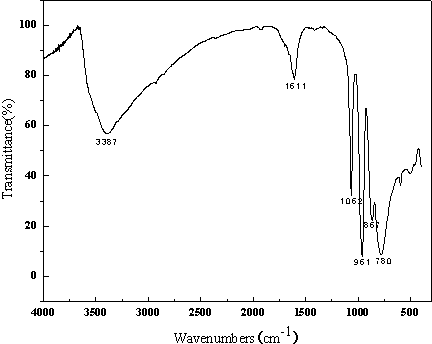
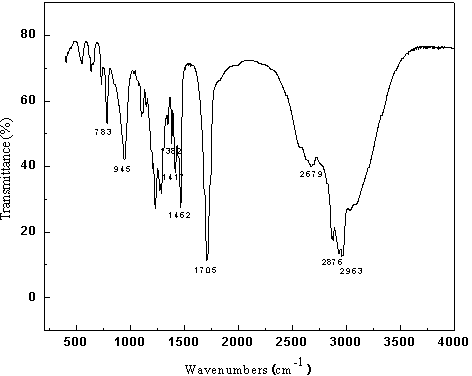
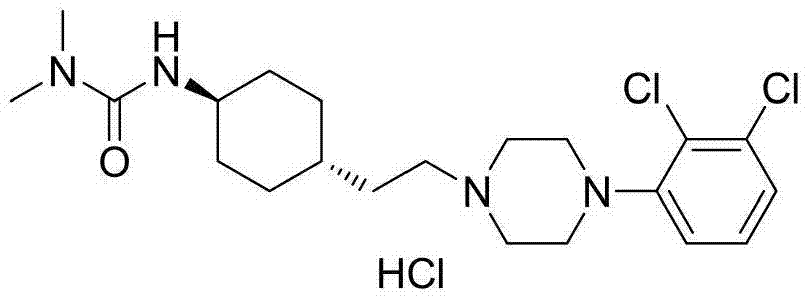
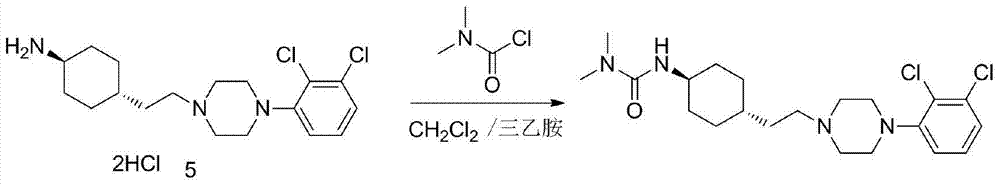
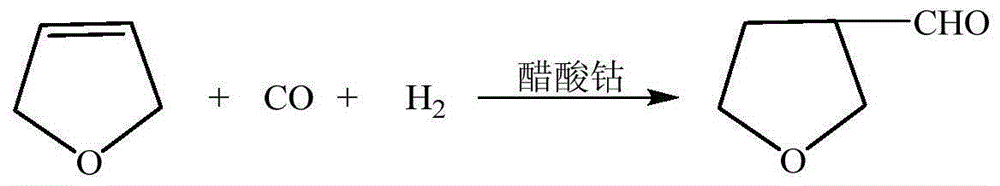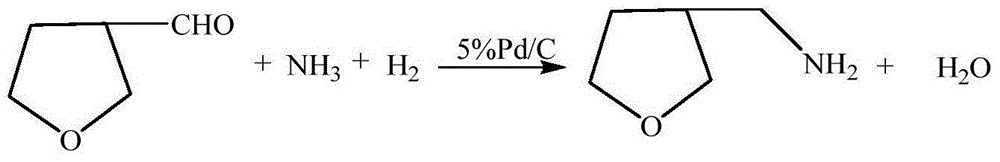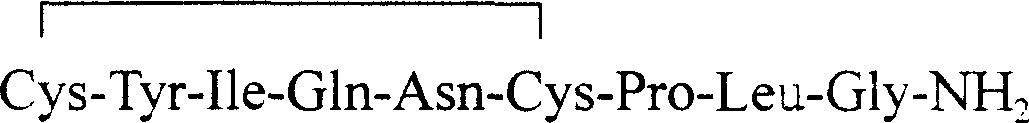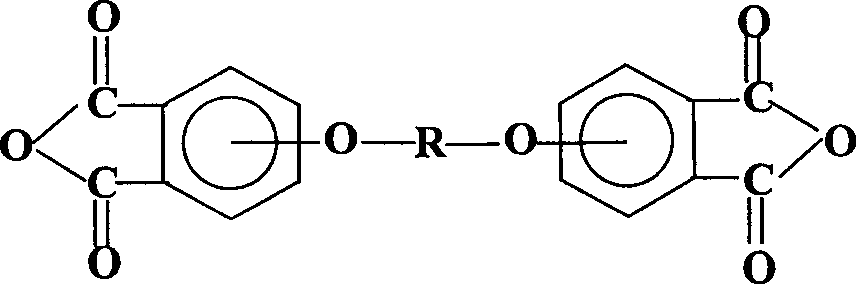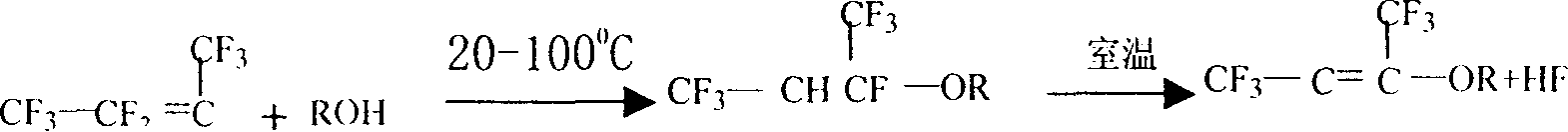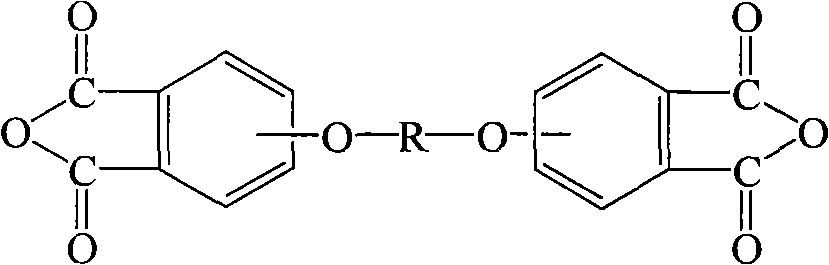Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
221results about How to "Three wastes less pollution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
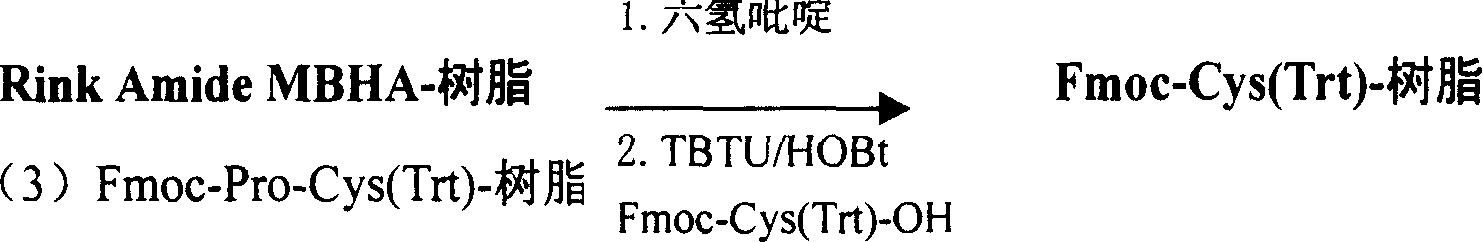
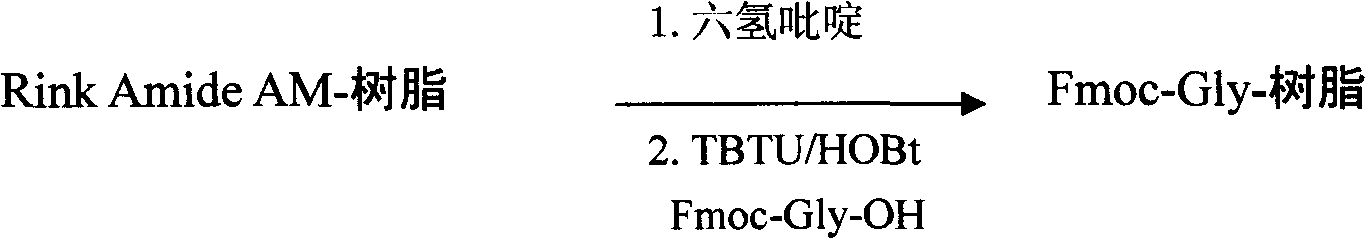
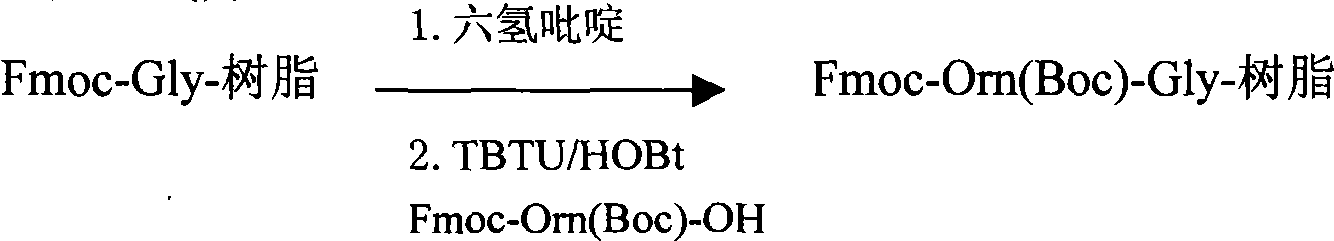
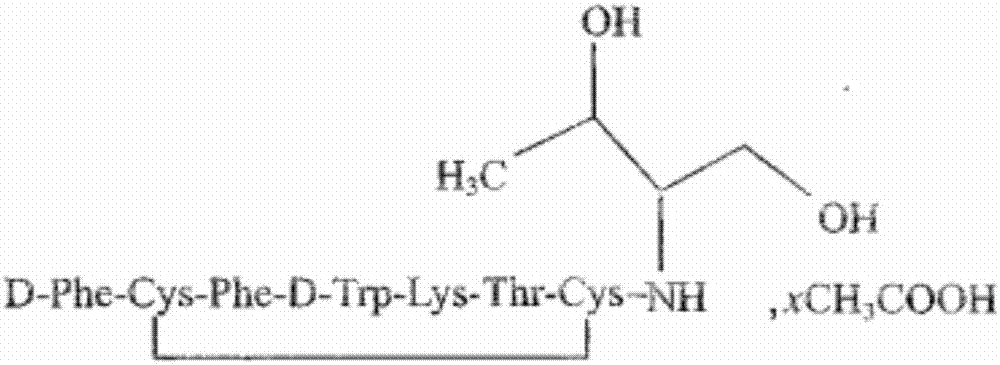
Preparation method of synthesizing bivalirudin from solid phase polypeptide
ActiveCN101033249AConvenient sourceReduce usagePeptide-nucleic acidsPeptide preparation methodsSide chainWang resin
This invention discloses a preparation method of solid-phase peptide synthesizing bivalirudin. It includes the following steps: taking any one of triphenyl methyl chloride resin, 4-methyl-triphenyl methyl chloride resin, 4-methoxy-triphenyl methyl chloride resin, 2-chlorine-triphenyl methyl chloride resin, or Wang resin as the starting raw materials, connecting amino acids in turn according to the method of solid-phase synthesis, to get a protective 28-peptide resin, removing Fmoc-protective group in turn, side-chain protecting group and cutting the peptide to get a crude, then purifying the crude through C18 (or C8) high-pressure column to get bivalirudin exquisite article. In this invention, the peptide yield of every step is more than 99%, and the total yield is 14%.
