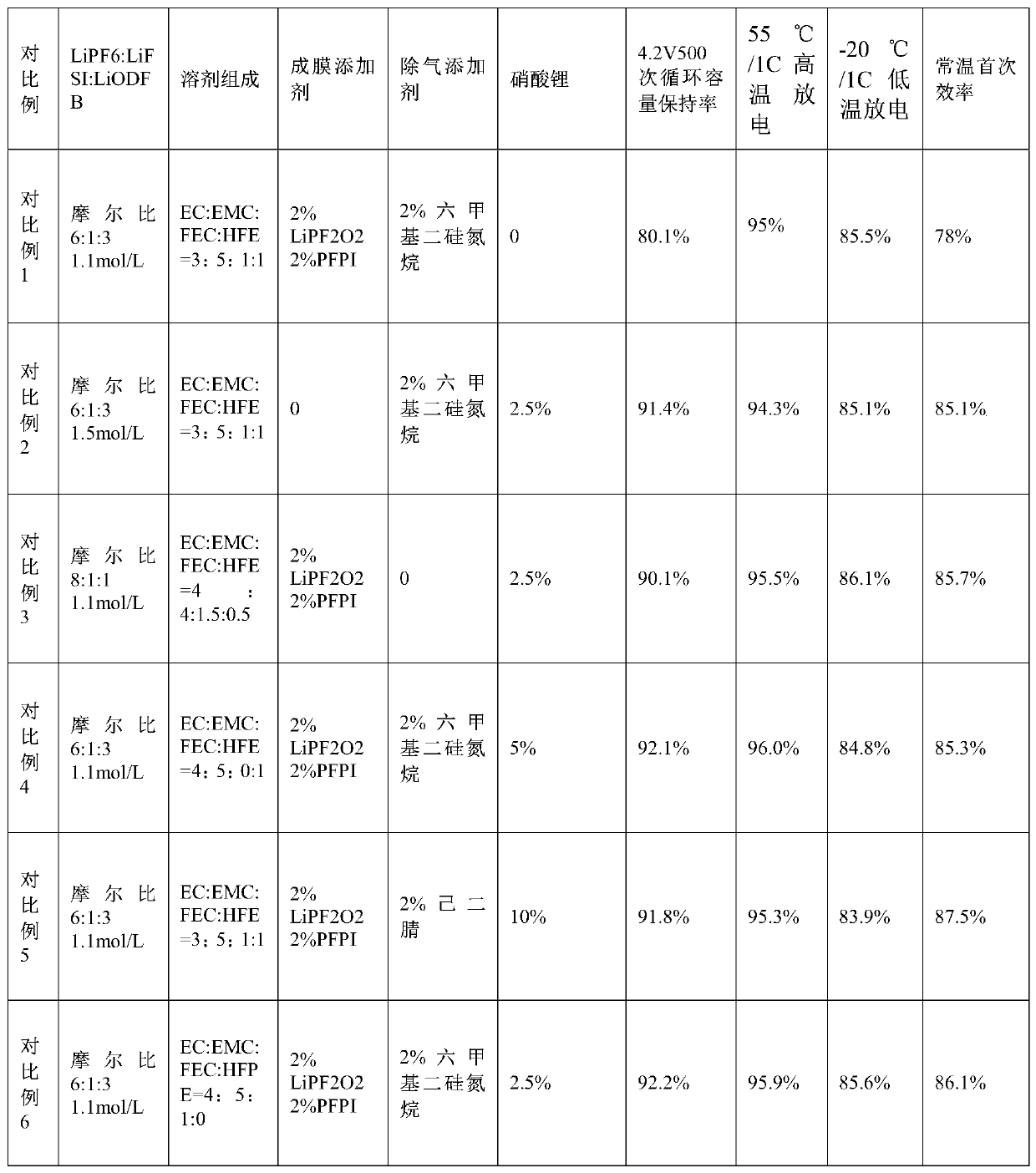
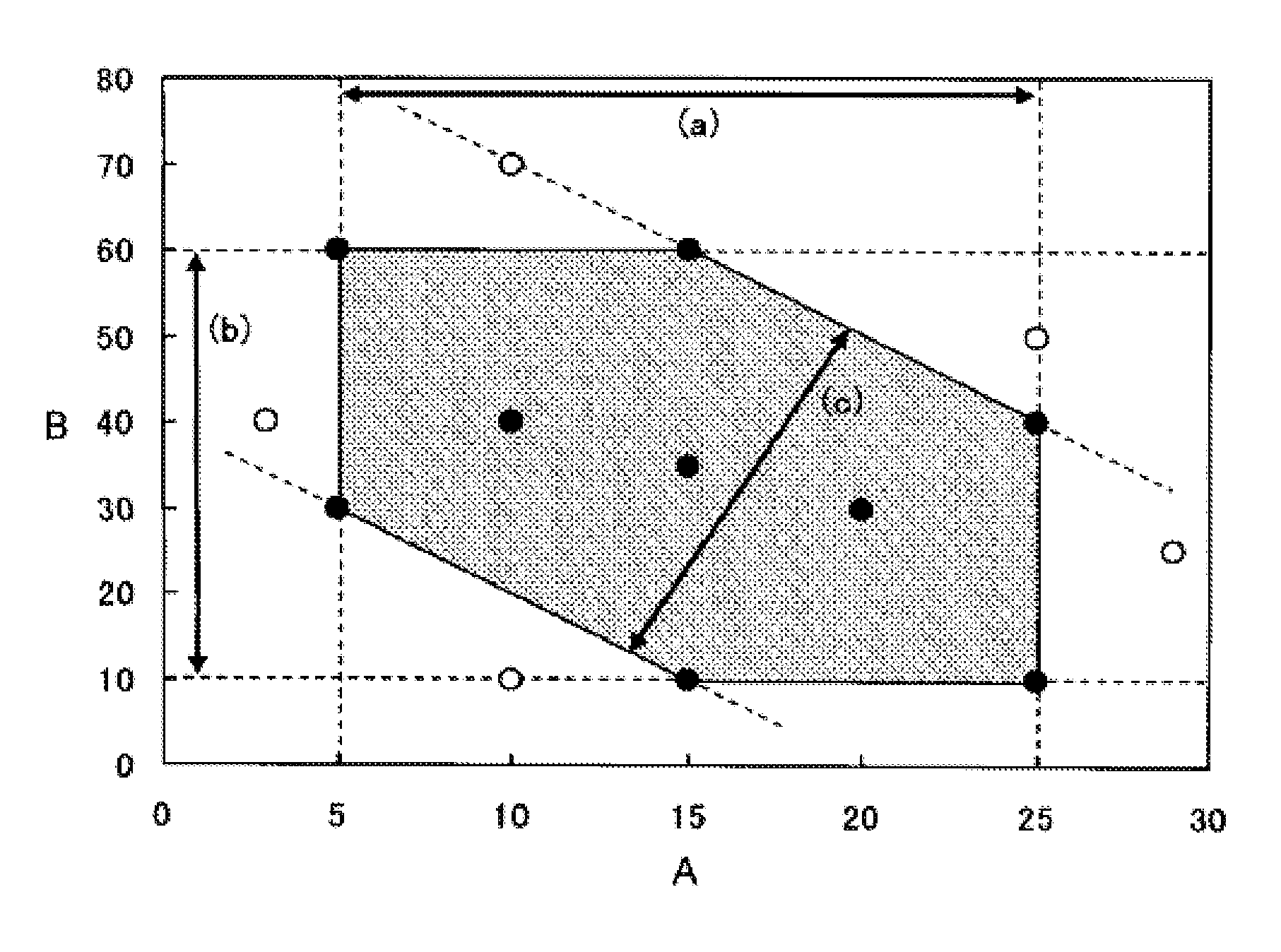
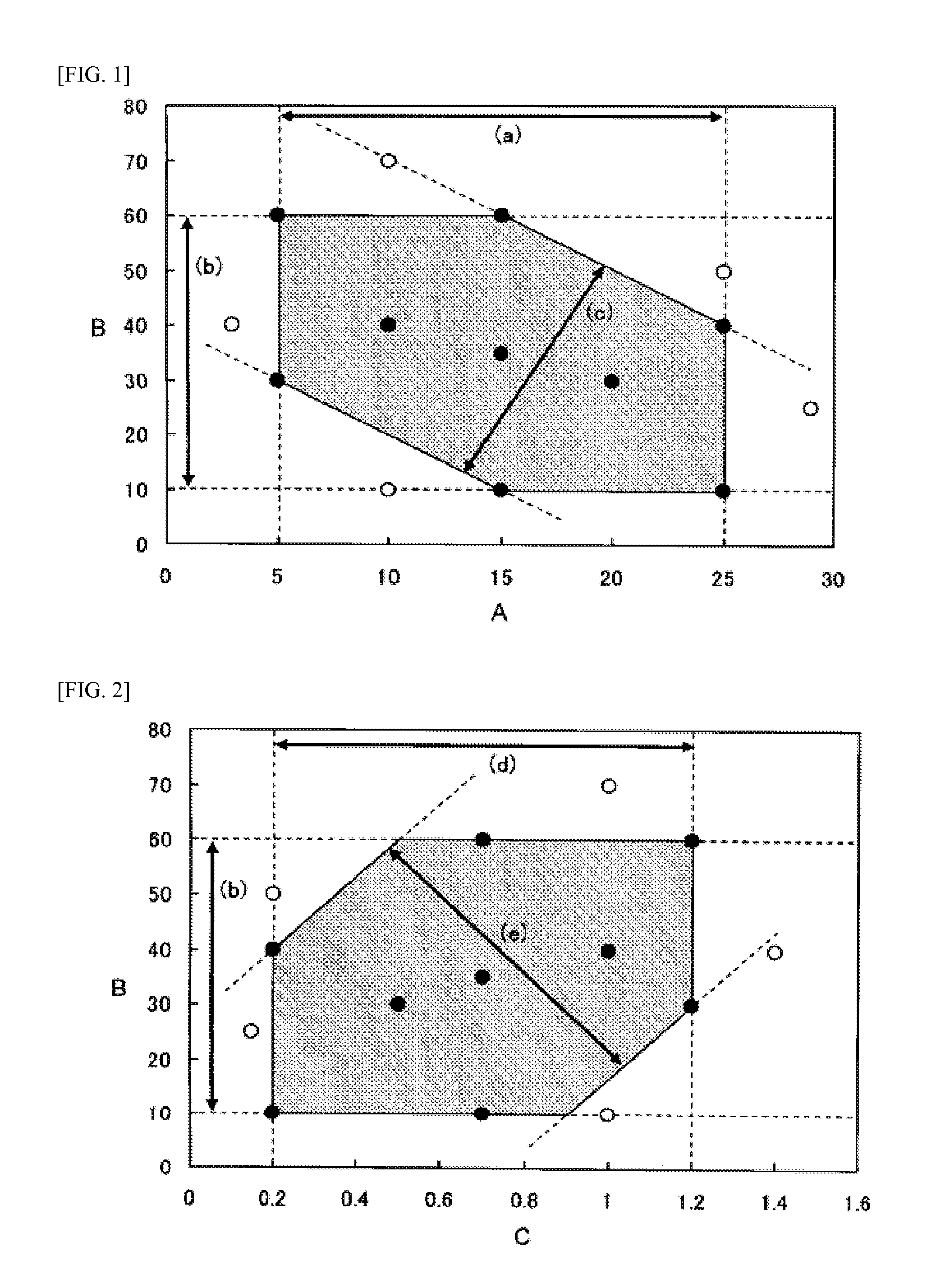
A lithium ion secondary battery which meets the requirement [1] of satisfying the formulae (a) to (c) or the requirement [2] of satisfying the formulae (b), (d), and (e): (a) 5≦A≦25, (b) 10≦B≦60, (c) 40≦2A+B≦90, (d) 0.2≦C≦1.2, and (e) −80 200C<3B≦150, wherein A (μm) represents an average particle size of a positive electrode active material; B (vol. %) represents a volume concentration of a fluorinated ether in a nonaqueous electrolyte solution; and C (m2 / g) represents a specific surface area of the positive electrode active material.
Owner:NEC ENERGY DEVICES LTD
Process for producing fluorinated alkyl ether
ActiveUS7193119B2Increase response rateHigh purityOrganic compound preparationEther preparation by compound additionAlcoholReaction rate
Owner:ASAHI GLASS CO LTD
Non-aqueous electrolyte solution for secondary batteries, and lithium ion secondary battery
InactiveUS20150037668A1Sufficient ion conductivityExcellent cycle characteristicsSecondary cellsVehicular energy storageAqueous solutionCarbonate
The object is to provide a non-aqueous electrolyte solution for secondary batteries, which has sufficient ion conductivity and which is provided also with excellent high voltage cycle properties and high voltage high temperature preserving properties, and a lithium ion secondary battery employing such a non-aqueous electrolyte solution.A non-aqueous electrolyte solution for secondary batteries, comprising a lithium salt and a liquid composition, wherein the liquid composition comprises from 5 to 50 vol % of a specific fluorinated ether compound, from 5 to 70 vol % of a specific fluorinated cyclic carbonate compound, and from 1 to 35 vol % of a specific sultone compound, and a lithium ion secondary battery employing such a non-aqueous electrolyte solution.
