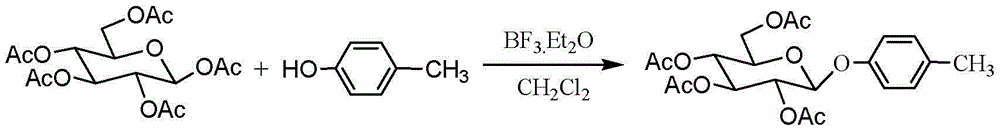Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
95results about How to "Simplify purification operations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
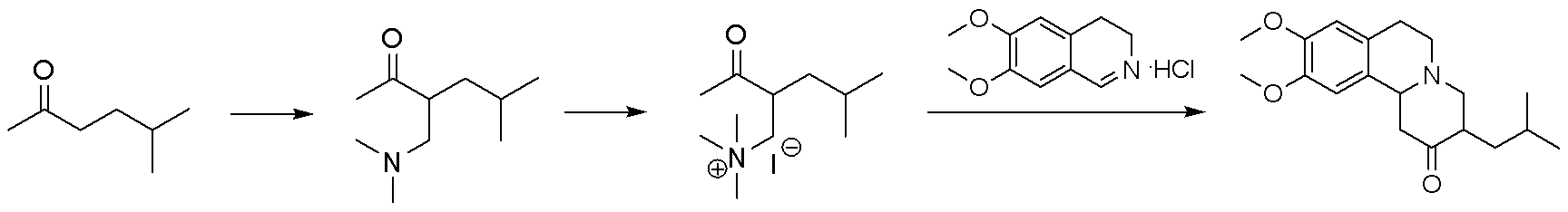
Method for synthesizing tetrabenazine
The invention discloses a method for synthesizing tetrabenazine. The method comprises: step one, using a dimethylamine aqueous solution and a formaldehyde aqueous solution as initial materials to be reacted1 to obtain tetramethyl methane diamine; step two, dissolving the tetramethyl methane diamine obtained in the step one in an organic solvent, dropwise adding acetyl chloride and 5- methyl-2-hexanone, and performing amine methylation to obtain an intermediate 3-[(dimethyl amino) methyl]-5-methyl-2-hexanone; and step three, reacting the 3-[(dimethyl amino) methyl]-5-methyl-2-hexanone obtained in the step two with 6,7-dimethoxy-3,4-dihydroisoquinoline hydrochloride to obtain the tetrabenazine. According to the method for synthesizing tetrabenazine, the cheap and accessible materials are used as the initial materials, imine salts serve as amine methylation reagents to perform amine methylation reaction on the 5- methyl-2-hexanone, and accordingly, the region-selectivity of chemical reactions is improved; and water serves as a reaction solvent to prepare the tetrabenazine, so that the operation is simple and convenient, no complex post-processing process exists, and good industrial application prospects are provided.
Owner:JIANGSU JIMING PHARMA TECH
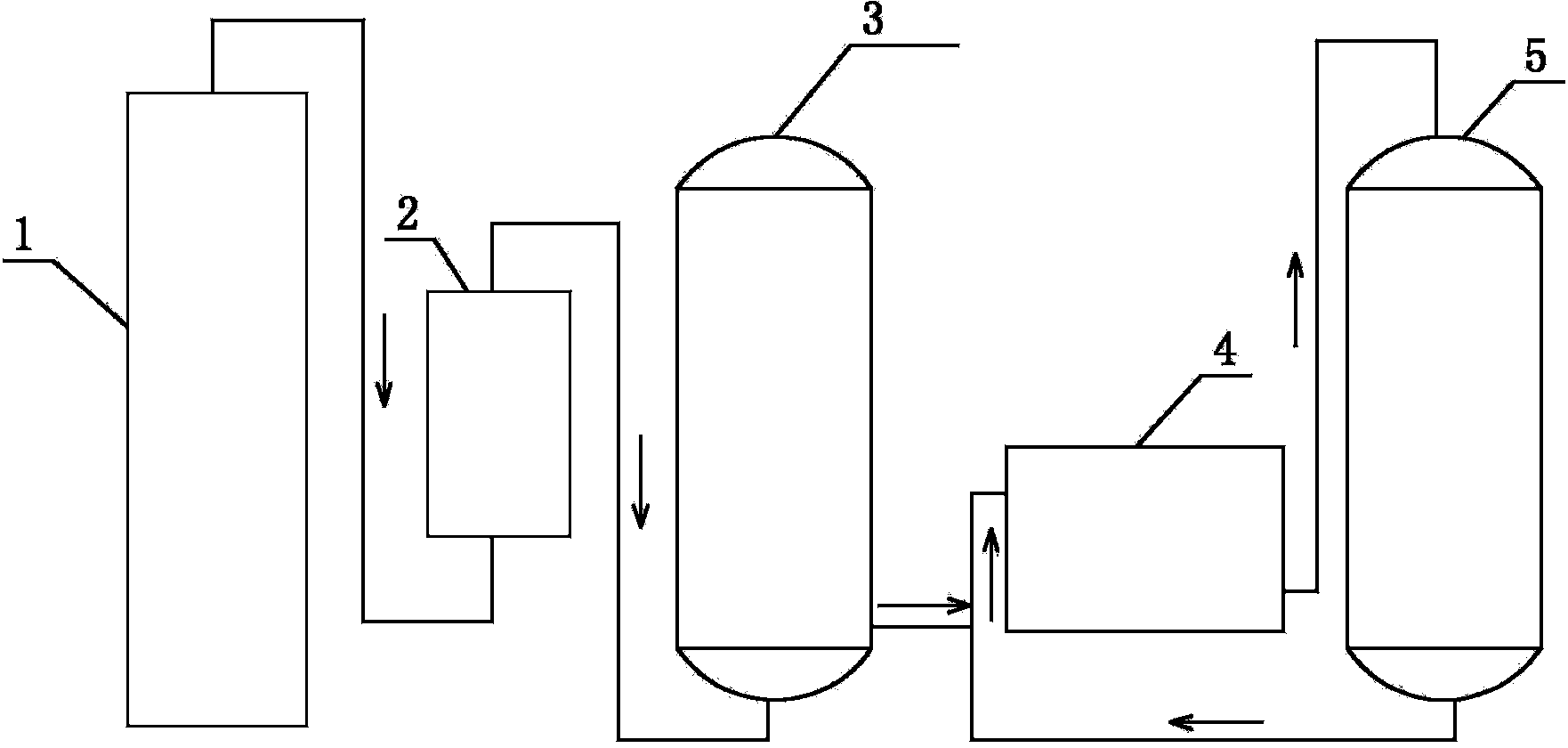
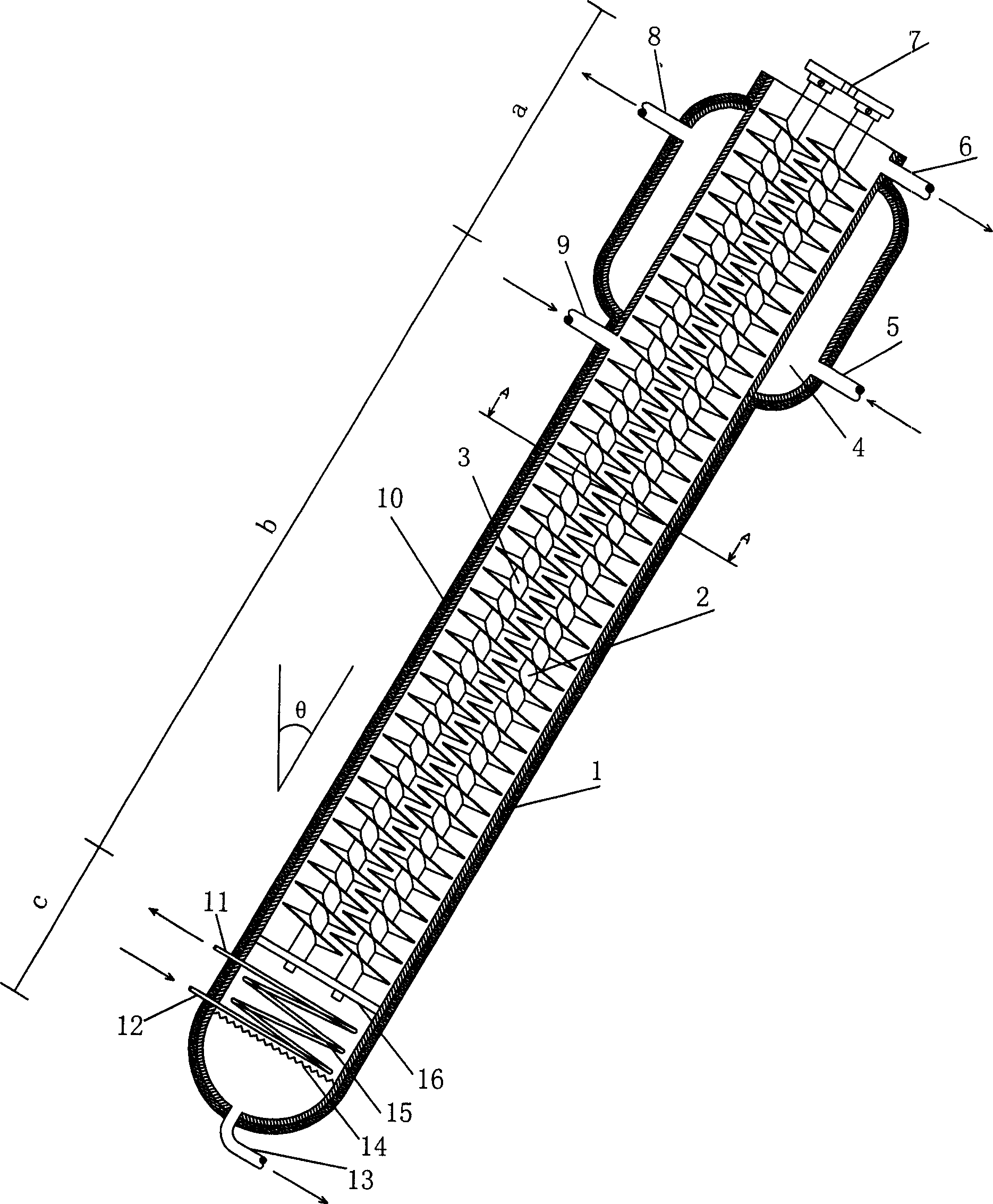
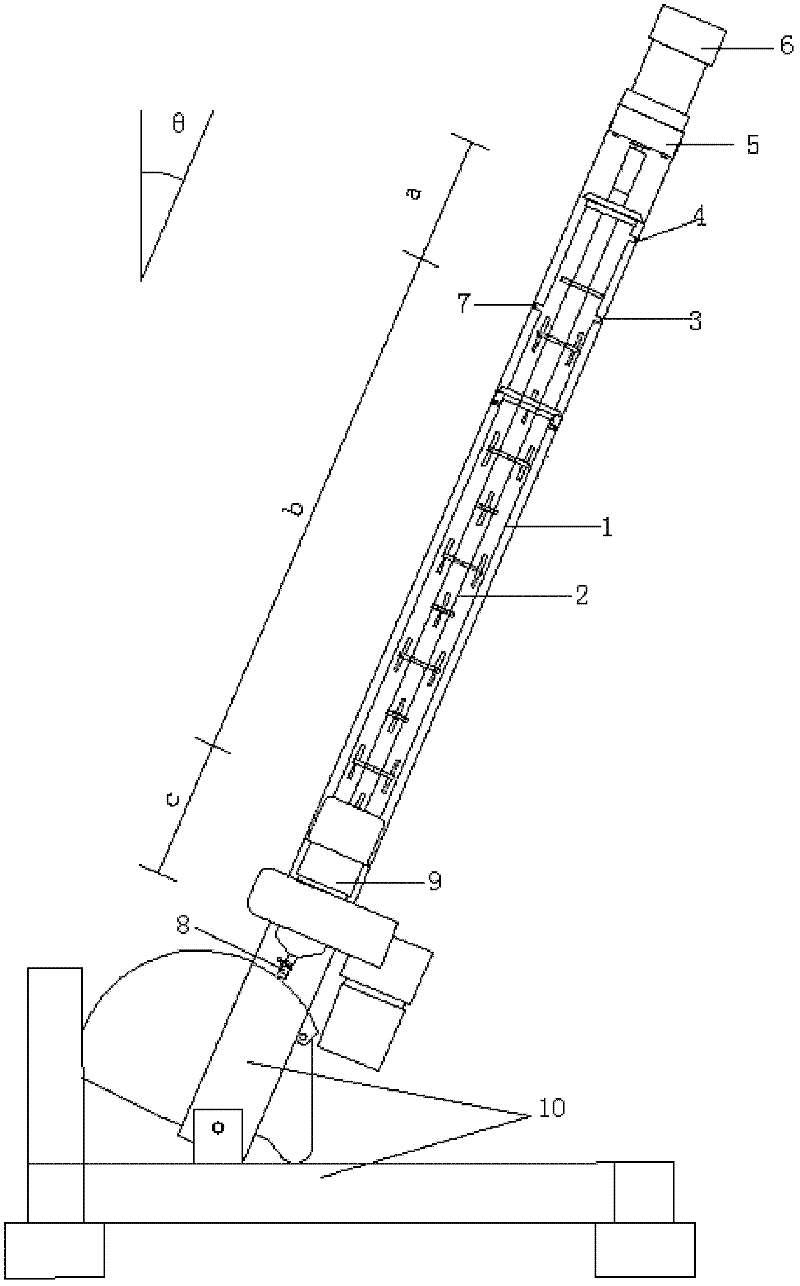
Equipment for preparing high pure organic matter by fusion-crystallization method
InactiveCN1792406AGuarantee continuous and stable operationReduce energy consumptionCrystallization separationCrystallisation purification/separationEngineeringOrganic matter
A device for preparing high-purity organic substance by fusion crystallizing method is a tower structure which is divided into upper cooling-crystallizing segment, middle separating-purifying segment and lower crystal fusing segment. Its tower body is inclined and is composed of casing consisting of intersected two cylinders, heater and parallel engaged two spiral stirrers.
