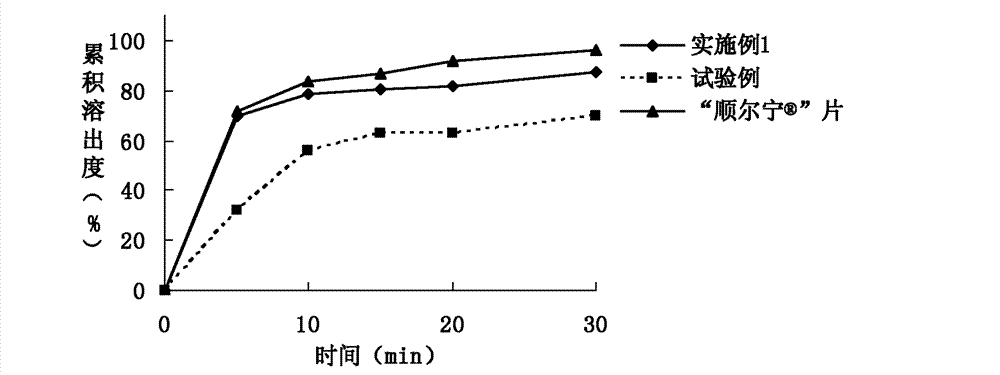
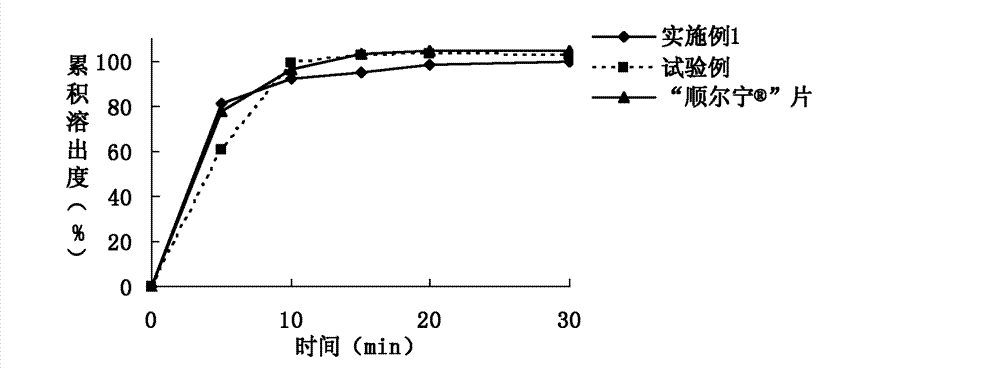
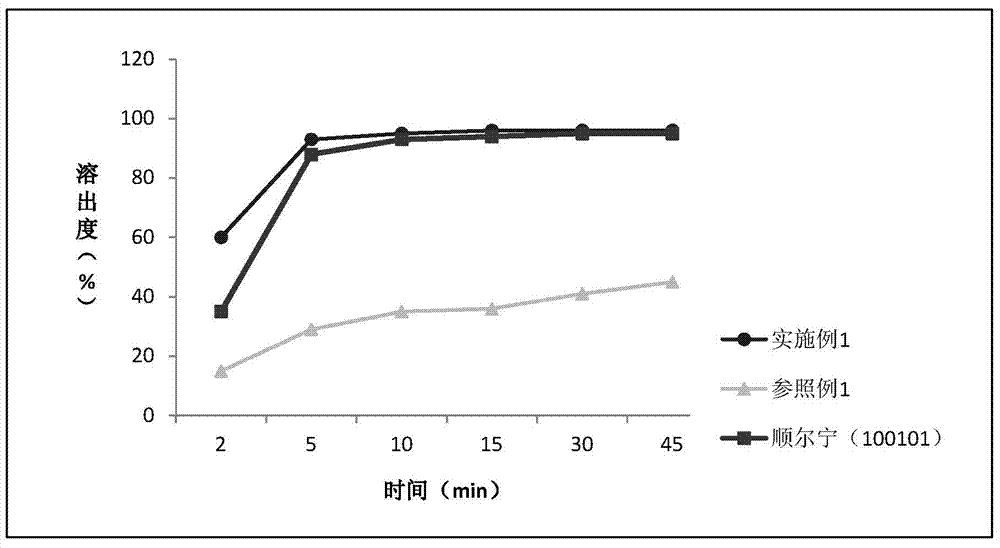
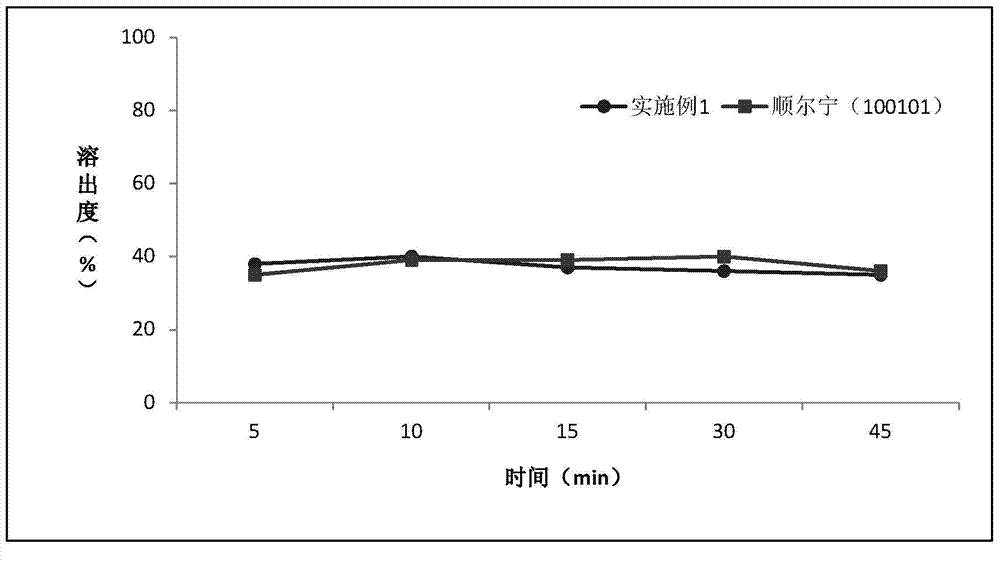
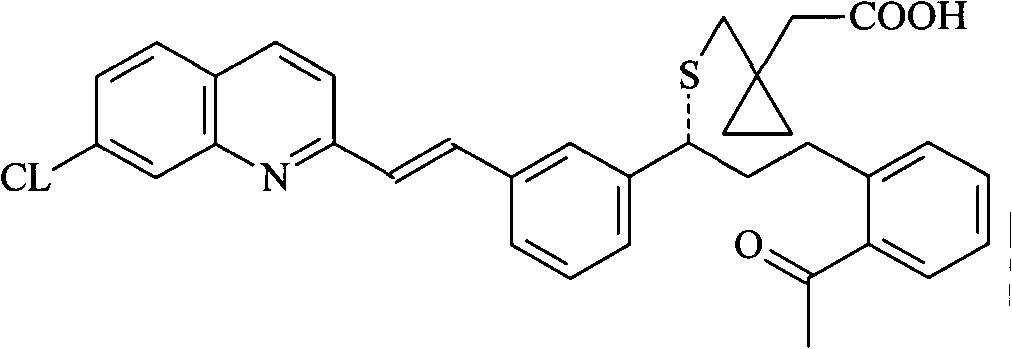
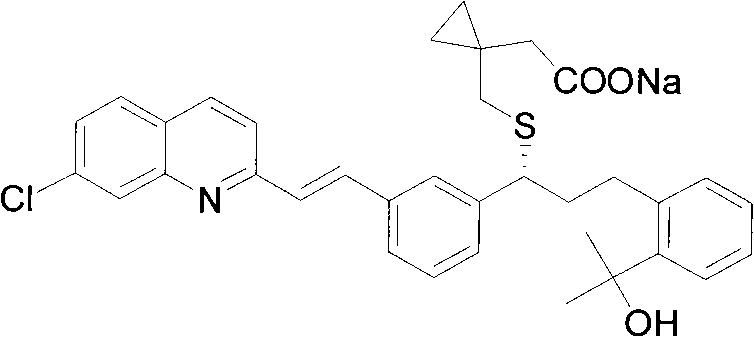
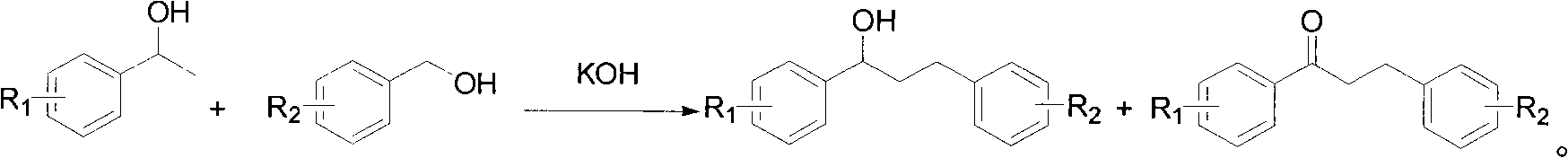
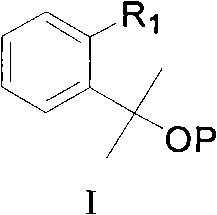
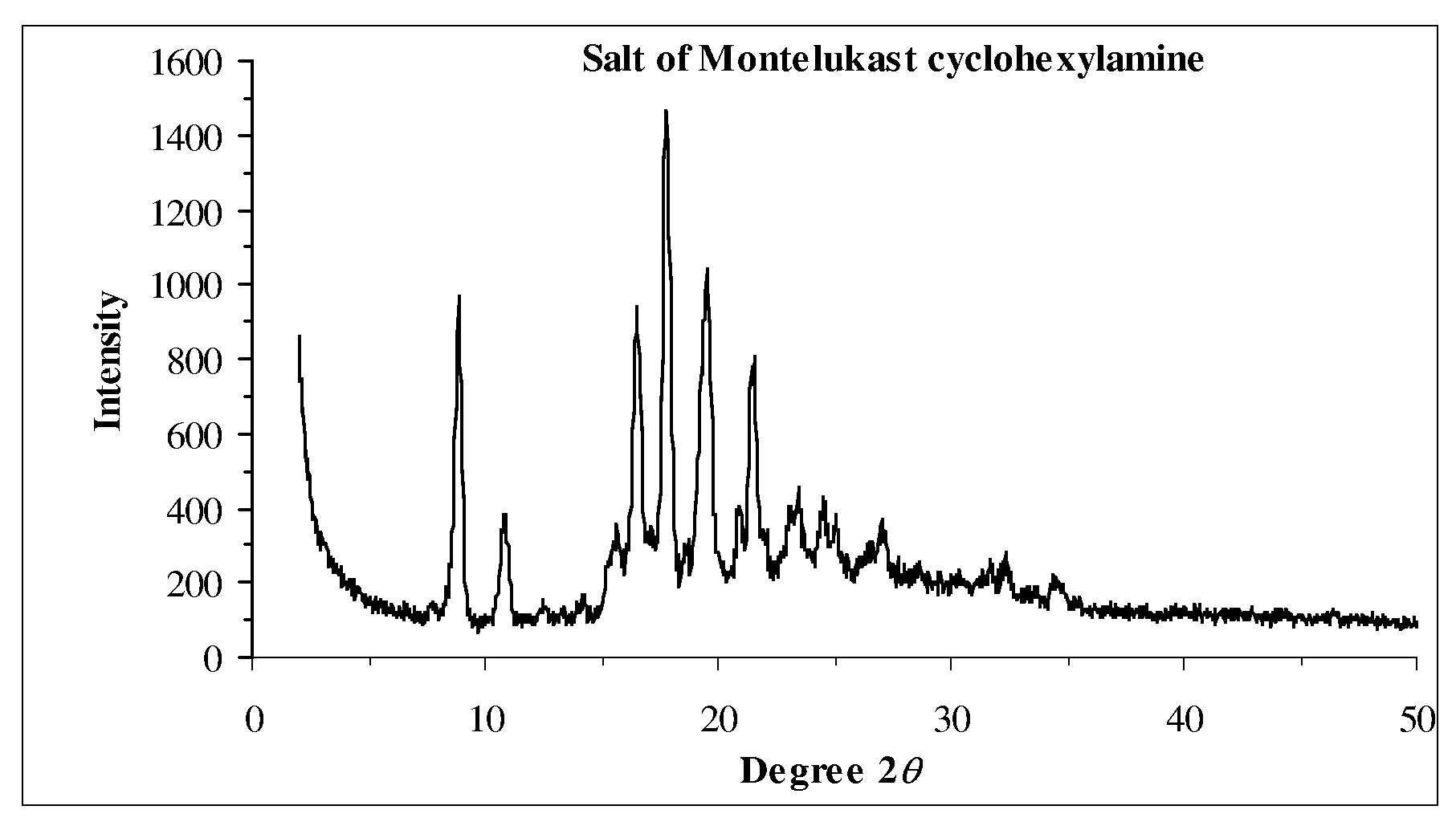
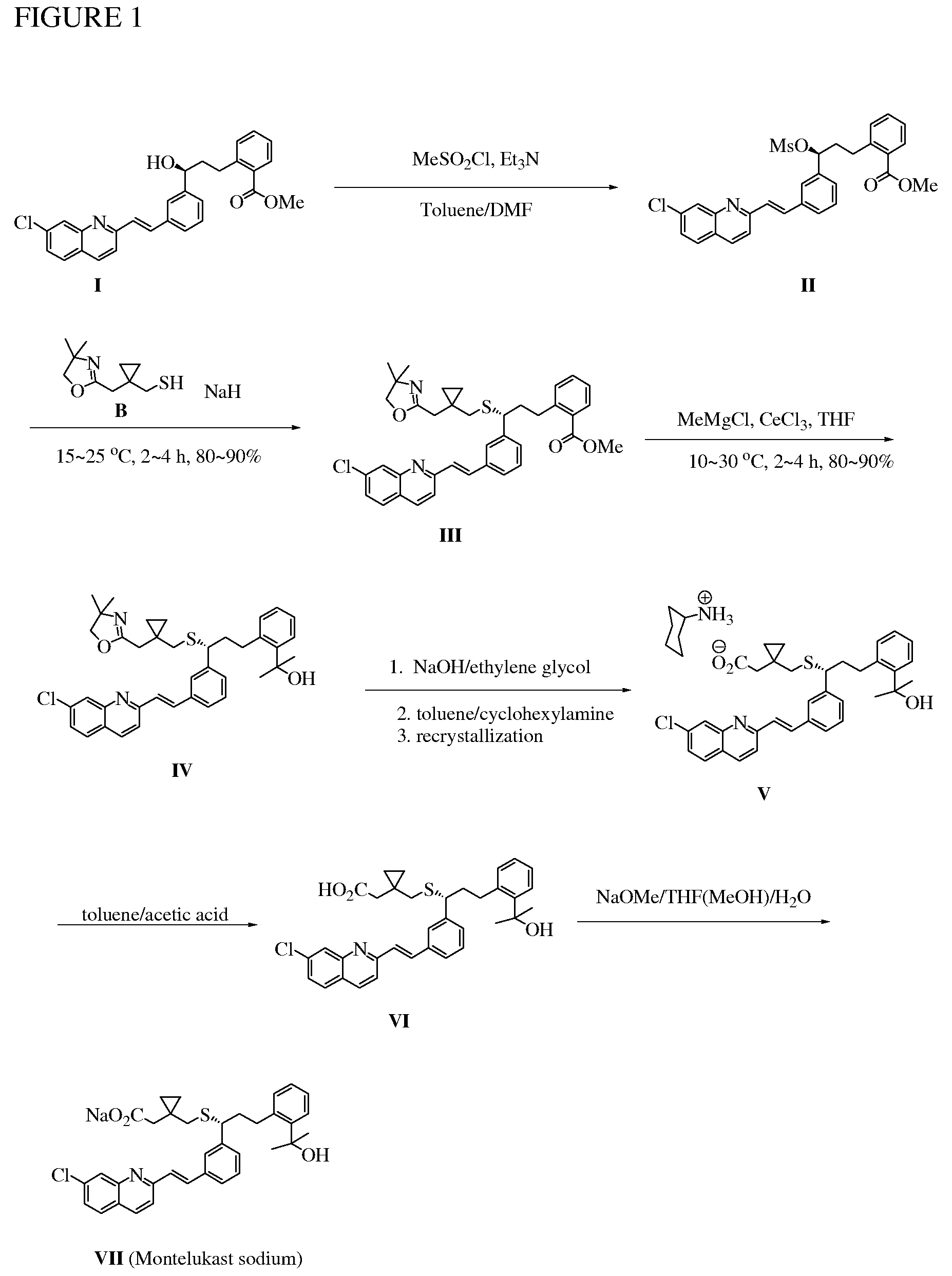
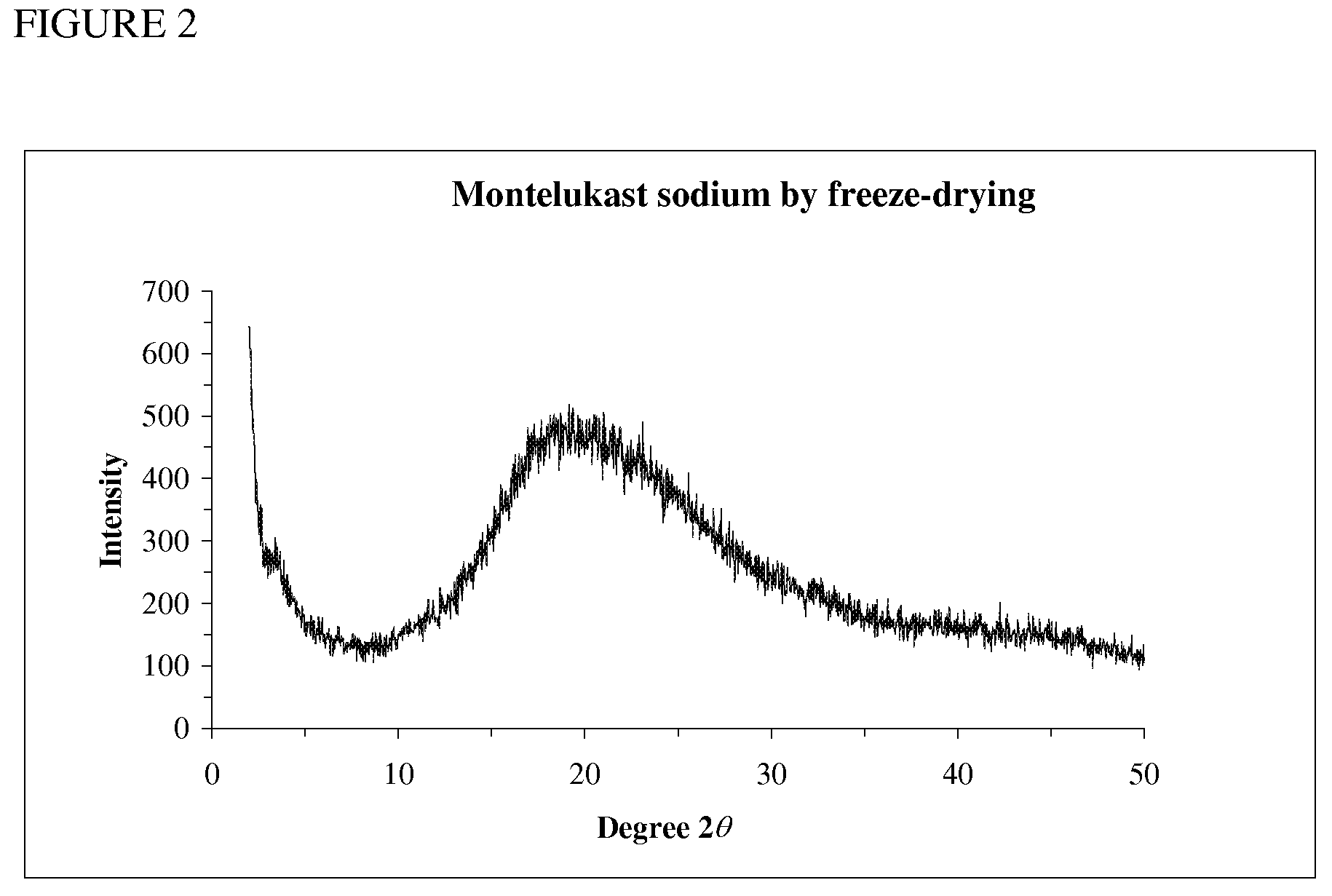
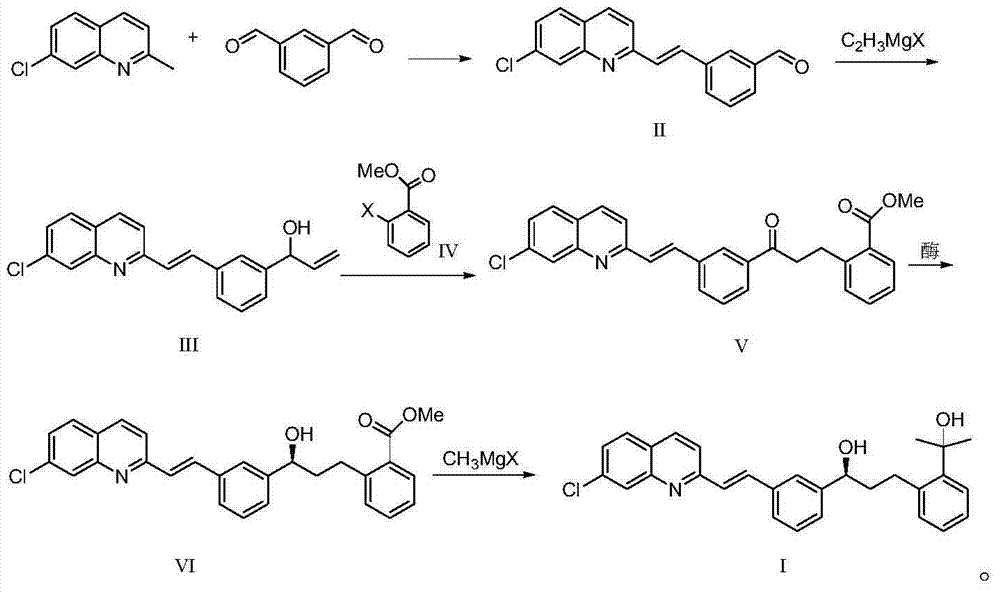
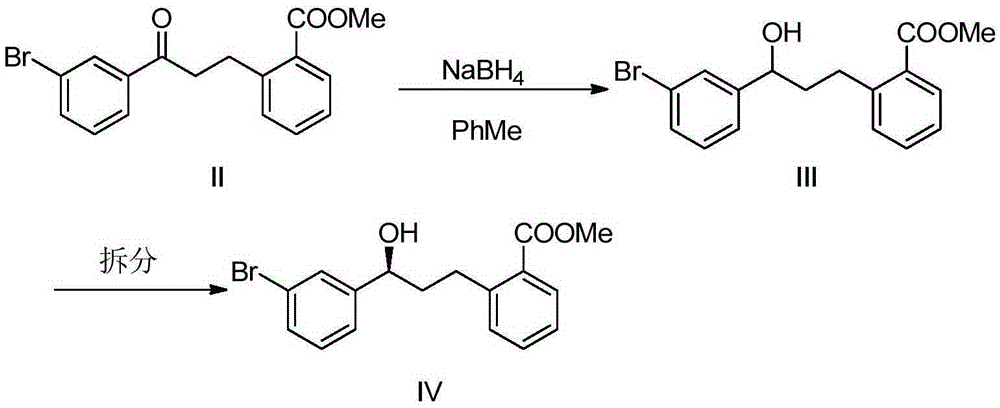
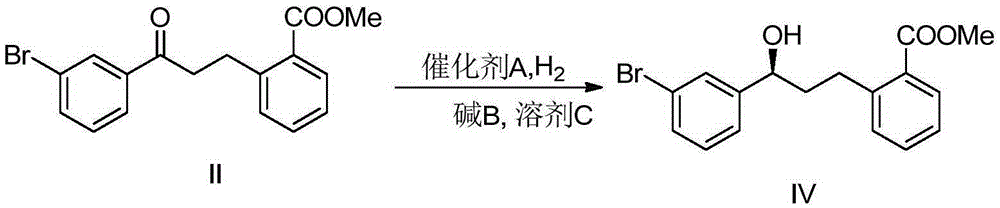
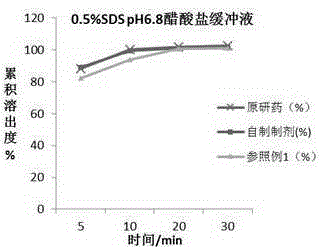
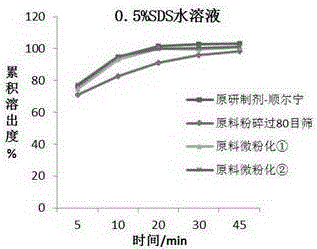
