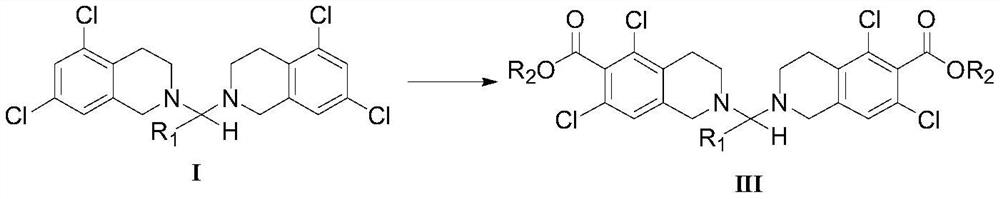
5, 7-dichlorotetrahydroisoquinoline acetal amine compound as well as preparation method and application thereof
A compound, C3-C20 technology, applied in the field of 5,7-dichlorotetrahydroisoquinoline aminals, its preparation, and the synthesis of key intermediates of ritazast, which can solve the problem of increasing recovery and processing Issues such as the cost of three wastes, great environmental hazards, and reduced production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Synthesis of two (5,7-dichloro-3,4-dihydroisoquinolin-2 (1H)-yl) methane (I-1) of embodiment 1
[0079]
[0080] At room temperature, 5,7-dichloro-1,2,3,4-tetrahydroisoquinoline hydrochloride (II-1) (4 g, 16.77 mmol) was added to 80 mL of water, and then added to the suspension 37% formaldehyde aqueous solution (0.680g, 8.38mmol) was stirred for 30 minutes, then sodium hydroxide (0.771g, 19.28mmol) aqueous solution (8mL) was added in portions, and the addition was completed in about 40 minutes. After the addition, the reaction solution was stirred at room temperature for 3 hours, then filtered, the filter cake was washed with water, and dried to obtain bis(5,7-dichloro-3,4-dihydroisoquinolin-2(1H)-yl)methane (I-1), white solid 3.278g, yield: 94%. 1 H NMR (400MHz, CDCl 3 ): δ7.23(d, J=1.8Hz, 2H), 6.96(s, 2H), 3.70(s, 4H), 3.28(s, 2H), 2.90~2.86(m, 4H), 2.84~2.81( m, 4H). 13 C NMR (400MHz, CDCl 3 ): δ138.3, 134.9, 131.6, 126.7, 125.7, 79.4, 53.9, 48.5, 26.7.
Embodiment 2
[0081] Example 2 Synthesis of (5,7-dichloro-3,4-dihydroisoquinolin-2(1H)-yl)methane (I-1)
[0082]
[0083] At room temperature, 5,7-dichloro-1,2,3,4-tetrahydroisoquinoline hydrochloride (0.5 g, 2.10 mmol) was added to 10 mL of water, and then paraformaldehyde ( 0.031g, 1.05mmol), and stirred for 30 minutes. The prepared aqueous sodium hydroxide solution (0.096 g of sodium hydroxide dissolved in 1 mL of water) was added to the suspension in batches, and the addition was completed in about 20 minutes. After the addition, the reaction solution was stirred at room temperature for 5 hours, then the reaction solution was filtered, the filter cake was washed with water, and dried to obtain bis(5,7-dichloro-3,4-dihydroisoquinolin-2(1H)-yl) Methane (I-1), white solid 0.38g. Yield: 87%. 1 H NMR (400MHz, CDCl 3 ): δ7.23(d,J=1.8Hz,2H),6.96(s,2H),3.70(s,4H),3.28(s,2H),2.90~2.86(m,4H),2.84~2.81( m, 4H). 13 C NMR (400MHz, CDCl 3 ): δ138.3, 134.9, 131.6, 126.7, 125.7, 79.4, 53.9, 4...
Embodiment 3
[0084] Example 3 Synthesis of two (5,7-dichloro-3,4-dihydroisoquinolin-2(1H)-yl)methane (I-1)
[0085]
[0086] At room temperature, 5,7-dichloro-1,2,3,4-tetrahydroisoquinoline hydrochloride (0.5 g, 2.10 mmol) was added to 10 mL of water, and then paraformaldehyde ( 0.031g, 1.05mmol), and stirred for 30 minutes. The prepared potassium carbonate aqueous solution (0.333g potassium carbonate dissolved in 2mL water) was added to the above suspension in batches, and the addition was completed in about 20 minutes. After the addition, the reaction solution was stirred at room temperature for 5 hours, then the reaction solution was filtered, the filter cake was washed with water, and dried to obtain bis(57-dichloro-3,4-dihydroisoquinolin-2(1H)-yl)methane (I-1), 0.35 g of white solid. Yield: 80%. 1 H NMR (400 MHz, CDCl 3 ): δ7.23(d,J=1.8Hz,2H),6.96(s,2H),3.70(s,4H),3.28(s,2H),2.90~2.86(m,4H),2.84~2.81( m, 4H). 13 C NMR (400MHz, CDCl 3 ): δ138.3, 134.9, 131.6, 126.7, 125.7, 79...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com