Owner:SICHUAN UNIV
Efficient production method and application of antibacterial peptide plectasin
InactiveCN101870986ASimplify purification operationsHigh expressionAntibacterial agentsFungiStreptococcus suisStaphylococcus aureus bacteria
The invention discloses an efficient production method and application of antibacterial peptide plectasin. The efficient production method comprises the following steps of: establishing a pGAPZaA-plectasin eukaryon expression vector, transforming the eukaryon expression vector into host cells, i.e. pichiapastoris to establish expression engineering bacteria, fermenting and culturing the expression engineering bacteria, centrifuging expression culture medium to obtain supernate, and purifying the supernate to obtain the antibacterial peptide plectasin. The antibacterial peptide plectasin can be applied to prepare antibacterial agents or feed additive or preservative, and has good resistance on staphylococcus aureus or streptococcus suis. The invention has the advantages of simple operation and lower cost.
Owner:SOUTH CHINA AGRI UNIV
Preparation of propacetamol hydrochloride
InactiveCN101353314ASimplify purification operationsHigh yieldOrganic compound preparationCarboxylic acid amides preparationAcetic acidPropacetamol
The invention provides a preparation method of propacetamol hydrochloride. The preparation method is characterized in that chloracetyl chloride and paracetamol carry out an acetylization chloride reaction in a polar aprotic solvent to obtain chloroactic acid-4-acetylamino phenyl ester which is directly aminated with diethylamine to obtain N, N'-diethylglycine 4-acetylamino phenyl ester, hydrochloric acid is used for adjusting the pH to be 4, and the propacetamol hydrochloride is obtained. The preparation method has the advantages of mild condition, convenient separation and purification, the total product yield is greatly increased, the use of the amount of the reaction solvent is low, and the preparation method reduces industrial pollution, and is applicable to industrialized production.
Owner:ANHUI PIOM PHARMA
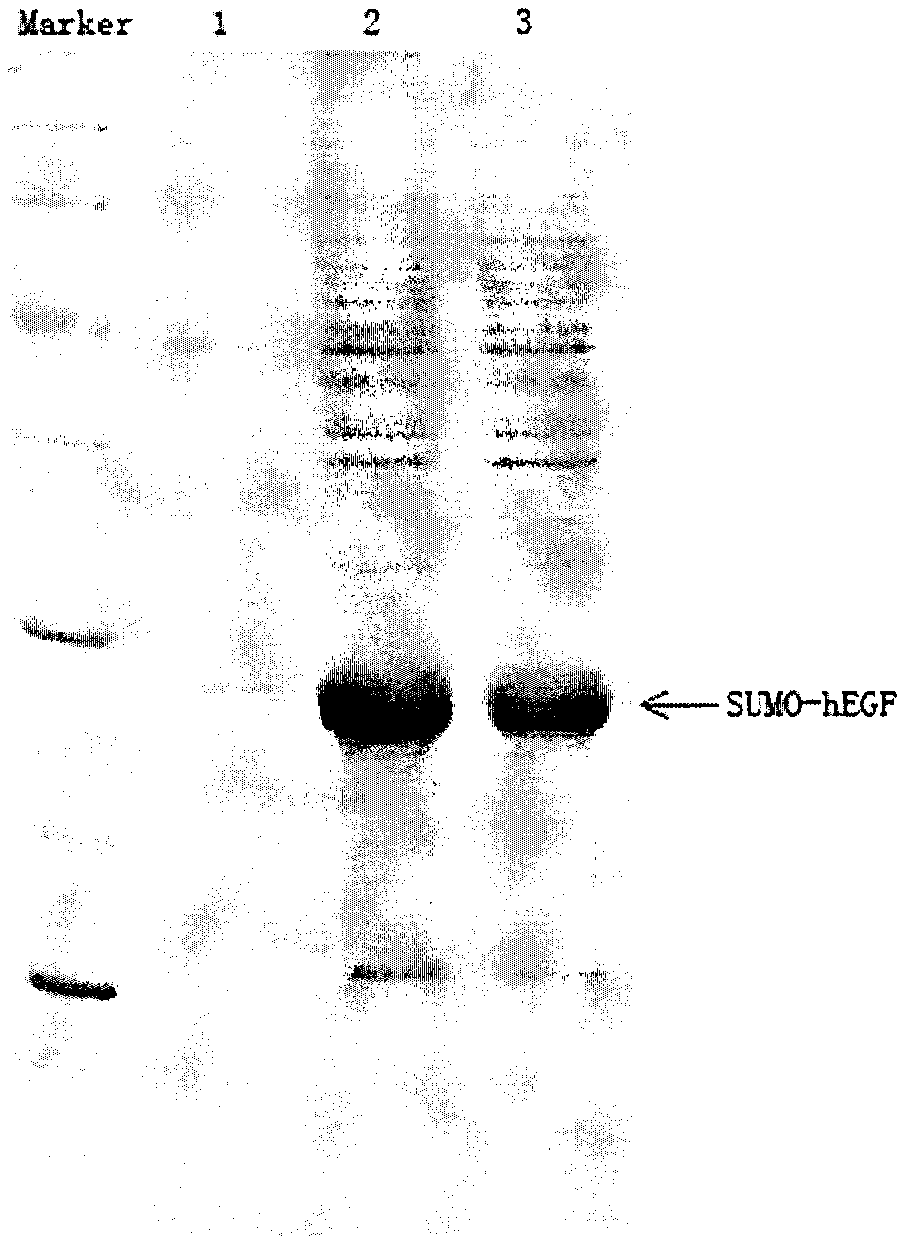
Construction and screening method for recombinant human epidermal growth factor engineering bacteria
InactiveCN102978231AGuaranteed homogeneityImprove stabilityBacteriaMicroorganism based processesChemical synthesisEscherichia coli
The present invention provides a construction and screening method for recombinant human epidermal growth factor (hEGF) engineering bacteria. The construction and screening method comprises the following main steps: 1, adopting a PCR technology to obtain kanamycin resistance gene, and adopting a chemical synthesis method to obtain an escherichia coli cspA gene function element; 2, respectively optimizing gene sequences encoding SUMO protein and hEGF, and then adopting chemical synthesis to obtain a large amount of expressed fusion protein gene sequences OPT-SUMO-hEGF; 3, linking the fragments obtained from the previous two steps into pET21 plasmid to obtain pET-Kan-cspA-SE plasmid; 4, adopting the pET-Kan-cspA-SE plasmid as a template to construct a transposition body; 5, transforming the transposition body into escherichia coli Origami 2 (DE3); and 6, screening a strain having high fusion protein SUMO-hEGF expression. According to the present invention, genome of the obtained strain is stable, a soluble rate of the expressed hEGF is high, and the method is suitable for large-scale industrial production.
Owner:天津强微特生物科技有限公司
Method for preparing alkoxy aluminum
ActiveCN102050700ASimplify purification operationsThorough responsePreparation of metal alcoholatesAlcoholBoiling point
The invention discloses a method for preparing alkoxy aluminum. The method comprises the following steps: aluminium powder and / or aluminium yarns and aluminium ingots are put in a reaction vessel; fatty alcohol between C3-C10 is used for reacting in an inert environment, and is divided into two parts; the weight of one part is 10-35 percent of the total weight of the fatty alcohol; the first partof the fatty alcohol is added into the reaction vessel, and is contacted with the aluminium powder or aluminium yarns at the temperature which is 5-25 DEG C lower than the boiling point of the fatty alcohol for initiating the reaction; the second part of the fatty alcohol which is left is continuously added into the reaction vessel after the reaction is initiated, and is continuously reacted withthe aluminium powder or aluminium yarns until the reaction is fully carried out at the temperature which is lower than the boiling point of the fatty alcohol; and the weight of the aluminium powder or aluminium yarns is 0.5-5.0 percent of the total weight of the aluminium powder and / or aluminium yarns and the aluminium ingots. The method has the advantages that a catalyst is not used, the preparation is simple, and the reaction can be carried out fully, stably and fast.
Owner:CHINA PETROLEUM & CHEM CORP +1
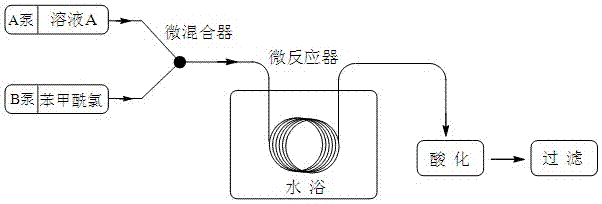
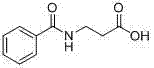
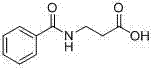
Method for synthesis of betamipron in continuous-flow microreactor
ActiveCN103588662AHigh yieldReduce hydrolysis side reactionsOrganic compound preparationCarboxylic acid amides preparationBenzoic acidSodium bicarbonate
The invention discloses a method for synthesis of betamipron in a continuous-flow microreactor. The method comprises the following steps: 1) injecting an aqueous solution containing 0.5-1 M of a mixture of beta-alanine, sodium hydroxide and sodium bicarbonate into a micro mixer through a connecting pipe at a flow rate of 4-48 [mu]l / min, at the same time, injecting benzoyl chloride into the micro mixer through a same-diameter connecting pipe at a flow rate of 0.35-4.14 [mu]l / min, mixing, then allowing the mixed solution to go into the micro-tube reactor for reaction, and thus obtaining a betamipron sodium salt solution, wherein the water bath temperature of the microreactor is -5 DEG C to 5 DEG C, the micro-tube diameter of the micro-tube reactor is 0.3-1.5 mm, and the tube length is 1-3 m; 2) acidizing the betamipron sodium salt solution by concentrated hydrochloric acid until the pH is 2, precipitating out a betamipron solid, filtering, washing, and drying to obtain the betamipron. The betamipron is synthesized in the continuous-flow microreactor; through precise control of mixing of the raw materials and strengthening condensation reaction conditions, the main reaction speed is accelerated, and generation of a benzoyl chloride hydrolysis side reaction is reduced, so that the content of benzoic acid in the crude product is reduced, the purification operation is simplified, and the betamipron yield is improved.
