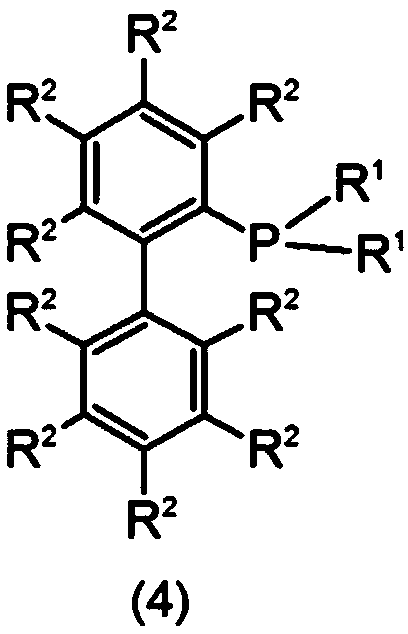
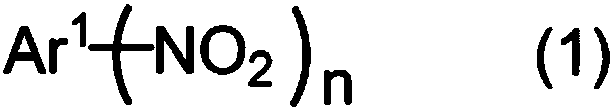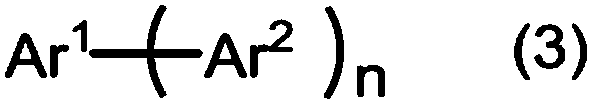Method for producing aromatic compound
A technology for aromatic compounds and manufacturing methods, which is applied in the field of cross-coupling reactions to manufacture aromatic compounds, can solve problems such as limitations and reaction substrate restrictions, and achieve excellent manufacturing processes, wide degrees of freedom/selection, and reduced environmental burdens Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Add a stirring bar, 92 mg (0.60 mmol) of 4-nitroanisole, 110 mg (0.90 mmol) of phenylboronic acid, 9.1 mg (0.030 mmol) of palladium (II) acetylacetonate, and 2 -Dicyclohexylphosphino-3,6-dimethoxy-2',4',6'-triisopropylbiphenyl 64mg (0.12mmol), tripotassium phosphate n-hydrate 480mg (1.8mmol), 18 -crown-616mg (0.060mmol), and 1,4-di Alkane 3mL. After the vial was tightly capped, it was heated and stirred at 130° C. for 24 hours. Next, the reaction solution was cooled to room temperature. Dichloromethane was added to the reaction liquid, and it was filtered through celite. The residue obtained by concentrating the filtrate was dissolved in diethyl ether (20 mL), and 30% aqueous hydrogen peroxide (5 mL) was added thereto. After stirring at room temperature for 1 hour, it was washed with distilled water (10 mL) and saturated aqueous iron(II) sulfate (10 mL). After extraction with diethyl ether (20 mL×3), the collected organic layer was washed with saturated brine (10 ...
Embodiment 2
[0061] Add a stirring bar, 119 mg (0.60 mmol) of 4-nitrobiphenyl, 110 mg (0.90 mmol) of phenylboronic acid, 9.1 mg (0.030 mmol) of palladium (II) acetylacetonate, and 2- Dicyclohexylphosphino-3,6-dimethoxy-2',4',6'-triisopropylbiphenyl 64mg (0.12mmol), cesium fluoride 270mg (1.8mmol), and 1,4- two Alkane 3mL. After the vial was tightly capped, it was heated and stirred at 150° C. for 24 hours. Next, the reaction solution was cooled to room temperature. Dichloromethane was added to the reaction liquid, and it was filtered through celite. The residue obtained by concentrating the filtrate was dissolved in diethyl ether (20 mL), and 30% aqueous hydrogen peroxide (5 mL) was added thereto. After stirring at room temperature for 1 hour, it was washed with distilled water (10 mL) and saturated aqueous iron(II) sulfate (10 mL). After extraction with diethyl ether (20 mL×3), the collected organic layer was washed with saturated brine (10 mL). After drying with anhydrous magnesium...
Embodiment 3
[0065] Add a stir bar, 115 mg (0.60 mmol) of 4-(trifluoromethyl) nitrobenzene, 110 mg (0.90 mmol) of phenylboronic acid, and 9.1 mg (0.030 mmol), 2-dicyclohexylphosphino-3,6-dimethoxy-2',4',6'-triisopropylbiphenyl 64mg (0.12mmol), cesium fluoride 270mg (1.8mmol), and 1,4-di Alkane 3mL. After the vial was tightly capped, it was heated and stirred at 130° C. for 24 hours. Next, the reaction solution was cooled to room temperature. Dichloromethane was added to the reaction liquid, and it was filtered through celite. The residue obtained by concentrating the filtrate was purified by medium-pressure column chromatography (using a Biotage SNAP Ultra column (particle size: 25 μm), developing solvent = hexane / ethyl acetate) to obtain the target 4-(trifluoromethyl Base) biphenyl 74mg (55% yield). pass 1 H and 13 C-NMR carried out the identification of the target substance.
[0066] 1 H-NMR(CDCL3)=δ7.70(s, 4H), 7.61(d, J=6.9Hz, 2H), 7.48(t, J=7.3Hz, 2H), 7.42(d, J=7.3Hz, 1H )...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com