Owner:ASAHI GLASS CO LTD
Refrigerator oil composition
ActiveUS20100029522A1Reduce coefficient of frictionGood energy saving effectAdditivesBase-materialsKetoneRefrigerated temperature
A refrigerator oil composition includes: a synthetic base oil; and a partial hydrocarbyl ether of an aliphatic polyhydric alcohol condensate, in which the aliphatic polyhydric alcohol condensate includes a condensate of 4 to 20 molecules of a hindered glycol and / or an aliphatic polyhydric alcohol having 3 to 6 hydroxyl groups. The refrigerator oil composition is preferably used in a compression refrigerator that uses a hydrofluorocarbon, a natural refrigerant such as a hydrocarbon, carbon dioxide, or ammonia, a mixed refrigerant of fluoroiodomethane and propene, an unsaturated fluorinated hydrocarbon, a fluorinated ether, a fluorinated alcohol, a fluorinated ketone, or a mixture thereof as a refrigerant, has a low coefficient of friction, and is excellent in energy-saving property.
Owner:IDEMITSU KOSAN CO LTD
Method of making fluorinated alkoxy carboxylic acids and precursors thereof
ActiveUS20110245520A1Organic compound preparationCarboxylic acid esters preparationOrganic solventCarboxylic acid
A method for preparing saturated partially fluorinated alkoxy carboxylic acids or salts thereof by treating a compound: (II), where Rf represents a fluorinated, linear or branched alkyl residue interruptible by one or more oxygen atoms, n is 0 or 1, with a Z-anion in a reaction medium comprising water and an organic solvent, where the Z-anion is selected from CN—, SCN— and OCN— or combinations thereof. A method of making partially fluorinated ethers of the general formula (I) wherein Rf is defined as above, n is 0 or 1, and Z is nitrile (—CN), azide (—N3), thiocyanate (—SCN) or cyanate (—OCN) group, said method comprising treating a fluorinated olefin of the general formula (II) wherein the Z-anion is CN—, OCN—, SCN— or N3−. A compound of the general formula (I) as previously described where Z is selected from SCN, OCN and N3.
Owner:3M INNOVATIVE PROPERTIES CO
Supercapacitor electrolyte and supercapacitor prepared by supercapacitor electrolyte
InactiveCN107481870AEnhanced fast delivery capabilitiesLow viscosityHybrid capacitor electrolytesCapacitanceFire resistance
The present invention discloses a supercapacitor electrolyte. The supercapacitor electrolyte comprises electrolyte salt and a non-aqueous solvent, the electrolyte salt at least comprises one ionic liquid, the non-aqueous solvent at least comprises one fluorinated ether, the viscosity of the fluorinated ether is lower than the viscosity of the ionic liquid, the fluorinated ether is employed as the solvent of the ionic liquid to change the ion transmission behavior of the electrolyte and improve the power features of the supercapacitor, and the fluorinated ether is taken as a flame retardant component to improve fire resistance and safety of the electrolyte. The weak polarity and the low solvability of the fluorinated ether allows lots of solions to exist as in a naked ion mode without a non-solvation mode so that more aperture structures being similar to the dimensions of the naked ions in the electrode materials to be employed, and the distance of a double electrode layer established at an electrode / electrolyte interface is smaller to cause the increasing of the capacitance of the double electrode layer. The present invention further provides a supercapacitor using the supercapacitor electrolyte. The supercapacitor is high in energy density, high power density and good in safety performance.
Owner:XIAN UNIV OF SCI & TECH
A non-aqueous electrolyte for a lithium ion battery and a lithium ion battery using the electrolyte
ActiveCN109148960AIncrease oxidation voltageImprove stabilitySecondary cellsOrganic electrolytesDecompositionElectrical battery
The invention discloses a non-aqueous electrolyte for lithium ion battery and a lithium ion battery using the electrolyte. A non-aqueous electrolyte for a lithium ion battery comprises a lithium salt,Organic solvents and additives, the lithium salt may be selected from the group consisting of LiPF 6, one or more of LiBF4, LiClO4 and the like, wherein the organic solvent is selected from the groupconsisting of chain carbonate, cyclic carbonate, carboxylic ester and a mixed organic solvent of a fluorinated ether compound represented by the general formula (I), and may further comprise a commonly used additive of a high voltage type. The non-aqueous electrolyte for lithium ion battery is added with fluorinated ether solvent, and the addition of fluorinated ether improves the oxidation decomposition voltage of the organic solvent and improves the working performance of the electrolyte under the condition of high voltage.
