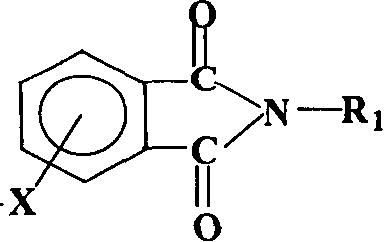
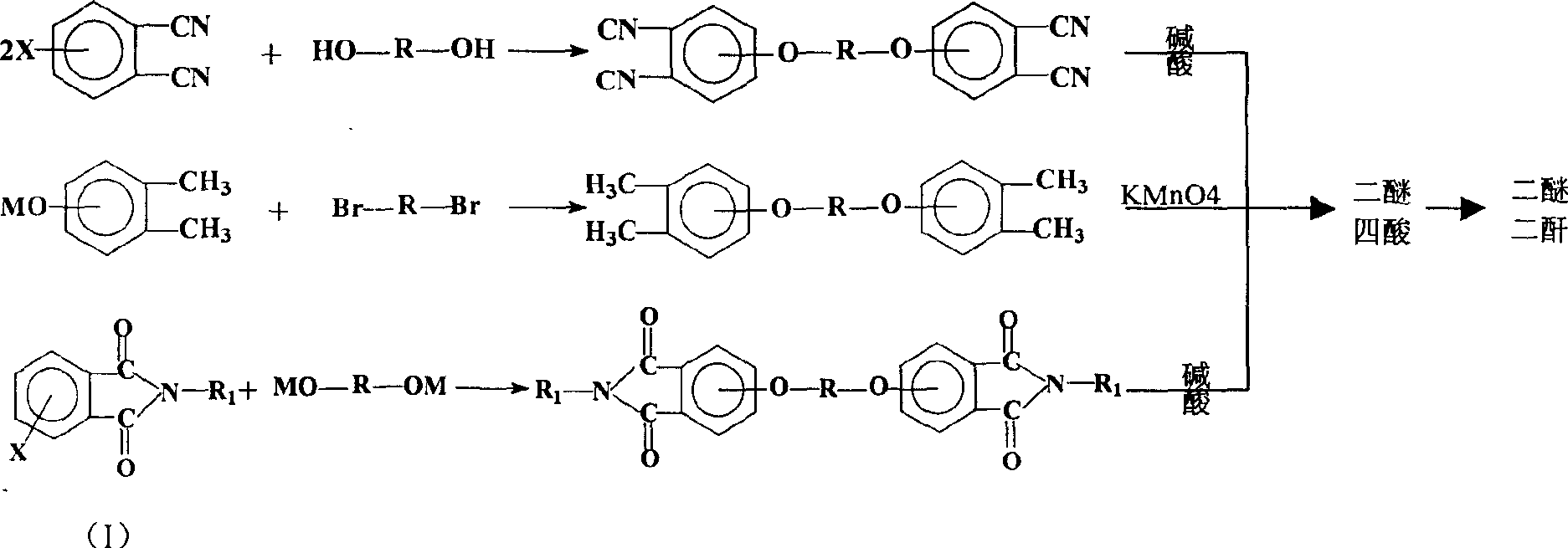
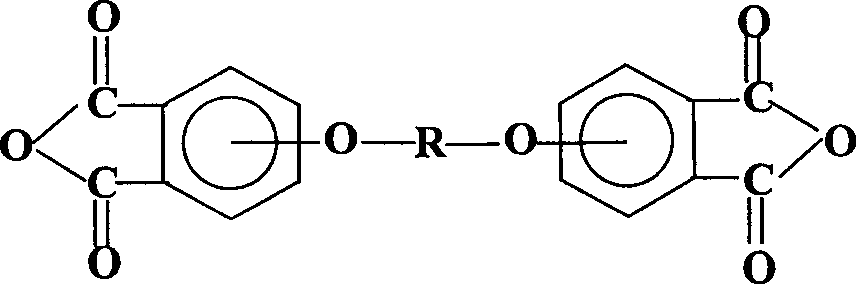Process for synthesis of aryl bis-ether dianhydrides monomer
A synthesis method and aryl diether technology are applied in the synthesis field of dianhydride monomers, which can solve the problems of large amount of solvent, waste of solvent, low solid content, etc., and achieve the improvement of reaction yield, the reduction of three-waste pollution, and the recovery of waste. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Add 11.0g of hydroquinone to a 500ml three-necked flask, blow nitrogen, add 50ml of dimethyl sulfoxide, 16g of 50% sodium hydroxide aqueous solution, and then add 20ml of benzene, reflux to remove water for 5 hours, and distill the benzene out. After cooling to 80°C, add 41.2g of 4-nitro-N-methylphthalimide, 1.8g of catalyst, and 300ml of dimethyl sulfoxide, and react at 80°C for 12 hours. After the reaction, the solvent was distilled off under reduced pressure. Add 400ml of hydrochloric acid, filter, and recrystallize the solid with ethylene glycol to obtain triphenylbis(ether) diphthalimide.
[0053] Weigh 42.8 g of the condensation product obtained above, add 267 g of 12% NaOH aqueous solution, heat to reflux for 24 hours under nitrogen flow, filter after cooling, add hydrochloric acid to the solution to make the pH ≤ 1, and filter to obtain triphenyl ether tetraacid.
[0054] Weigh 43.8 g of the triphenylenedither tetraacid obtained above, add 20.4 g of acetic anh...
Embodiment 2
[0056] Add 25.0g bisphenol S to a 1000ml three-necked flask, blow nitrogen, add 120ml dimethyl sulfoxide, 16g 50% sodium hydroxide aqueous solution, then add 40ml benzene, reflux to remove water for 5 hours, and steam the benzene out after the water separation is completed . After cooling to 70°C, add 41.2g of 4-nitro-N-methylphthalimide, 1.2g of catalyst, and 320ml of dimethyl sulfoxide, raise the temperature to 90°C, and react for 18 hours. After the reaction, the solvent was distilled off under reduced pressure. Add 400ml of hydrochloric acid, filter, and recrystallize the solid from methanol.
[0057] Weigh 56.8g of the condensation product obtained above, add 210g of 15% NaOH aqueous solution, heat to reflux for 24 hours under nitrogen, filter after cooling, add hydrochloric acid to the solution to make the pH ≤ 1, and filter to obtain bisphenol S-type diether tetraacid.
[0058] Weigh 57.8 g of the diether tetraacid obtained above, add 25 g of acetic anhydride and 70 g...
Embodiment 3
[0060] Add 18.6g of biphenol into a 1000ml three-necked flask, blow nitrogen into it, add 90ml of dimethyl sulfoxide, 16g of 50% sodium hydroxide aqueous solution, and then add 30ml of benzene, reflux to remove water for 5 hours, and evaporate the benzene after the water separation is completed. out. After cooling to 70°C, add 41.2g of 4-nitro-N-methylphthalimide, 1.0g of catalyst, and 400ml of dimethyl sulfoxide, raise the temperature to 90°C, and react for 24 hours. After the reaction, the solvent was distilled off under reduced pressure. Add 400ml of hydrochloric acid, filter, and recrystallize the solid with methanol to obtain biphenyl-type diether diphthalimide.
[0061] Weigh 50.4 g of the condensation product obtained above, add 420 g of 12% NaOH aqueous solution, heat and reflux for 24 hours under nitrogen flow, filter after cooling, add hydrochloric acid to the solution to make the pH ≤ 1, and filter to obtain biphenyl bis-ether tetra-acid.
[0062] Weigh 51.4 g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com