Owner:ZHEJIANG UNIV
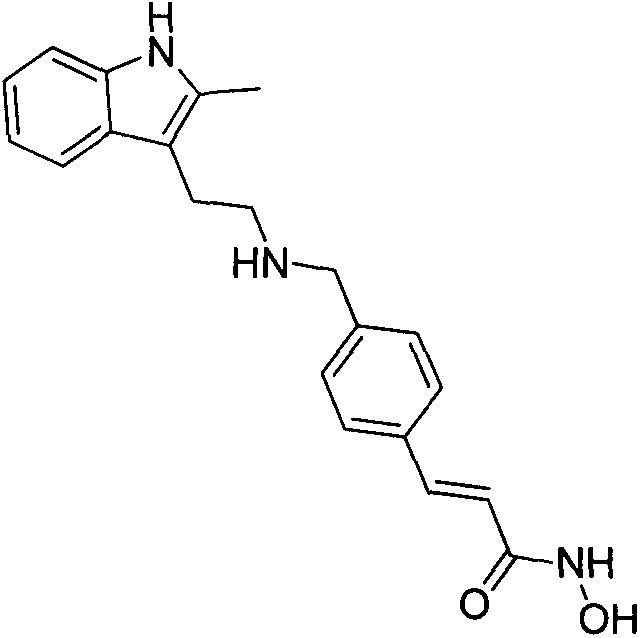
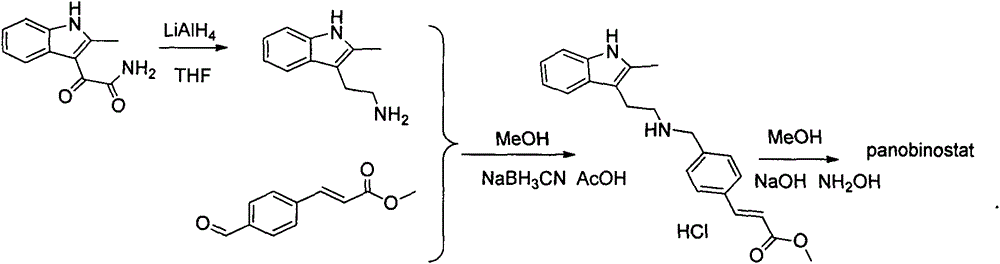
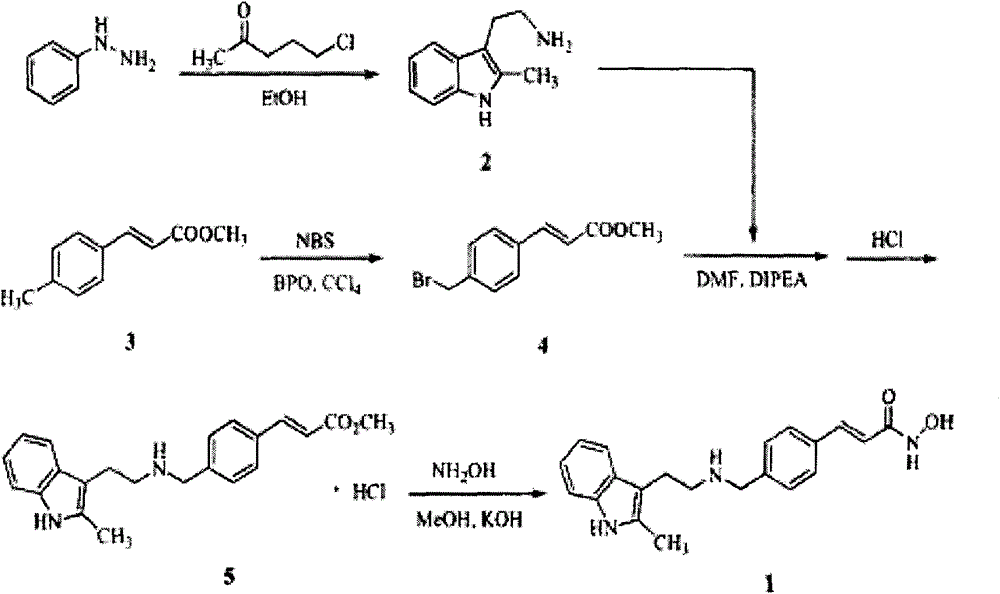
Synthesis method of panobinostat
InactiveCN106674079ASecurity impactSuitable for industrial productionOrganic chemistryTryptaminePanobinostat
The invention discloses a synthesis method of panobinostat. The synthesis method comprises a step of synthesizing 2-methyl tryptamine or hydrochloride thereof, namely taking 2-methylindole as a raw material, conducting reaction with chloroacetyl chloride or bromoacetyl bromide, and conducting reaction with potassium phthalimide to obtain the 2-methyl tryptamine. According to the technical scheme adopted by the invention, influence of toxic raw materials on the safety of a panobinostat product is avoided; meanwhile, the invention provides the synthesis method applicable to industrial production.
Owner:NANJING CAVENDISH BIO ENG TECH +1
Synthetic method of saponin
InactiveCN104693266AEasy to operateStrategies are simpleSugar derivativesGlycoside steroidsPtru catalystOrganic synthesis
The invention belongs to the field of organic synthesis, and involves a synthesis method of saponin, and in particular a method to construct glycosidic bond in saponin. The method includes the following steps: directly reacting an excessive amount of aldose or ketose with aglycone in an appropriate organic solvent under the action of an acid catalyst and reflux temperature of 30 DEG C to obtain the target product of saponin.
Owner:YANGZHOU BLUE BIOMEDICAL TECH CO LTD
Method for producing aromatic compound
ActiveCN108017479AReduce the burden onSimple manufacturing processOrganic compound preparationCarboxylic acid esters preparationNitro compoundHydrogen
The invention provides a method for producing an aromatic compound. In a cross coupling reaction, in a case where a halogen atom is selected as the leaving group of the raw material compound, a harmful halogen waste forms as a by-product after the reaction, and disposal process of the waste liquid is complicated and environmental burden is high. In a carbon-hydrogen activation cross coupling reaction which requires no halogen atom as the leaving group, although no halogen waste forms as a by-product, the reaction substrate is considerably restricted, and the reaction remains a limited molecular construction method. A method for producing an aromatic compound, which comprises subjecting an aromatic nitro compound and a boronic acid compound to a cross coupling reaction in the presence of ametal catalyst.
Owner:TOSOH CORP +1
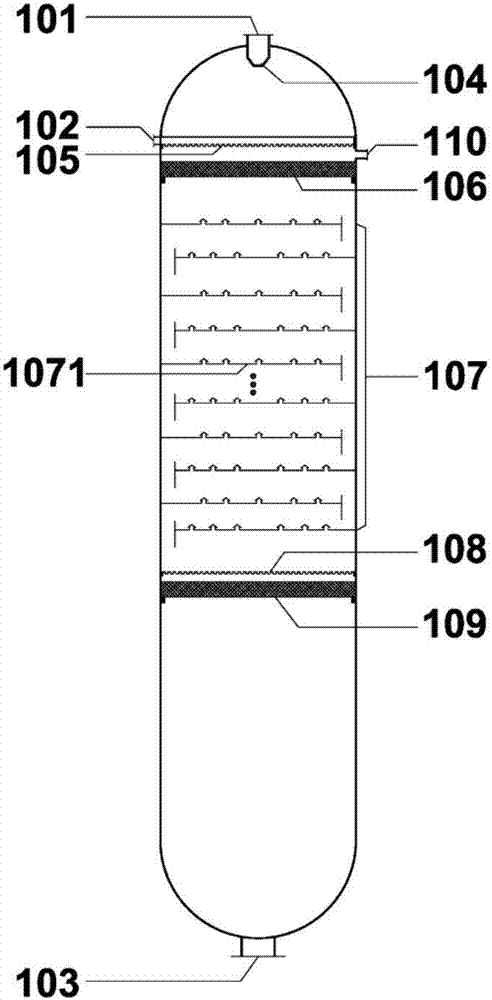
Crystallization tower for purifying phosphoric acid
ActiveCN102580345AHigh removal rateFully meet the requirements of sweating mechanismCrystallization separationPhosphorus compoundsPhosphoric acidEngineering
The invention relates to a crystallization tower for purifying phosphoric acid. The crystallization tower comprises a tower body, one stirrer, a heater and a driving mechanism, wherein the tower body is divided into a cooling crystallization section, a separating purification section and a crystal melting section; the cooling crystallization section is arranged at the upper part of the tower body; the crystal melting section is arranged at the lower part of the tower body; the separating purification section is arranged between the cooling crystallization section and the crystal melting section; a feeding hole and a coolant inlet are arranged at the lower part of the cooling crystallization section of the tower body; the coolant inlet is arranged above the feeding hole; the upper part of the cooling crystallization section of the tower body is provided with a raffinate and coolant discharge hole; the bottom of the crystal melting section of the tower body is provided with a purified phosphoric acid outlet; the main body part of the stirrer is arranged in the tower body; one end of the stirrer extends out of the tower body and is connected with a speed reducer of the driving mechanism; and the heater is arranged on the tower body and is positioned on the crystal melting section of the tower body.
Owner:SICHUAN UNIV
Spray tower tray reactor, and system and technology for producing propylene glycol monomethyl ether through using reactor
ActiveCN107162882AProduction applicableImprove conversion rateEther separation/purificationEther preparation from oxiranesAlcoholMonomethyl ether
The invention provides a spray tower tray reactor, and a system and a technology for producing propylene glycol monomethyl ether through using the reactor. The spray tower tray reactor comprises a reactor body, and a cavity is arranged in the reactor body; the top of the reactor body is provided with a first material inlet, the sidewall of the reactor body is provided with a second material inlet, and the bottom of the reactor body is provided with a material outlet; and an atomizing nozzle cooperating with the first material inlet, and a first distributor, a second distributor, a multistage tower tray, a liquid collector and a third distributor which cooperate with the second material inlet are sequentially arranged in the cavity from top to bottom. The system comprises the spray tower tray reactor. When the spray tower tray reactor is used to produce the propylene glycol monomethyl ether, the epoxy propane conversion rate is improved, the propylene glycol monomethyl ether selectivity is improved, the alcohol proportion is reduced, and the production efficiency is improved.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Preparation method and intermediate of Pazopanib
The invention discloses a preparation method and an intermediate of Pazopanib. The preparation method comprises the steps of carrying out two-step condensation reaction on 2,4-dichloro-5-nitropyrimidine so as to obtain a compound of a formula 5 (shown in the description), and carrying out reduction on the compound of the formula 5, so as to obtain Pazopanib. The preparation method has the beneficial effects that the yield of Pazopanib is increased, reaction conditions are mild, column chromatography purification is not required, and the preparation method is applicable to industrial production.