Owner:산산어드밴스드머테리얼스(취저우)컴퍼니리미티드
High Voltage Electrolyte for Li-ion Batteries
ActiveCN104659417BImprove securityLow viscositySecondary cellsElectrolytesElectrical batteryManganese oxide
The invention discloses high-voltage electrolyte for a lithium ion battery. The high-voltage electrolyte comprises high-voltage-resistance 5V electrolyte for the lithium ion battery, and comprises fluoro carbonate compounds, fluorinated ether compounds, lithium oxalyldifluroborate and lithium hexafluorophosphate, wherein 100 parts by weight of a solvent of the electrolyte, the electrolyte comprises, 30-70 parts by weight of the fluoro carbonate compounds, 30-70 parts by weight of the fluorinated ether compounds, 1-5 parts by weight of lithium oxalyldifluroborate; the mole concentration of electrolyte lithium salt which does not contain lithium oxalyldifluroborate in the solvent is 0.9-1.5mol / L. The invention provides the multifunctional lithium ion battery electrolyte with the self-extinguish time less than 5 seconds, the viscosity less than 2cp and high voltage stability, and the multifunctional lithium ion battery electrolyte is applied to commercial positive electrode materials, such as lithium nickel manganese oxide, lithium cobalt phosphate, lithium vanadium phosphate, high-voltage nickel cobalt manganese ternary materials, lithium ferric manganese phosphate, and high-voltage lithium cobalt oxide, and negative electrode materials, such as graphite and lithium titanate.
Owner:HUBEI JIUBANG NEW ENERGY TECH
Lithium secondary battery