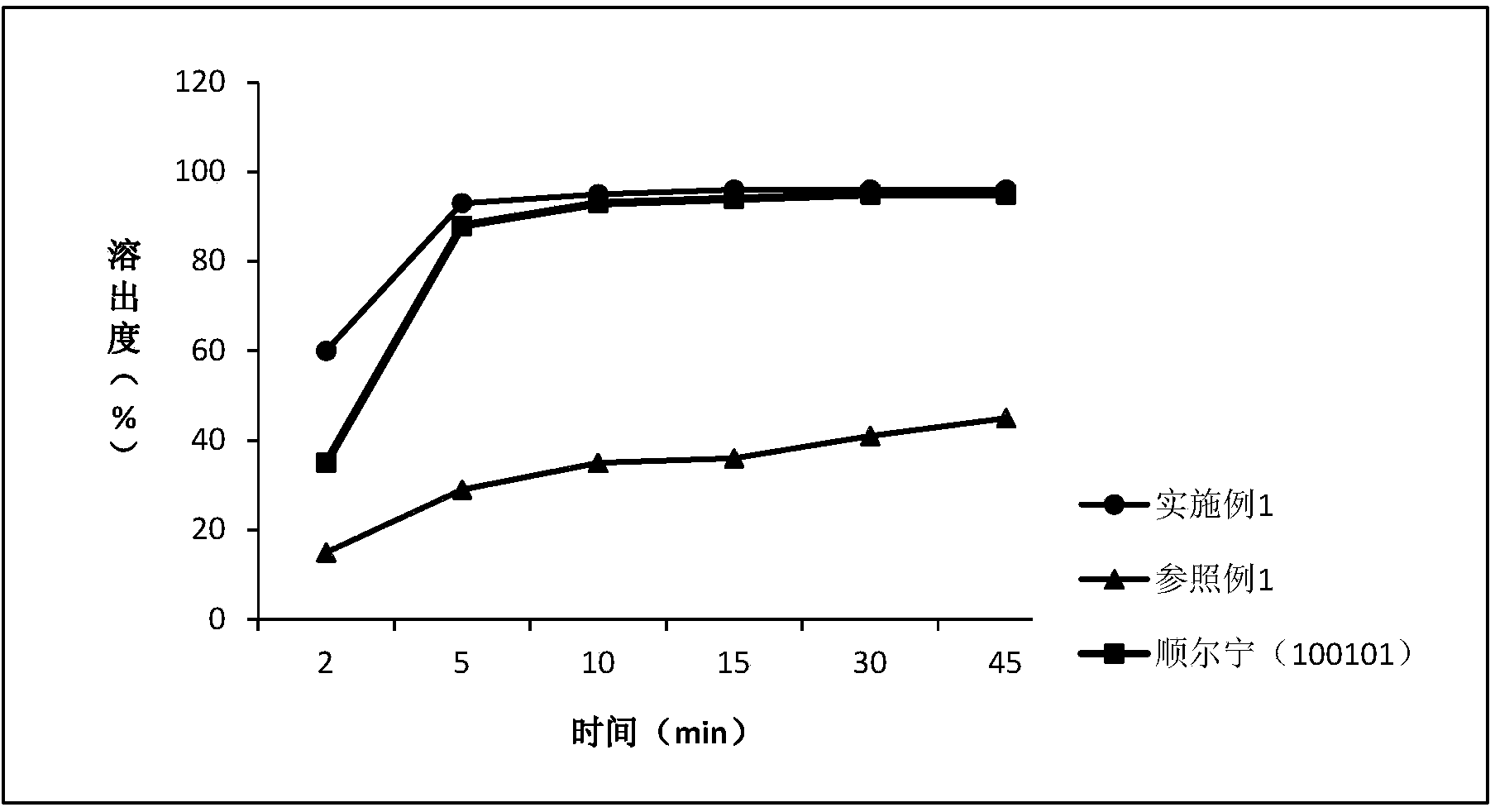
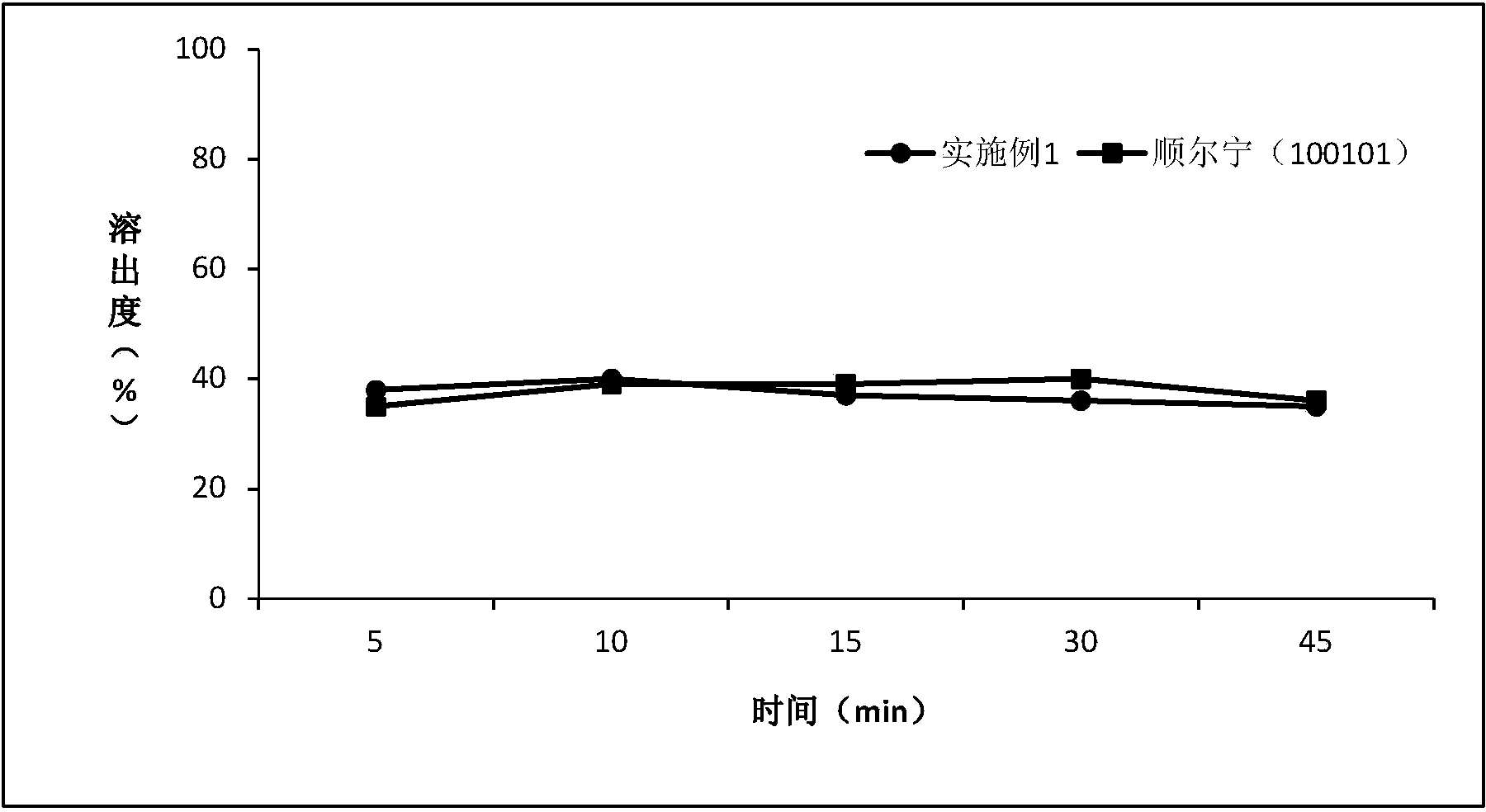
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
202 results about "Montelukast Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
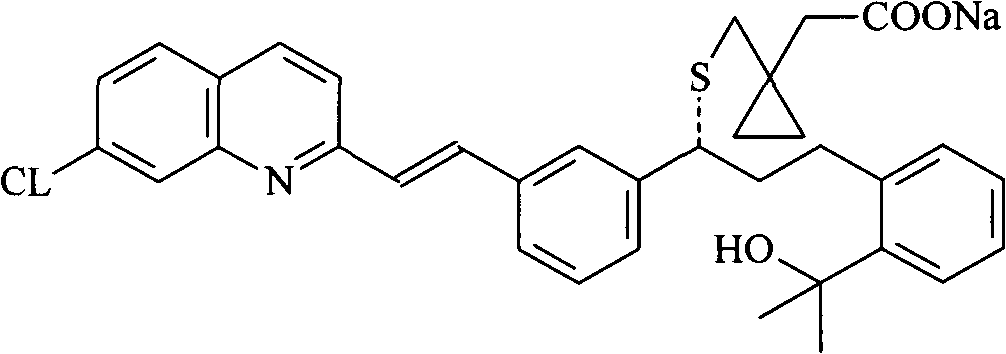
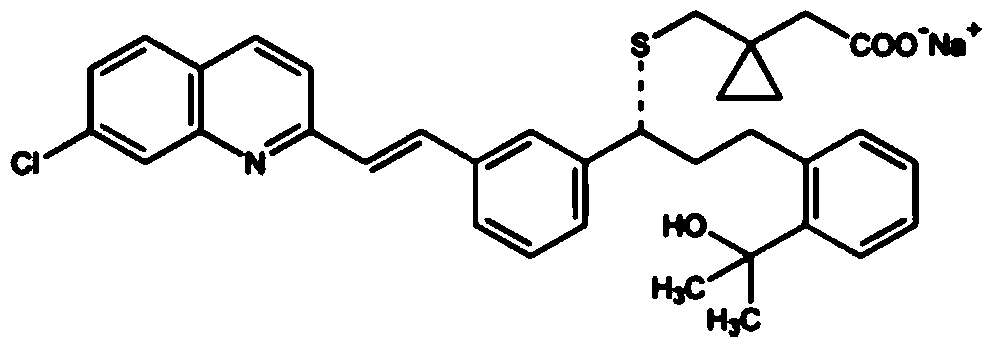
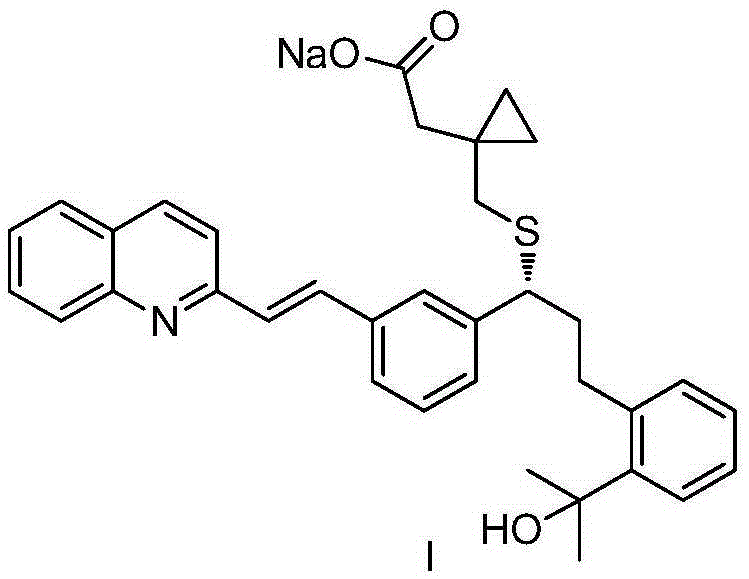
The orally bioavailable monosodium salt of montelukast, a selective cysteinyl leukotriene receptor antagonist with anti-inflammatory and bronchodilating activities. Montelukast selectively and competitively blocks the cysteinyl leukotriene 1 (CysLT1) receptor, preventing binding of the inflammatory mediator leukotriene D4 (LTD4). Inhibition of LTD4 activity results in inhibition of leukotriene-mediated inflammatory events including: migration of eosinophils and neutrophils; adhesion of leukocytes to vascular endothelium, monocyte and neutrophil aggregation; increased airway edema; increased capillary permeability; and bronchoconstriction. The CysLT1 receptor is found in a number of tissues including spleen, lung, placenta, small intestine, and nasal mucosa, and in a variety of cell types including monocyte/macrophages, mast cells, eosinophils, CD34-positive hemopoietic progenitor cells, neutrophils and endothelial cells.
