Preparation methods of anticoagulant and key intermediate of anticoagulant
A technology of anticoagulant drugs and intermediates, which is applied in the field of preparation of anticoagulant drugs and their key intermediates, can solve the problems of complex components, many side reactions, and affecting the reaction yield, and achieve simple and easy process. The effect of the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
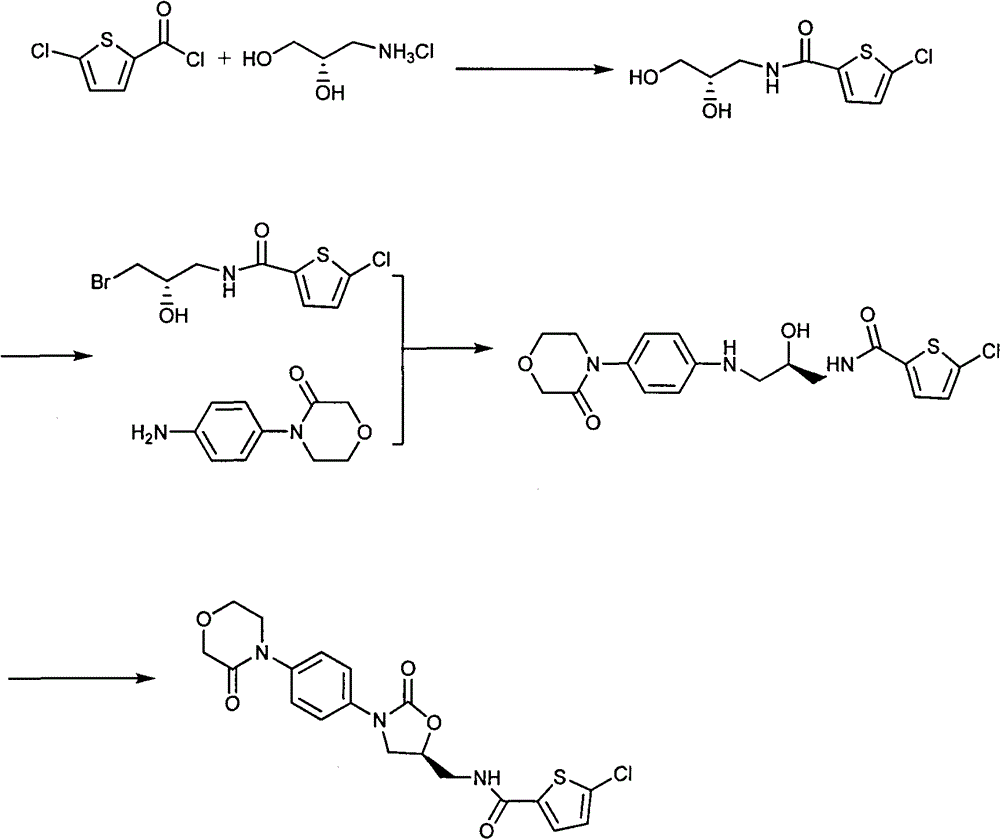
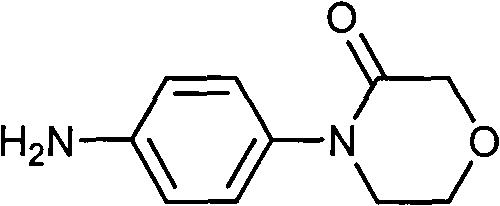
[0029] Embodiment 1: the preparation of 4-[(4-aminophenyl)-]3-morpholinone (II)
[0030] 1.1 Preparation of N-{2-(O-chloroacetyl)-hydroxyethyl}-chloroacetamide
[0031] Suspend 255g of potassium carbonate in 1400ml of dichloromethane, cool to 0°C, add 173.7g of chloroacetyl chloride dropwise for about 1 hour, after the addition is complete, add 100ml of ethanolamine in dichloromethane solution (containing 40g of ethanolamine), dropwise Adding time is about 3 hours. After the addition is complete, react at 0°C for 30 minutes, then raise the temperature to 20°C for 3 hours. After the reaction is completed, cool to 0°C, add 400ml of water, separate layers, wash the organic layer with 400ml×2 water, dry over anhydrous sodium sulfate, filter, concentrate to dryness, add 200ml of methyl tert-butyl ether, heat to dissolve, Crystallize at 0°C for 1 hour, filter, and dry to obtain 118 g of a white solid, with a yield of 83%. 1 H-NMR (CDCl 3 ): δ3.6-3.7 (m, 2H) δ4.09 (s, 2H) δ4.11 (s...
Embodiment 2
[0040] Embodiment 2: the preparation of rivaroxaban
[0041] 2.1 Preparation of (5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidin-5-yl]-phenyl}-morpholin-3-one hydrogensulfate
[0042] 26g 2-({(5S)-2-oxo-3-[4-(3-oxo-4-morpholine)phenyl]-1,3-oxazolidin-5-yl}methyl)-1H-iso Indole-1,3(2H)-dione was suspended in 195ml of absolute ethanol, 31.2g of 25% methylamine aqueous solution was added to the reaction solution, the temperature was raised to 65°C, the temperature was kept for 2 hours, 5g of activated carbon was added, and filtered. Add 10 g of concentrated sulfuric acid dropwise, after the addition is complete, cool to 20° C. for crystallization for 1 hour, filter, beat with 50 ml of ethanol, and dry to obtain 20.9 g of white solid with a yield of 87%.
[0043] 2.2 The preparation method of rivaroxaban (1)
[0044] 167g 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidin-5-yl]-phenyl}-morpholin-3-one sulfhydryl Suspend in 500ml of dichloromethane at room temperature, add 135g of triethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com