A lithium secondary battery including: a cathode including a high-voltage cathode active material; an anode; and an electrolyte disposed between the cathode and the anode, wherein the high-voltage cathode active material has a charge cut-off voltage of about 4.2 Volts or greater with respect to a lithium (Li) counter electrode, and wherein the electrolyte includes an organic fluorinated ether compound represented by Formula 1, an organic solvent, and a lithium salt:R1—O—CFnH2-n—R2 Formula 1wherein, in Formula 1, R1 is a C1-C10 alkyl group, a C3-C10 cycloalkyl group, a C1-C10 fluorinated alkyl group, or a C3-C10 fluorinated cycloalkyl group; R2 is hydrogen, fluorine, a C1-C10 alkyl group, a C3-C10 cycloalkyl group, a C1-C10 fluorinated alkyl group, or a C3-C10 fluorinated cycloalkyl group; and n is 1 or 2.
Owner:SAMSUNG ELECTRONICS CO LTD +1
Secondary circulation cooling system
InactiveUS20070235681A1Reduce power consumptionImprove efficiencyHeat-exchange elementsRefrigeration componentsEnvironment effectAlcohol
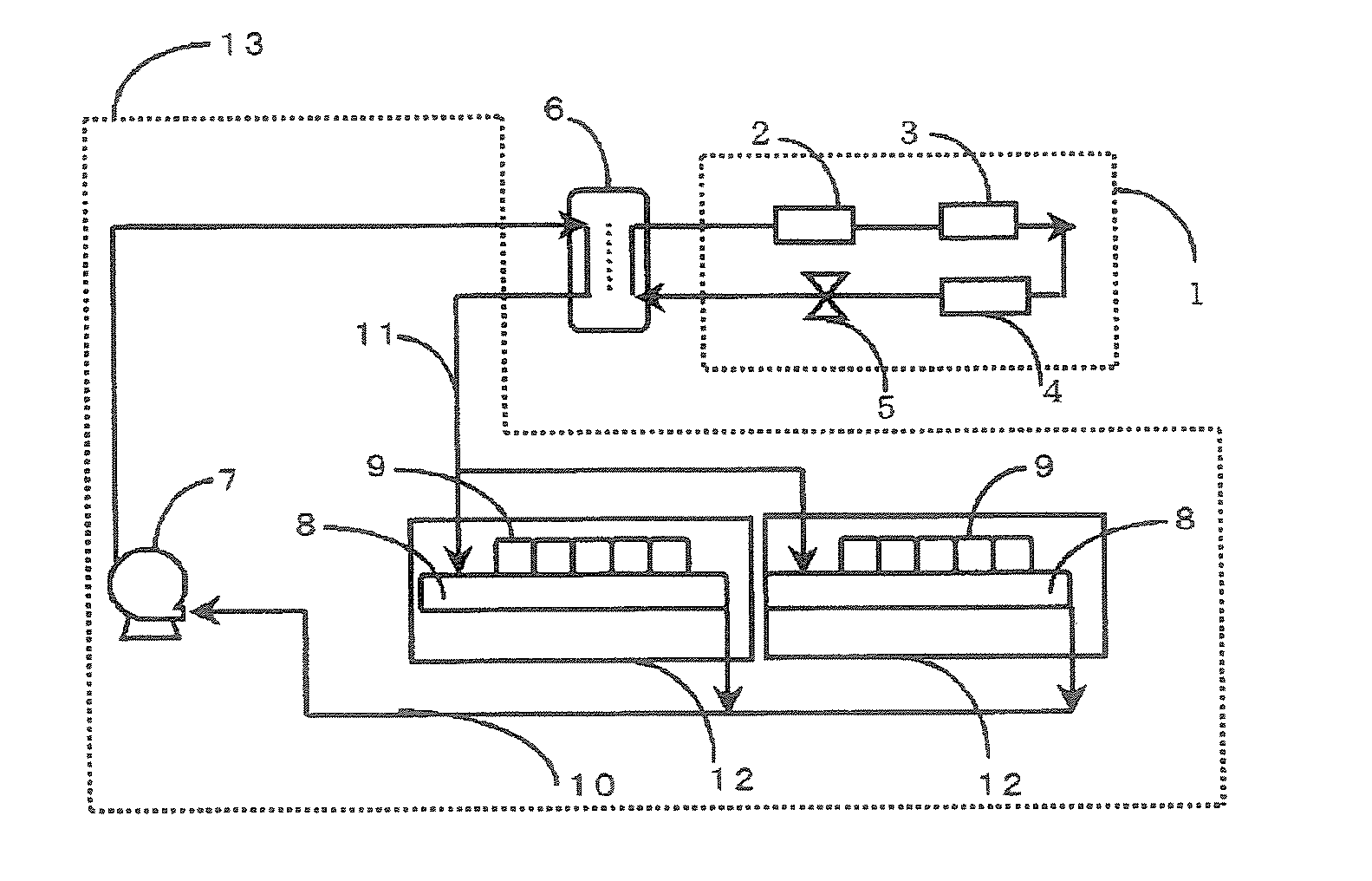
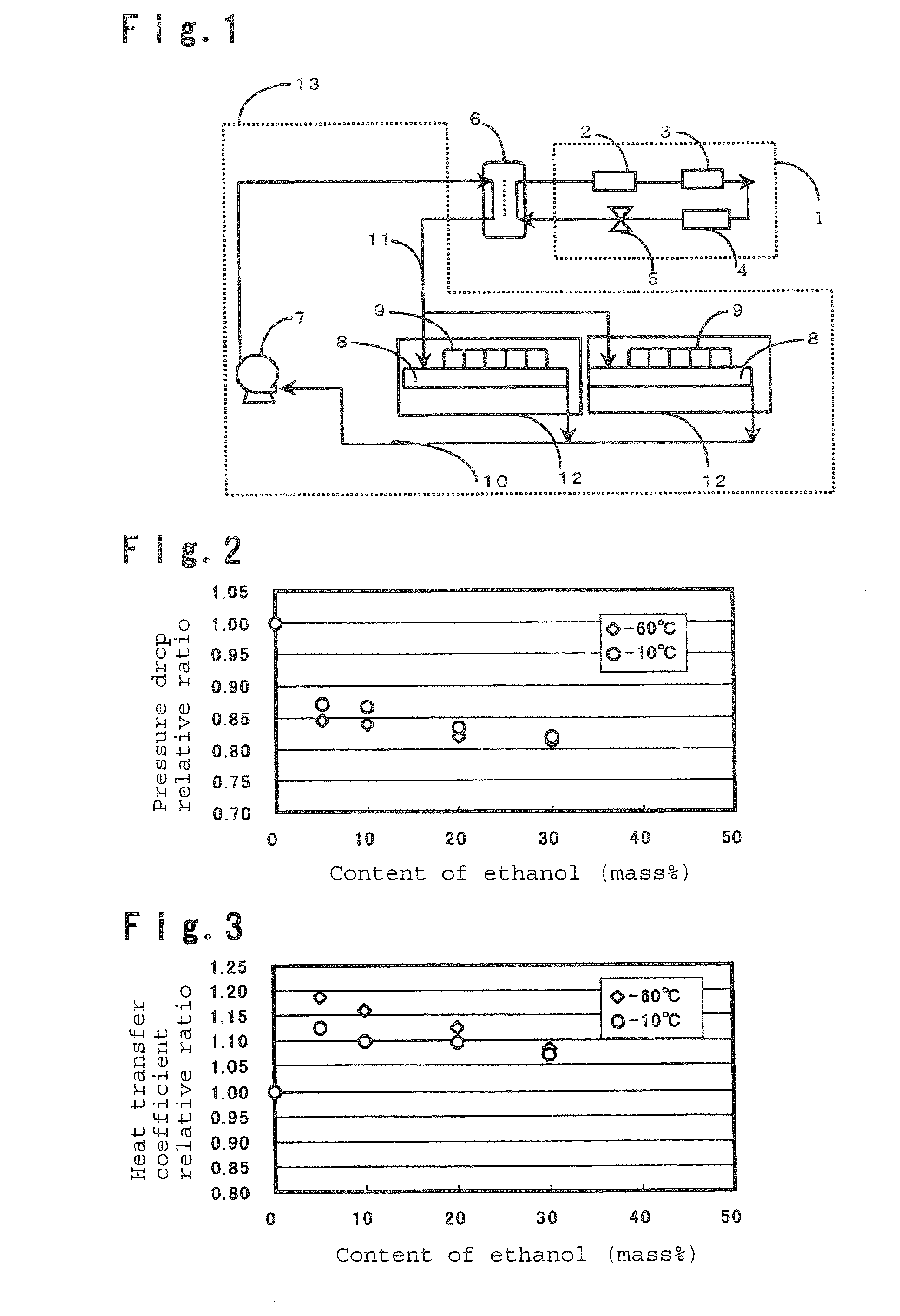
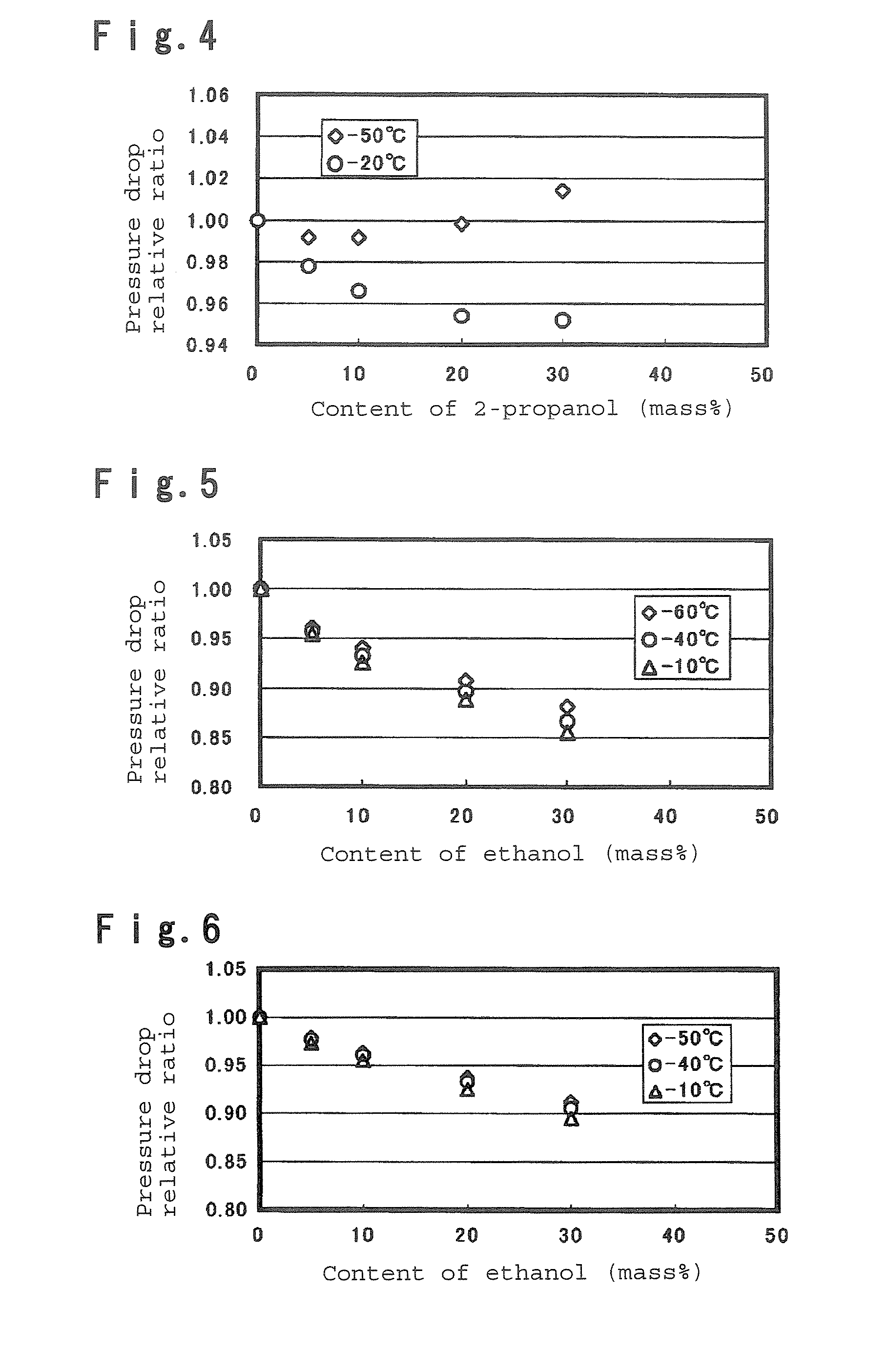
To provide a secondary circulation cooling system using a composition which is less influential over the environment as a secondary coolant, is non-inflammable, has a small pressure drop particularly at a low temperature and has a large heat transfer coefficient. A secondary circulation cooling system comprising a primary cooling means 1 using a primary coolant, a secondary