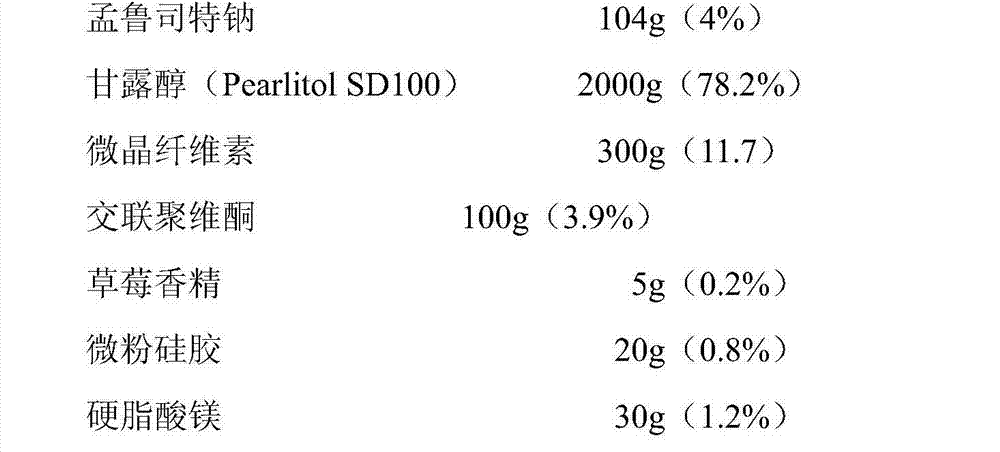
Chewable tablet containing montelukast sodium
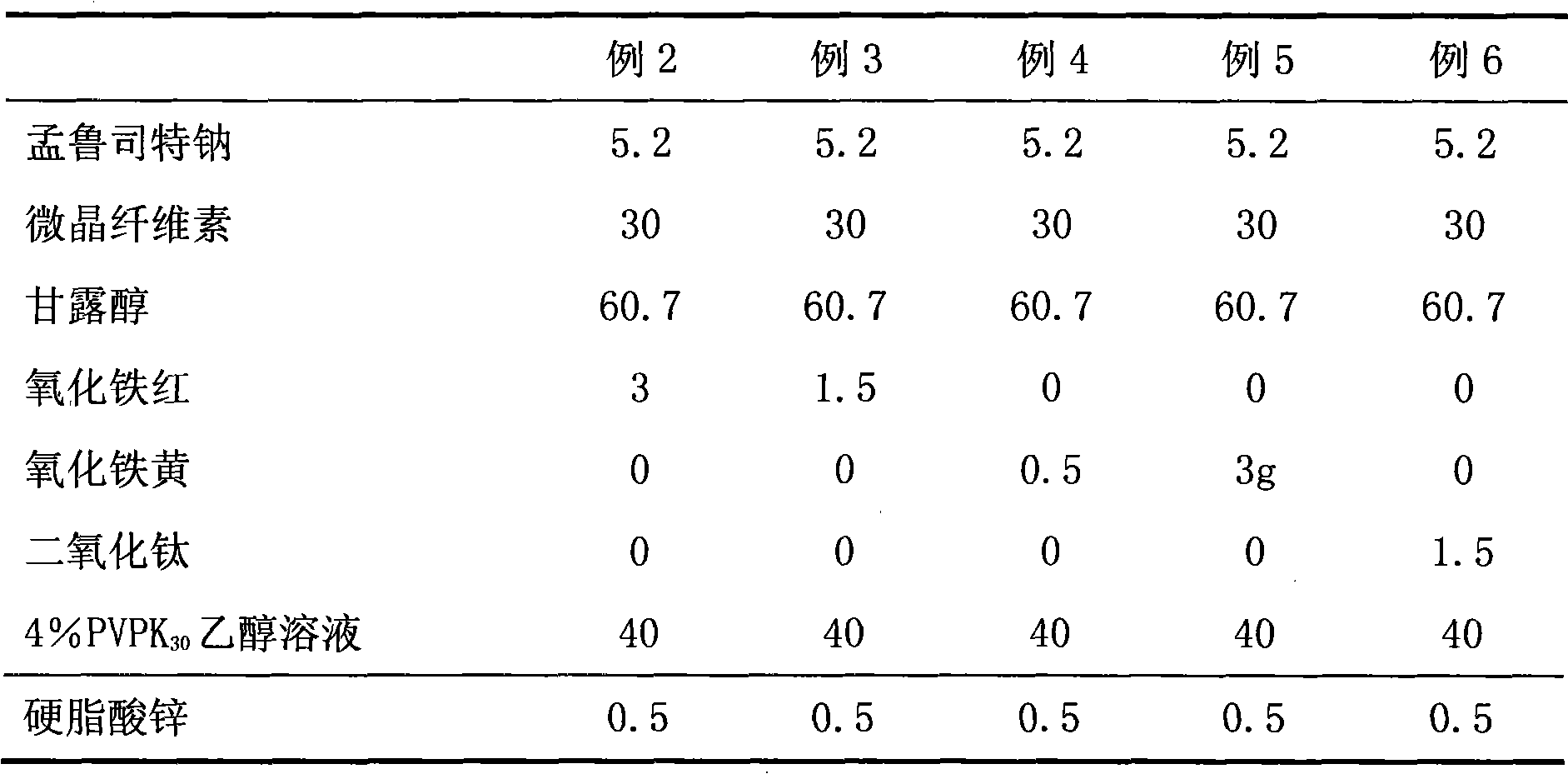
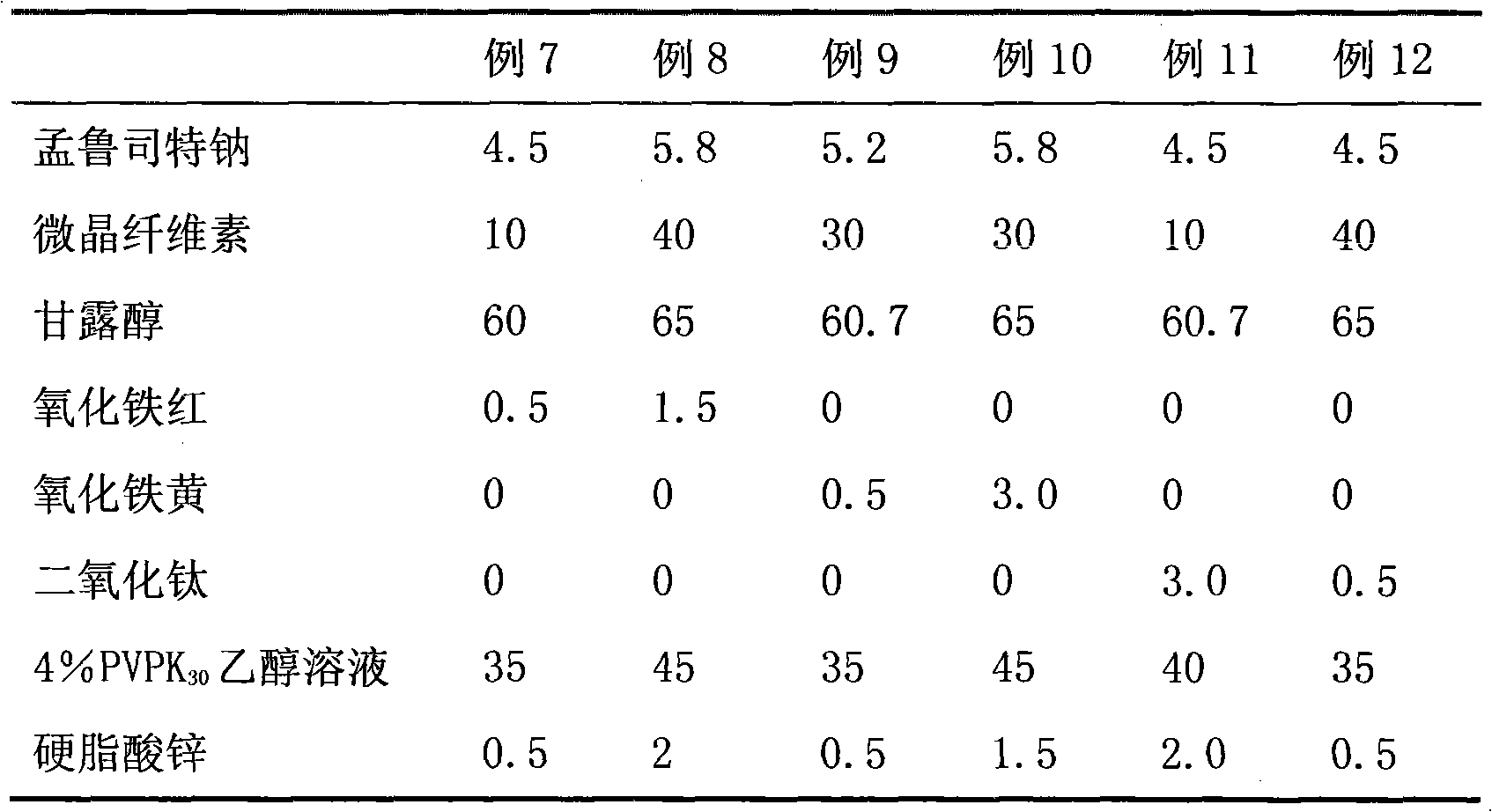
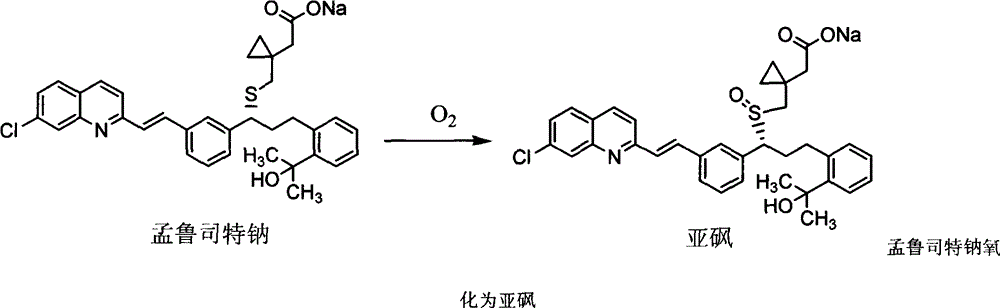
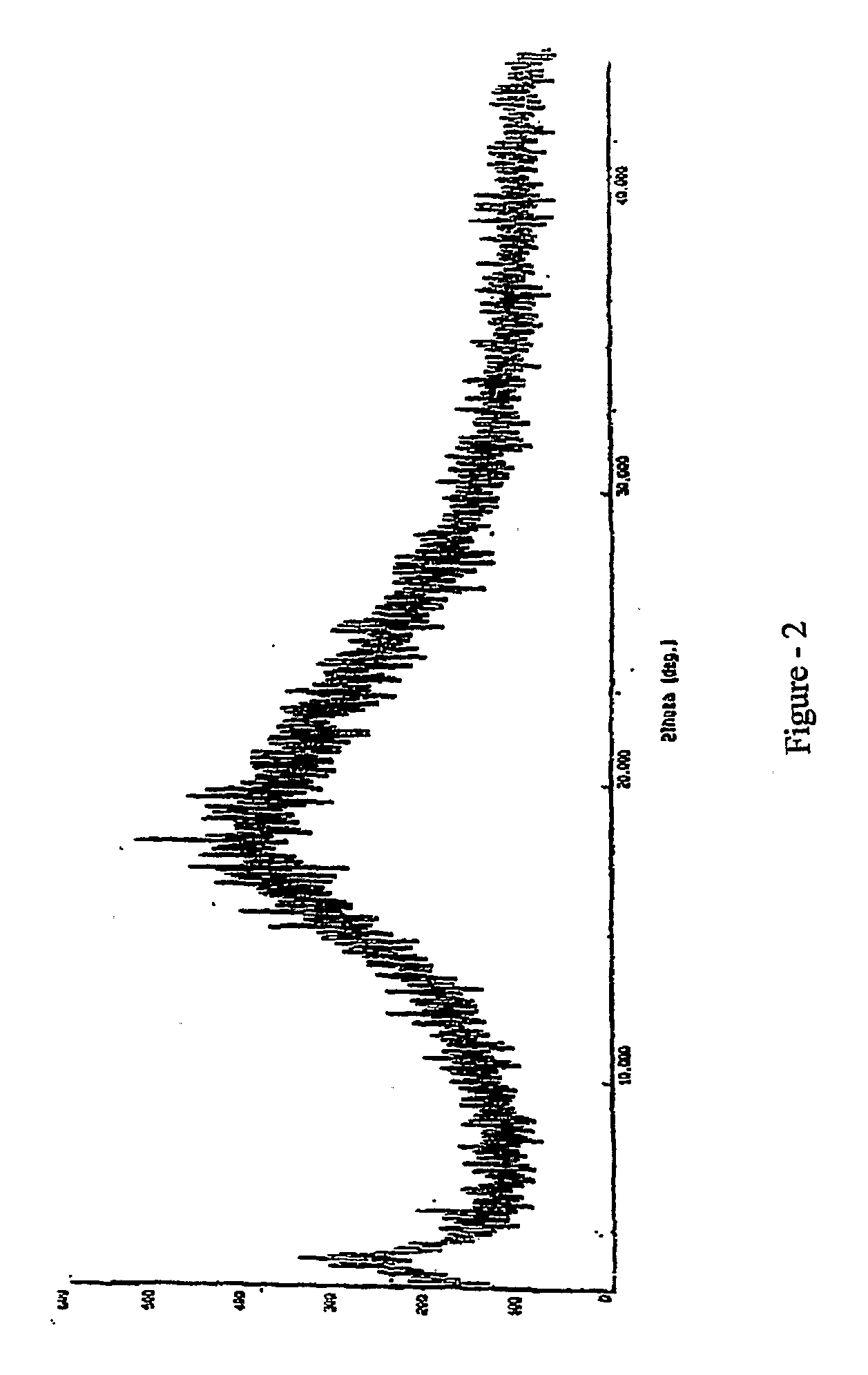
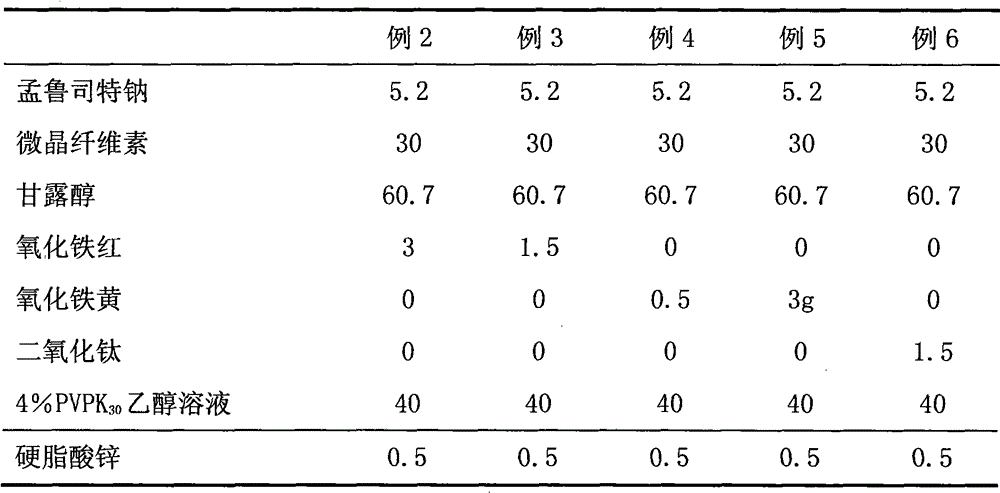
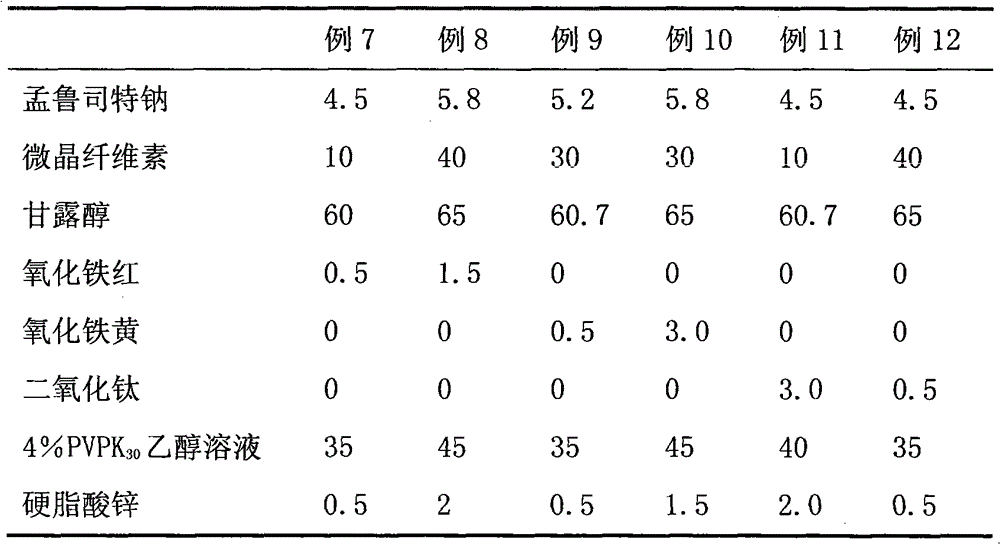
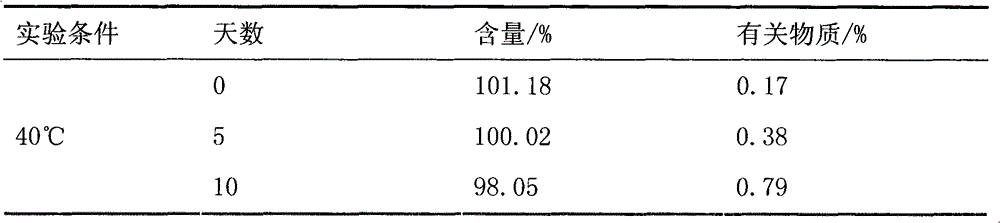
ActiveCN101773481AImprove stabilityAvoid photolysisInorganic non-active ingredientsPill deliveryMedicineMontelukast Sodium
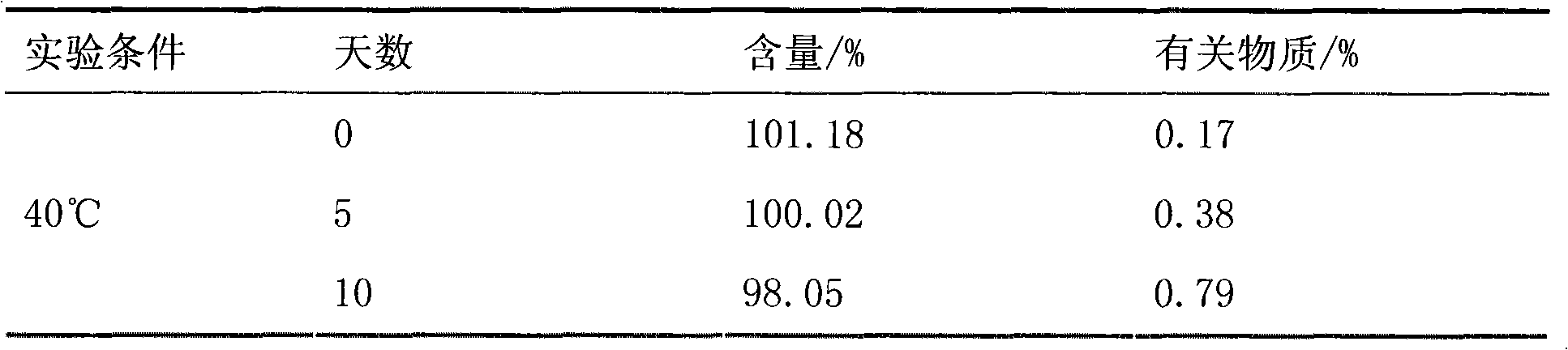
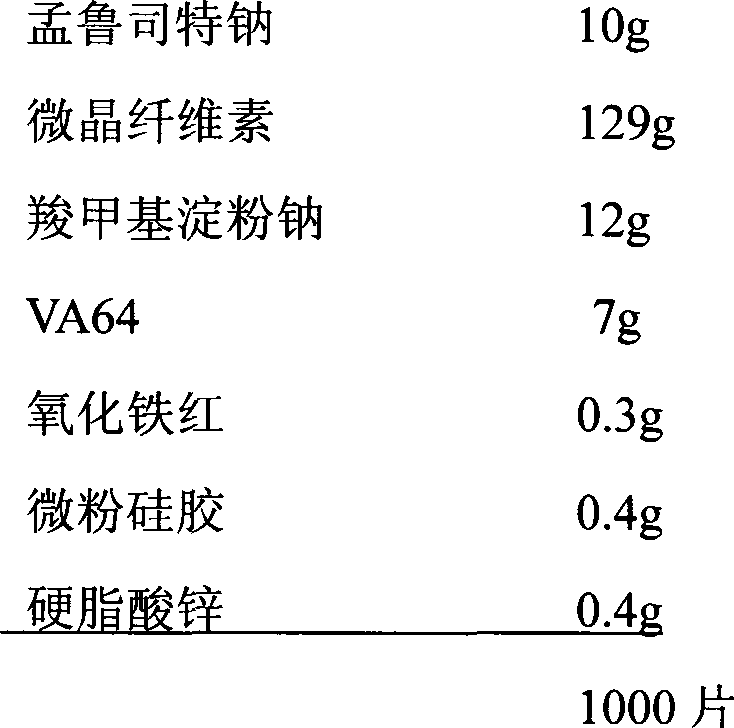
The invention belongs to the field of medicine preparations, which particularly relates to a chewable tablet of montelukast sodium. Because the production process of the chewable tablet of the montelukast sodium needs the light shielding operation at present, the mass production is inconvenient, and the chewable tablet of the montelukast sodium has the defect of poorer stability. In the invention, zinc stearate and an opacifier of iron oxide red, iron oxide yellow and titanium dioxide are added into the auxiliary materials of a chewable tablet of montelukast, so that the content of relevant substances can be lowered, and the stability of the chewable tablet is enhanced.
Owner:LUNAN PHARMA GROUP CORPORATION
Rapidly-dissolving and stabile montelukast oral solid preparation and preparation method thereof
ActiveCN103239450ADissolution rate is fastImprove stabilityPharmaceutical non-active ingredientsPill deliveryAdhesiveMedicine
The invention relates to a rapidly-dissolving and stabile montelukast oral solid preparation and a preparation method thereof, wherein the preparation uses montelukast as a raw material drug, is added with a diluents, a disintegrating agent, an additive, an adhesive and a lubricant as auxiliary materials, and uses a pharmaceutically acceptable preparation technology. The preparation and the preparation technology can raises a dissolving speed of the oral preparation, enables the oral preparation to substantially raise the dissolving speed in a dissolving mediums of pH1.0 and pH 4.5, and can raises the medicament stability.
Owner:QILU PHARMA HAINAN
Montelukast sodium chewable tablet and preparation method thereof
InactiveCN103494785AUniform and bright colorImprove stabilityPill deliveryPharmaceutical non-active ingredientsAlcoholAdhesive
The invention belongs to the technical field of medicines and particularly relates to a montelukast sodium chewable tablet and a preparation method thereof. The montelukast sodium chewable tablet provided by the invention is composed of montelukast sodium, a diluting agent, a lubricating agent, a binding agent, a disintegrating agent, a coloring agent, a sweetening agent and a corrigent. A preparation process of the montelukast sodium chewable tablet