Owner:苏州东南药业股份有限公司
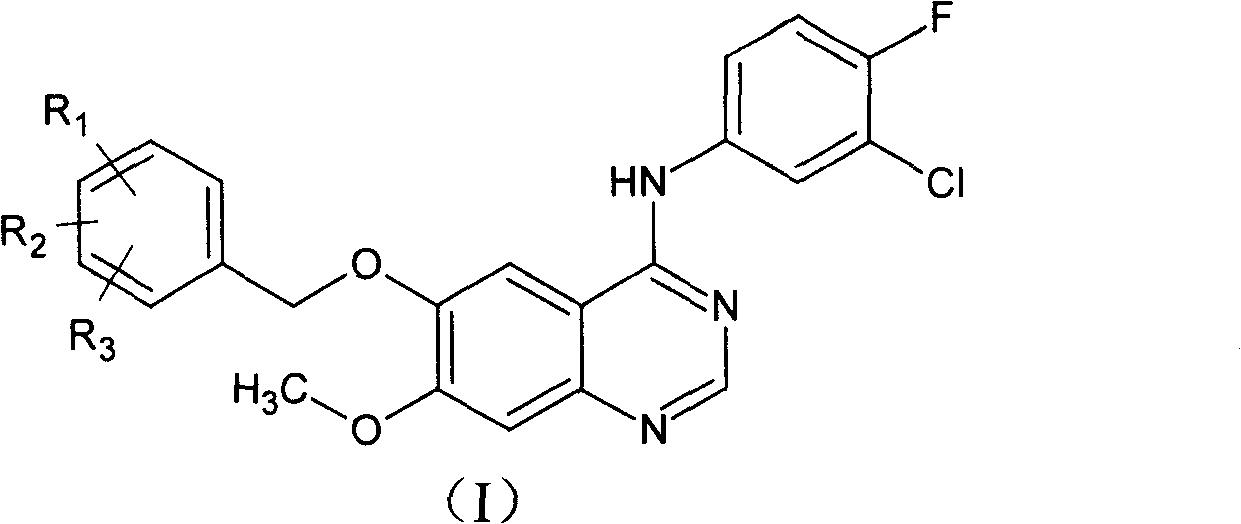
Gefitinib synthesis intermediate, and its preparing method and use
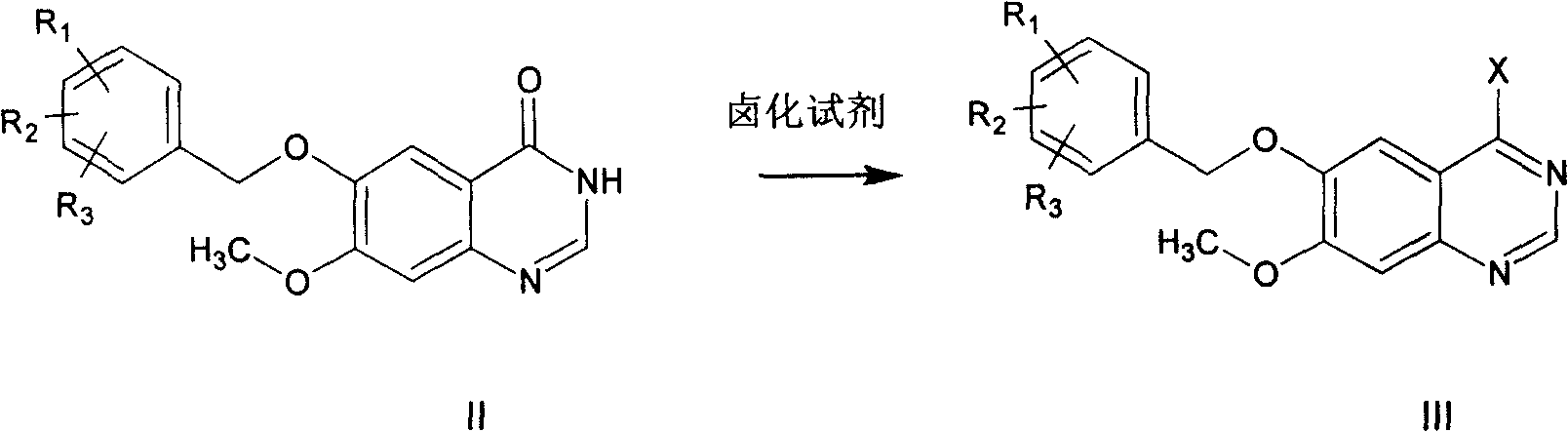
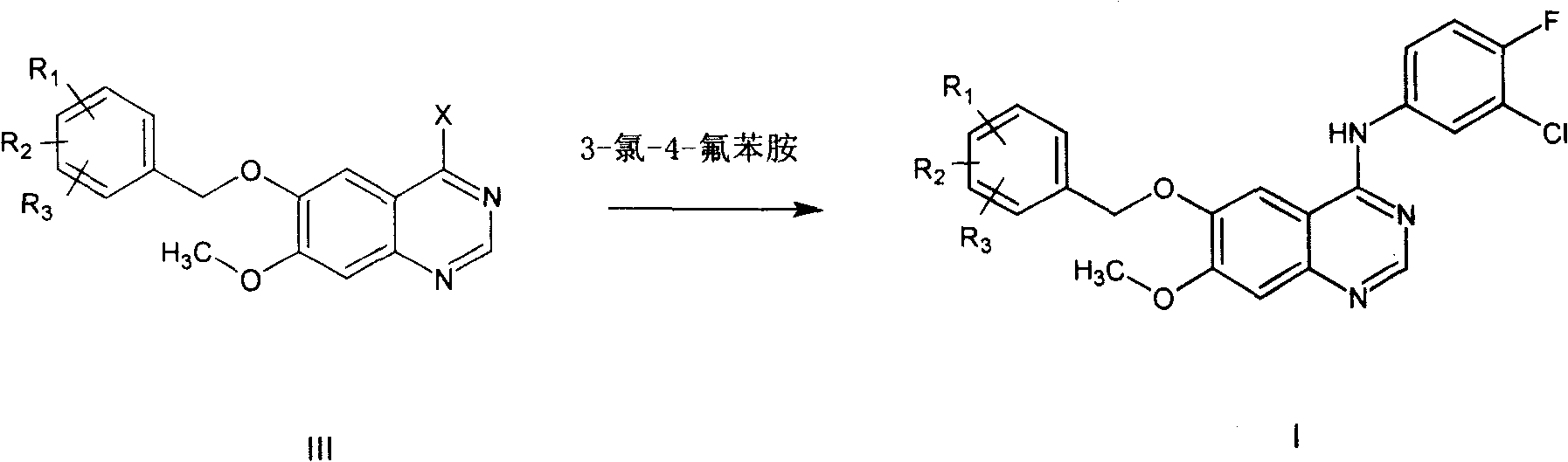
ActiveCN100420676CQuality improvementReduced two-step reactionOrganic chemistryCombinatorial chemistryQuinazoline
This invention relates to gefetinib synthesis intermediate and its preparation method and application. Compound of formula (II) is halogenated to make formula (III) compound, then condensed with 3-chlorine-4-fluoroaniline to make formula (I) compound. The formula (I) compound is new intermediate compound that used to synthesize the gefetinib, another important intermediate compound 6-hydroxy group- 7-methoxyl group-4-(3'-chlorine-4'-fluoroaniline) quinazoline (2) that used to synthesize gefetinib can be easily made by (I) compound.
Owner:ZHEJIANG HISUN PHARMA CO LTD
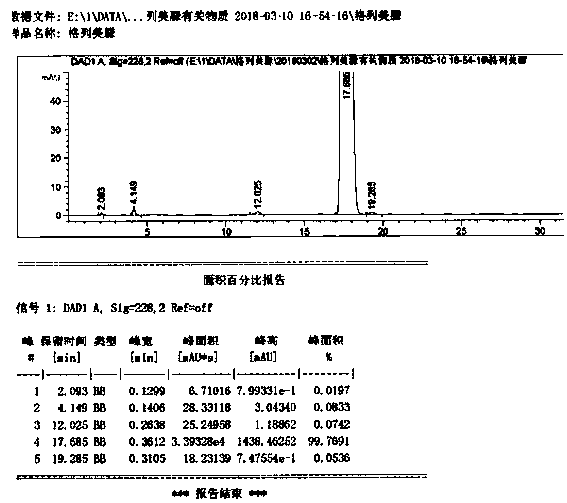
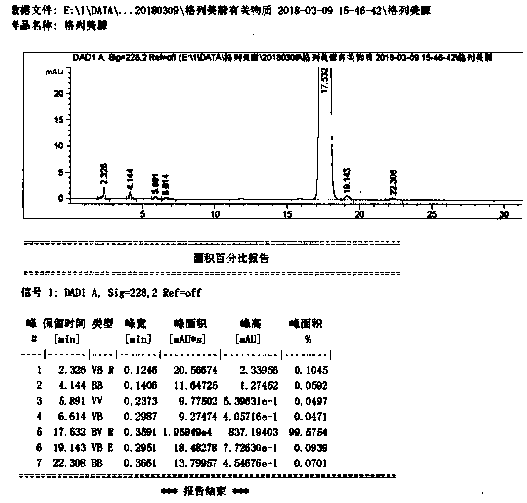
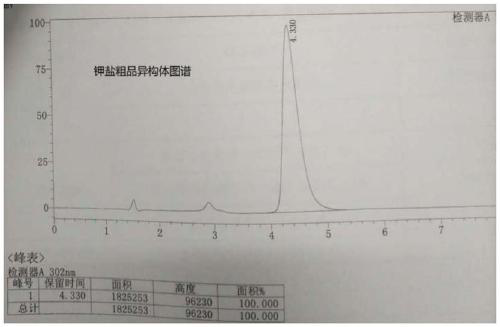
Synthetic method for 1-{2-floro-4-[5-(4-isobutyl phenyl)-1,2,4-oxadiazol-3-yl]-benzyl}-3-azetidinecarboxylic acid
ActiveCN105348276ALow costImprove efficiencyOrganic chemistryAzetidinecarboxylic AcidCarboxylic acid
The invention provides a synthetic method for a compound 1-{2-floro-4-[5-(4-isobutyl phenyl)-1,2,4-oxadiazole-3-yl]-benzyl}-3-azetidinecarboxylic acid represented by a formula 2. The synthetic method is simple in reaction condition, simple and convenient in post-treatment, easy to operate, high in yield, stable in process and suitable for industrial production.
Owner:SUZHOU CONNECT BIOPHARMACEUTICALS LTD
Preparation method of calcium D,L-2-hydroxy-4-methylthiobutyrate having high purity and high bulk density
ActiveCN103641756AStable in natureLong storage timeAnimal feeding stuffSulfide preparationChemical industryMixed gas
The invention aims at the chemical industry field, and relates to a preparation method of calcium D,L-2-hydroxy-4-methylthiobutyrate having a high purity and a high bulk density. The method comprises the following steps: fully reacting a hydrocyanic acid mixed gas prepared through an Andrussow method with methylthiopropionaldehyde under the catalysis of an alkali to obtain a 2-hydroxy-4-methylthiobutyronityile system; hydrating the 2-hydroxy-4-methylthiobutyronityile system in the presence of an inorganic acid to obtain D,L-2-hydroxy-4-methylthiobutyramide; and chelating D,L-2-hydroxy-4-methylthiobutyramide with calcium oxide or calcium hydroxide to prepare calcium D,L-2-hydroxy-4-methylthiobutyrate. The method has the advantages of simple synthesis technology and easily available raw materials, the above obtained product has a large bulk density, a high purity and a good fluency, can be effectively mixed with feeds as an animal feed additive to supplement the calcium element and amino acids in the daily ration and improve the endozoic production and immunity performances, and can also be used as a medicinal reagent.
Owner:NINGXIA UNISPLENDOUR TIANHUA METHIONINE CO LTD
Process for synthesizing glimepiride raw material medicine
InactiveCN108383768AReduce lossesReduce generationOrganic active ingredientsOrganic chemistryKetoneGlimepiride
The invention discloses a process for synthesizing a glimepiride raw material medicine. A compound A, namely 3-ethyl-4-methyl-3-pyrroline-2-ketone and a compound B, namely 2-phenethyl isocyanate are taken as start raw materials. The process is characterized in that when an intermediate 1 is synthesized, filtrate is applied mechanically, so that the loss of the intermediate 1 can be reduced, the yield can be increased, and the production efficiency can be improved; when an intermediate 2 is synthesized, hydrochloric ether is adopted as a solvent, so that isomer impurities can be greatly reduced, the content of the isomer impurities can be reduced to 0.5% or less from 8%, and later purification procedures can be simple to operate; when a glimepiride metallic salt is synthesized, acetonitrileis adopted as a solvent, sufficient reactions can be achieved, the reaction time can be greatly shortened, the residue of an intermediate 3 is reduced to 0.2% or less from 5-10%, in addition, a highsolvent recycling rate can be achieved. The process disclosed by the invention is simple and safe, low in production cost, high in yield, stable in intermediate and finished product quality and applicable to industrial large-scale production and hypoglycemic drug, namely glimepiride, synthesis processes with relatively great social, economic and environmental-friendly benefits.