circulation cooling means 13 using a secondary coolant and a heat exchange means 6 to carry out heat exchange between the primary coolant and the secondary coolant, characterized in that a composition comprising a fluorinated ether such as CF2HCF2OCH2CF3, and an alcohol such as ethanol, is used as the secondary coolant.
Owner:ASAHI GLASS CO LTD
Lithium-sulfur battery electrolyte and application thereof
InactiveCN108933274AReduce contact resistanceDissolution inhibitionLi-accumulatorsOxide ceramicLithium–sulfur battery
The invention relates to a lithium sulfur battery electrolyte and application thereof. The lithium sulfur battery electrolyte comprises an oxide ceramic electrolyte and a liquid electrolyte, wherein the liquid electrolyte is an electrolyte containing fluorinated ether solvents, and the electrolyte containing fluorinated ether solvents comprises a lithium salt, a solvent, and at least one fluorinated ether solvent; and the general chemical formula of the fluorinated ether solvents is (MF<x>H<3-x>)<m>-O-(MF<y>H<3-y>)<n>, M is C or Si , x is no less than 0 and no more than 3, y is no less than0 and no more than 3, the sum of x and y is no less than 1, m is no less than 1 and no more than 10, n is no less than 1 and no more than 10, and i is no less than 1 and no more than 10. According tothe invention, the electrolyte containing the fluorinated ether solvents is used as an interface modification layer, so the contact resistance between the ceramic electrolyte and electrodes is reduced, the dissolution