adopts absolute ethyl alcohol as a solvent to prepare the binding agent and then the binding agent is mixed with powder of other raw materials and auxiliary materials to carry out wet-method granulation. After being dried, wet grains are mixed with the corrigent and the lubricating agent and then a mixture is tabletted. According to the montelukast sodium chewable tablet prepared by using the process method provided by the invention, the color and the luster of the appearance of the tablet are uniform and bright, the stability is good, the disintegrating speed is high and the bioavailability is high.
Owner:FUJIAN HUAHAI PHARMA
Montelukast sodium tablet and preparation method thereof
ActiveCN101732268AWell mixedSolve production problems that require dark operationPill deliveryPharmaceutical non-active ingredientsTemperature controlAdhesive
The invention belongs to the field of medicine preparations, in particular to a montelukast sodium tablet and a preparation method thereof. Because the montelukast sodium tablet adopts a wet method granulation process and has the problems of long drying time, difficult temperature control and easy causing of content difference, content descent and relevant substance increase between particles, the invention provides the montelukast sodium tablet and the preparation method thereof in order to enhance the stability and the operability of the montelukast sodium tablet. The montelukast sodium tablet provided by the invention comprises montelukast sodium, a filling agent, a disintegrating agent, an adhesive, a colorant, a lubricant and a flow aid. The preparation method of the montelukast sodium tablet provided by the invention adopts direct tabletting after dry method granulation and uses the colorant, the colorant can act as a very good light shielding function in the operational process, the operation is convenient, the influence of a drying process and moist heat to the product quality is avoided, and the product stability is enhanced.
Owner:LUNAN PHARMA GROUP CORPORATION
Montelukast sodium chewing tablet prescription and preparation process thereof
ActiveCN103494781AImprove complianceQuality improvementPill deliveryPharmaceutical non-active ingredientsMontelukast SodiumMedical prescription
The invention relates to montelukast sodium chewing tablets and a preparation process thereof. The tablets comprise montelukast sodium and a filling agent, a diluting agent, a bonding agent, a disintegrating agent, and a flavoring. The tablets are prepared with a powder direct tabletting method. The fluidity and compressibility are good. The chewing tablets have long-term storage stability. The tablets are used in prevention and long-term treatment of asthma of children aged 2 to 14, and are used in relieving symptoms caused by allergic rhinitis.
