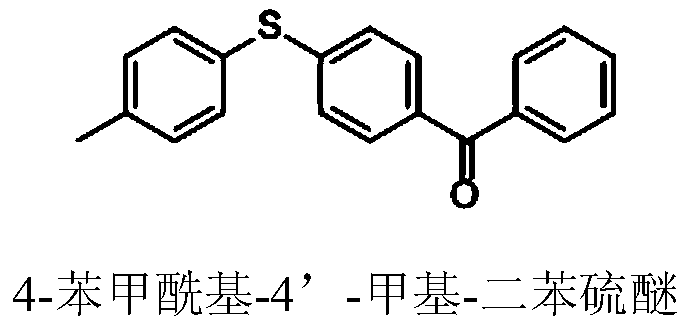
Method for preparing 4-benzoyl-4'-methyl-diphenyl sulfide
A technology of benzoyl and diphenyl sulfide, which is applied in the field of preparation of photoinitiator 4-benzoyl-4'-methyl-diphenyl sulfide, can solve the problems of unavailable raw materials, market shortage, and impact on industrial sustainability. Production and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of 4-benzoyl-4'-methyl-diphenylsulfide
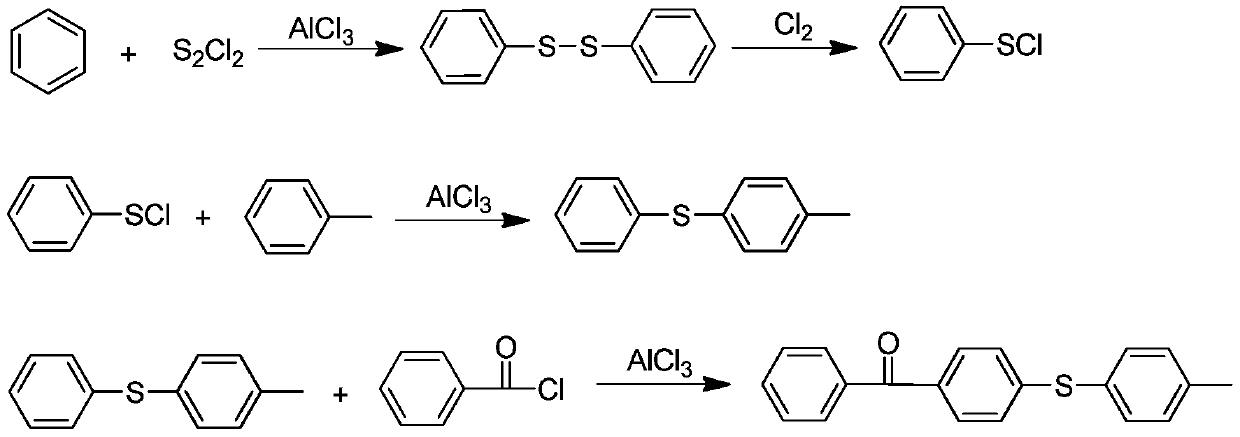
[0029] 1) Preparation of diphenyl disulfide
[0030] After mixing 200mL of benzene and aluminum trichloride (43g, 0.4mol) evenly, connect the tail gas absorption device, slowly add sulfur monochloride (54g, 0.4mol) dropwise while stirring, and keep the reaction temperature at 30-35°C. After dripping and keeping warm, monitor the reaction by TLC or GC. After the reaction is complete, pour it into ice water for hydrolysis reaction. After the reaction, let it stand and separate layers. The organic phase is washed with water and precipitated. Recrystallization was carried out to obtain 77.7 g of a white solid with a GC content of 99.0% and a melting point of 58-59°C.
[0031] 2) Preparation of phenylthionyl chloride
[0032] Take the above-prepared diphenyl disulfide (65.5g, 0.3mol) and dissolve it in 180mL of dichloroethane, connect the tail gas absorption device, then slowly introduce chlorine gas, c...
Embodiment 2
[0037] Embodiment 2: the preparation of 4-benzoyl-4'-methyl-diphenylsulfide
[0038] 1) Preparation of phenylsulfenyl chloride
[0039] Dissolve the diphenyl disulfide (65.5 g, 0.3 mol) prepared by the method in Example 1 in 200 mL of chlorobenzene, connect the tail gas absorption device, and then slowly introduce chlorine gas, control the reaction temperature 0-5 ° C, after the reaction is complete , first remove the solvent, then distill and purify phenylthionyl chloride under reduced pressure, and collect 41.6g of red liquid at b.p.82-83℃@10mmHg.
[0040] 2) The preparation method of 4-benzoyl-4'-methyl-diphenyl sulfide
[0041]Mix 250mL dichloroethane, aluminum trichloride (40.0g, 0.3mol) and toluene (32.2g, 0.35mol) evenly, connect the tail gas absorption device, and slowly add the benzene prepared by step 1) dropwise under vigorous stirring. Sulfenyl chloride (43.4g, 0.3mol), the reaction temperature is maintained at 25-35 ° C, 1h drop, TLC or GC monitoring reaction, a...
Embodiment 3
[0042] Embodiment 3: the preparation of 4-benzoyl-4'-methyl-diphenylsulfide
[0043] 1) Preparation of phenylsulfenyl chloride
[0044] Dissolve the diphenyl disulfide (65.5 g, 0.3 mol) prepared by the method in Example 1 in 180 mL of dichloroethane, connect the tail gas absorption device, then slowly feed chlorine gas, control the reaction temperature at 0-5 ° C, and react After completion, remove the solvent first, then distill and purify phenylthionyl chloride under reduced pressure, and collect 40.8g of red liquid at b.p.82-83°C@10mmHg.
[0045] 2) The preparation method of 4-benzoyl-4'-methyl-diphenyl sulfide
[0046] Mix 250mL dichloroethane, aluminum trichloride (72.0g, 0.54mol) and toluene (32.2g, 0.35mol) evenly, connect the tail gas absorption device, and slowly add the benzene prepared by step 1) dropwise under vigorous stirring. Sulfenyl chloride (43.4g, 0.3mol), the reaction temperature was maintained at 25-35°C, and the drop was completed after 1h, and the reac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com