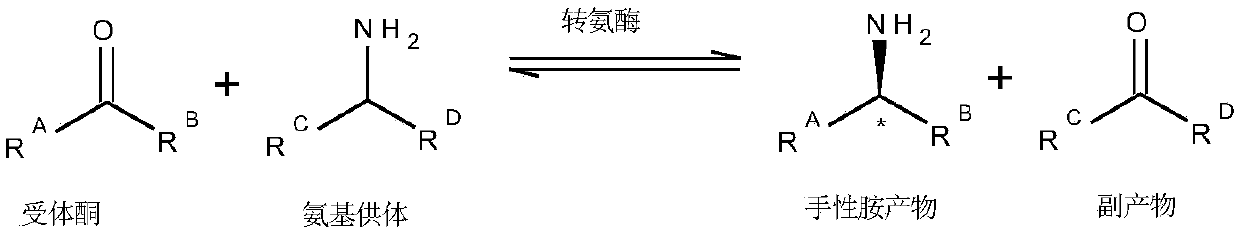
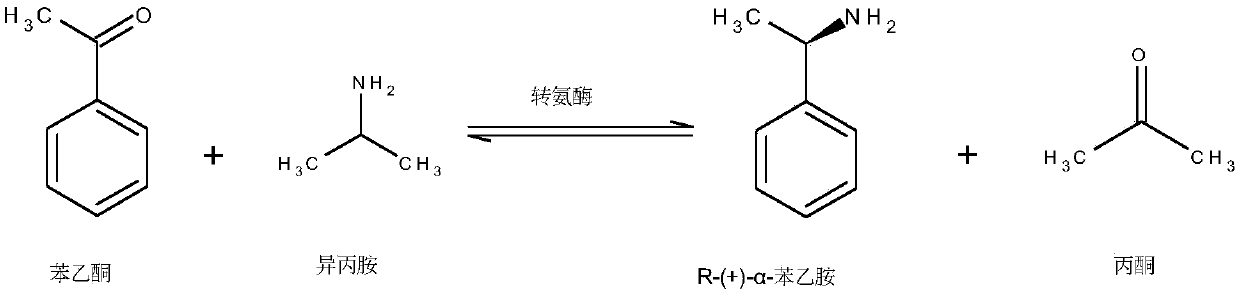
Engineered transaminase polypeptide and application thereof
A transaminase and engineering technology, applied in the field of engineering transaminase polypeptides, can solve the problem of limited cost of natural raw materials and high
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0295] Embodiment 1: the construction of gene cloning and expression vector
[0296] The amino acid sequence of the wild-type transaminase derived from Aspergillus fumigatus can be retrieved from NCBI, and then its corresponding nucleic acid is synthesized by common techniques in the art and cloned into the expression vector pACYC-Duet-1. Transform the recombinant expression plasmid into competent cells of E.coil BL21(DE3), the transformation condition is 42°C, heat shock for 90 seconds, the transformation solution is spread on the LB plate containing chloramphenicol, and cultured upside down at 37°C overnight, that is Recombinant transformants were obtained.
Embodiment 2
[0297] Embodiment 2: Shake flask expression of transaminase polypeptide
[0298] The recombinant E.coli BL21 (DE3) transformant obtained in Example 1 was inoculated into 50 mL of chloramphenicol-containing LB medium (peptone 10 g / L, yeast extract powder 5 g / L, sodium chloride 10 g / L, pH 7.0±0.2, 25°C) in a 250mL Erlenmeyer flask, placed at 30°C, shaking at 250rpm and culturing overnight. When the OD600 of the culture medium reaches 2, insert 250mL TB medium (tryptone 12g / L, yeast extract powder 24g / L, disodium hydrogen phosphate 9.4g / L , dipotassium hydrogen phosphate 2.2g / L, pH value 7.2±0.2, 30°C) into a 1000mL Erlenmeyer flask with a final concentration of 6g / L lactose as an inducer, placed at 30°C, 250rpm shaker shaking culture. After induction at 30°C for 20 h, the culture medium was centrifuged to collect the cells, washed twice with PBS buffer (pH 7.4), and the supernatant was centrifuged to obtain wet cells, which were placed in a -20°C refrigerator until use. If en...
Embodiment 3
[0299] Embodiment 3: Construction of transaminase mutant library
[0300] All the reagents used here are commercial reagents, preferably Quikchange kit (supplier: Agilent). The sequence design of the mutant primers was carried out according to the instructions of the kit. The construction of a saturation mutation library at a single residue position is now described as an example. The PCR system is: 5xBuffer 10uL, 10mM dNTP 1uL, plasmid DNA template 1uL (50ng / uL), upstream and downstream primers 0.75uL (10uM), high-fidelity enzyme 0.5uL, ddH2O 36uL. The codon of the PCR primer at the mutation position is NNK.
[0301] PCR amplification steps are: (1) 98°C, pre-denaturation for 3min; (2) 98°C, denaturation for 10s; (3) 72°C annealing and extension for 3min; steps (2)-(3) were repeated 25 times; (5) Continue to extend at 72°C for 10min, and cool to 4°C. Add 2uLDpnI to the PCR product, and digest overnight at 37°C to eliminate the plasmid template. The digested PCR product w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com