Owner:哈药集团股份有限公司 +1
Stable montelukast sodium tablet and preparation method thereof
InactiveCN102973532AInorganic non-active ingredientsPill deliveryMontelukast SodiumStabilizing Agents
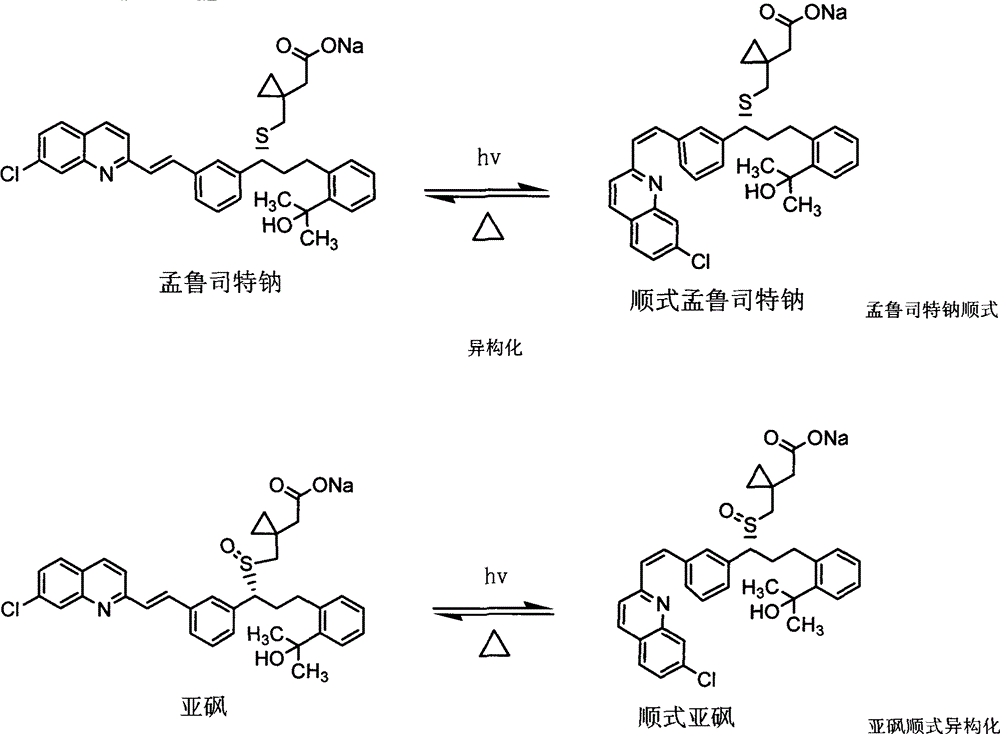
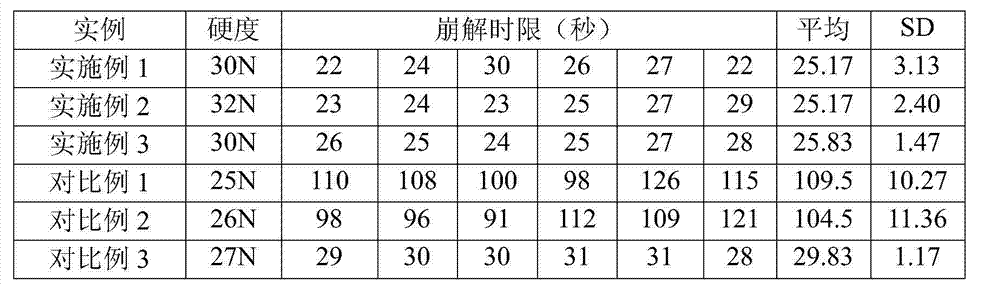
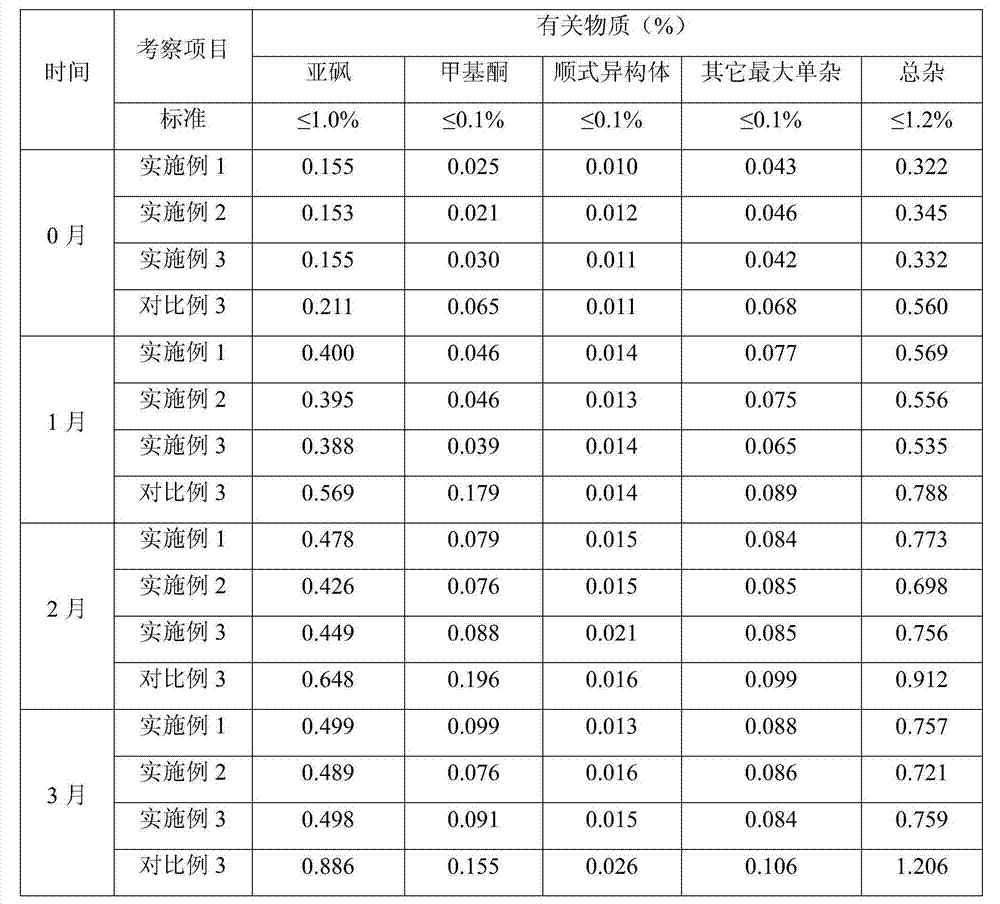
The invention relates to a stable pharmaceutical composition containing montelukast or salt of the montelukast and a method for preparing the composition. The montelukast sodium is easily affected by moist heat and light and degraded into sulfoxide and cis-isomer, so as to become a problem for development of a pharmaceutical preparation. A stabilizer is added to the stable montelukast sodium tablet disclosed by the invention to coexist with the montelukast sodium. Therefore, increase of the sulfoxide and the cis-isomer can be restrained to the maximal extent.
Owner:NANJING REAL PHARMA
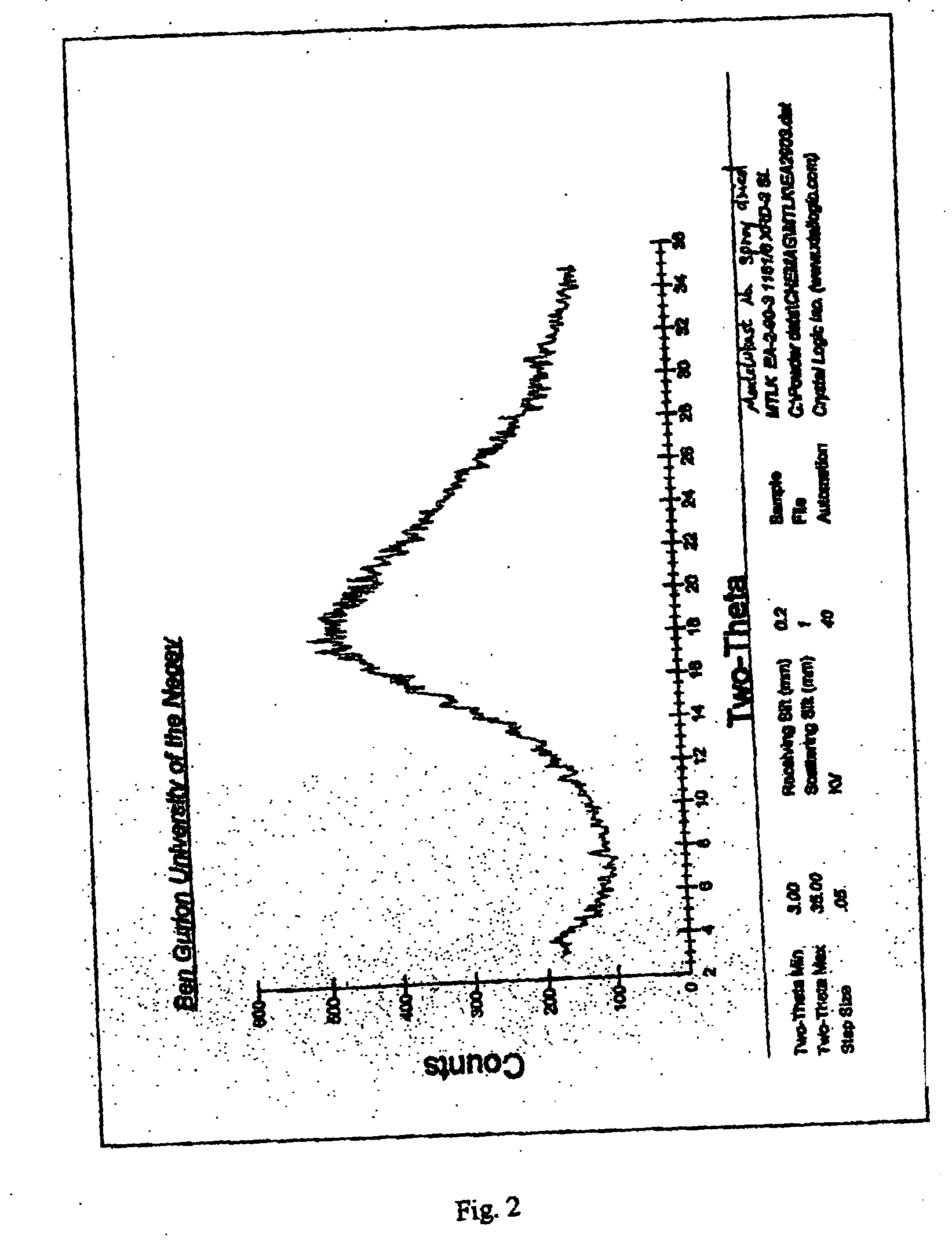
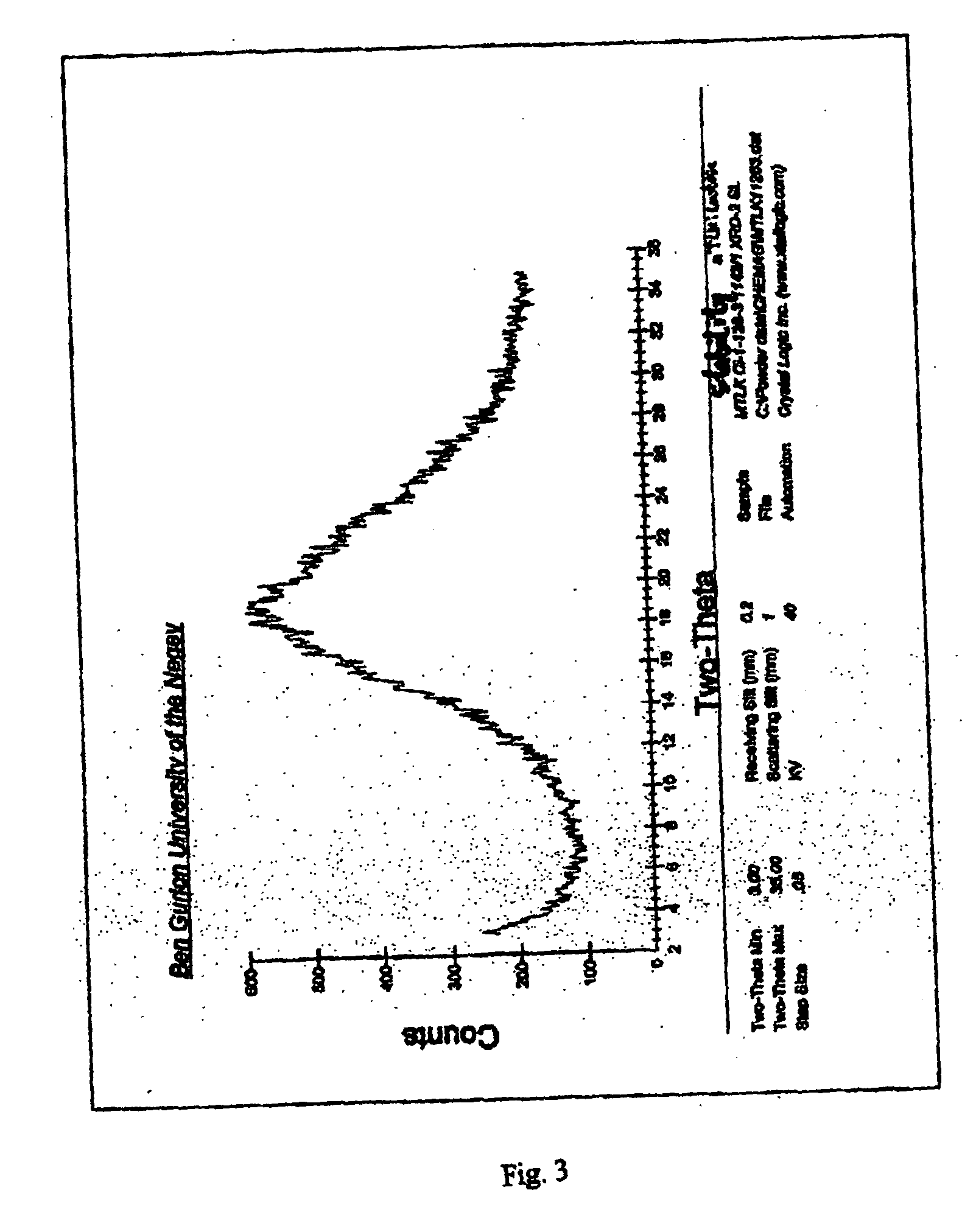
Stable amorphous forms of montelukast sodium
An amorphous form of montelukast sodium and a montelukast sodium lactose co-precipitate, processes for producing same, pharmaceutical compositions containing same and methods of treatment utilizing same are disclosed.