Owner:江西博雅欣和制药有限公司
Preparation method of medicinal D,L-2-hydroxy-4-methylthiobutyric acid metal chelate
ActiveCN103641754AStable in natureLong storage timeOrganic compound preparationSulfide preparationChemical industryMetal chelate
The invention aims at the chemical industry field, and relates to a preparation method of a medicinal D,L-2-hydroxy-4-methylthiobutyric acid metal chelate. The method comprises the following steps: carrying out a reaction of a hydrocyanic acid mixed gas prepared through an Andrussow process and methylthiopropionaldehyde as initial raw materials to obtain a 2-hydroxy-4-methylthiobutyronityile system, hydrating the 2-hydroxy-4-methylthiobutyronityile system to obtain D,L-2-hydroxy-4-methylthiobutyramide, and hydrolyzing to obtain D,L-2-hydroxy-4-methylthiobutyrate; and chelating D,L-2-hydroxy-4-methylthiobutyrate with a trace metal element salt to obtain the D,L-2-hydroxy-4-methylthiobutyrate. The method has the advantages of mild technological conditions, few side reactions, low production cost and simple purifying operation, and the obtained D,L-2-hydroxy-4-methylthiobutyrate has a high purity and a large bulk density, and can be used as an animal feed additive or a medicinal reagent.
Owner:NINGXIA UNISPLENDOUR TIANHUA METHIONINE CO LTD
Novel preparation method of esprazole magnesium trihydrate and intermediate thereof
The invention relates to a novel preparation method of esprazole magnesium trihydrate and an intermediate thereof, and belongs to a pharmaceutical technology. The preparation method comprises the following steps: (1) performing condensation reaction to prepare omeprazole sulfide; and (2) performing chiral oxidation reaction to prepare esprazole, performing potassium salt forming reaction to prepare esprazole potassium, and performing ion exchange reaction to obtain the esprazole magnesium trihydrate. The method is high in product yield, high in product purity, mild and safe in reaction condition, low in energy consumption, easy to control and suitable for industrial mass production.
Owner:浙江康德药业集团股份有限公司 +1
Process for converting hydrocarbon feedstocks with electrolytic recovery of halogen
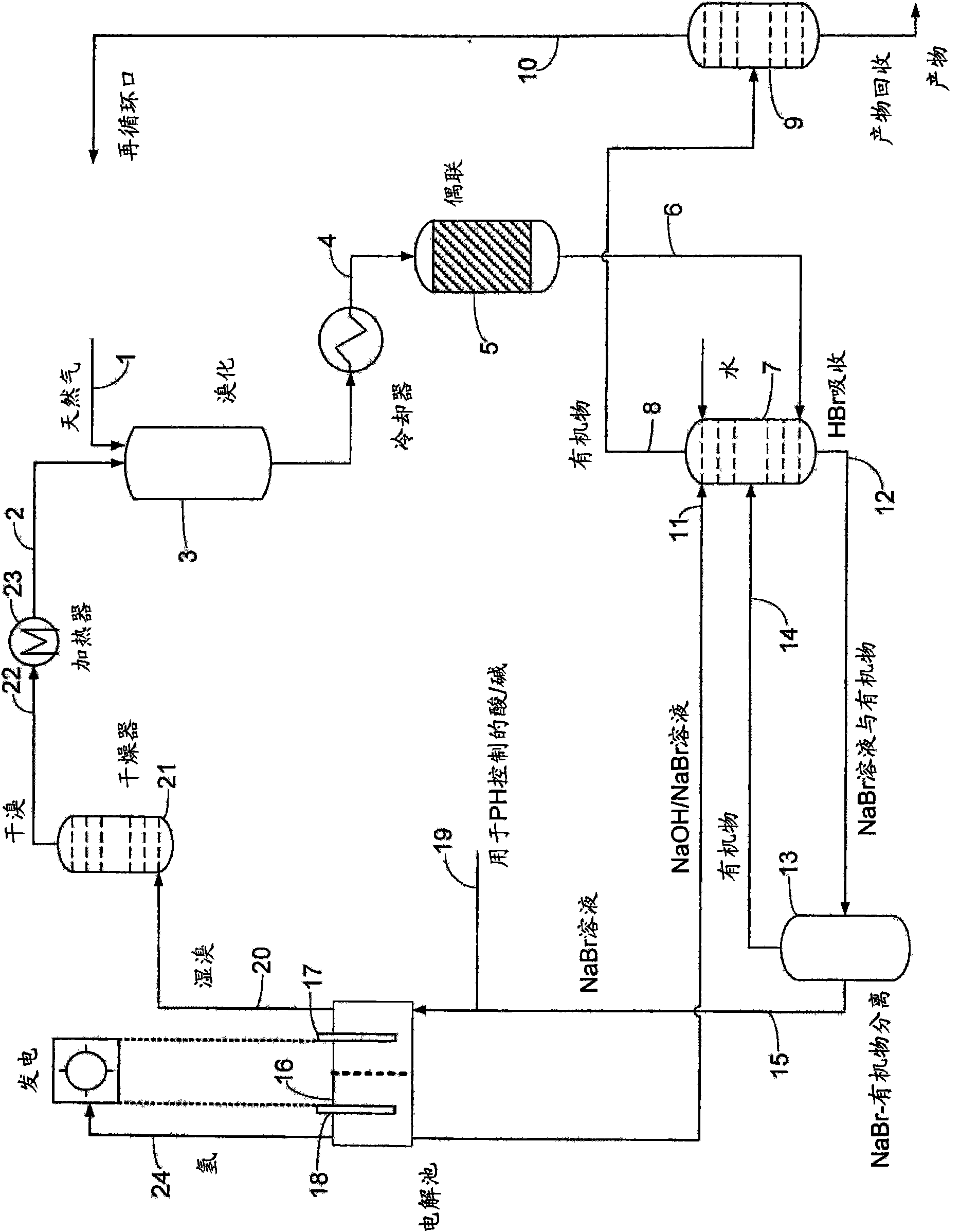
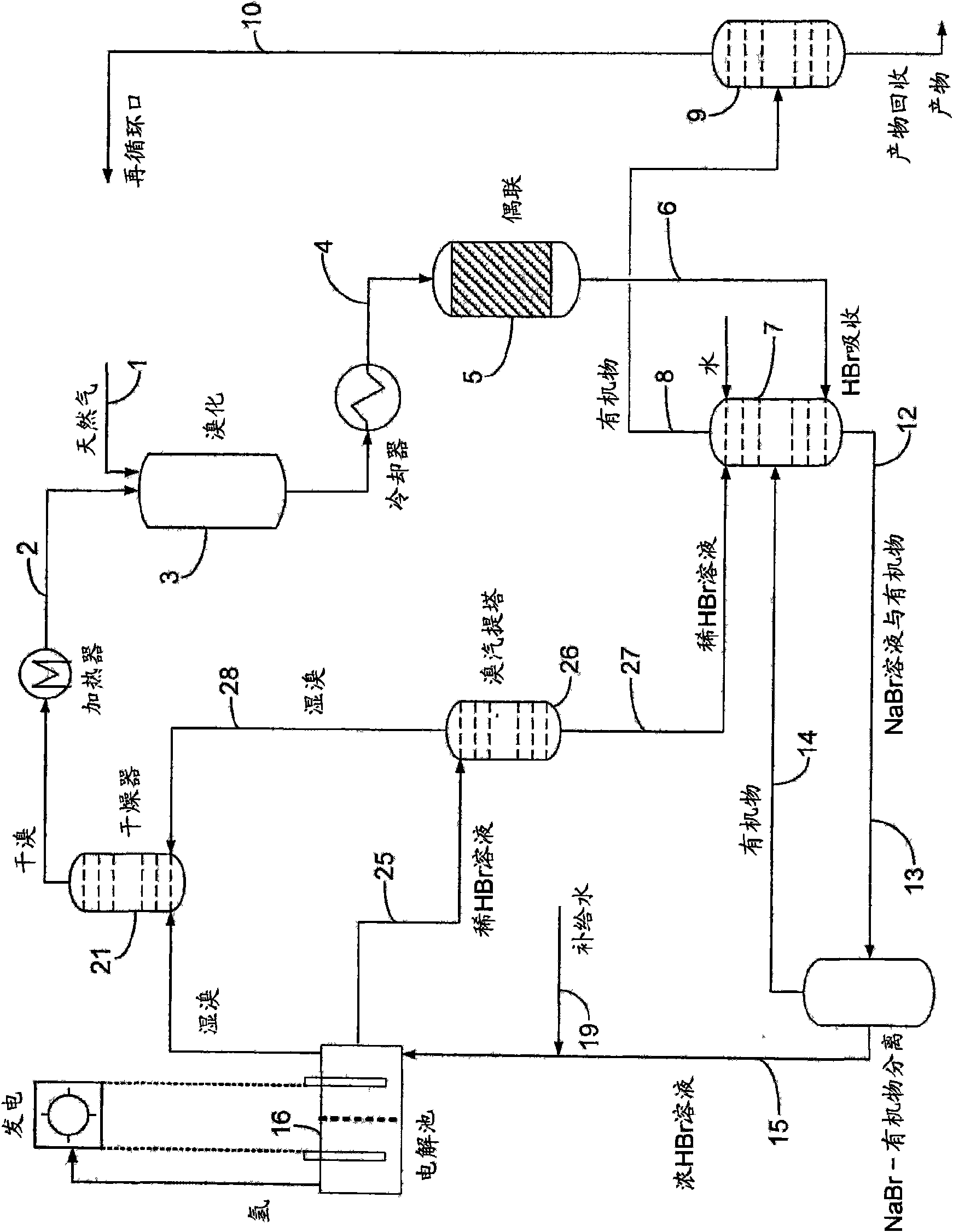
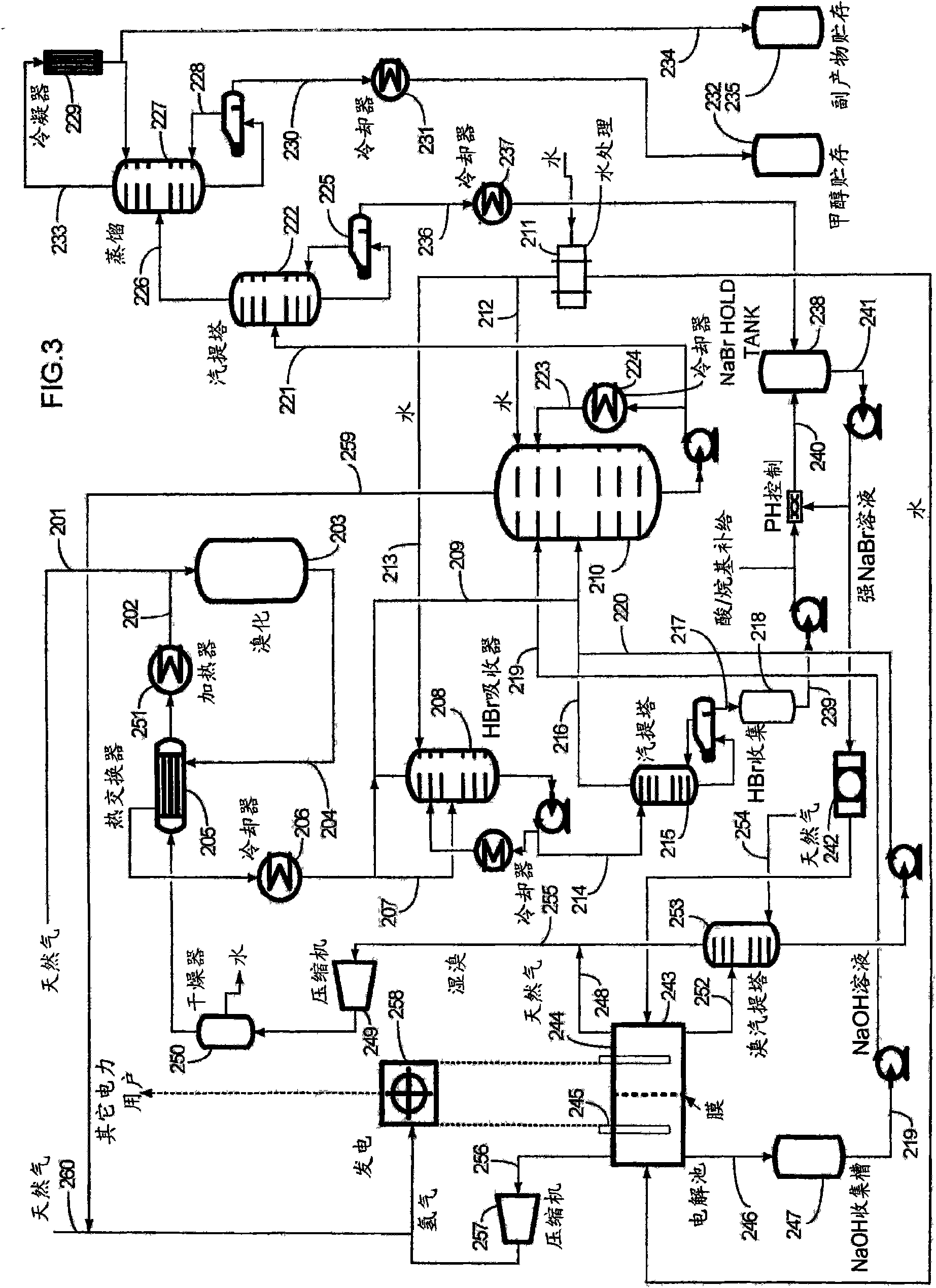
InactiveCN101687725AHigh value productLow operating pressureElectrolysis componentsSolid-state devicesHalogenProduct formation
Owner:GRT
Novel physical hydrogel and usage thereof
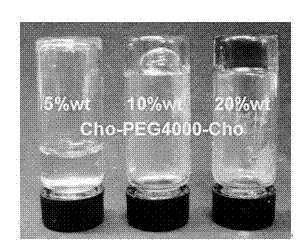
InactiveCN102863630ASimplify purification operationsEasy to manufactureAerosol deliverySurgeryCholesterolPolyethylene glycol
The invention discloses a novel physical hydrogel which is characterized by containing cholesterol-polyethylene glycol-cholesterol three-block compound. When the mass percent of the cholesterol-polyethylene glycol-cholesterol three-block compound in the hydrogel is 5-50%wt, the gel is formed; when the double-substituted substance has the concentration of 10-30%wt according to the different lengths of the chain segment of polyethylene glycol, the gel is formed at the room temperature; meanwhile, when the chain segment of the polyethylene glycol is 1.5-3kDa, the sol with the polymer content of 10-20%wt has sol-gel transition phenomenon at the temperature of 35-70 DEG C. The novel physical hydrogel is simple in preparation process, and the product is simple in purification operation; the prepared gel has controllable gel property and better biocompatibility, thus being used as biodegradation material; and the novel physical hydrogel has better biocompatibility, can be discharged outside by circulatory system and is free from long-term toxicity to the human body, thus being used as medicine gel formulation, embolic material and tissue engineering material.