Owner:SHANGHAI SOHO YIMING PHARMA
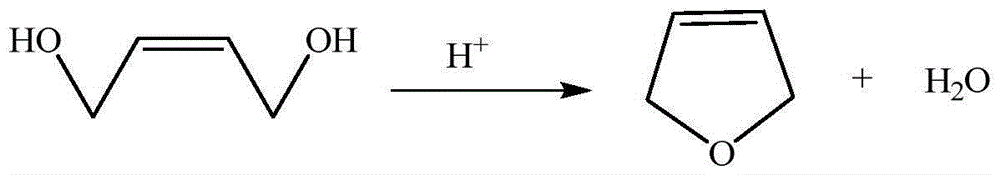
3-methylamine tetrahydrofuran preparation method
ActiveCN106397372ARaw materials are cheap and easy to getThe synthesis process is simpleOrganic chemistryCobalt acetateHydrogenation reaction
The invention provides a 3-methylamine tetrahydrofuran preparation method, wherein 1,4-butenediol is adopted as a raw material and is subjected to cyclization dehydration under the catalysis effect of a solid acid to produce 2,5-dihydrofuran, the 2,5-dihydrofuran reacts with water gas under the catalysis effect of cobalt acetate to produce 3-tetrahydrofuran formaldehyde, and the 3-tetrahydrofuran formaldehyde, ammonia gas and hydrogen gas are subjected to a hydrogenation reaction under the catalysis effect of 5% Pd / C to prepare the target product3-methylamine tetrahydrofuran, wherein the purity is 98.12%, and the total yield is 74.16%. According to the present invention, the preparation method has advantages of cheap and easily available raw materials, less steps, simple operation, high product purity, high yield, less three-waste pollution and production cost reducing, and is suitable for industrial production.
Owner:浙江捷达科技有限公司
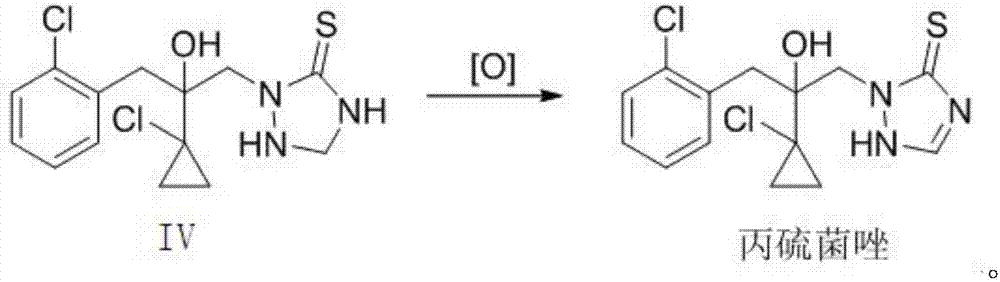
Preparation method of prothioconazole
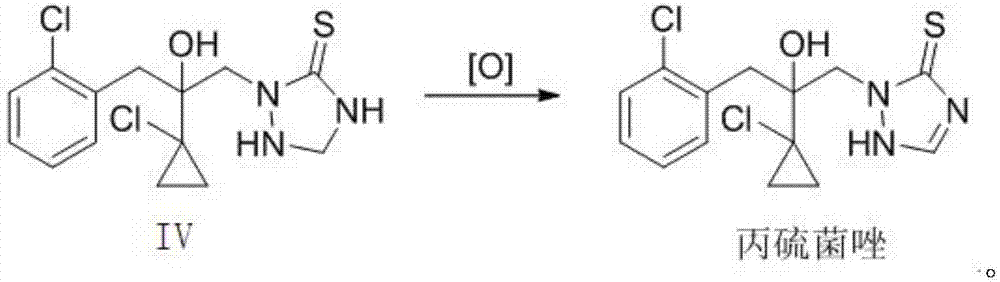
The invention relates to a preparation method of prothioconazole. The preparation method comprises that a compound IV undergoes a reaction at 20 to 120 DEG C in the presence of an oxidizing agent and a solvent, and after the reaction, the product is treated to form prothioconazole. The preparation method has mild reaction conditions, utilizes cheap and easily available raw materials, has simple processes, is environmentally friendly and clean in regents and reaction processes, greatly reduces three wastes, is suitable for industrial production, and realizes a high yield of a final product and high content.
Owner:JIANGSU SEVENCONTINENT GREEN CHEM CO LTD
Method for synthesizing triptorelin from solid phase polypeptide
ActiveCN101357936AConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionSide chainFreeze-drying
The invention discloses a preparation method of solid phase peptide synthesis triptorelin, which includes the following steps: with Rink Amide AM resins or Rink Amide MBHA resins as starting materials, amino acids with protective groups are sequentially connected according to solid phase synthesis, so as to obtain protective decapeptide resins, and meanwhile crude products are obtained by sequentially removing Fmoc-protective groups and synchronously removing side-chain protective groups and cutting peptides, and triptorelin elaborate products are prepared after the crude products are separated and purified by C18 (or C8 ) column and freeze-dried. The preparation method is stable in technology, convenient in raw and auxiliary material sources, short in production cycle, high in yield, stable in quality, low in production cost and high in transpeptidase yield. Besides, as the preparation method avoids using poisonous reagents, such as hydrogen fluoride, and the like, the pollution of three wastes is low, purification yield is over 25 percent and each step of transpeptidase yield is above 98 percent; the yield after cutting peptides is 78.8 percent and the total yield is 25.4 percent.
Owner:SHANGHAI SOHO YIMING PHARMA
Method for synthesizing triazine ring
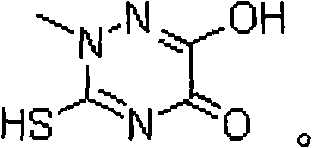
The invention discloses a method for synthesizing triazine ring, which is implemented by reacting aqueous methylhydrazine solution with ammonium sulfocyanate, and then preparing amino methyl sulfourea methanol solution by using methanol; performing cyclization reaction on amino methyl sulfourea methanol solution with dimethyl oxalate and sodium methylate, adjusting pH value with hydrochloric acid after finishing cyclization reaction, removing excessive sodium methylate, and filtering to prepare triazine ring sodium salt; aciding out the triazine ring sodium salt to obtain a crude triazine ring product; agitating the crude triazine ring product with hot water, washing, cooling, crystallizing, and drying to obtain a fine triazine ring product. The invention has high yield and low material cost, reduces labor intensity and pollution of waste gas, waste water and industrial residue.
Owner:NANTONG NABAIYUAN CHEM
Method for preparing 1-(4-chlorphenyl)-2-cyclopropyl-1-acetone
ActiveCN101786948ACheap and easy to getSimple stepsOrganic compound preparationCarbonyl compound preparationOxygenPhenyl group
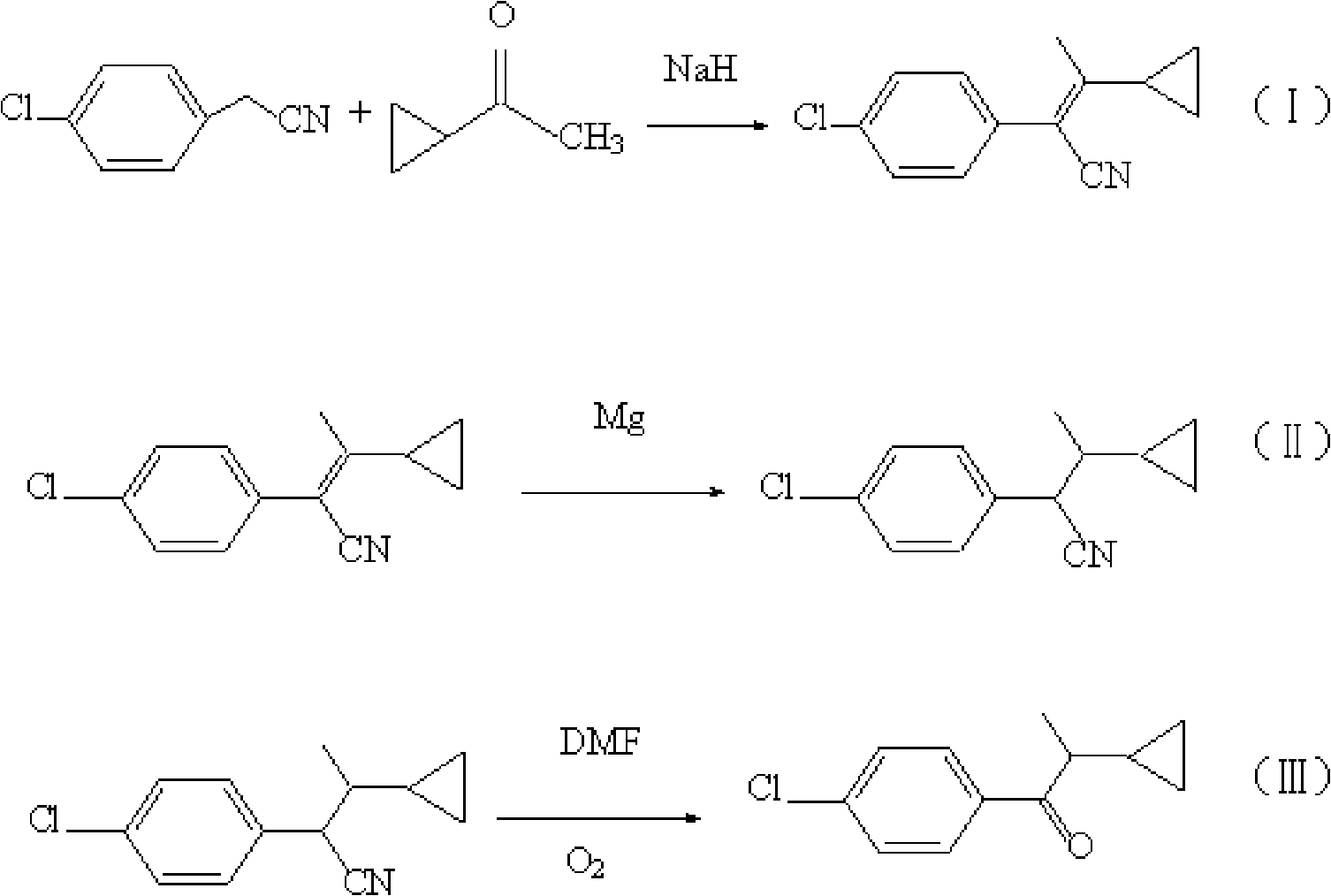
The invention discloses a method for preparing 1-(4-chlorphenyl)-2-cyclopropyl-1-acetone. The method comprises the following steps of: firstly, carrying out reaction on cyclopropyl methyl ketone and p-chlorobenzyl for 2-4 hours at the temperature ranging from 90 DEG C to 110 DEG C, and obtaining a compound I after post treatment, wherein the cyclopropyl methyl ketone and the p-chlorobenzyl are used as reactants, sodium hydride is used as a catalyst and toluol is used as solvent; secondly, leading the compound I to react with magnesium in a isopropanol dissolvent at the temperature ranging from 45 DEG C to 60 DEG C, and obtaining a compound II after post treatment; and thirdly, adding the compound II to DMF (Dimethyl Formamide), then adding sodium hydroxide, importing oxygen for 8 hours to15 hours under stirring for reaction, controlling the temperature to range from 10 DEG C to 25 DEG C, and finally obtaining the 1-(4-chlorphenyl)-2-cyclopropyl-1-acetone after post treatment. The preparation method has the advantages of simple steps, cheap and easily obtained raw materials, no deadly poison, low cost; less three-waste (waste gas, waste water and industrial residue) pollution, suitability for industrial production and higher yield and content of end products.