Owner:CHEMAGIS
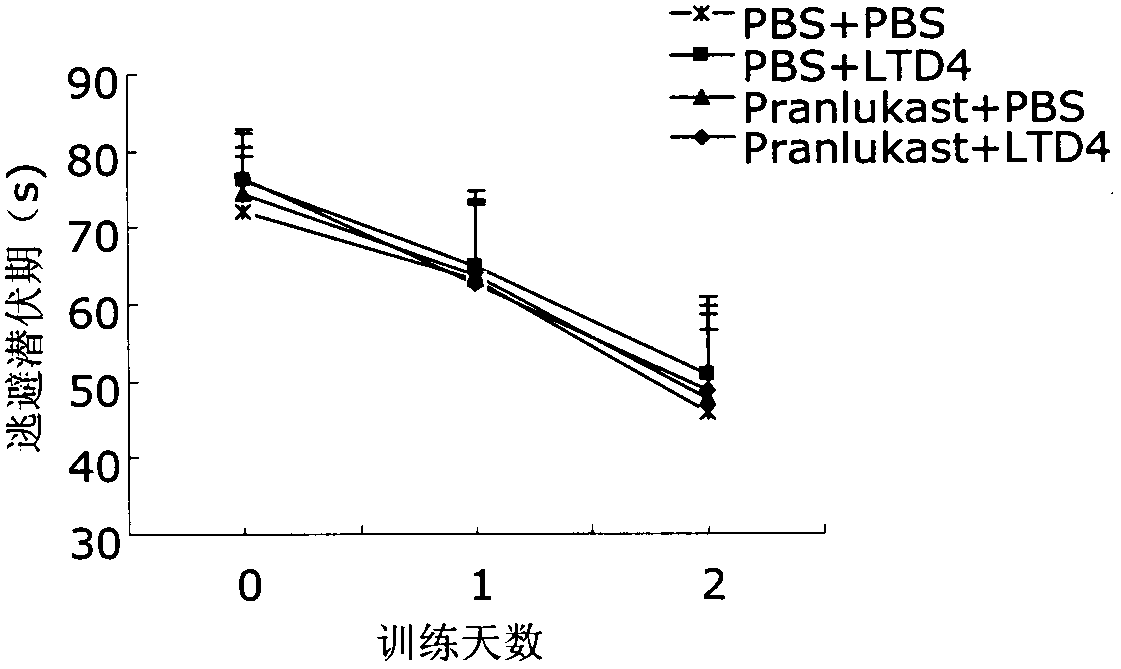
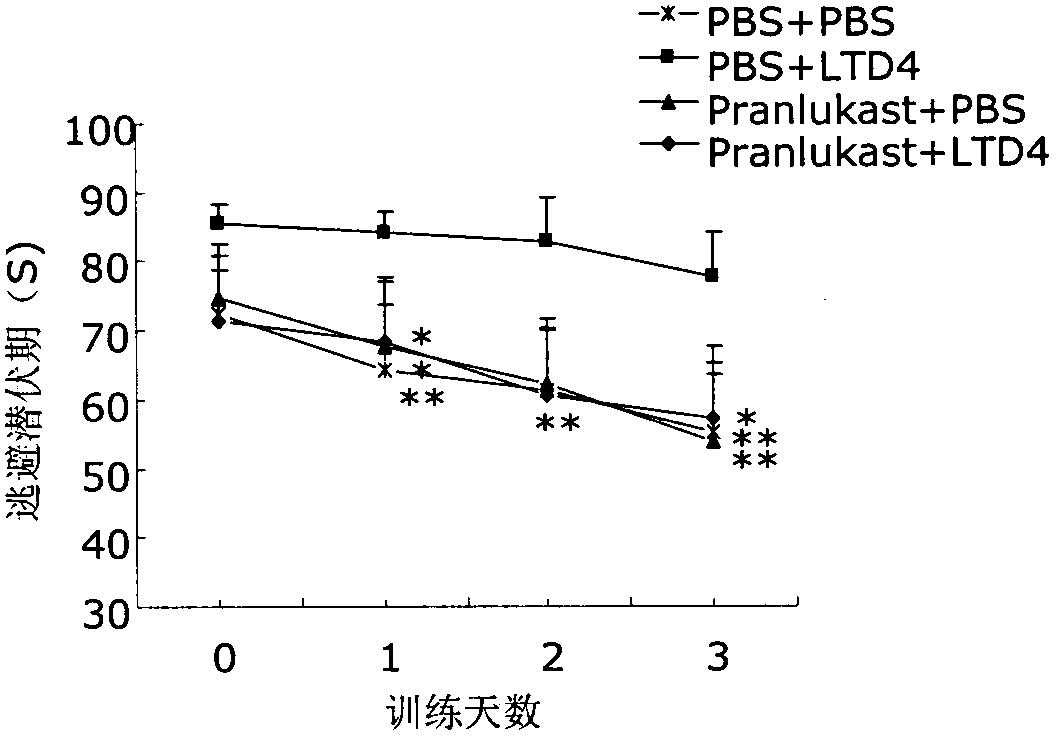
Applications of 1 type cysteinyl leukotriene receptor antagonist on preparation of medicament for treating the Alzheimer disease
InactiveCN103505731ADecreased memory functionIncreased Aβ levelsNervous disorderHeterocyclic compound active ingredientsCysteinyl leukotrienesOral medication
The invention relates to applications of a cysteinyl leukotriene receptor antagonist (CysLT1R) on preparation of a medicament for treating the Alzheimer disease. Research finds that intracerebral CysLT1R mediates A beta aggregation and dysmnesia; and by oral medication, CysLT1R antagonists such as pranlukast, zafirlukast and montelukast can improve mice memory impairment caused by A beta. The CysLT1R antagonists such as pranlukast, zafirlukast and montelukast, and analogs thereof such as iralukast, ablukast, imitrodast, pobilukast, cinalukast, verlukast, pemirolast and ibudilast, have significant protection effect on primarily-cultured mice neuron damage caused by A beta. The CysLT1R antagonists such as pranlukast, zafirlukast and montelukast, and the analogs thereof such as iralukast, ablukast, imitrodast, pobilukast, cinalukast, verlukast, pemirolast and ibudilast, can be used for preparing anti-AD medicaments.
Owner:CHINA PHARM UNIV
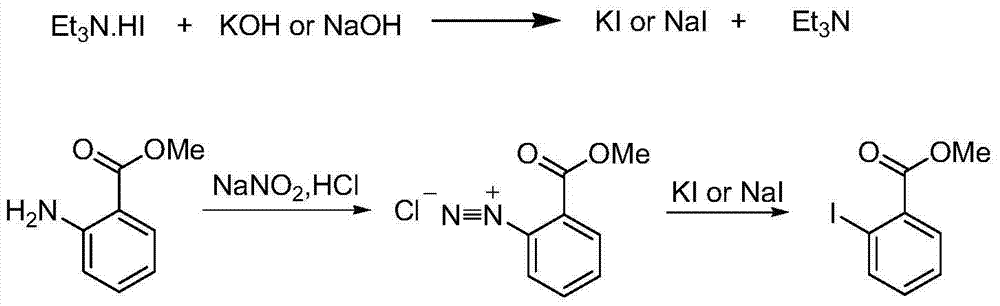
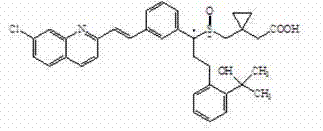
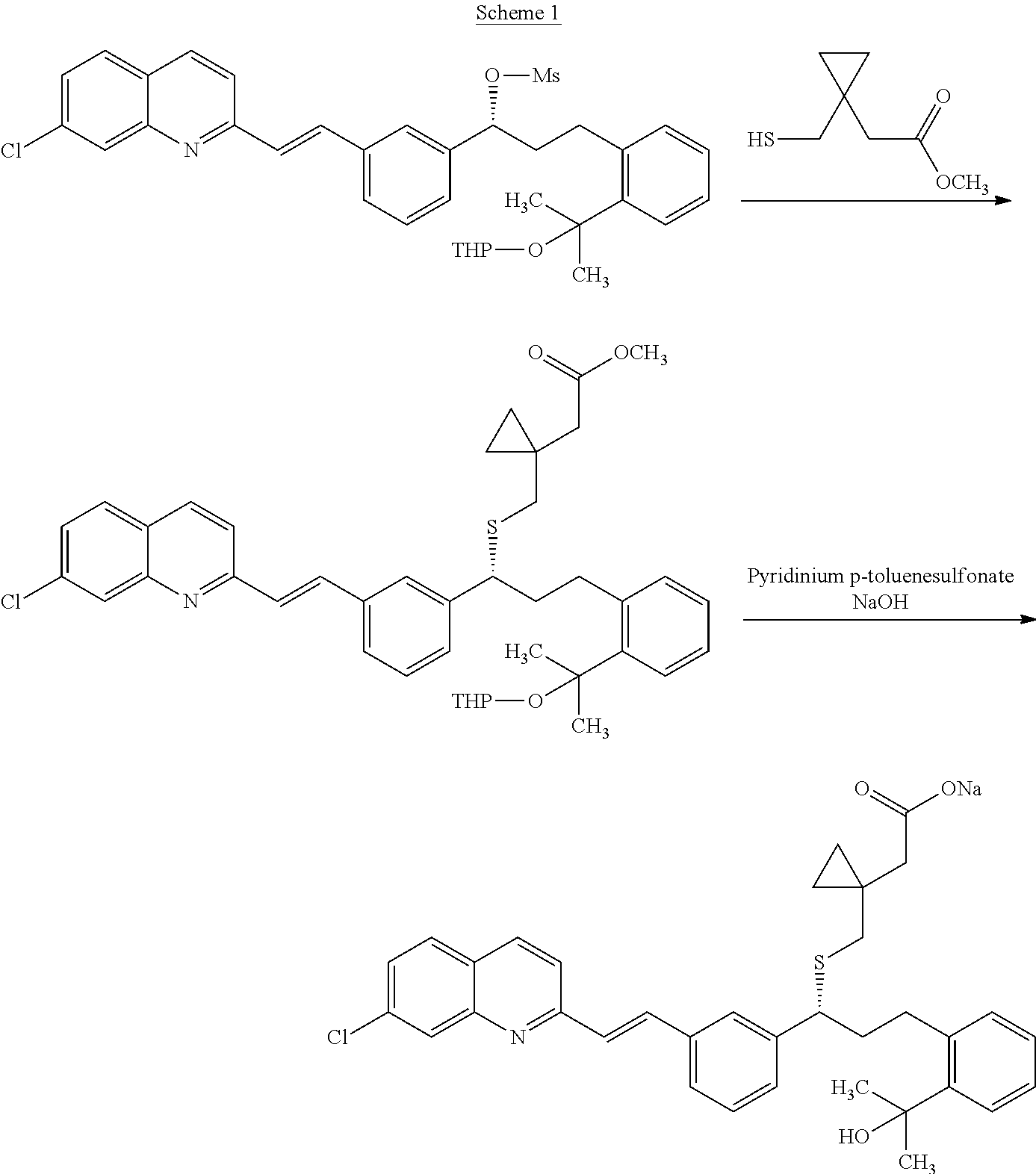
Method for preparing Montelukast sodium
InactiveCN104119270AReduce dosageSimple process conditionsOrganic chemistryAcetic acidPurification methods
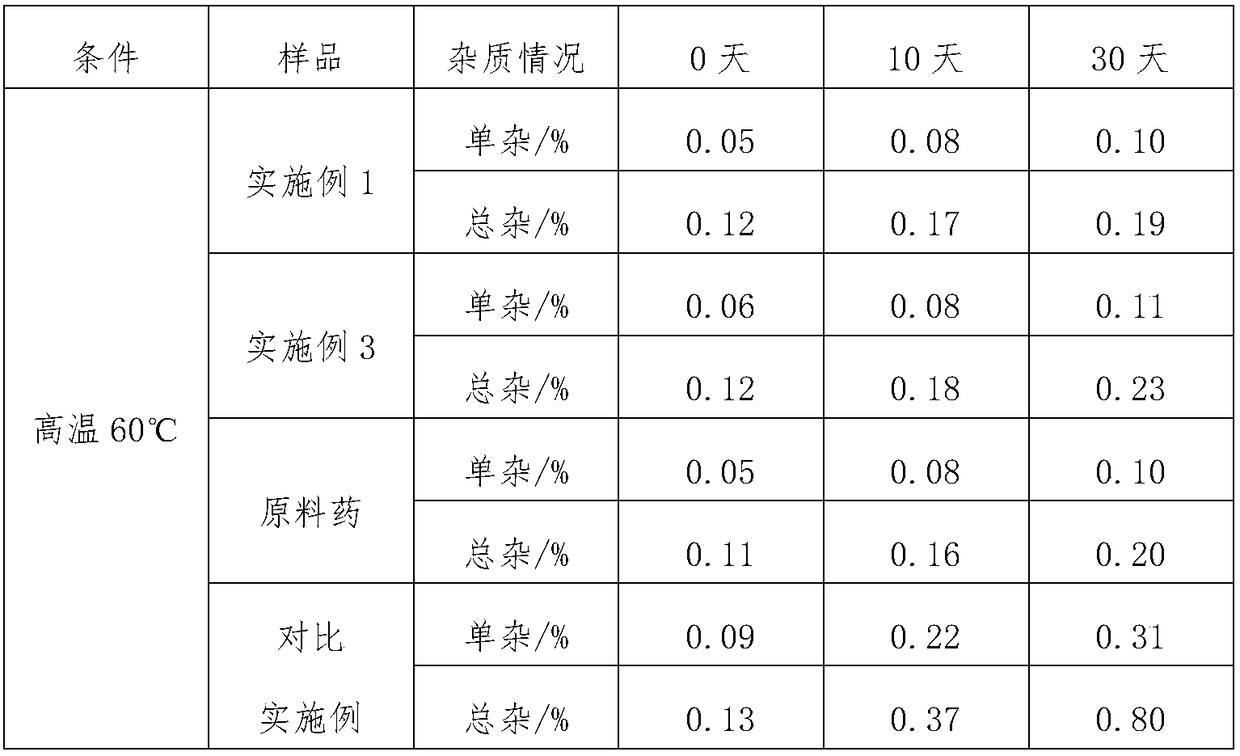
The invention provides a method for preparing and purifying Montelukast sodium. The method comprises the following steps: A. preparing a disodium salt of 2-[1-(mercapto methyl) cyclopropyl] acetic acid; B. preparing an Montelukast free acid; C. preparing an Montelukast di-n-propylamine salt; D. preparing an Montelukast dicyclohexylamine salt; E. converting the Montelukast dicyclohexylamine salt into an Montelukast sodium salt. The synthesis method is simple in process conditions, good in impurity removal effect and small in risk factor, the sulfoxide impurity is less than 0.1%, other impurities are less than 0.05%, and even a certain impurity cannot be detected, and the purity of the Montelukast sodium is larger than 99.8%. All impurities of the Montelukast sodium can be effectively controlled to be less than 0.05%.