Owner:WUHAN UNIV
Preparation method for 2-fluoropyridine-4-boric acid
InactiveCN104478913AHigh yieldRaw materials are cheap and easy to getGroup 3/13 element organic compoundsN-ButyllithiumIodine
The invention discloses a preparation method for 2-fluoropyridine-4-boric acid. The preparation method comprises the following steps: firstly, reacting 2-fluoropyridine with iodine in the presence of LDA (lithium diisopropylamide) to obtain an intermediate A; secondly, reacting the intermediate with water in the presence of LDA to obtain an intermediate B; thirdly, reacting the intermediate B with triisopropyl borate in the presence of n-butyl lithium to obtain the target product-2-fluoropyridine-4-boric acid. According to the preparation method, raw materials are simple and easily available, the process is safe and reliable, and the target product-2-fluoropyridine-4-boric acid is an important common intermediate in pharmaceutical chemistry.
Owner:DALIAN NETCHEM CHIRAL TECH
Albumin magnetic nanoparticles for magnetic resonance imaging (MRI) and preparation method thereof
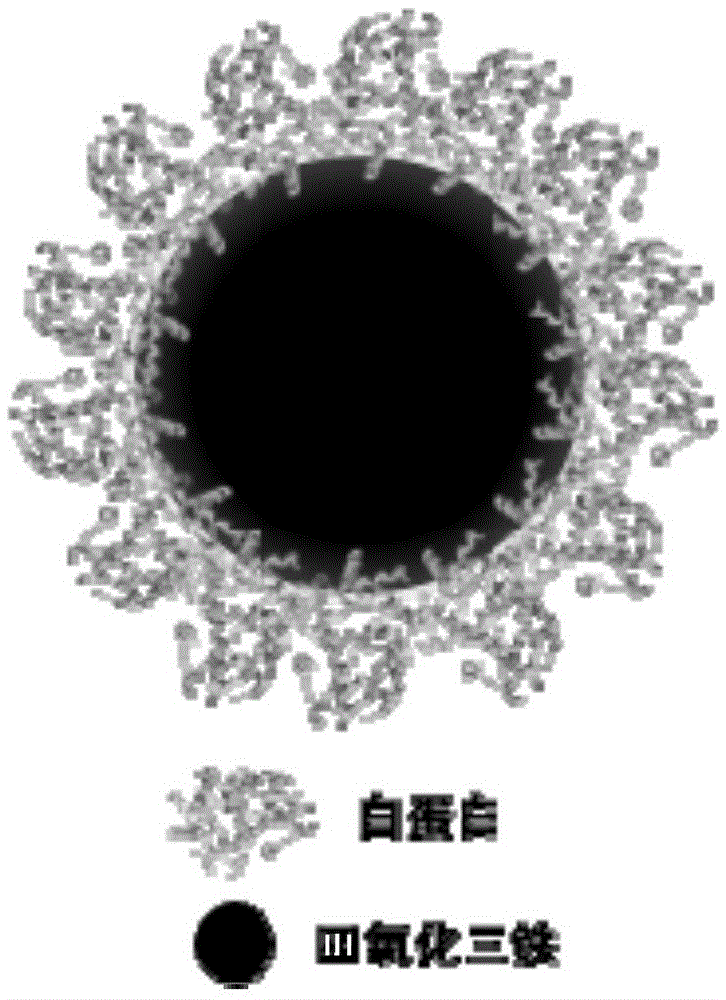
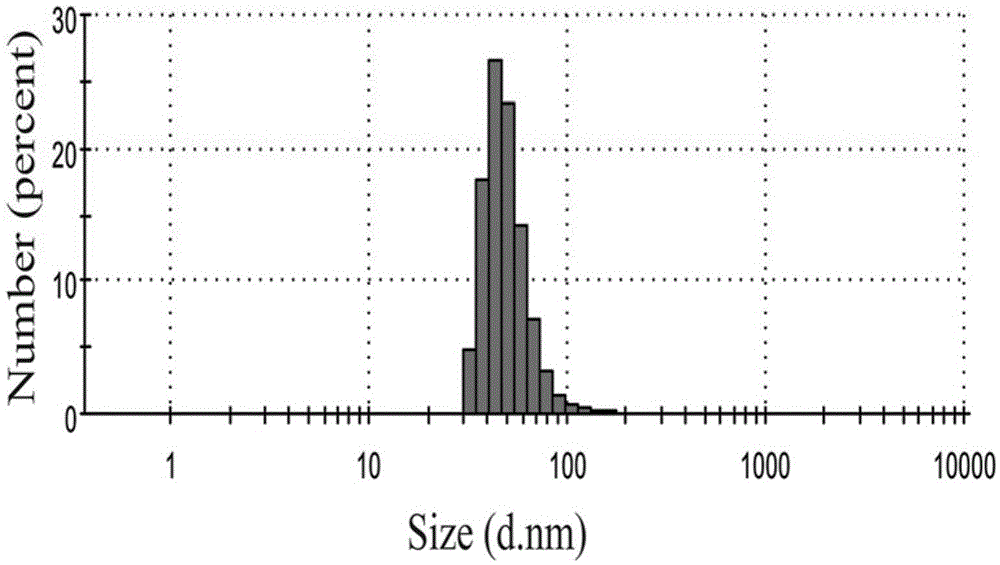
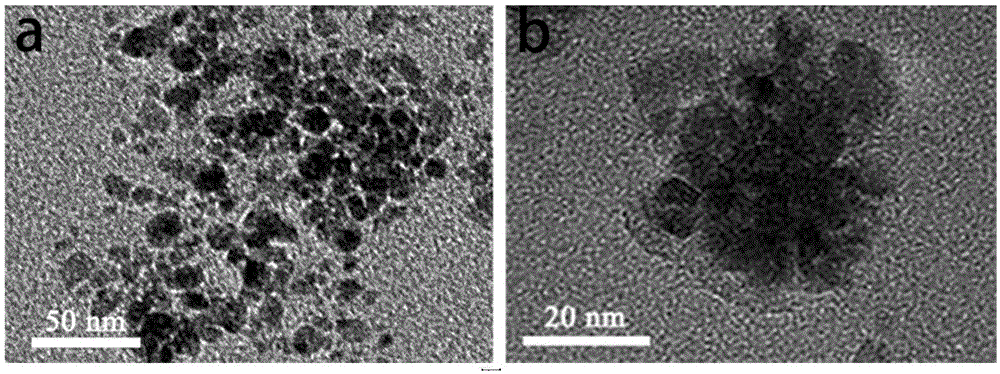
ActiveCN105617408ASimplify purification operationsStabilized Magnetic NanoparticlesEmulsion deliveryIn-vivo testing preparationsChemistryAmmonium hydroxide
The invention relates to albumin magnetic nanoparticles for magnetic resonance imaging (MRI) and a preparation method thereof. The preparation method includes the steps of dissolving albumin in deoxidized water, leading in nitrogen gas to remove oxygen in a reaction system, adding in dilute ammonia solution dropwise to regulate pH value of the system to be 10-12, adding in anhydrous ferric chloride and ferrous sulfate mixed solution dropwise, stirring on a condition of nitrogen protection, raising heat to 65-75 DEG C for 30-60 minutes, and then cooling to the room temperature and subjecting the mixture to purification after finishing reaction, and finally dialyzing the prepared nanoparticles by dialysis bags with molecular weight of 8kD-14kD for 24 hours to obtain the pure albumin magnetic nanoparticles. In anhydrous ferric chloride and ferrous sulfate mixed solution, the total Fe concentration is up to 1.4-1.8M / L, and molar ratio of Fe to protein is equal to (2.4*104-3.1*104):1. The hydrodynamic size of the nanoparticles ranges from 50 micrometers to 90 micrometers, and thus the nanoparticles are adaptable to biological application such as MRI, drug carriers and immunoassay, and are perfect materials for biomedicine application.
Owner:TIANJIN MEDICAL UNIV
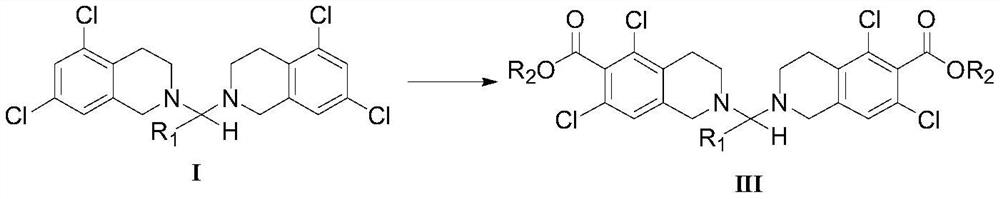
5, 7-dichlorotetrahydroisoquinoline acetal amine compound as well as preparation method and application thereof
PendingCN112409256AAtom utilization is highIncrease productivityOrganic chemistrySide productQuinoline
The invention provides a 5, 7-dichlorotetrahydroisoquinoline acetal amine compound as shown in a formula III, and a preparation method and an application thereof. The 5, 7-dichlorotetrahydroisoquinoline compound as shown in the formula III can be used for synthesizing a key intermediate of the rotinostat. The method has the advantages of high atom economy, few byproducts and the like, conforms tothe concept of green chemistry, and is suitable for being developed into an industrial production process.