Owner:江苏省农用激素工程技术研究中心有限公司
Ultrafine fiber with high color fastness and manufacturing method thereof
InactiveCN101445973AAvoid wastingEliminate heavy pollutionFilament/thread formingConjugated synthetic polymer artificial filamentsPolyesterSpinning
The invention relates to ultrafine fiber with high color fastness and a manufacturing method thereof. The method comprises the steps of adding 3-40% coloring agent into a polymer powder to make into colored stock particles; adding the colored stock particles into spinning polymer polyester and / or polyamide in a ratio of 0.2-15%, and mixing; melt extruding the spinning polymer with a conjugate spinning machine, and coiling to obtain colored POY composite fiber; stretching and false-twist texturing to obtain colored fiber with high color fastness; and splitting with a basic hydrolysis method to obtain colored ultrafine fiber with high color fastness. The colored ultrafine fiber with high color fastness has excellent handfeel and color fastness to washing and light, and contains mass-colored dyeing components, so as to dispense with dyeing after being made into a textile. The colored ultrafine fiber has the advantages of simple process, low cost, low pollution, small product color difference, good color fastness, low fading liability, wide color spectra, and vivid and bright color.
Owner:段宏伟
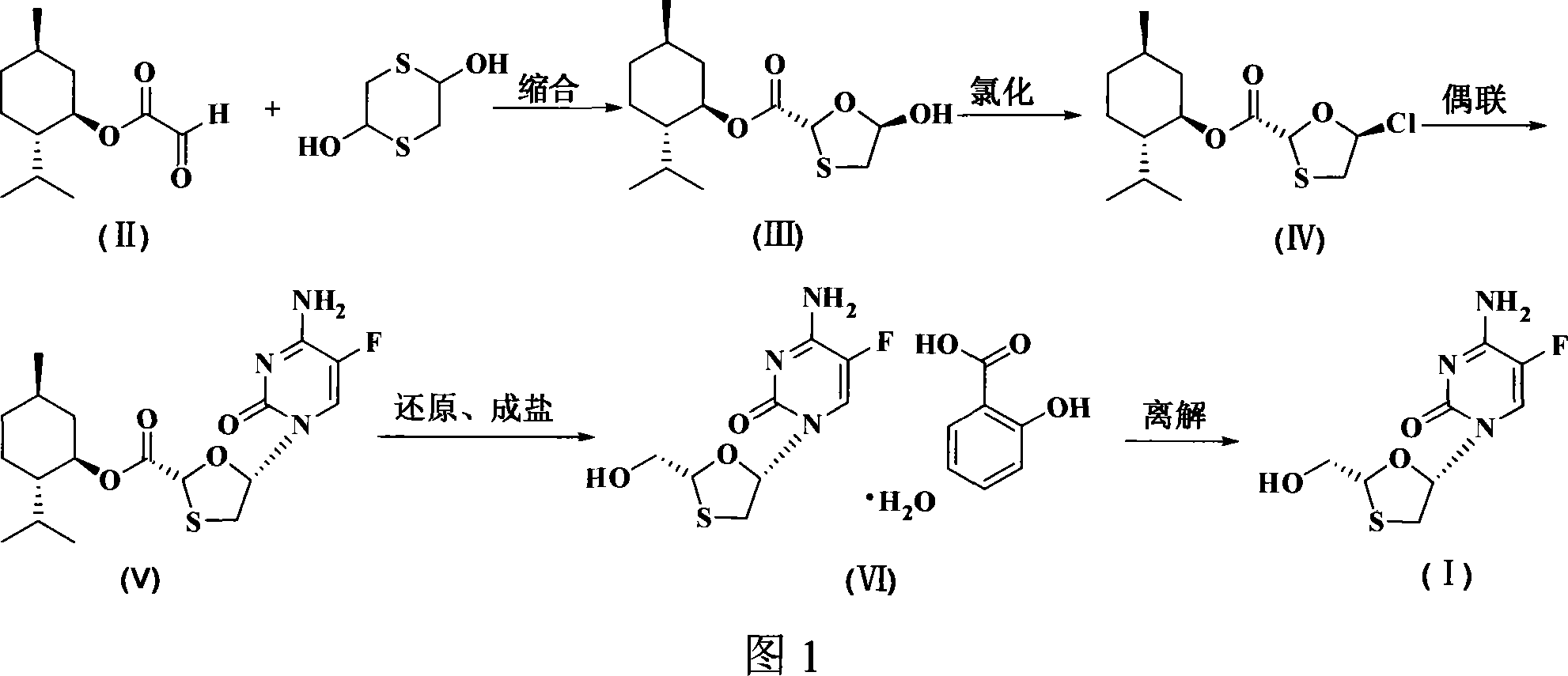
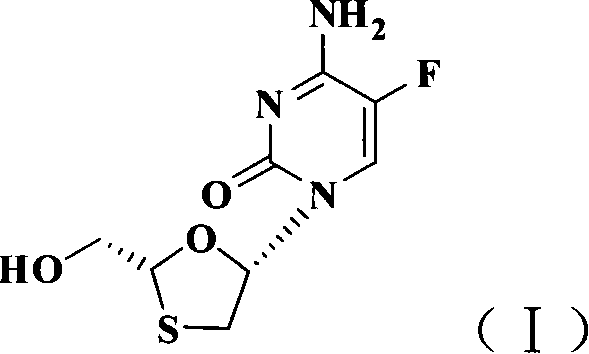
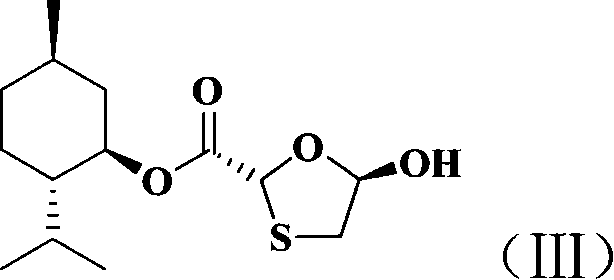
Non-enantioselective prepn process of emtricitabine
InactiveCN101066971AMild reaction conditionsSimple and fast operationOrganic chemistryDithianeAlcohol
The present invention discloses non-enantioselective preparation process of emtricitabine. The preparation process includes condensation of glyoxalic acid as the initial material and 2, 5-dihydroxy-1, 4-dithiane under the asymmetric induction of optically active alcohol ester to obtain trans-5-hydroxy-1, 3-oxythiacyclopentane-2-carboxylate; halogenating and coupling with silylated 5-flucytosine, and reducing to obtain initial product; reaction with salicylic acid to form salt, purification and separation to obtain optically pure emtricitabine. The present invention has mild reaction condition, simple operation, low cost, less environmental pollution and high product purity reaching medicinal standard, and is suitable for industrial production. The product is used in treating hepatitis B and AIDS.
Owner:葛建利
Method for preparing 2-ethyl hexanoic acid by catalytically oxidizing 2-ethylhexanal by molybdovanadophosphoric acid
InactiveCN102701944AStable chemical propertiesImprove thermal stabilityPhysical/chemical process catalystsOrganic compound preparation2-Ethylhexanoic acidReaction temperature
The invention discloses a method for preparing 2-ethyl hexanoic acid by catalytically oxidizing 2-ethylhexanal by molybdovanadophosphoric acid, and relates to a method for preparing a chemical preparation. The method comprises the following steps: weighing molybdovanadophosphoric acid and dissolving the molybdovanadophosphoric acid in distilled water at first and then in hydrochloric acid to prepare a catalyst solution; adding 2-ethylhexanal and the prepared catalyst in an ordinary-pressure reaction device, slowly heating and stirring the mixture, regulating oxygen flux, and starting to introduce oxygen when the temperature rises to the reaction temperature; after the reaction is ended, separating out the aqueous phase solution on the lower layer to obtain organic phase matter on the upper layer; and depressurizing and distilling the organic phase matter to obtain colorless transparent and pungent oily liquid, 2-ethyl hexanoic acid. By changing the ratio of molybdenum to vanadium of the catalyst and optimizing the reaction condition, the conversion rate of the 2-ethylhexanal reaches above 99%, and the selection and yield of the 2-ethyl hexanoic acid reach above 98% respectively.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
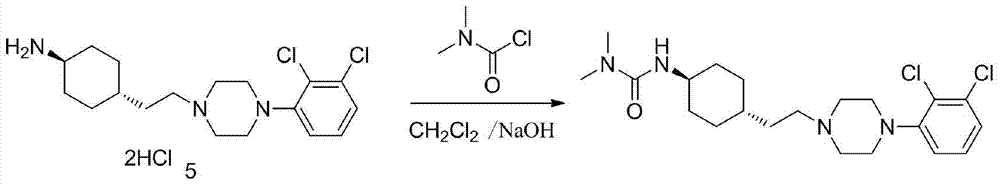
3-cyclohexyl-1,1-dimethylurea compound as well as preparation method and application thereof
ActiveCN104496854AAtom economy is highHigh purityUrea derivatives preparationOrganic compound preparationCariprazineDimethylurea
