Preparation method of prothioconazole intermediate
A technology for prothioconazole and intermediates, which is applied in the field of preparation of fungicide prothioconazole intermediates, can solve the problems of increasing solid waste in reaction routes, increasing operation steps, etc., achieves less pollution of three wastes, simplifies operation steps, and saves The effect of high rate and content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
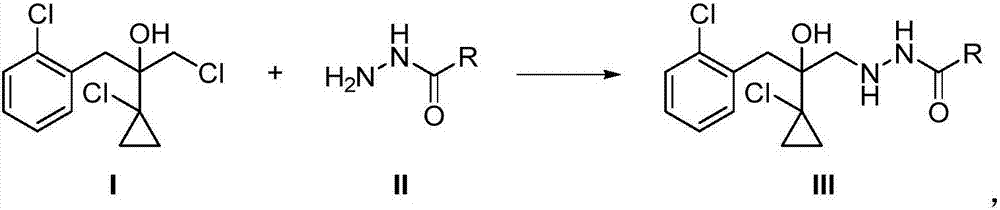
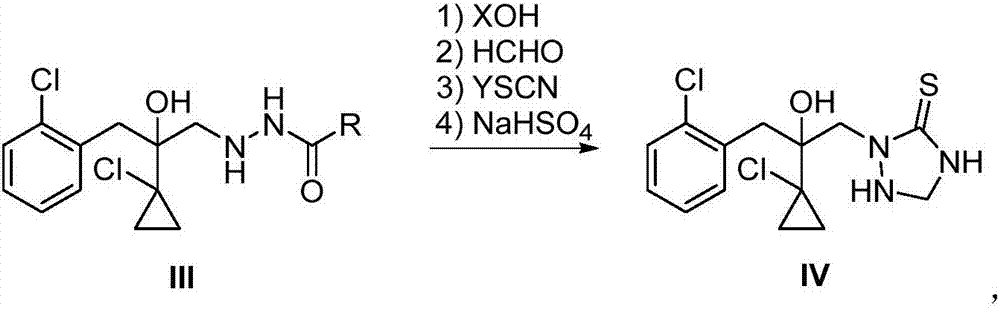
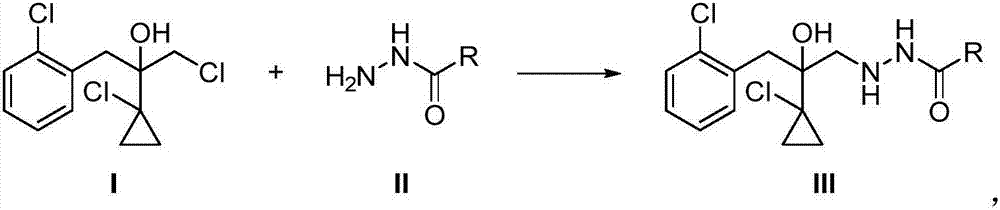
[0033] The 2-(1-chloro-cyclopropan-1-yl)-1-(2-chlorophenyl)-2-hydroxyl-3-(1,2,4-triazolidine-5-thione of this embodiment The preparation method of -1-yl)-propane has the following steps:
[0034] Step (1), 2-(1-chlorocyclopropyl)-3-chloro-1-(2-chlorophenyl)-2-propanol I (28.0g, 0.10mol) was dissolved in acetonitrile (50mL) , followed by addition of potassium carbonate (13.8 g, 0.10 mol) and ethyl carbazate II (11.5 g, 0.11 mol). The reaction solution was stirred at 80°C for 4 hours. After the reaction, water (50 mL) was added to the reaction solution, followed by extraction with ethyl acetate (extraction three times, 50 mL each time), the organic phases were combined, dried over sodium sulfate and concentrated. 36.8 g of crude product was obtained, with a content of 83.4%. The obtained crude product was recrystallized from ethanol to obtain compound III (30.7 g), a pale yellow solid with a content of 95% and a yield of 84%. The NMR data of compound III are as follows:
[...
Embodiment 2
[0039] The 2-(1-chloro-cyclopropan-1-yl)-1-(2-chlorophenyl)-2-hydroxyl-3-(1,2,4-triazolidine-5-thione of this embodiment The preparation method of -1-yl)-propane has the following steps:
[0040] Step (1), 2-(1-chlorocyclopropyl)-3-chloro-1-(2-chlorophenyl)-2-propanol I (28.0g, 0.10mol) was dissolved in acetonitrile (50mL) , followed by addition of triethylamine (10.1 g, 0.10 mol) and ethyl carbazate II (11.5 g, 0.11 mol). The reaction solution was stirred at 80° C. for 3 hours. After the reaction, water (50 mL) was added to the reaction solution, followed by extraction with ethyl acetate (extraction three times, 50 mL each time), the organic phases were combined, dried over sodium sulfate and concentrated. 37.5 g of crude product was obtained, with a content of 86%. The obtained crude product was recrystallized from ethanol to obtain compound III (33.1 g), a pale yellow solid with a content of 95% and a yield of 91%.
[0041] Step (2), sodium hydroxide (3.60 g, 0.09 mol) ...
Embodiment 3
[0043] The 2-(1-chloro-cyclopropan-1-yl)-1-(2-chlorophenyl)-2-hydroxyl-3-(1,2,4-triazolidine-5-thione of this embodiment The preparation method of -1-yl)-propane has the following steps:
[0044] Step (1), 2-(1-chlorocyclopropyl)-3-chloro-1-(2-chlorophenyl)-2-propanol I (28.0g, 0.10mol) was dissolved in acetonitrile (50mL) , followed by the addition of triethylamine (10.1 g, 0.10 mol) and acetylhydrazide II (8.15 g, 0.11 mol). The reaction solution was stirred at 80° C. for 3 hours. After the reaction, water (50 mL) was added to the reaction solution, followed by extraction with ethyl acetate (extraction three times, 50 mL each time), the organic phases were combined, dried over sodium sulfate and concentrated. 36.3 g of crude product was obtained, with a content of 81%. The obtained crude product was recrystallized from ethanol to obtain compound III (30.0 g), a pale yellow solid with a content of 95% and a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com