Owner:TOPHARMAN TANCHENG CO LTD +1
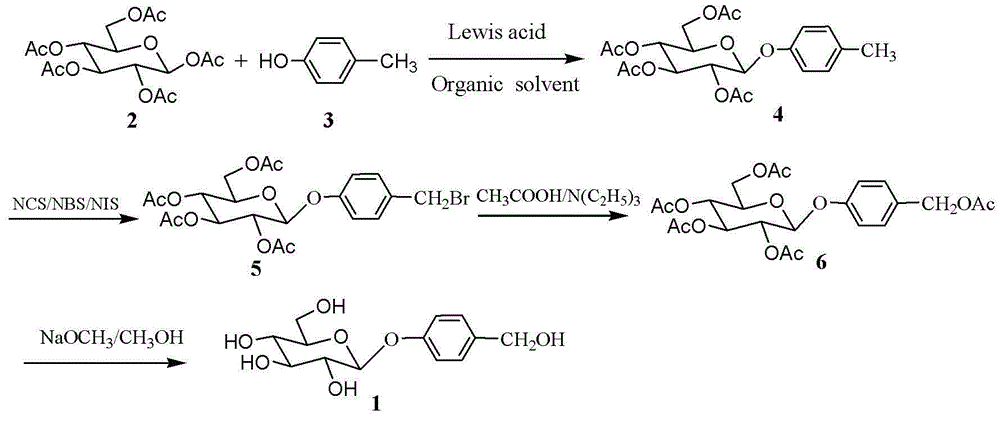
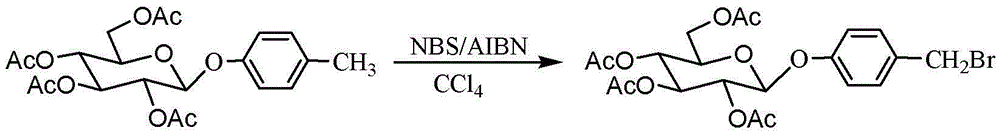
Method for chemically synthesising gastrodin
InactiveCN102977161BImprove stabilityAvoid harmSugar derivativesSugar derivatives preparationChemical synthesisLewis acid catalysis
The invention discloses a method for chemically synthesising gastrodin, comprising the following steps of: in the presence of a molecular sieve, under the catalysis of Lewis acid, and performing glycosylation reaction on pent-acetyl-b-D-glucose and p-cresol in an organic solvent with to generate 4-methylphenyl-2,3,4,6-O-tetra-acetyl-b-D-glucopyranoside; then preparing 4-halomethylphenyl-2,3,4,6-O-tetra-acetyl-b-D-glucopyranoside from 4-methylphenyl-2,3,4,6-O-tetra-acetyl-b-D-glucopyranoside and N-halosuccinimide in the presence of an initiator, and then reacting 4-halomethylphenyl-2,3,4,6-O-tetra-acetyl-b-D-glucopyranoside with the mixed solution of glacial acetic acid and tertiary amine to obtain 4-acetoxylmethylphenyl-2,3,4,6-O-tetra-acetyl-b-D-glucopyranoside; and finally removing the acetyl protecting group from the 4-acetoxylmethylphenyl-2,3,4,6-O-tetra-acetyl-b-D-glucopyranoside in an alkaline condition to obtain gastrodin. Compared with the traditional method, the method disclosed by the invention has easily available raw materials, and is short in reaction time, capable of preparing the reaction product in each step via recrystallization, simple, and more suitable for industrialized production for gastrodin.
Owner:QINGDAO AGRI UNIV
A kind of preparation method of tranexamic acid
ActiveCN103172528BReduce transformation operationsReduce consumptionOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidCyclic Amino Acids
The invention relates to a tranexamic acid high-efficiency production method which comprises the following steps: performing hydrogenation reduction on 4-(acetylaminomethyl)benzoic acid used as an initial raw material, filtering the hydrogenation solution to remove a catalyst, distilling until the reaction solution is cured, heating, and baking to perform transformation; and dissolving with water, regulating the reaction solution to neutral with acid, adding p-toluenesulfonic acid to perform salification, filtering, performing resin exchange with a weak base anion-exchange resin, distilling the eluate until a large amount of solids are precipitated, adding a right amount of ethanol to crystallize, cooling, filtering, washing, and drying to obtain tranexamic acid. For the tranexamic acid produced by the method, the cis-compound content is lower than 0.05%, and the quality completely meets pharmacopeia standards at home and abroad and is higher than the quality of products currently reported in China. The production route provided by the invention has the advantages of fewer steps, simple operation process, high yield, energy saving, environment friendliness and the like.
Owner:VALIANT CO LTD
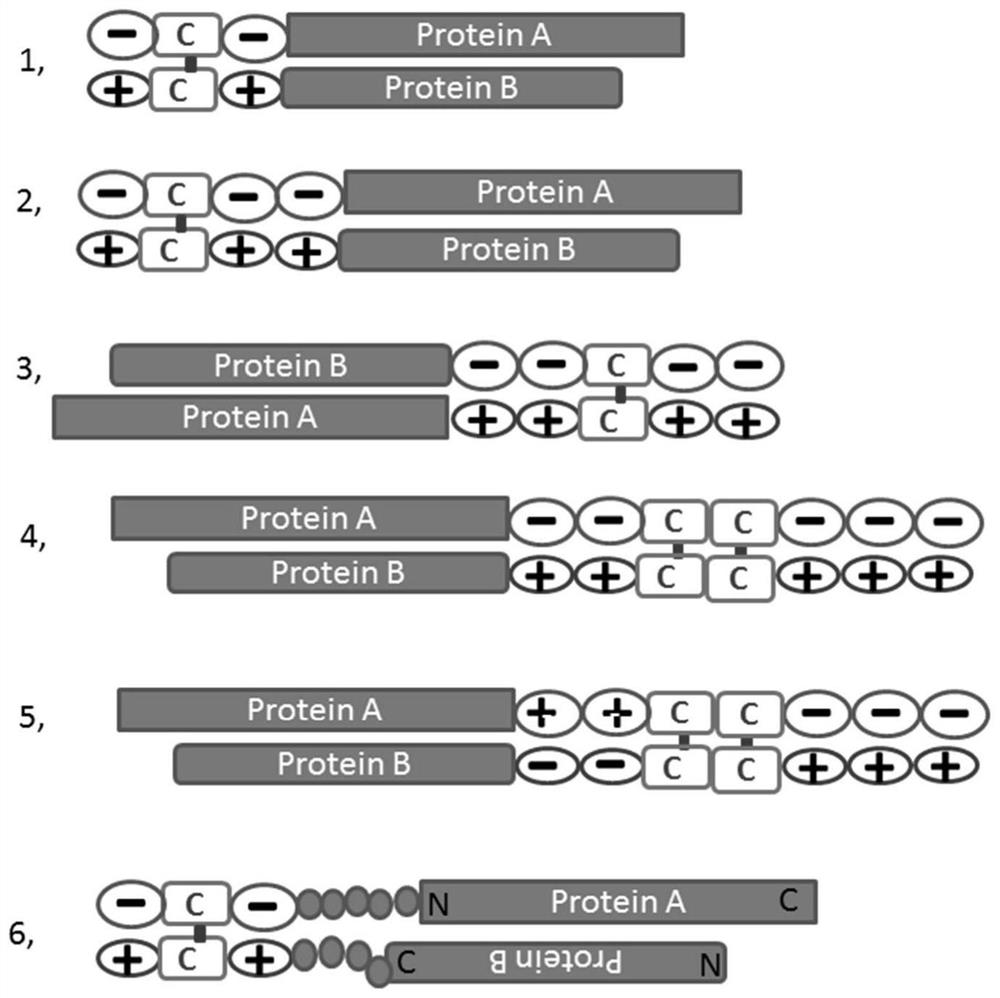
Zipper fastener structure for promoting formation of protein dimers and application thereof
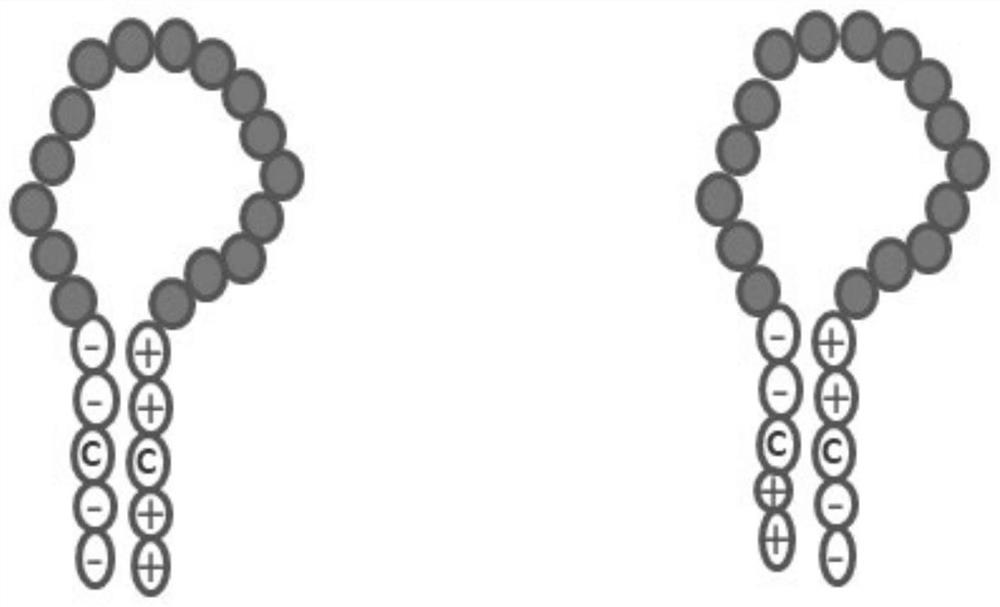
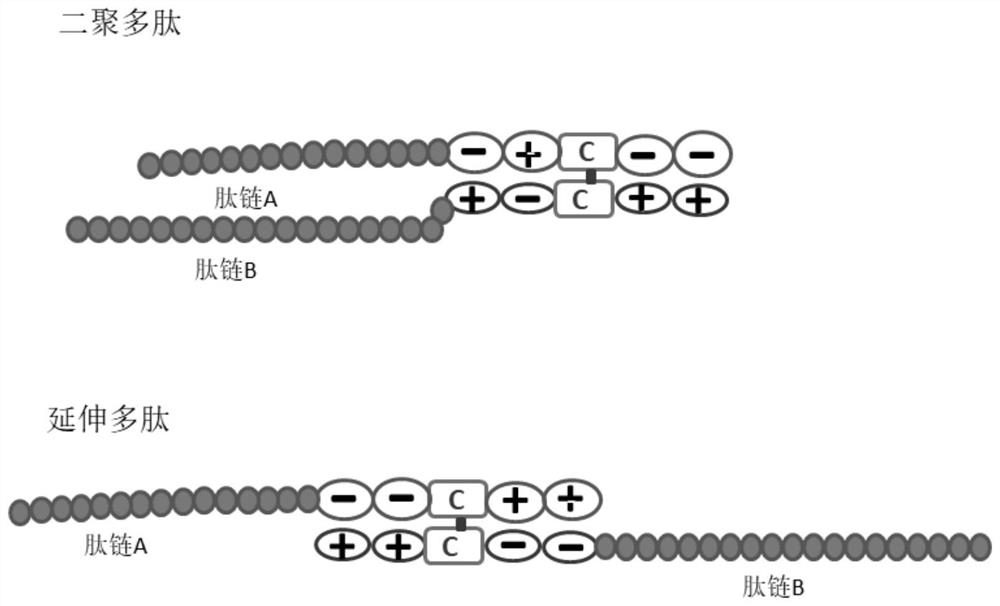
PendingCN111655734AReduce the chance of formationAuxiliary formationAntibody mimetics/scaffoldsDepsipeptidesDimerAntiendomysial antibodies
The invention belongs to the field of gene engineering, and relates to a zipper fastener structure for promoting formation of protein dimers and an application thereof, and the zipper fastener can beused for dimerization of homologous proteins and dimerization of heterologous proteins, and can also be used for polypeptide cyclization, dimerization of polypeptides and extension of polypeptides. Some examples can obtain an ESAT6-CFP10 dimer close to native conformation, wherein the dimer has better solubility and has a better stimulation effect on memory T cells than ESAT6-CFP10 protein expressed by linear fusion. The inventor also finds that the dimer zipper fastener can help to form more stable cyclic polypeptide, and the CCP polypeptide added with the dimer fastener can increase the detection rate of citrullinated antibody in serum of rheumatoid arthritis patients.