The invention discloses a 3-cyclohexyl-1,1-dimethylurea compound as well as a preparation method and application thereof. The 3-cyclohexyl-1,1-dimethylurea compound is used for preparing an antischizophrenic drug cariprazine. Compared with the prior art and report literatures, the preparation method of the 3-cyclohexyl-1,1-dimethylurea compound has the remarkable advantages of being free of removal of protecting groups such as Boc group, high in atom economy, low in cost and easy in getting of raw materials, mild in reaction condition, stable in yield, simple and convenient to operate, controllable in product quality, high in product purity, less in three waste pollution and easy to produce industrially. The structure formula of the 3-cyclohexyl-1,1-dimethylurea compound is as shown in (I) in the specification.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
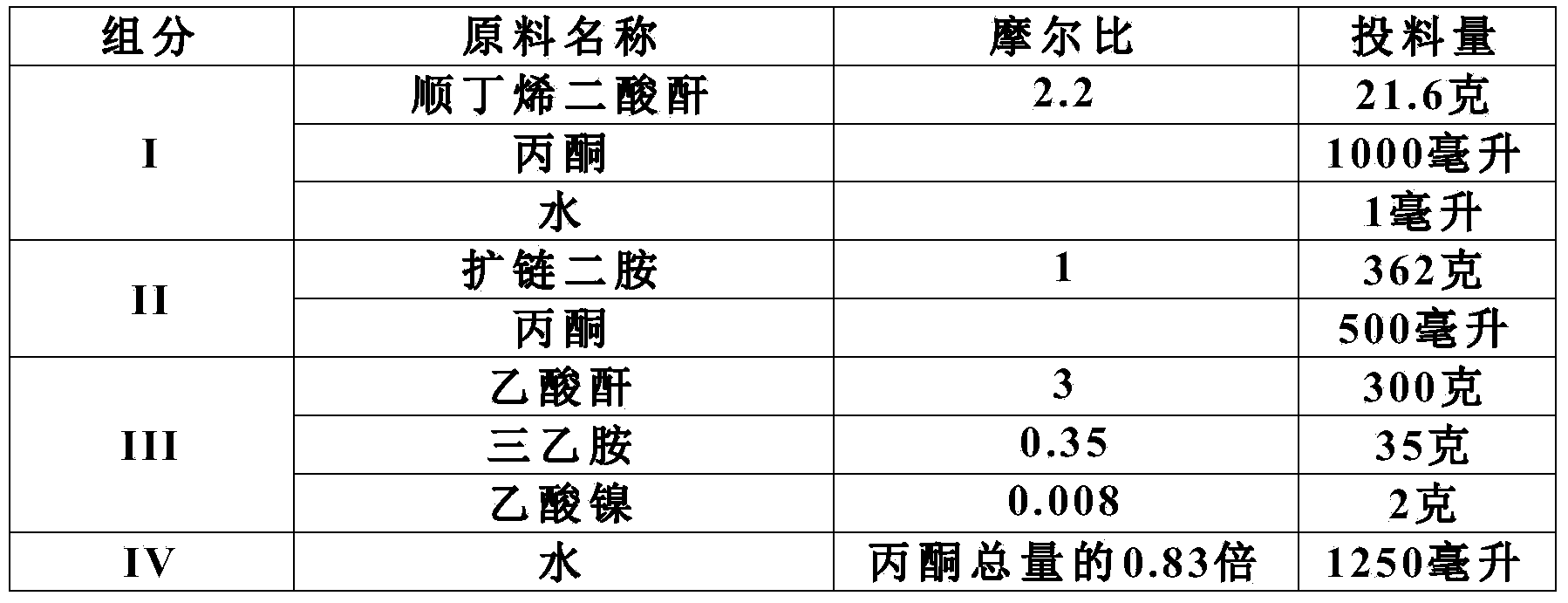
High-temperature resistant polyimide wire enamel and preparation method thereof
The invention discloses high-temperature resistant polyimide wire enamel and a preparation method thereof. The wire enamel comprises the following components in percent by weight based on total mass of enamel liquor: 50-80 percent of polyimide acid solution with solid content of 30 percent and 0-40 percent of chain-extended bismaleimide prepolymer solution with solid content of 30 percent. The preparation method of the high-temperature resistant polyimide wire enamel comprises the steps of mixing the polyimide acid solution and the chain-extended bismaleimide prepolymer solution according to proportions, adding an organic solvent to adjust the solid content and viscosity of the enamel liquor, and stirring to uniformly mix, thus obtaining claret-red transparent viscose polyimide wire enamel. The wire enamel disclosed by the invention is good in storage stability, resistant to high temperature, uniform in enamel film, good in flexibility, simple and easy to operate in an enamel and wire preparation process, high in raw material utilization rate, little in three-waste pollution and easy to realize industrialization; the soft breakdown voltage and the breakdown voltage of the wire enamel are remarkably higher than 240-level polyimide enamelled round copper wire standard in China respectively. The high-temperature resistant polyimide wire enamel can be used for insulation of a high-load, high-power and refrigerant-resistant motor and has broad market prospects.
Owner:HUAWEI TEHCHNOLOGIES CO LTD
Preparing process for synthesizing oxytocin from solid-phase polypeptide
ActiveCN1990501AReduce usageSimple processOxytocins/vasopressinsPeptide preparation methodsProduction rateRink amide resin
The invention discloses a method for preparing oxytocin through solid phase polypeptide synthesis, comprising following steps: taking Rink Amide resin (comprising Rink Amide MBHA resin, Rink Amide Am resin) as raw material, taking amino acid protected by Fmoc, TBTU or HBTU / HOBt as condensing agent, making up amino acid in sequence; adding peptide cutting agent for peptide cutting, adding for precipitation and getting reduced coarse product; adding basic matter, feeding air for oxidation or oxiding with H2O2 with pH being 7.5- 10.0, getting oxidized coarse product; separating and purifying by using C18 or C 8 column and getting final product. The method is characterized by low production cost, simple process, little pollution, high production rate and convenience for industrial production.
Owner:SHANGHAI SOHO YIMING PHARMA
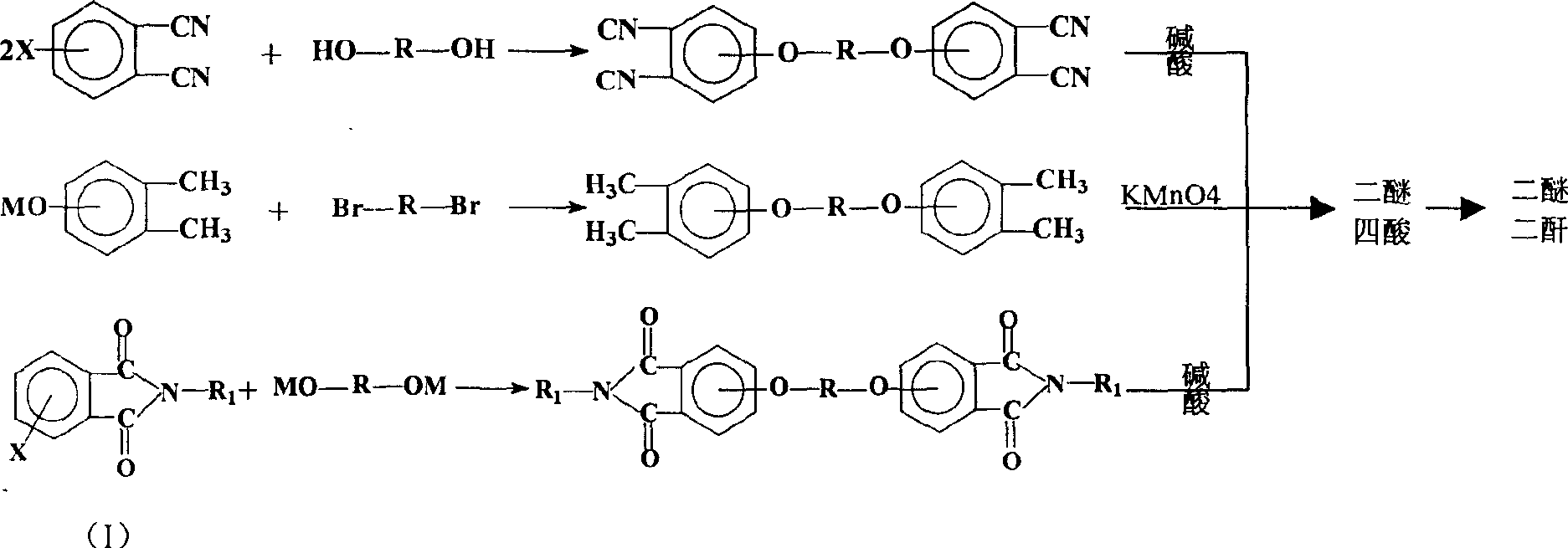
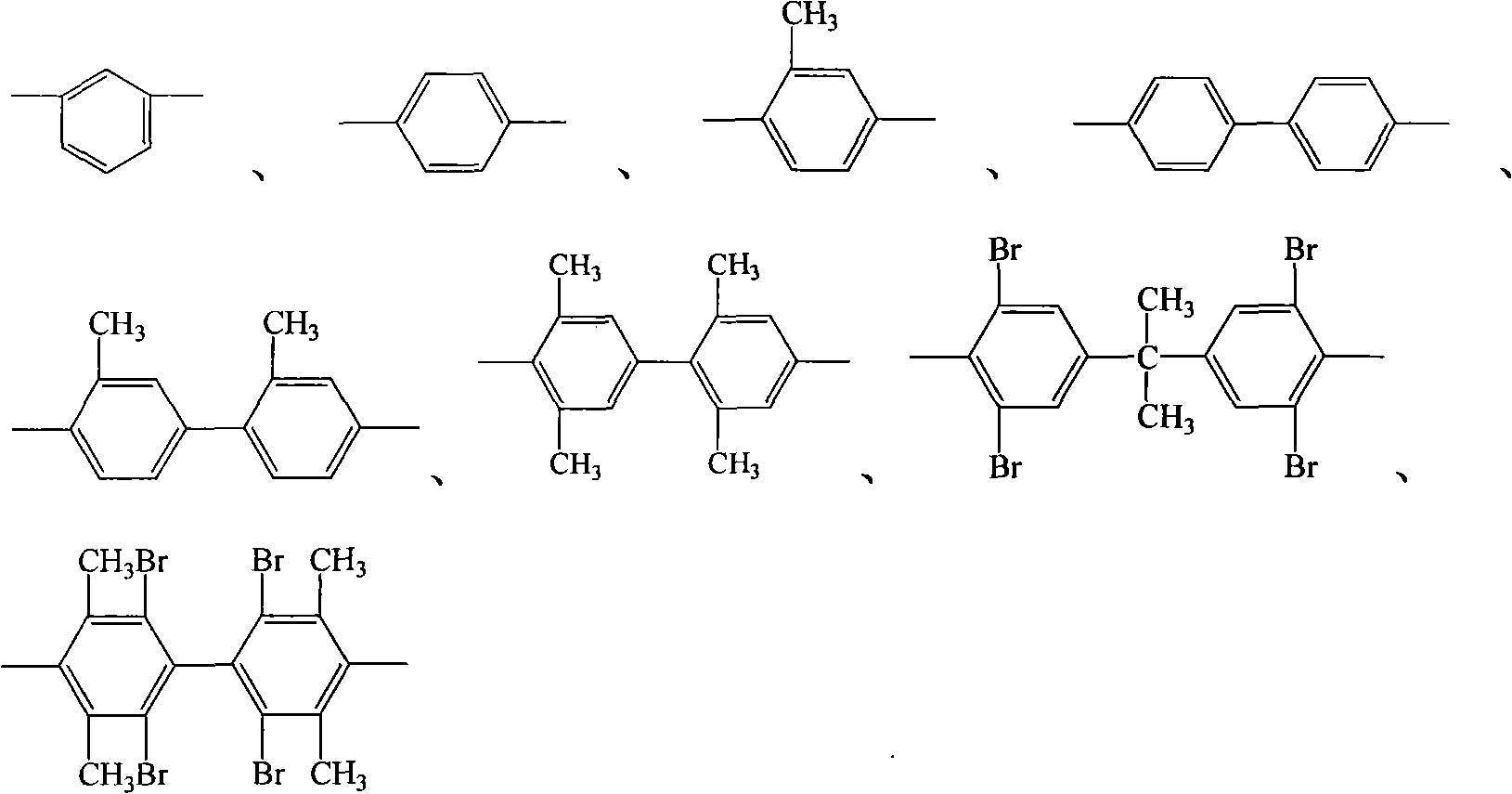
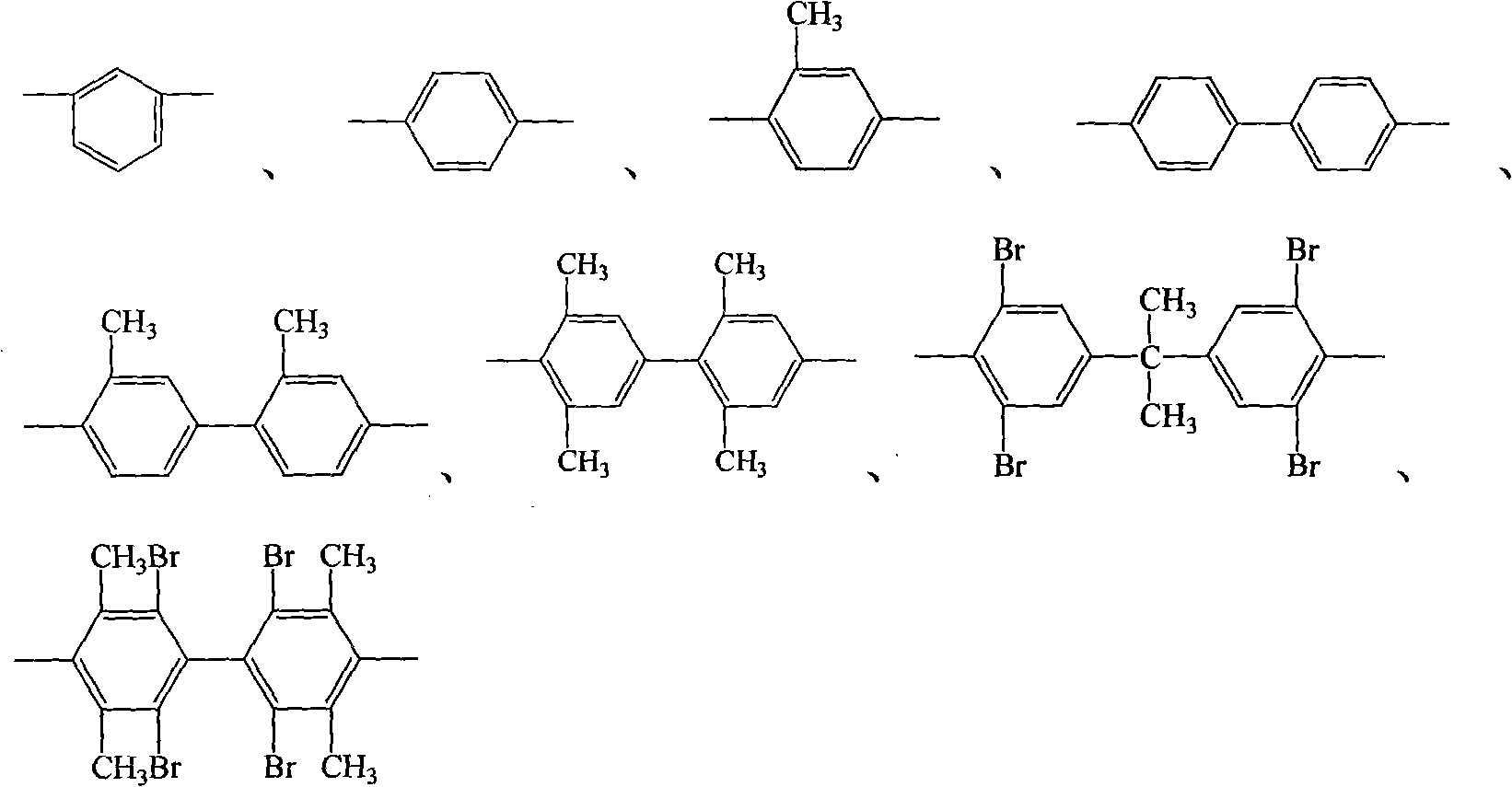
Process for synthesis of aryl bis-ether dianhydrides monomer
InactiveCN1634904AReduce usageShort melting rangeOrganic chemistryAcetic anhydrideReaction temperature
The invention relates to a method for synthesizing dianhydride monomer, especially a method for synthesizing aryl diether dianhydride by condensation, hydrolysis, acidification, and dehydration. In the invention, catalyst is added in the process of condensation for improving the yield of product; the solid content is 10-20%, reaction temperature is 7-100C., and reaction time is 15-48 hours; and the solvent consumption is reduced for reducing the impurity in condensed product. And diether tetracid in the diether dianhydride treated by dehydration is removed by dehydration in toluene or acetic acid and acetic anhydride and high temperature dehydration in vacuum oven sequentially, and the targeted product processed by said method has melting range less than 1C. and high purity.
