Diosgenin anti-tumor derivative and synthesizing method thereof
A technology of diosgenin and a synthesis method, which is applied in the field of medicine, can solve the problems of low oral availability, poor antitumor activity, high fat solubility of diosgenin, etc., and achieves the effects of high inhibitory activity and low cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
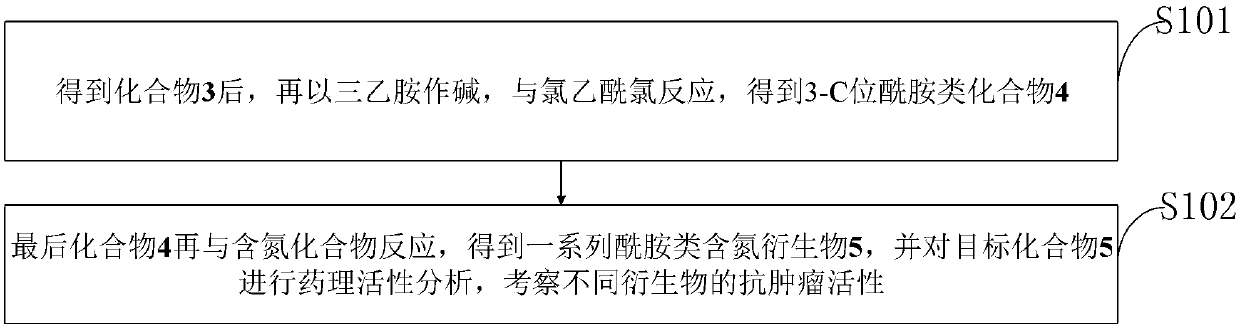
[0026] In order to make the object, technical solution and advantages of the present invention more clear, the present invention will be further described in detail below in conjunction with the examples. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
[0027] In the prior art, the application of diosgenin in medicine is limited; by transforming the 3-position of diosgenin A ring into a compound with an amide nitrogen-containing structure, there are few reports on the synthesis of similar derivatives.
[0028] Diosgenin molecular formula of the present invention is:
[0029]
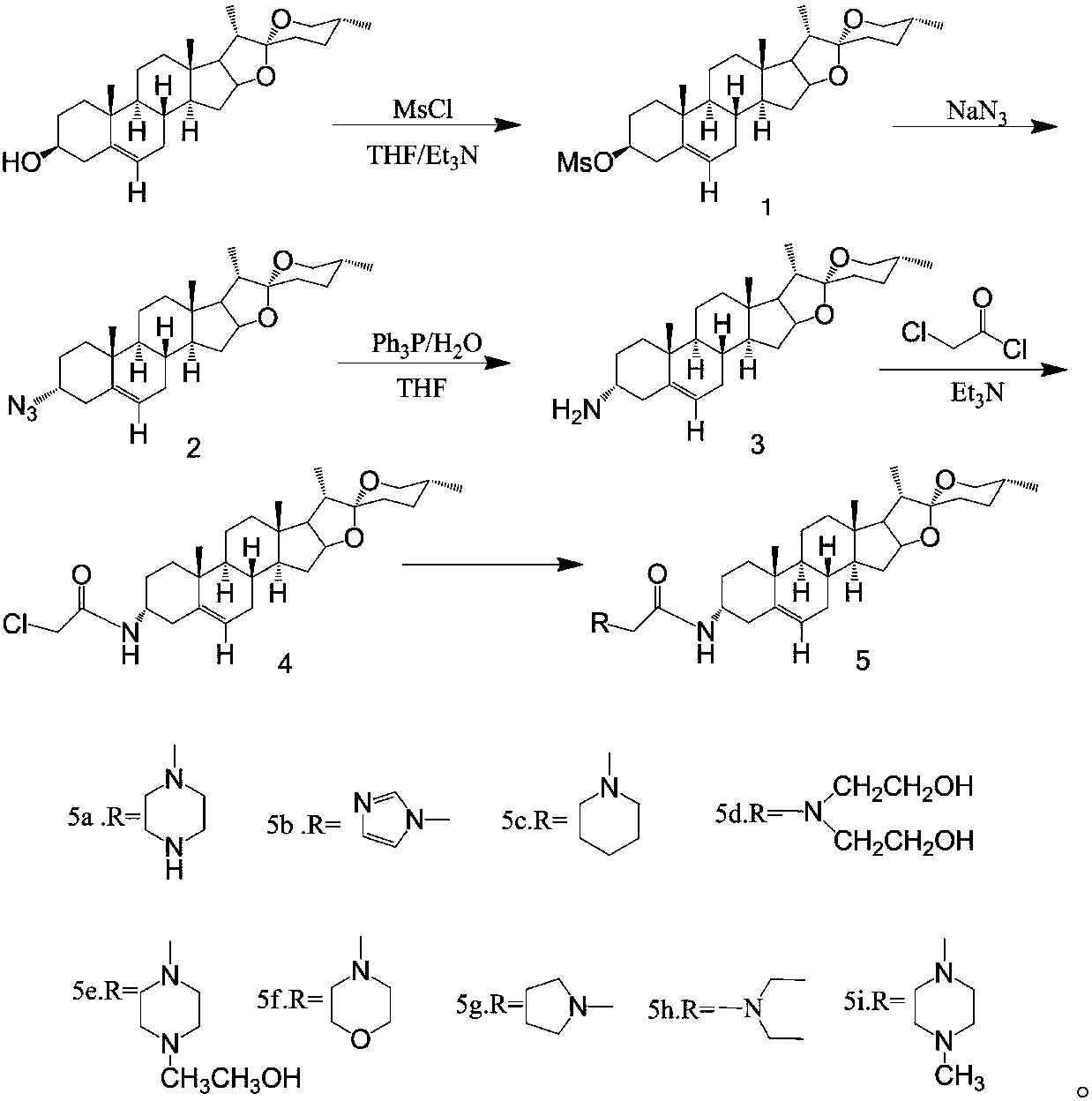
[0030] The application principle of the present invention will be described in detail below in conjunction with the accompanying drawings.
[0031] The synthesis method of diosgenin anti-tumor derivatives provided in the examples of the present invention uses diosgenin as a substrate, azides the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com