Owner:GUANGZHOU LDEBIO TECH CO LTD
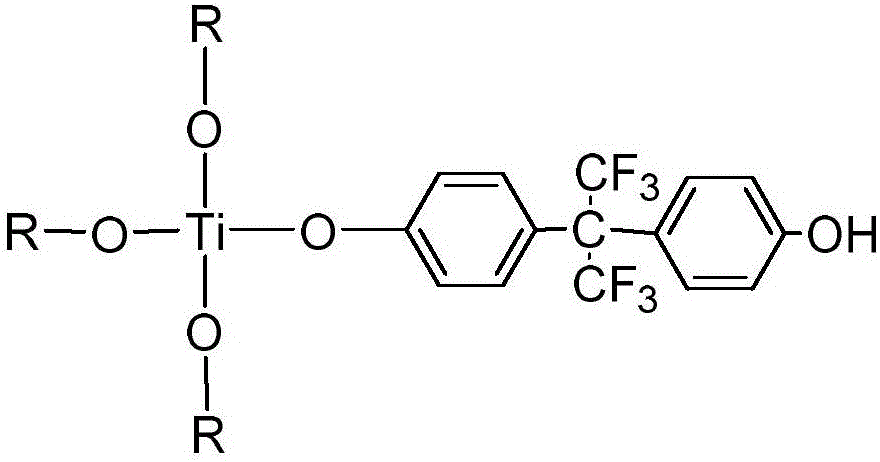
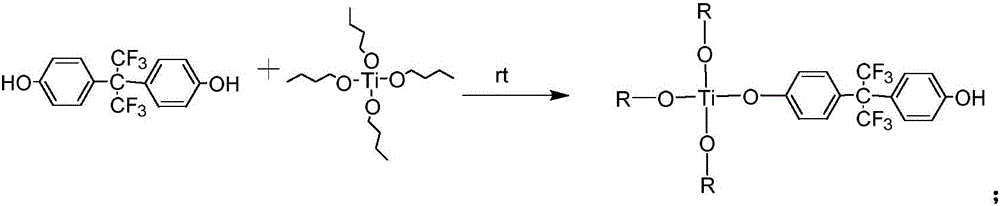
Fluorine titanium hybrid flame retardant, and preparation method and application thereof
ActiveCN106751469ASimple and mild reaction conditionsHigh yieldTitanium organic compoundsEnvironmental resistanceEpoxy
The invention discloses a fluorine titanium hybrid flame retardant, and a preparation method and application thereof. The fluorine titanium hybrid flame retardant is characterized in that the fluorine titanium hybrid flame retardant has the molecular structural formula shown in the description, wherein R is CH3CH2CH2CH2 or shown as the description. When the fluorine titanium hybrid flame retardant is used for preparing flame-retardant epoxy resin, the reaction conditions are simple and mild; the yield is high; the product purification operation is simple; the industrialization is easy; the prepared epoxy resin has high transparency; on the premise of ensuring the excellent heat resistance and mechanical property, the flame retardant effect is good; the application range is wide; the environment protection requirements are met.
Owner:XIAMEN UNIV
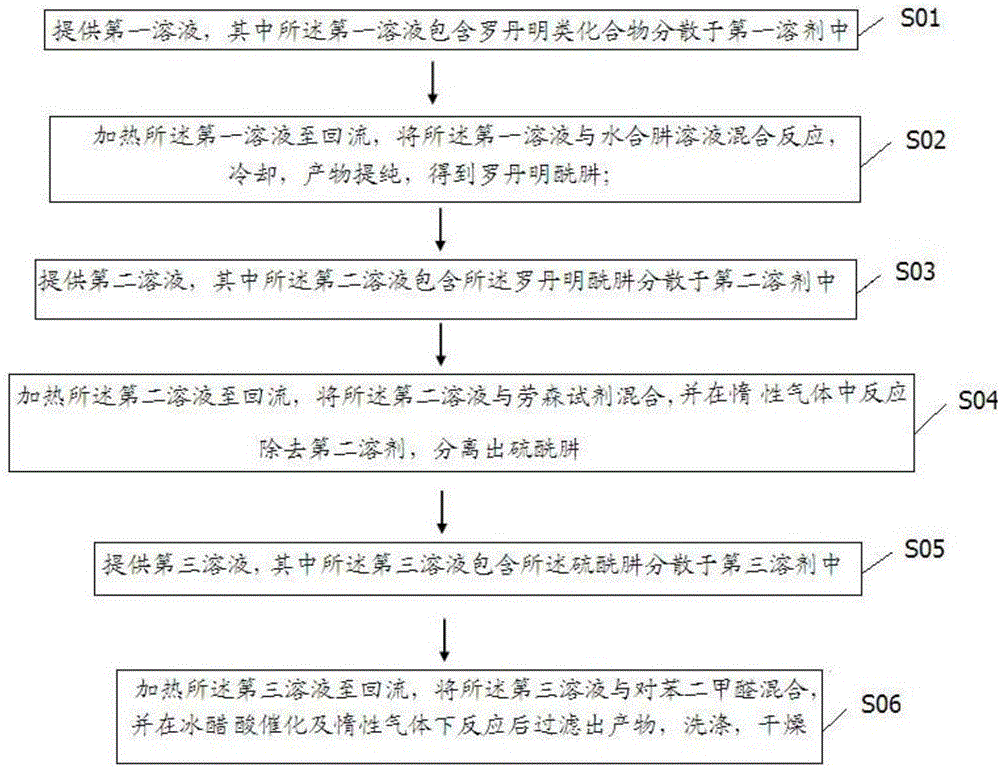
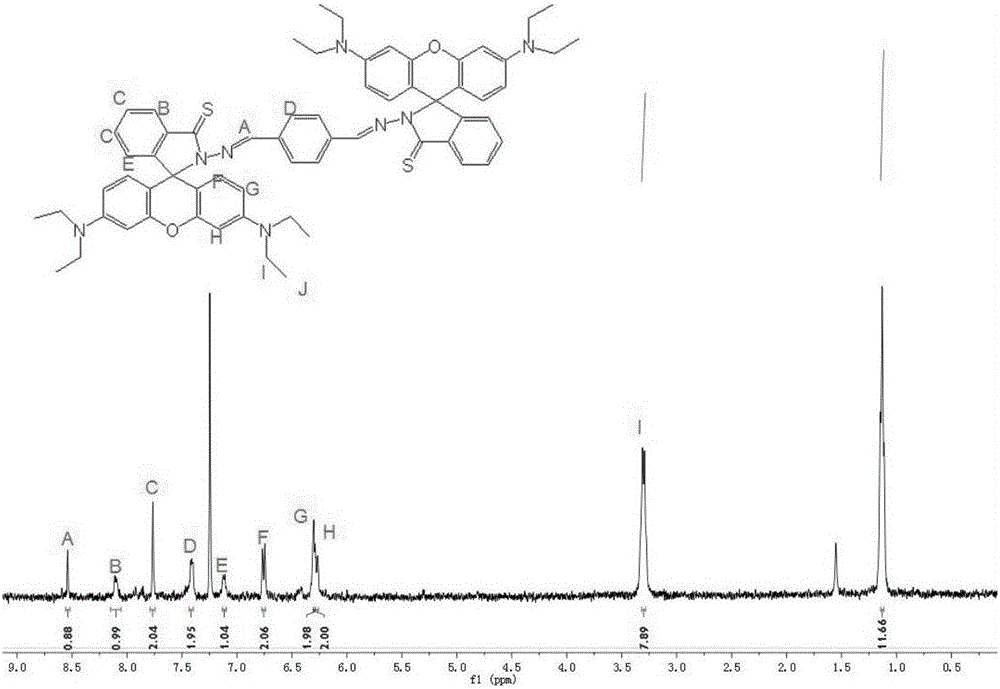
Compound selectively recognizing mercury ions and preparation method and application thereof
ActiveCN106349250ASimplify purification operationsGood selective identificationOrganic chemistryFluorescence/phosphorescenceHydrazine compoundSolvent
The invention mainly aims to provide a compound selectively recognizing mercury ions and a preparation method and application thereof. The preparation method includes following steps: 1), providing a first solution which contains a rhodamine compound dispersed in a first solvent; 2), heating the first solution, and mixing the first solution with a hydrazine hydrate solution for reaction to obtain rhodamine hydrazide; 3), providing a second solution which contains rhodamine hydrazide dispersed in a second solvent; 4), heating the second solution, mixing the second solution with a Lawson reagent, reacting in an inertial gas atmosphere, and separating out sulfur hydrazide; 5), providing a third solution which contains sulfur hydrazide dispersed in a third solvent; 6), heating the third solution, mixing the third solution with terephthalaldehyde, reacting under catalysis of glacial acetic acid and in inertial gas, and filtering to obtain a product. The synthesis process is free of byproduct generation, and the compound is simple in purifying operation and has good selective recognition effect on Hg2+.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Method for preparing alkoxy aluminum
ActiveCN102050700BSimplify purification operationsThorough responsePreparation of metal alcoholatesAlcoholBoiling point
The invention discloses a method for preparing alkoxy aluminum. The method comprises the following steps: aluminium powder and / or aluminium yarns and aluminium ingots are put in a reaction vessel; fatty alcohol between C3-C10 is used for reacting in an inert environment, and is divided into two parts; the weight of one part is 10-35 percent of the total weight of the fatty alcohol; the first partof the fatty alcohol is added into the reaction vessel, and is contacted with the aluminium powder or aluminium yarns at the temperature which is 5-25 DEG C lower than the boiling point of the fatty alcohol for initiating the reaction; the second part of the fatty alcohol which is left is continuously added into the reaction vessel after the reaction is initiated, and is continuously reacted withthe aluminium powder or aluminium yarns until the reaction is fully carried out at the temperature which is lower than the boiling point of the fatty alcohol; and the weight of the aluminium powder or aluminium yarns is 0.5-5.0 percent of the total weight of the aluminium powder and / or aluminium yarns and the aluminium ingots. The method has the advantages that a catalyst is not used, the preparation is simple, and the reaction can be carried out fully, stably and fast.
Owner:CHINA PETROLEUM & CHEM CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com












![Synthetic method for 1-{2-floro-4-[5-(4-isobutyl phenyl)-1,2,4-oxadiazol-3-yl]-benzyl}-3-azetidinecarboxylic acid Synthetic method for 1-{2-floro-4-[5-(4-isobutyl phenyl)-1,2,4-oxadiazol-3-yl]-benzyl}-3-azetidinecarboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c21b9f10-3d3c-49eb-9ae9-8a98ea128392/BDA0000558580880000021.PNG)
![Synthetic method for 1-{2-floro-4-[5-(4-isobutyl phenyl)-1,2,4-oxadiazol-3-yl]-benzyl}-3-azetidinecarboxylic acid Synthetic method for 1-{2-floro-4-[5-(4-isobutyl phenyl)-1,2,4-oxadiazol-3-yl]-benzyl}-3-azetidinecarboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c21b9f10-3d3c-49eb-9ae9-8a98ea128392/BDA0000558580880000022.PNG)
![Synthetic method for 1-{2-floro-4-[5-(4-isobutyl phenyl)-1,2,4-oxadiazol-3-yl]-benzyl}-3-azetidinecarboxylic acid Synthetic method for 1-{2-floro-4-[5-(4-isobutyl phenyl)-1,2,4-oxadiazol-3-yl]-benzyl}-3-azetidinecarboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c21b9f10-3d3c-49eb-9ae9-8a98ea128392/BDA0000558580880000031.PNG)