Owner:NANJING UNIV OF TECH
A process for extraction and apparatus thereof
InactiveCN1546195AOvercome unresolvable flawsIncrease profitSolid solvent extractionFood preparationAdditive ingredientSolvent
The invention discloses a kind of extraction method and device. The method includes following steps: crushing and immersing the materials, carries on extraction under pressure of 25MPa-35MPa and frequency between 18 KHz-33 KHz by using water as solvent, the extraction liquid with material active ingredients can be gotten. The invention can protect the biology activity, and it can realize nearly fully extraction of ingredients.
Owner:曹培生
Method for preparing ethyl levulinate based on solid superacid catalysis and furfuryl alcohol alcoholysis
InactiveCN103274942ALow costEasy to separateOrganic compound preparationCarboxylic acid esters preparationAlcoholEthyl ester
The invention discloses a method for preparing ethyl levulinate based on solid superacid catalysis and furfuryl alcohol alcoholysis, and relates to ethyl levulinate. The method comprises the steps of adding furfuryl alcohol, a catalyst and a reaction solvent to a reaction kettle, reacting, and obtaining ethyl levulinate. The catalyst is selected from at least one of solid superacid of SO4<2-> / TiO2, SO4<2-> / ZrO2, SO4<2-> / Fe2O3, SO4<2-> / SnO2, and SO4<2-> / Al2O3, or at least one of solid superacid of S2O8<2-> / TiO2, S2O8<2-> / ZrO2, S2O8<2-> / Fe2O3, S2O8<2-> / SnO2, and S2O8<2-> / Al2O3; the reaction solvent is ethyl alcohol; and reaction conditions are that the temperature is 100-150 DEG C, the rotating speed is 300-500 rpm (revolutions per minute), the time is 0.5-2.5h, a mass ratio of furfuryl alcohol to solid superacid is (1-4):1, and a mass ratio of furfuryl alcohol to ethyl alcohol is (0.01-0.2):1.
Owner:XIAMEN UNIV
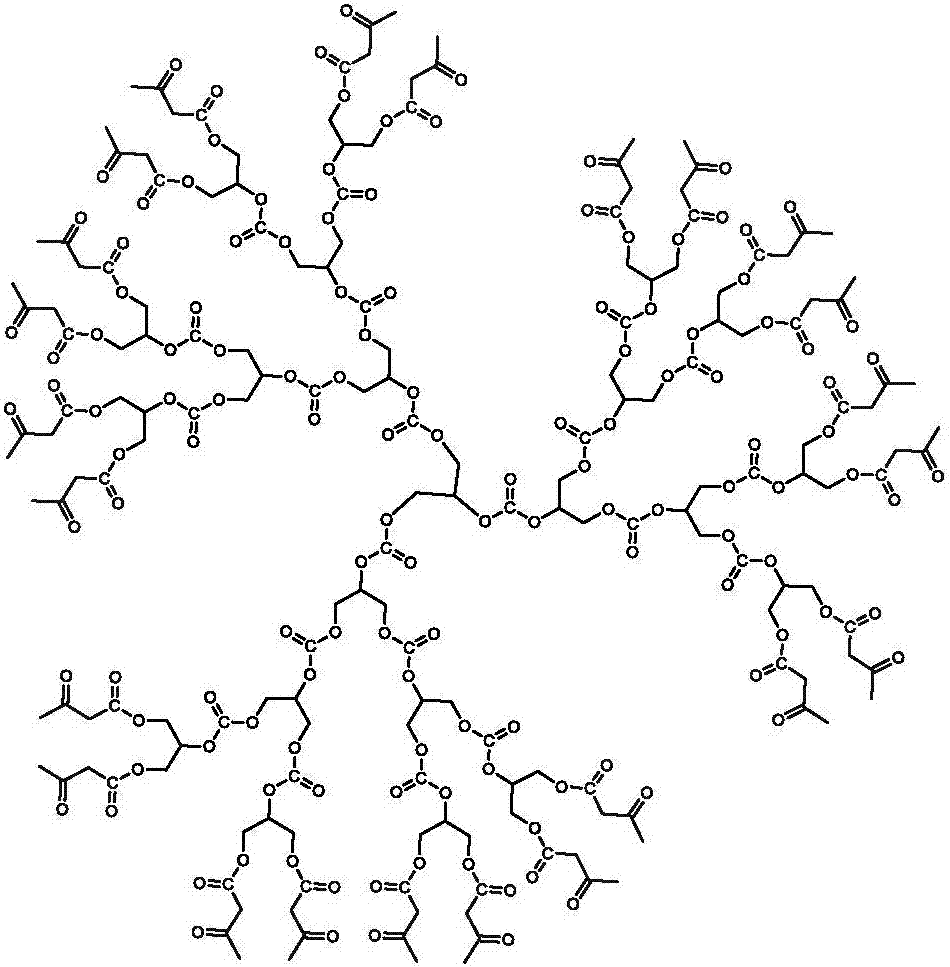
Carbonyl terminated hyperbranched polycarbonate capable of emitting bright fluorescence and preparation method thereof
The invention relates to carbonyl terminated hyperbranched polycarbonate capable of emitting bright fluorescence. Glycerol and diethyl carbonate are used as raw materials; a simple and controllable ester exchange condensation method is used for synthesizing the terminated hyperbranched polycarbonate; then, tert-Butyl acetoacetate is used for termination to obtain the hyperbranched polycarbonate. The synthesized hyperbranched polycarbonate does not contain pi keys such as benzene rings; the addition of fluorescent powder is not needed; the bright blue fluorescence can be given out; the characteristics of simple process, controllable process and low three-waste pollution are realized; in addition, the product stability is high; the toxicity is high; the biodegradability is high; the light intensity is high; the application range is wide.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
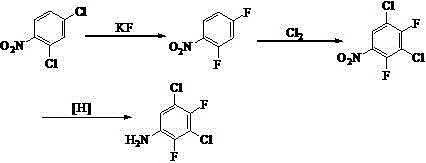
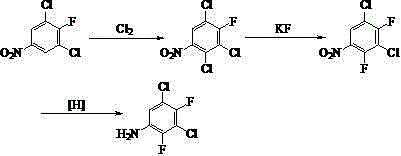
Synthetic method of 3, 5-dichloro-2, 4-difluoroaniline
ActiveCN102617360AQuality is not affectedObvious cost advantageOrganic compound preparationAmino compound preparationManufacturing technologyResource utilization
The invention discloses a synthetic method of 3, 5-dichloro-2, 4-difluoroaniline. The synthetic method takes distillation residues which are generated in the manufacturing technology that 2,6-dichlor fluorbenzene is nitrified to prepare 2, 4-dichloro-3-fluoronitrobenzene as raw material, and the distillation residues are chlorinated through chlorine gas and fluorated through potassium fluoride so as to prepare 3, 5-dichloro-2, 4-difluoro nitrobenzene; and finally, 3, 5-dichloro-2, 4-difluoro nitrobenzene is reduced to obtain 3, 5-dichloro-2, 4-difluoroaniline. The synthetic method adopts by-products that are generated in the conventional manufacturing technology line as raw material, is directly used for synthesizing 3, 5-dichloro-2, 4-difluoroaniline without any treatment, is characterized by low price of raw material, full resource utilization, mild reaction conditions, high yield coefficient of products, low manufacturing cost and the like compared with a conventional method for preparing 3,5-dichloro-2,4-difluoroaniline, is easy to get raw material, and is very suitable for industrialized production.
