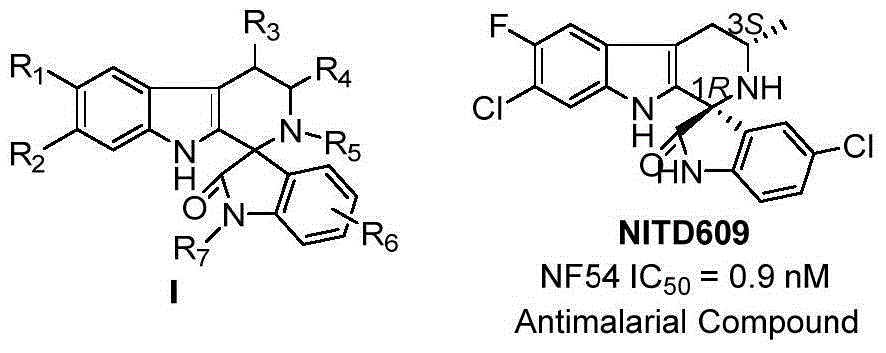
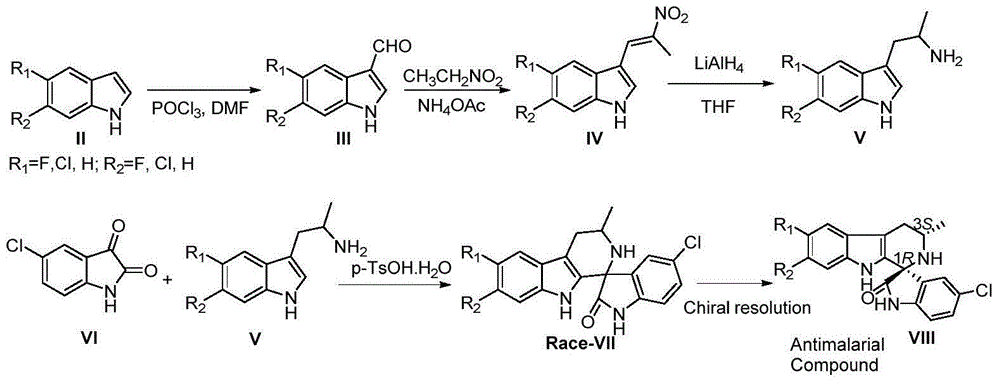
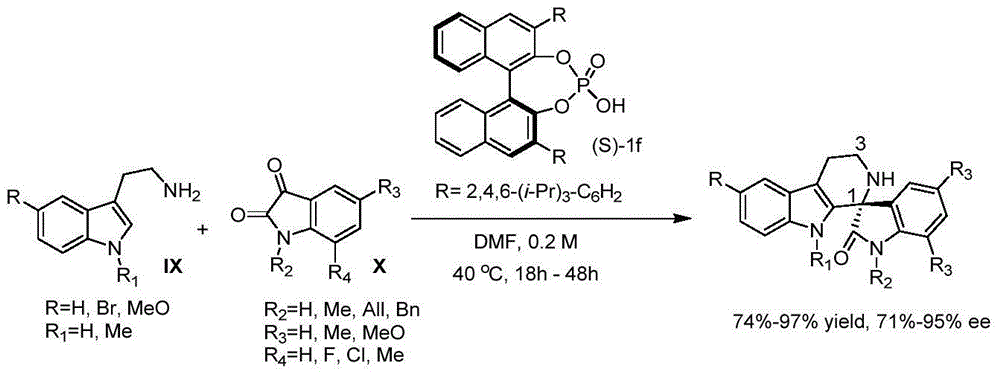
Method for asymmetric catalytic synthesis of spirocyclic tetrahydrocarbazoline compound
A technology of carbazoline and compound, which is applied in the field of synthesizing spiro tetrahydrocarbazoline compounds, can solve the problems of narrow types of spiro tetrahydrocarbazoline compounds, cannot be constructed at the same time, and high cost, and achieve a simple and clean reaction system Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Different chiral amine oxides and nickel trifluoromethanesulfonate [Ni(OTf) 2 ] Catalyzed reaction of unsymmetrical 3-alkenylindole with isatin-derived ketimine aza-Diels-Alder.
[0048]
[0049] Add nickel trifluoromethanesulfonate [Ni(OTf) 2 ] (0.01mmol), chiral amine oxide L (0.01mmol), isatin-derived ketimine 2a (0.1mmol), stirrer, add dichloromethane (15.5mmol), activate at 30°C for 30min. Take 1mL round bottom flask B, weigh compound 1a (0.15mmol) into it, add 3.1mmol dichloromethane to dissolve. Add the B reaction solution dropwise to A, stir for 48 hours, add 0.2 mL HCl (6.0 mol / L solution) to the reaction solution, stir for 6 hours at 30°C, add saturated NaHCO 3 The solution was neutralized to neutral, dichloromethane extracted, anhydrous Na 2 SO 4 Dry, filter with suction, concentrate, and pass through a column with petroleum ether / ethyl acetate (or heptane, or hexane / ethyl acetate, or heptane / ethyl acetate, etc.) to obtain compound I-a with ...
Embodiment 2
[0052] Example 2: Chiral amine oxide L-RaPr at -10°C 3 With nickel trifluoromethanesulfonate [Ni(OTf) 2 ] Catalyzed reaction of asymmetric 3‐alkenylindole with isatin-derived ketimine aza-Diels-Alder
[0053]
[0054] Add nickel trifluoromethanesulfonate [Ni(OTf) 2 ] (0.01mmol), chiral amine oxide L-RaPr 3 (0.01mmol), isatin-derived ketimine 2a (0.1mmol), stirrer, add dichloromethane (15.5mmol), activate at 30°C for 30min, and cool the reaction solution in test tube A to -10°C. Take 1mL round bottom flask B, weigh compound 1a (0.15mmol) into it, add 3.1mmol dichloromethane to dissolve, and cool to -10°C. Add the reaction liquid from bottle B to A dropwise, stir and react at -10°C for 192h, add 0.2mL HCl (6.0mol / L solution) to the reaction liquid, stir at 30°C for 6h, add saturated NaHCO 3 The solution was neutralized to neutral, dichloromethane extracted, anhydrous Na 2 SO 4 Dry, filter with suction, concentrate, and use petroleum ether / ethyl acetate (or heptane, or h...
Embodiment 3
[0055] Example 3: Chiral amine oxide L-RaPr 3 With nickel trifluoromethanesulfonate [Ni(OTf) 2 ] Catalytic aza-Diels-Alder reactions of unsymmetrical 3-alkenylindoles with different isatin-derived ketimines
[0056]
[0057] Add nickel trifluoromethanesulfonate [Ni(OTf) 2 ] (0.01mmol), chiral amine oxide L-RaPr 3 (0.01mmol) (chiral amine oxide L-RaPr 3 With nickel trifluoromethanesulfonate Ni(OTf) 2 molar ratio of 1.0:1.0), isatin-derived ketimine (0.1mmol), stirrer, add dichloromethane (15.5mmol), activate at 30°C for 30min, cool the reaction solution in test tube A to -10°C . Take 1mL round bottom flask B, weigh compound 1a (0.15mmol) into it, add 3.1mmol dichloromethane to dissolve, and cool to -10°C. Add the reaction liquid in B dropwise to A, stir and react at -10°C for 96-192h, add 0.2mL HCl (6.0mol / L solution) to the reaction liquid, stir at 30°C for 6h, add saturated NaHCO 3 The solution was neutralized to neutral, dichloromethane extracted, anhydrous Na 2 S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com