of an intermediate product from a positive electrode is inhibited, the utilization ratio of active materials is improved, and the capacity performance and cycle stability of the battery are enhanced; and a preparation method is simple and feasible.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
Lithium ion secondary battery
ActiveCN111066188AImprove life characteristicsOrganic chemistryCell electrodesElectrolytic agentBattery cell
Owner:NEC CORP
Nonaqueous electrolyte solution for secondary batteries and lithium ion secondary battery
InactiveCN104335409AFull ionic conductivityExcellent high voltage cycle characteristicsPrimary cellsLi-accumulatorsPhysical chemistryCarbonate
The object is to provide a non-aqueous electrolyte solution for secondary batteries, which has sufficient ion conductivity and which is provided also with excellent high voltage cycle properties and high voltage high temperature preserving properties, and a lithium ion secondary battery employing such a non-aqueous electrolyte solution. A non-aqueous electrolyte solution for secondary batteries, comprising a lithium salt and a liquid composition, wherein the liquid composition comprises from 5 to 50 vol % of a specific fluorinated ether compound, from 5 to 70 vol % of a specific fluorinated cyclic carbonate compound, and from 1 to 35 vol % of a specific sultone compound, and a lithium ion secondary battery employing such a non-aqueous electrolyte solution.
Owner:ASAHI GLASS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com