Owner:江西吉翔医药化工有限公司
Process for preparing solid phase polypeptide synthetic eptifibatide
ActiveCN1858060AConvenient sourceHigh peptide yieldPeptide preparation methodsPropanoic acidRink amide resin
The preparation process of solid phase polypeptide synthesized eptifibatide includes the following steps: (1) connecting amino acids one by one with Rink Amide resin, Rink Amide MBHA resin or Rink Amide AM resin as initial material, and Fmoc protected amino aids as monomer, with the last peptide chain being S-benzyl mercapto propionic acid Map(SBzl); (2) adding peptide cutting agent (TFA / HBr / HAc / TIS / EDT) to cut peptide; (3) precipitating and collecting coarse reductant eptifibatide product in ether solvent; (4) dissolving coarse reductant eptifibatide product in water, regulating pH value with ammonia water to 7.5-10.0, introducing air for oxidation, and collecting coarse eptifibatide product; and (5) separating and purifying the coarse product in C18 column to obtain the target product. The present invention has high yield, and is suitable for industrial production.
Owner:SHANGHAI SOHO YIMING PHARMA
Method for synthesizing atosiban acetate from solid phase polypeptide
ActiveCN101357937AConvenient sourceHigh peptide yieldPeptide preparation methodsBulk chemical productionPreparative hplcSide chain
The invention discloses a preparation method of solid phase peptide synthesis atosiban which includes the following steps: taking Rink Amide resins, Rink Amide MBHA resins or Rink Amide AM resins as starting materials and taking Fmoc amino acids as monomers, amino acids are grafted one by one and mercaptopropionic acids (Map(SX)) are protected by the last peptide chain; after protected nonapeptide resins are obtained, the acellular side-chain protective groups and cutting peptides are synchronized; then cutting peptides is carried out, and reduced crude atosiban is collected; the pH value is adjusted to 7.5 to 10.0, and oxidized crude atosiban is collected; target products are obtained by the separation and purification by preparative HPLC(C18 or C8 column). The preparation method is convenient in material source, simplifies technology and reduces cost; and the preparation method is low in the pollution of three wastes and is high in yield, and the preparation method is convenient for being industrialized and has good industrialization prospect.
Owner:SHANGHAI SOHO YIMING PHARMA
Polypeptide synthesis method for octreotide acetate
ActiveCN103351426AConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionFluoroacetic acidAceric acid
The invention relates to a polypeptide synthesis method for octreotide acetate. The method comprises the following steps of: taking chloromethyl resin as a starting raw material, preparing a cesium salt from Boc-Thr(tBu)-OH, sequentially connecting amino acids with protecting groups according to a solid-phase synthesis method so as to obtain protected octapeptide resin, meanwhile, removing Boc protecting groups by sequentially using HCl / isopropyl alcohol, carrying out peptide connecting reaction in a manner of taking DIC and HOBT as condensing agents, carrying out reduction by using palladium carbon / hydrogen gas, meanwhile, cutting off peptide chains so as to obtain reduced octreotide, introducing air at the Ph of 7.8-9 so as to cyclize disulfide linkages, then, obtaining a crude octreotide product, and carrying out separation and purification through a C18 column, thereby preparing a fine octreotide acetate product. The method disclosed by the invention has the advantages that threoninol and Fmoc-threoninol are not adopted, the production cost is very low, the method has large-scale production capacity, the process is stable, the raw and auxiliary materials are convenient to obtain, the production cycle is short, the yield of connected peptide is high, the quality is stable, the use of highly-toxic reagents, such as hydrogen fluoride, trifluoroacetic acid and the like, is avoided, and the pollution caused by waste gas, waste water and waste residues is little.
Owner:SHANGHAI SOHO YIMING PHARMA
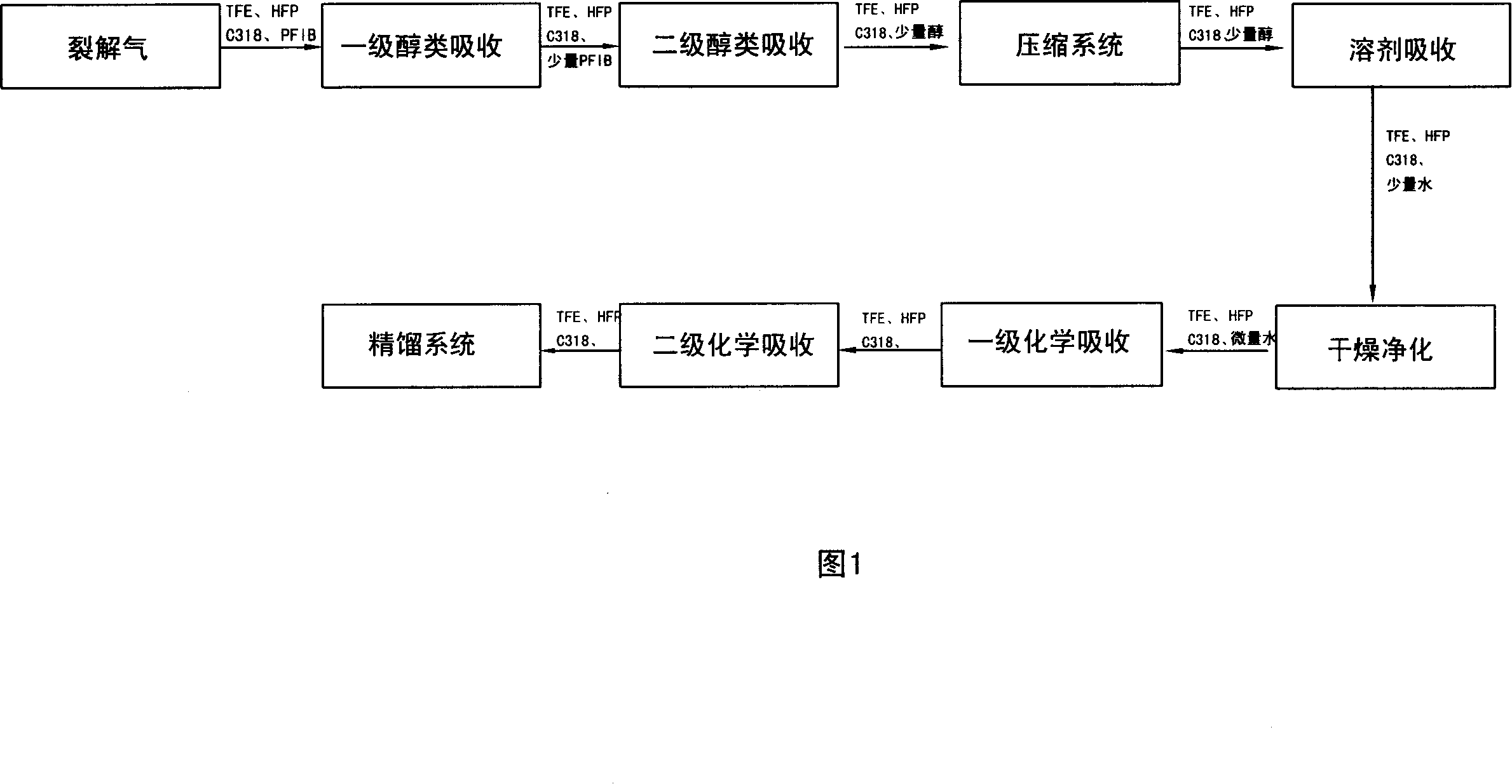
Removed method for poisonous component of cleaved product in hexafluoropropylene manufacturing technique
ActiveCN1923775AGuaranteed smooth productionEasy to separateHalogenated hydrocarbon preparationAlcoholManufacturing technology
The invention discloses a removing method of toxic component in the hexafluoropropylene manufacturing technology, which comprises the following steps: adopting cracking gas in the manufacturing course to enter into distilling system before adsorbed by alcohol material to remove toxic component; proceeding solvent adsorption; purifying; proceeding chemical adsorption; adopting alcohol material as adsorbant; detoxifying octafluoroisobutylene in the cracking gas.
Owner:SHANDONG DONGYUE POLYMER MATERIAL
Process for producing aromatic diaether dianhydride monomer
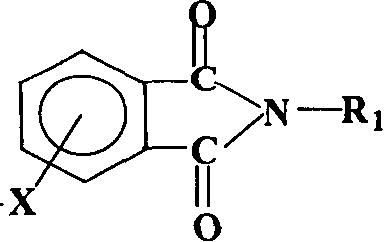
The invention relates to a method for synthesizing a dianhydride monomer, in particular to a method for synthesizing an aromatic HQEDA monomer. The method adopts a 3(4) substituted phthalimide to prepare HQEDA and provides novel middle steps for preparing the HQEDA. The method comprises the following steps: preparing a 3 (4) substituted-N-alkyl (aryl) phthalimide from the 3(4) substituted phthalimide through Gabriel reaction principle or by adding a salifying agent and halocarbon into an apolar aprotic solvent to react; and using the prepared 3 (4) substituted-N-alkyl (aryl) phthalimide to prepare bis imide and the bis ether anhydride. The method has the advantages that raw materials are easily available, preparation method is simple and easy to operate, and the purity and yield of the prepared 3 (4) substituted-N-alkyl (aryl) phthalimide are obviously higher than that of the prior method.
Owner:NANJING UNIV OF TECH
Process of producing sodium glutamate
ActiveCN101033478AGoodwill cleaner productionReduce the pollution of three wastesFermentationCentrifugationNitrogen source
This invention provides a new technology of producing sodium glutamate, including the following steps: first, nitrogen source and alkali sodium are mixed and added into broth to get a broth containing sodium glutamate crude product and its pH is 6.8 to 7.2. Then the broth is filtered through the membrane and followed by centrifugation to get a centrifuged broth. The broth is isolated and purified through the ion exchange resin for, and the effluent is collected and condensed to get glutamate sodium after crystallization.
