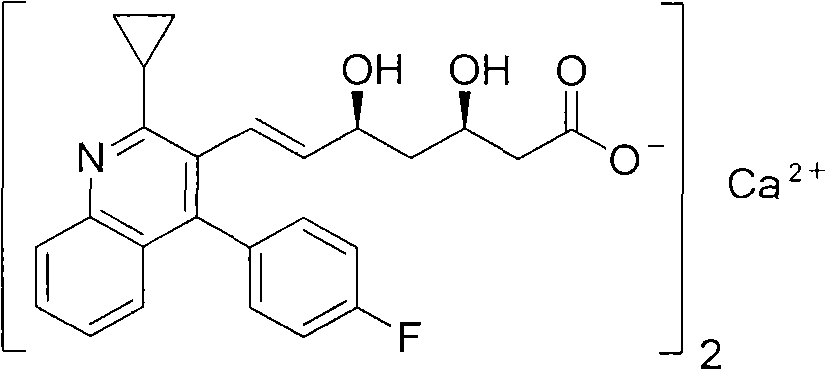
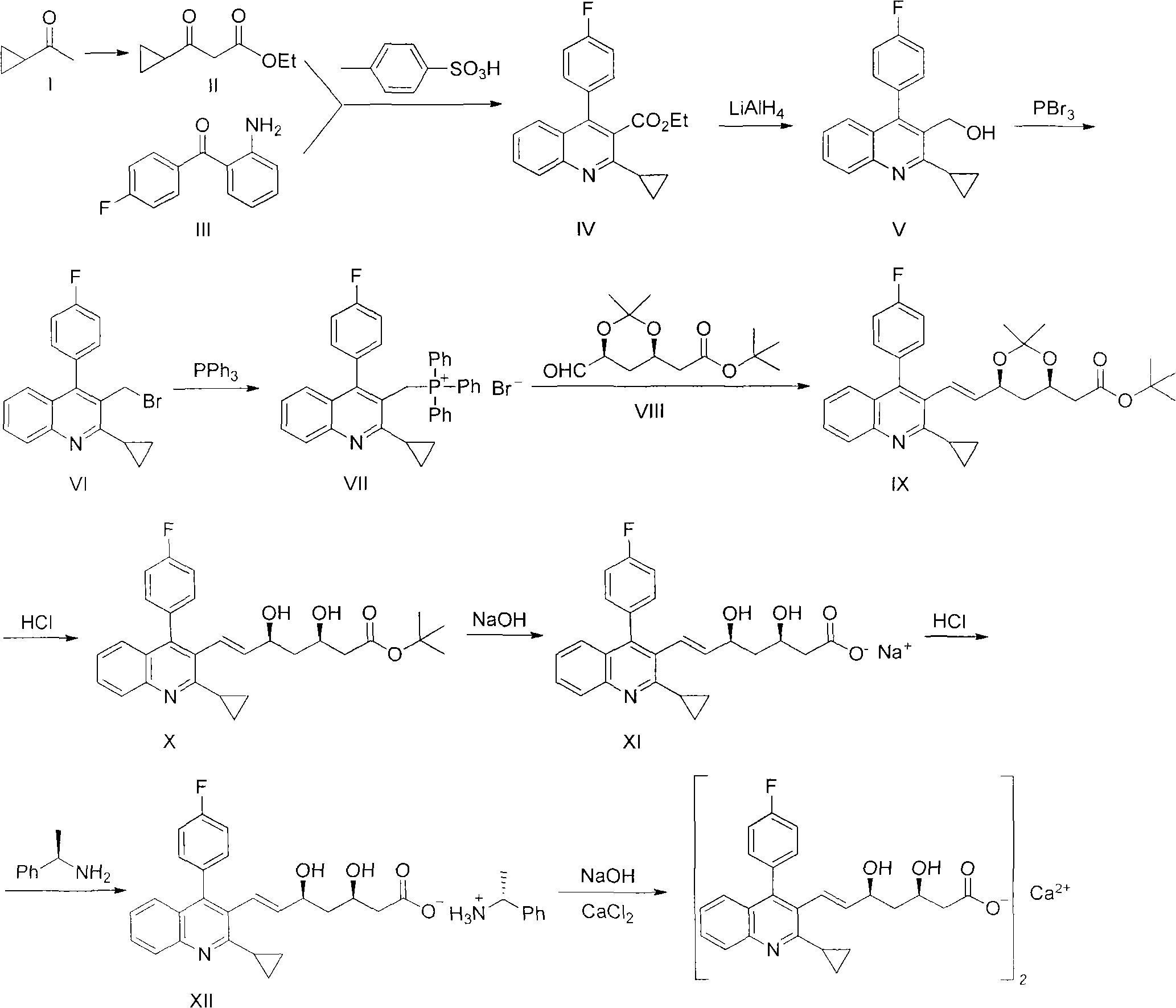
Preparation method of pitavastatin calcium
A technology of pitavastatin calcium and calcium salt, applied in the field of medicine, can solve the problems of rising cost and operation difficulty, and achieve the effects of reasonable design, high yield and concise process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: Preparation of 3-cyclopropyl-3-oxo-propionic acid ethyl ester (compound II)
[0022] Start stirring, add 4.3L ether into the reaction kettle, lower the temperature, and add 355g of 80% (weight) sodium hydride. First add 2.8kg of diethyl carbonate into the reaction kettle, then slowly drop in the mixed solution of 455g of cyclopropylmethyl ketone and 1.1L of ether. After the dropwise addition, the ice-water bath was removed and stirred for 48 hours. Add 4.3 L of 3 mol / L hydrochloric acid dropwise to the reaction solution for acidification and hydrolysis. The organic phase was separated, and the aqueous phase was extracted 3 times with ether. Combine the organic phases, wash them with saturated saline for 3 times, dry them with anhydrous magnesium sulfate, recover diethyl ether under normal pressure, and then change to vacuum distillation to collect fractions at 69-72°C to obtain about 605 g of the product, with a yield ranging from 65% to 75%. .
Embodiment 2
[0023] Example 2: Preparation of ethyl 2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinecarboxylate (compound IV)
[0024] Put 8L of toluene into the reaction kettle, add 760g of 2-amino-4'-fluoro-benzophenone, about 605g of compound II and 465g of p-toluenesulfonic acid, stir and heat to reflux for 30 hours, and remove the water formed in the reaction from time to time. Cooled to room temperature, the reaction solution was washed 3 times with saturated aqueous sodium bicarbonate solution, the organic layer was dried with anhydrous sodium sulfate, crystallized with petroleum ether after concentration, suction filtered, and dried to obtain about 1.06 kg of light yellow solid product, the yield range was 75% to 85%, melting point: 73.3-75.0°C.
Embodiment 3
[0025] Example 3: Preparation of 2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinemethanol (compound V)
[0026] Put 240g of lithium aluminum hydride and 5.4L of tetrahydrofuran into the reaction kettle, and start stirring. After stirring for 30 minutes, about 1.06 kg of the product from the previous step (compound IV) was dissolved in 2.6 L of anhydrous tetrahydrofuran and added dropwise to the reaction kettle. After the dropwise addition was completed, the reaction was continued at room temperature for 2 hours. Concentrate the reaction solution, add dropwise ice water solution to decompose unreacted lithium aluminum hydride, stir for 3 to 5 hours; filter, and dry the solid; dissolve the solid with ethanol, filter out the insoluble matter, and cool the filtrate to crystallize, filter and dry to obtain a white solid product of about 765g, the yield range is 78%-88%, melting point: 73.2-75.2°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com