Method for preparing alpha-(di-n-butylaminomethyl)-2,7-dichloro-4-fluorenemethanol and the hydrochloride thereof
A technology of di-n-butylaminomethyl and fluorene methanol is applied in chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxy compounds, etc., and can solve the problems of unfavorable industrialized production, cumbersome post-processing methods, and many raw materials consumed. , to achieve the effect of reduced production cost, easy operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
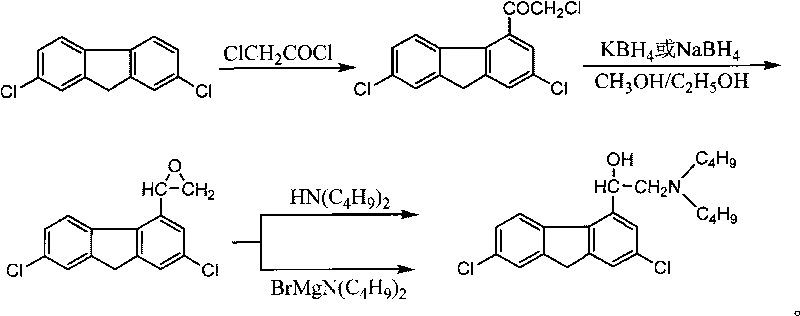
[0028] Weigh 20.2g (0.151mol) of anhydrous aluminum trichloride and 12g (0.106mol) of chloroacetyl chloride into 300ml of 1,2-dichloroethane, cool the reactant to 0-5°C, and further Stir the reactant at 5°C for 30 minutes, add 20g (0.085mol) of 2,7-dichlorofluorene, stir the reactant at 0-5°C for 2-3 hours, then allow it to naturally heat up and react for 2-3 hours; After completion, the reactant was cooled to 0-5°C, then 400ml of ice water containing 120ml of concentrated hydrochloric acid was slowly added to the reactant, fully stirred for 30 minutes, and then the organic layer was separated and washed with 50ml of 1,2-dichloroethane The aqueous layer was extracted; the obtained organic layers were combined, washed with water until almost no acidity, and then the solvent was evaporated to dryness, and the residue was 2,7-dichloro-4-chloroacetylfluorene. Add 400ml of absolute ethanol to the residue, control the temperature at 40-45°C, add 8g (0.148mol) of potassium borohydrid...
Embodiment 2
[0030]Weigh 20.2g (0.151mol) of anhydrous aluminum trichloride and 12g (0.106mol) of chloroacetyl chloride into 300ml of dichloromethane, cool the reactant to 0-5°C, and further stir at 0-5°C Add 20g (0.085mol) of 2,7-dichlorofluorene to the reactant for 30 minutes, stir the reactant at 0-5°C for 2-3 hours, then allow it to naturally heat up and react for 2-3 hours; after the reaction is completed, the reaction The mixture was cooled to 0-5°C, and then 400ml of ice water containing 120ml of concentrated hydrochloric acid was slowly added to the reactant, stirred thoroughly for 30 minutes, then the organic layer was separated, and the aqueous layer was extracted with 50ml of dichloromethane; the organic layers obtained were combined, It was washed with water until it was not acidic, and then the solvent was evaporated to dryness, and the residue was 2,7-dichloro-4-chloroacetylfluorene. Add 400ml of absolute ethanol to the residue, control the temperature at 40-45°C, add 8g (0.1...
Embodiment 3
[0032] Add 50.5g (0.379mol) of anhydrous aluminum trichloride and 30g (0.266mol) of chloroacetyl chloride into 900ml of dichloromethane, cool the reactant to 0-5°C, and then stir the reaction at 0-5°C After 30 minutes, add 50 g (0.213 mol) of 2,7-dichlorofluorene, stir the reactant at 0-5°C for 2-3 hours, then allow it to naturally heat up and react for 2-3 hours; after the reaction is completed, the reactant Cool to 0-5°C, then slowly add 1000ml of ice water containing 300ml of concentrated hydrochloric acid to the reactant, stir thoroughly for 30 minutes, then separate the organic layer, extract the aqueous layer with 100ml of dichloromethane; combine the obtained organic layers, and water Wash until no acidity, then evaporate the solvent to dryness, the residue is 2,7-dichloro-4-chloroacetylfluorene. Add 1000ml of absolute ethanol to the residue, control the temperature at 40-45°C, add 20g (0.371mol) of potassium borohydride, maintain the temperature at 40-45°C for 18 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com