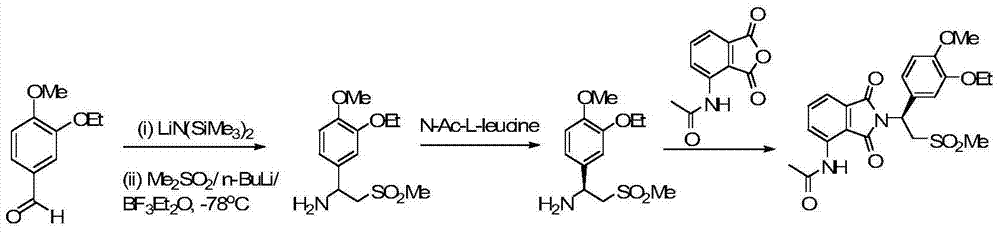
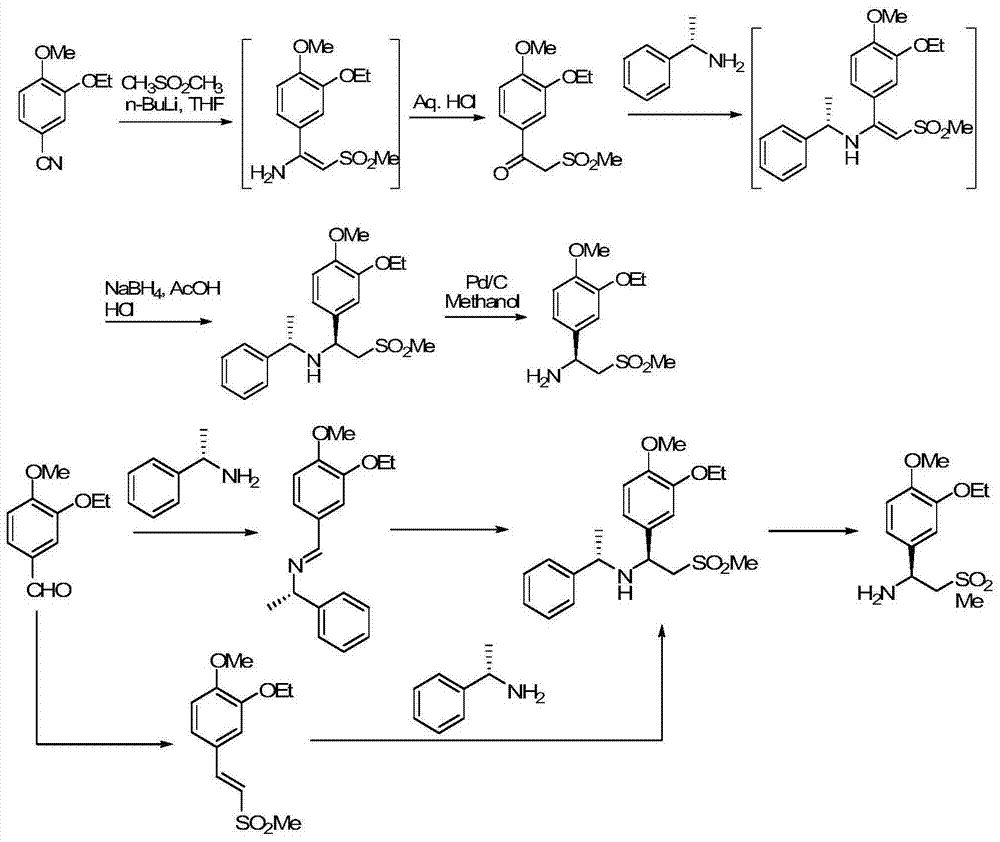
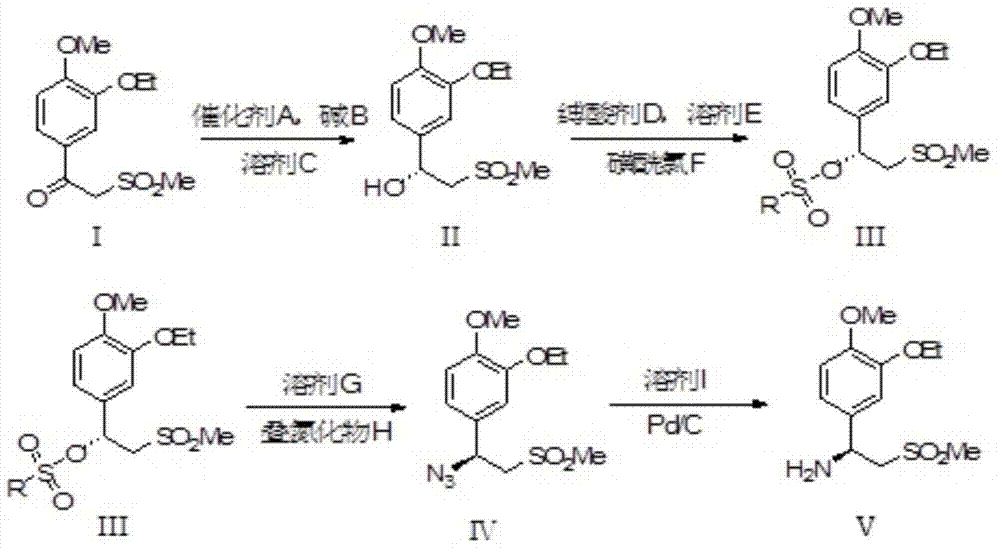
Synthetic method of apremilast chiral amine intermediate
A synthetic method and intermediate technology, applied in the field of pharmaceutical and chemical intermediate synthesis, can solve the problems of difficult industrialization, long process cycle, high reagent price, etc., and achieve the effect of simple synthetic route, stable reaction process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In a 250ml dry Shrek bottle, 2.72g (10mmol) of 1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanone was dissolved in 30ml of anhydrous toluene, After bubbling with argon for 15 min, transfer to a 100 mL hydrogenation tube, add 10 mg of DIOP-RuCl 2 -Me-BIMAH catalyst and 70mg (0.6mmol) of potassium tert-butoxide, passed through 3MPa of hydrogen, stirred at 25°C for 16h. Filtrate, and remove the solvent by rotary evaporation to obtain 2.70 g of off-white solid (R)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanol, the reaction yield is 99.4% , the enantiomeric excess value was 98.2%, and it was directly carried out to the next reaction without purification.