Owner:JIANGNAN UNIV +1
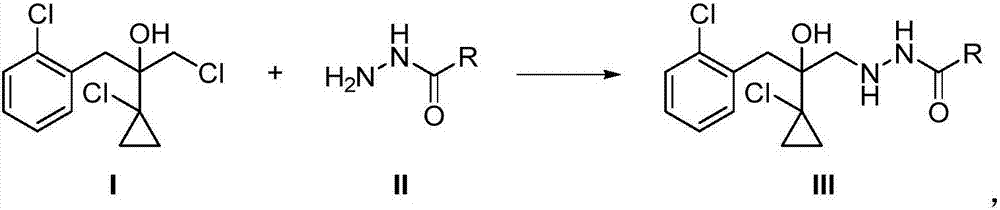
Preparation method of prothioconazole intermediate
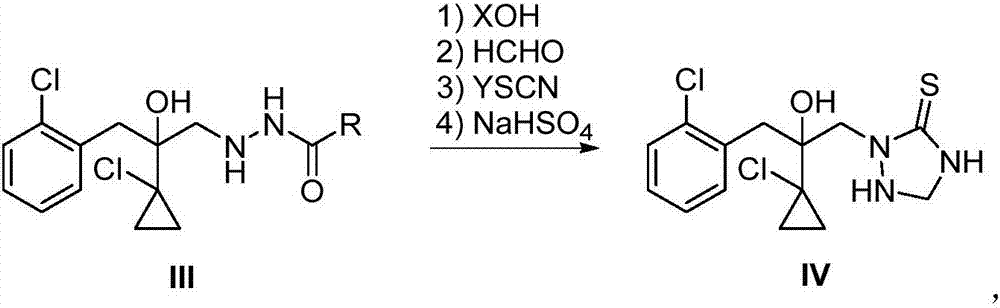
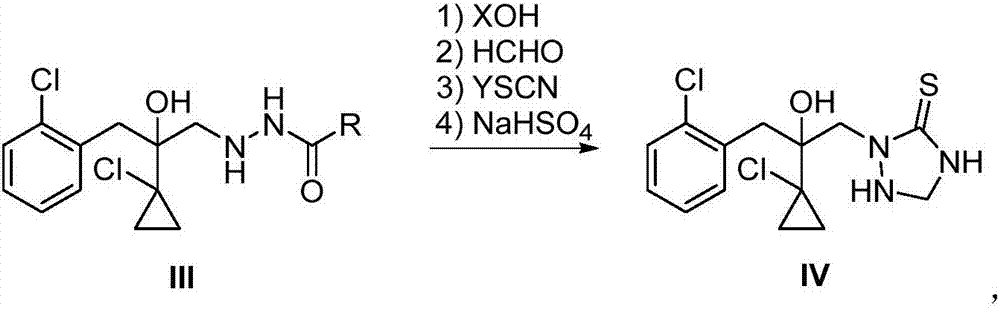
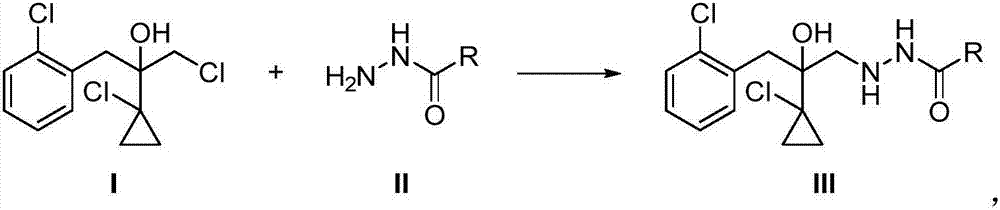
The invention relates to a preparation method of a prothioconazole intermediate. The method sequentially comprises the following steps: enabling a compound I and a compound II to react at the temperature of 20-120 DEG C in presence of a solvent; after the reaction is finished, treating to obtain a compound III; enabling the compound III to react with XOH, formaldehyde, YSCN and sodium hydrogen sulfate at the temperature of 10-80 DEG C in presence of a solvent so as to obtain a compound IV 2-(1-chloro-cyclopropyl-1-yl)-1-(2-chlorphenyl)-2-hydroxyl-3-(1,2,4-triazolidine-5-thioketone-1-yl)-propane, namely, the prothioconazole intermediate. Compared with the reported methods, the preparation method provided by the invention not only enables the intermediate to be more stable, but also reduces the amount of reagents, simplifies the operation steps, avoids the occurrence of side effects, increases the reaction efficiency and is less in pollution caused by the three wastes, thus being environmentally friendly and suitable for industrial production.
Owner:JIANGSU SEVENCONTINENT GREEN CHEM CO LTD
Industrial production method for ethoxy quinoline
The invention relates to an industrial production method for ethoxy quinolone. The industrial production method comprises the following steps: adding p-phenetidine, a diluent and a home-made chelate catalyst serving as raw materials into a reaction kettle; heating to 125-165 DEG C; dropwise adding acetone to perform a synthetic reaction; preserving heat for half an hour after finishing dropwise adding of the acetone; recovering the diluent by distilling; fractionating under a reduced pressure to obtain the ethoxy quinolone. The ethoxy quinolone is over 98 percent in purity, is less than 0.5 percent in raw material residues, and is over 90 percent in total yield.
Owner:张加明 +1
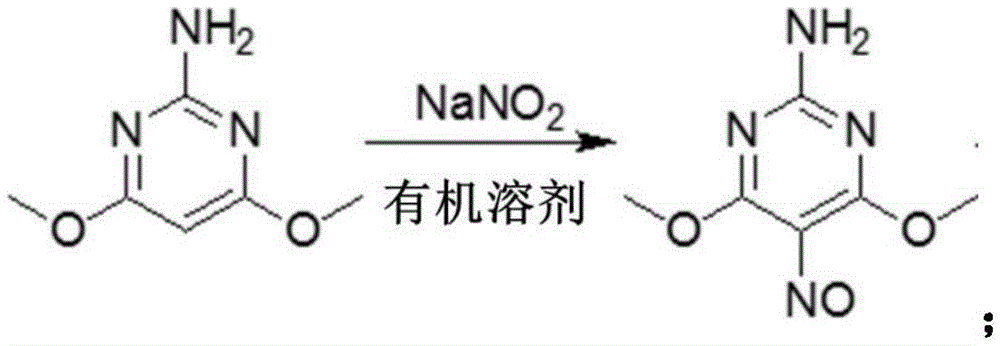
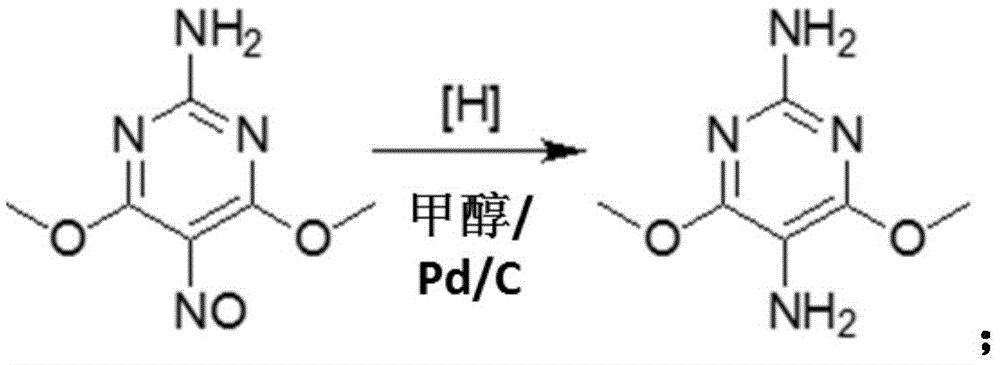
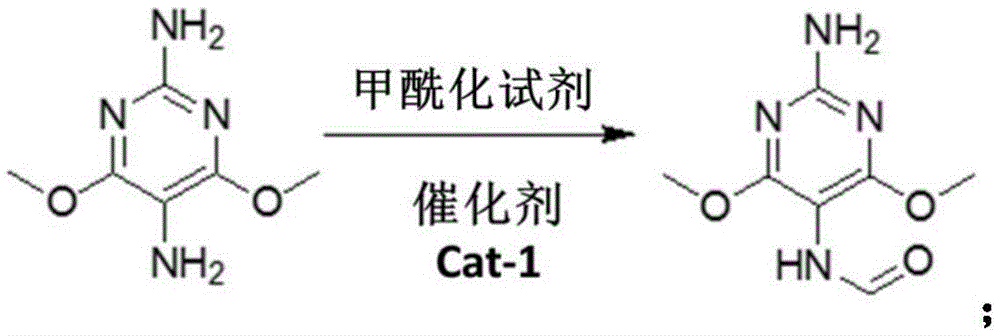
Preparation method of 2-amino-4,6-dichloro-5-formamine pyrimidine
The invention provides a preparation method of 2-amino-4,6-dichloro-5-formamine pyrimidine. According to the preparation method of the 2-amino-4,6-dichloro-5-formamine pyrimidine, 2-amino-4,6-dimethoxy pyrimidine is used as a basic raw material, and the 2-amino-4,6-dichloro-5-formamine pyrimidine is synthesized through reactions of nitrosation, hydrogenation reduction, formylation, alkaline hydrolysis, chlorination and the like. By using the method provided by the invention, a new process route for preparing the FADCP (2-amino-4,6-dichloro-5-formamine pyrimidine) is established; the preparation method has innovative significance and a utility value; in addition, the preparation method provided by the invention has the characteristics that a process is smooth, a reaction condition is mild, a productive cycle is quick, the consumed time is shorter, three wastes are easily treated, the pollution by the three wastes are reduced, and the like; moreover, product purity is more than 98 percent; a total yield is up to above 79 percent; the preparation method is suitably applied to industrial production.