[0038] After dissolving 2.74g (10mmol) (R)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanol in 30ml of dichloromethane, transfer it to a 100mL three-necked flask 4.0 mL (30 mmol) of triethylamine was added, and a solution of 1.16 mL of methanesulfonyl chloride dissolved in 10 mL of dichloromethane was...
Embodiment 2
[0042] In a 250ml dry Shrek bottle, 5.45g (20mmol) of 1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanone was dissolved in 60ml of anhydrous toluene, After bubbling with argon for 15 min, transfer to a 250 mL hydrogenation tube, add 17 mg of DIOP-RuCl 2 -Me-BIMAH catalyst and 135 mg (1.2 mmol) of potassium tert-butoxide, passed through 1 MPa of hydrogen, stirred at 45° C. for 10 h. Filtrate, and remove the solvent by rotary evaporation to obtain 5.42 g of off-white solid (R)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanol, with a reaction yield of 98.8% , the enantiomeric excess value was 98.0%, and it was directly carried out to the next reaction without purification.
[0043] After dissolving 2.74g (10mmol) (R)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanol in 30ml of dichloromethane, transfer it to a 100mL three-necked flask 6.0 mL (40 mmol) of pyridine was added, and a solution of 2.2 mL of benzenesulfonyl chloride dissolved in 10 mL of dichloromethane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com