Owner:INSIGHT FINECHEM +1
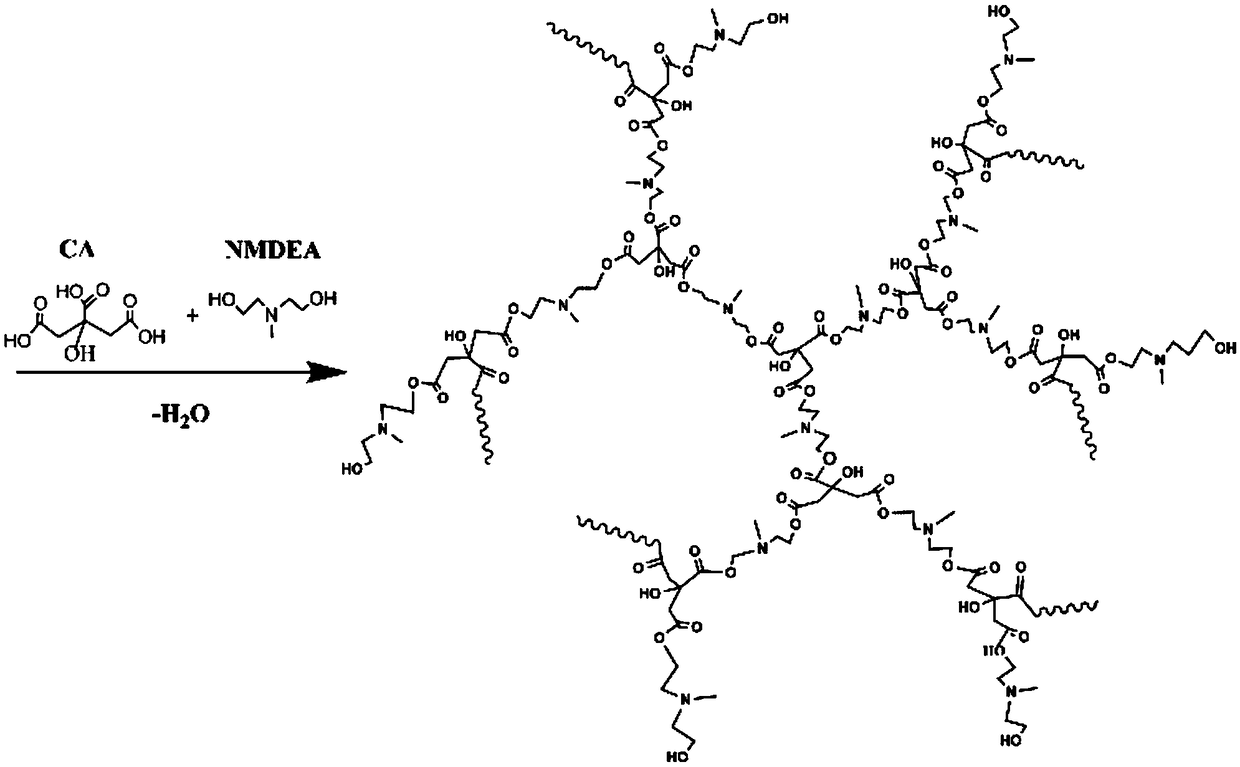
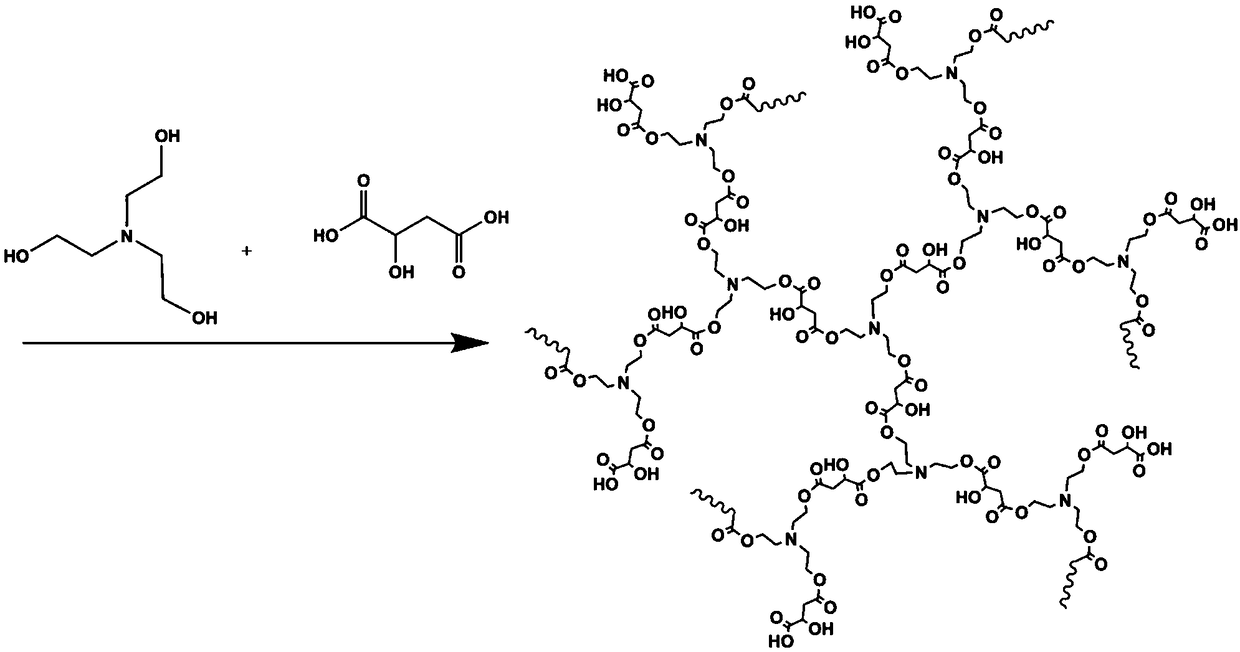
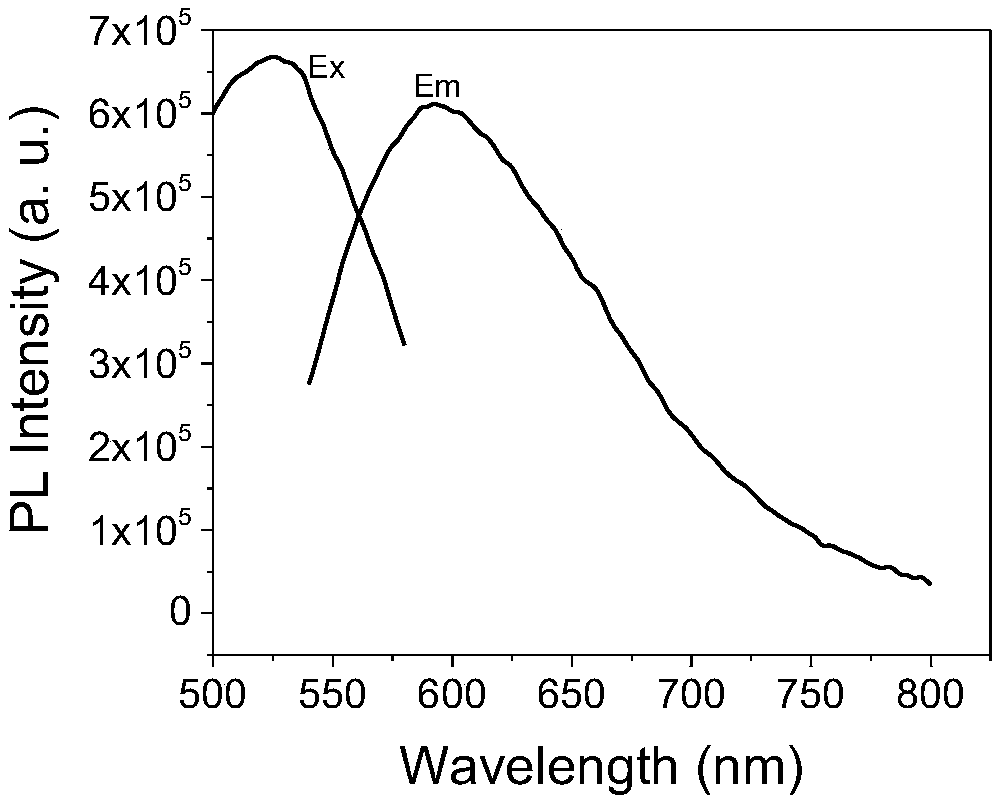
Hyperbranched polyaminoester capable of emitting multicolor fluorescence and preparation method thereof
InactiveCN108727576AEasily biodegradableThe synthesis process is simpleLuminescent compositionsUltraviolet lightsPollution
The invention relates to hyperbranched polyaminoester capable of emitting multicolor fluorescence and a preparation method thereof. Citric acid and N-methyldiethanolamine (or malic acid and triethanolamine) are used as raw materials to be synthesized into the hyperbranched polyaminoester by a simple controllable polycondensation method. The synthesized hyperbranched polyaminoester does not containbenzene rings and only contains ester groups; the biodegradation is easy; the hyperbranched polyaminoester can emit the multicolor fluorescence. The synthesized hyperbranched polyaminoester can emitbright fluorescence of different colors (including red, yellow, blue and the like) under the irradiation of different wavelength ultraviolet light. The method has the characteristics that the synthesis process is simple and convenient; the process is controllable; the environment-friendly effect is achieved and the like; the three-waste pollution is little; the product stability is high; the toxicity is low; the biodegradability is high; the fluorescence intensity is high; the colors are rich; the application range is wide, and the like.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Superfine fiber one-step functional producing method
InactiveCN1888155AReduce manufacturing costGood antibacterial functionMelt spinning methodsMonocomponent polyesters artificial filamentFiberMetallurgy
The present invention relates to a production method of super fine fibre, in particular it relates to a production method of colored functional super fine fibre. Said production method includes the following several steps: directly mixing super fine fibre raw material with coloring material, adjuvant and functional additive according to a certain mixing ratio to obtain colored anti-bacterial mother materi, melting said mother material at high temperature, extruding said molten mother material by using twin-screw extruder and making basic hydrolysis so as to obtain the invented product.
Owner:宁波新顺化纤有限公司
Preparation of octreotide acetate and octreotide acetate injection pharmaceutical composition
ActiveCN106866788AConvenient sourceReduce usagePeptide/protein ingredientsDigestive systemDisulfide bondSolid-phase synthesis
The invention relates to preparation of octreotide acetate and an octreotide acetate injection pharmaceutical composition. Specifically, the invention relates to a method for preparing the octreotide acetate; the method comprises the following steps: taking merrifield resin as a starting raw material and preparing Boc-Thr(tBu)-OH into cesium salt; sequentially connecting amino acid with protecting groups according to a solid-phase synthesis method to obtain protected octapeptide resin; removing Boc-protecting groups in sequence by utilizing HCl / isopropyl alcohol and carrying out peptide linking reaction by utilizing a condensing agent; reducing with palladium-carbon / hydrogen; meanwhile, chopping off a peptide chain to obtain reduced octreotide; ventilating air under the condition that the pH (Potential of Hydrogen) is 8 to 9 to form a ring by a disulfide bond, so as to obtain an octreotide crude product; separating and purifying the octreotide crude product through a C18 column to prepare refined octreotide. The invention further relates to the octreotide acetate injection pharmaceutical composition. The method for preparing the octreotide acetate and the octreotide acetate injection pharmaceutical composition, provided by the invention, have the excellent technical effects shown in the description.
Owner:CHENGDU TIANTAISHAN PHARMA
Method for synthesizing 3,5-diamido benzoic acid by industrial continuous hydrogenation
ActiveCN102249942AMeet conversion rate requirementsMild reaction conditionsOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidPtru catalyst
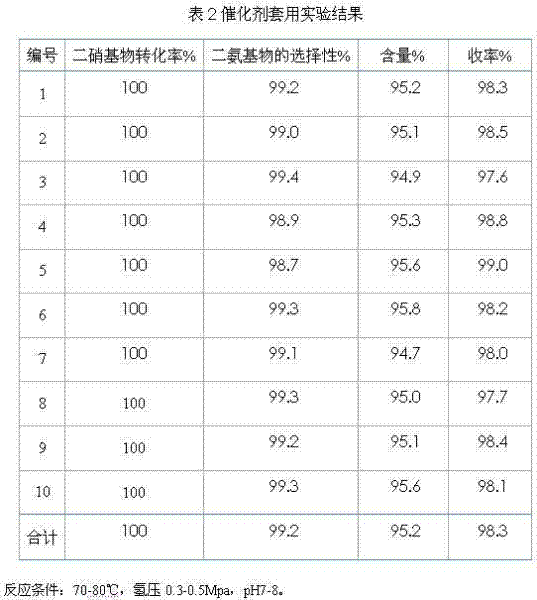
The invention discloses a method for synthesizing 3,5-diamido benzoic acid by industrial continuous hydrogenation. The method comprises the following steps: by taking m-dinitrobenzoic acid and hydrogen as raw materials and water as a reaction solvent, adding water and a catalyst in a reduction kettle, in addition, respectively adding the raw material, namely m-dinitrobenzoic acid and hydrogen for reduction reaction; carrying out further reaction on a reaction liquid in second and third reduction kettles until the conversion rate of the raw materials reaches 100%; and then crystallizing the reaction liquid so as to obtain the product 3,5-diamido benzoic acid. In the method, three reduction kettles which are successively connected are adopted so as to complete the reduction reaction, the contact time of the raw materials is long, the conversion rate of the raw materials can reach 100%, the appearance of the obtained product is of grey white powder, the chromatograph purity of the product is above 99%, the content of the product is above 95%, and the yield of the product is above 97%.
Owner:济宁市金泰利华化工科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com