Owner:MUDANJIANG HENGYUAN PHARMA CO LTD
Montelukast sodium composition
ActiveCN102552921AImprove stabilityReduce hidden dangersPharmaceutical non-active ingredientsRespiratory disorderMontelukast SodiumKetone
The invention discloses a montelukast sodium composition, which comprises montelukast sodium and polyacrylic resin L100-55. The weight of the montelukast sodium accounts for 50-95 percent of the total weight of the composition, and the weight of the polyacrylic resin L100-55 accounts for 5-50 percent of the total weight of the composition. By adopting the technical scheme, the stability of the montelukast sodium can be improved significantly, and the risk of the montelukast sodium oxidized into montelukast ketone can be reduced.
Owner:NANJING ZENKOM PHARMA
Montelukast sodium intermediate and method for synthesizing montelukast sodium thereof
ActiveCN102424673AEconomic synthesisEasy to synthesizeOrganic chemistryOrganic compound preparationChemical synthesisMontelukast Sodium
The invention relates to a chemical synthesis drug technology, especially relates to a novel technology for synthesizing montelukast sodium, the invention also relates to a novel intermediate of the synthesized montelukast sodium. The method has the advantages of cheap raw material and easy acquisition, short synthesis route and simple operation, and is suitable for industrial production.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Novel Compounds and Preparation for Montelukast Sodium
Owner:FORMOSA LAB
Preparation method for montelukast sodium intermediate
ActiveCN103936671AAchieve recyclingSimple purification processOrganic chemistryPhthalaldehydeMontelukast Sodium
The invention discloses a preparation method for a montelukast sodium intermediate. The preparation method comprises the steps of reacting 7-chloroquinaldine with m-phthalaldehyde to generate a compound shown in formula II; reacting the compound shown in formula II with vinyl magnesium halide to generate a compound shown in formula III; reacting the compound shown in formula III with a compound shown in formula IV to generate a compound shown in formula V; carrying out a reduction reaction on the compound shown in formula V to generate a compound shown in formula VI; and reacting the compound shown in formula VI with methyl magnesium halide to generate a compound shown in formula I. According to the preparation method disclosed by the invention, production cost is further reduced, and a technological basis is laid for large-scale industrialized production for the intermediate.
Owner:启东东岳药业有限公司 +1
Montelukast sodium tablet and preparation method thereof
ActiveCN103989645ADisintegrates quicklySimple preparation processPill deliveryPharmaceutical non-active ingredientsAlcoholMontelukast Sodium
The invention provides a montelukast sodium tablet which is prepared by the following method: dissolving montelukast sodium and mycose in alcohol, drying to remove alcohol, and sieving dried substances; then, mixing with alginic acid and pharmaceutically common auxiliary materials, and pressing into tablets. Compared with the prior art, the preparation process disclosed by the invention is simple, and the tablet is quick to disintegrate and good in stability.
Owner:LUNAN PHARMA GROUP CORPORATION
Montelukast sodium tablet and preparation method thereof
ActiveCN101732268BWell mixedSolve production problems that require dark operationPharmaceutical non-active ingredientsPill deliveryTemperature controlAdhesive
The invention belongs to the field of medicine preparations, in particular to a montelukast sodium tablet and a preparation method thereof. Because the montelukast sodium tablet adopts a wet method granulation process and has the problems of long drying time, difficult temperature control and easy causing of content difference, content descent and relevant substance increase between particles, the invention provides the montelukast sodium tablet and the preparation method thereof in order to enhance the stability and the operability of the montelukast sodium tablet. The montelukast sodium tablet provided by the invention comprises montelukast sodium, a filling agent, a disintegrating agent, an adhesive, a colorant, a lubricant and a flow aid. The preparation method of the montelukast sodium tablet provided by the invention adopts direct tabletting after dry method granulation and uses the colorant, the colorant can act as a very good light shielding function in the operational process, the operation is convenient, the influence of a drying process and moist heat to the product quality is avoided, and the product stability is enhanced.
Owner:LUNAN PHARMA GROUP CORPORATION
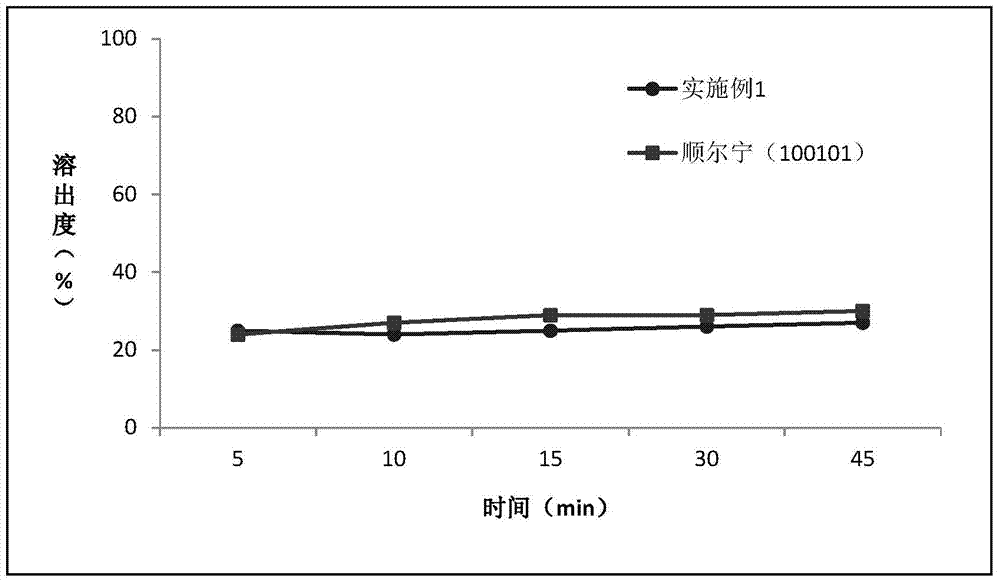
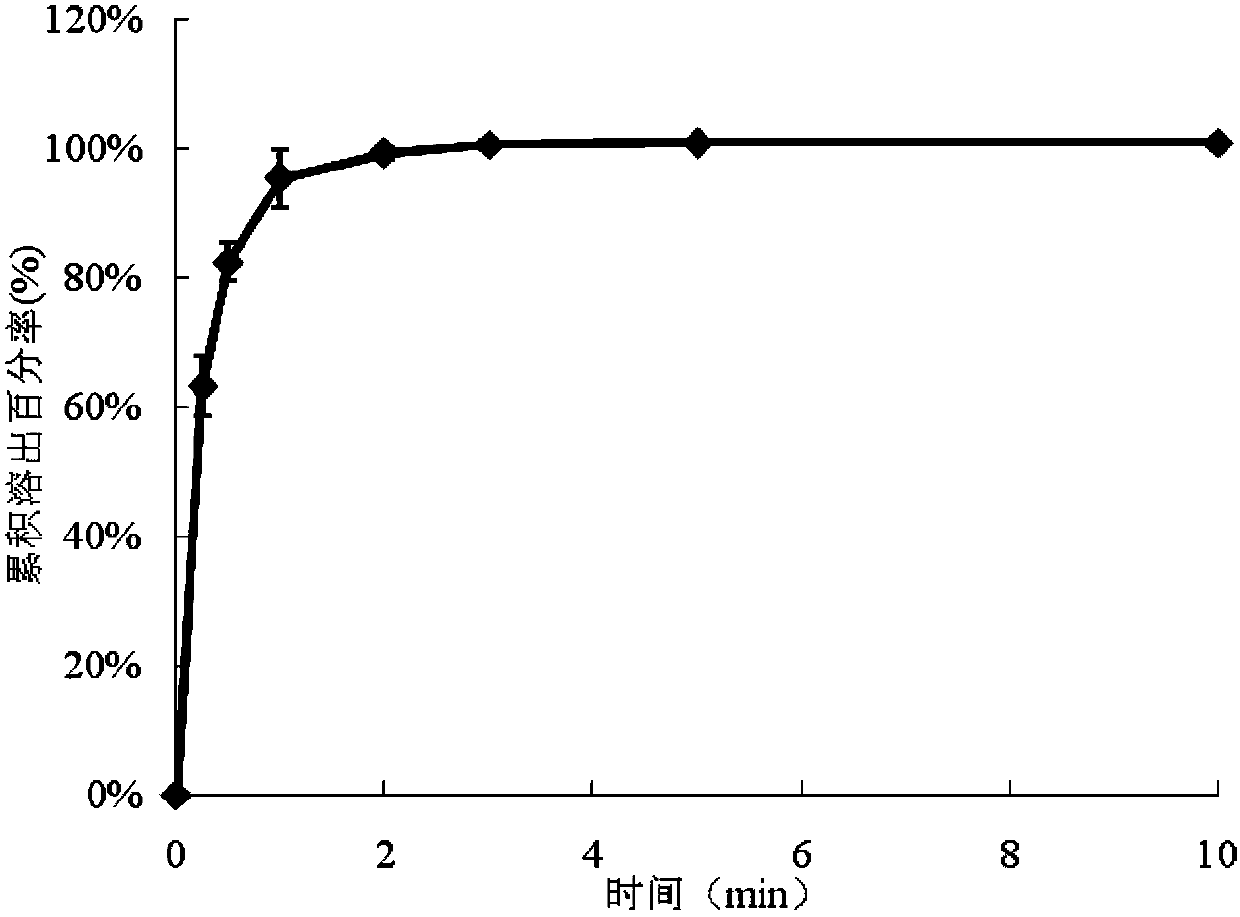
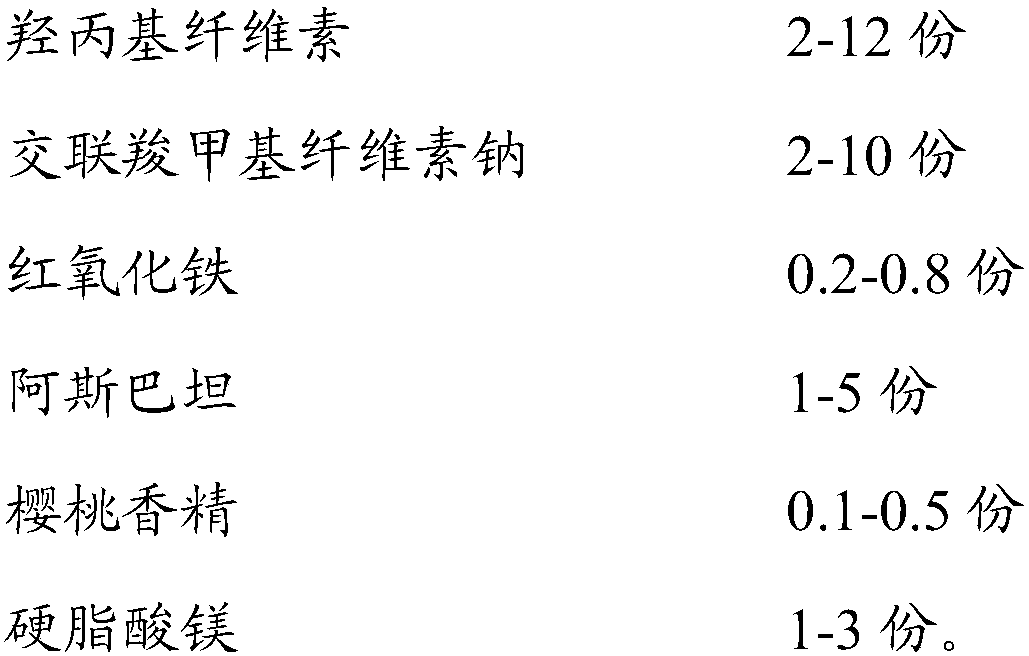
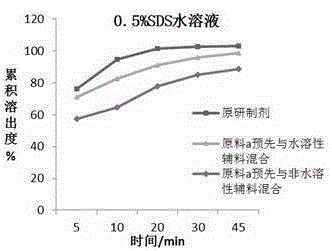
Montelukast sodium chewable tablet, preparation method and determination method of dissolution rate
InactiveCN104146975AImprove in vitro dissolutionGuaranteed stabilityAntipyreticComponent separationMANNITOL/SORBITOLDissolution
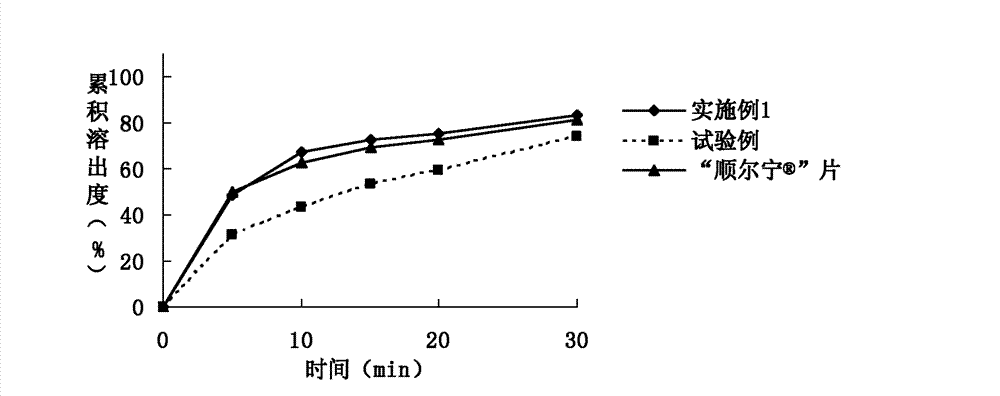
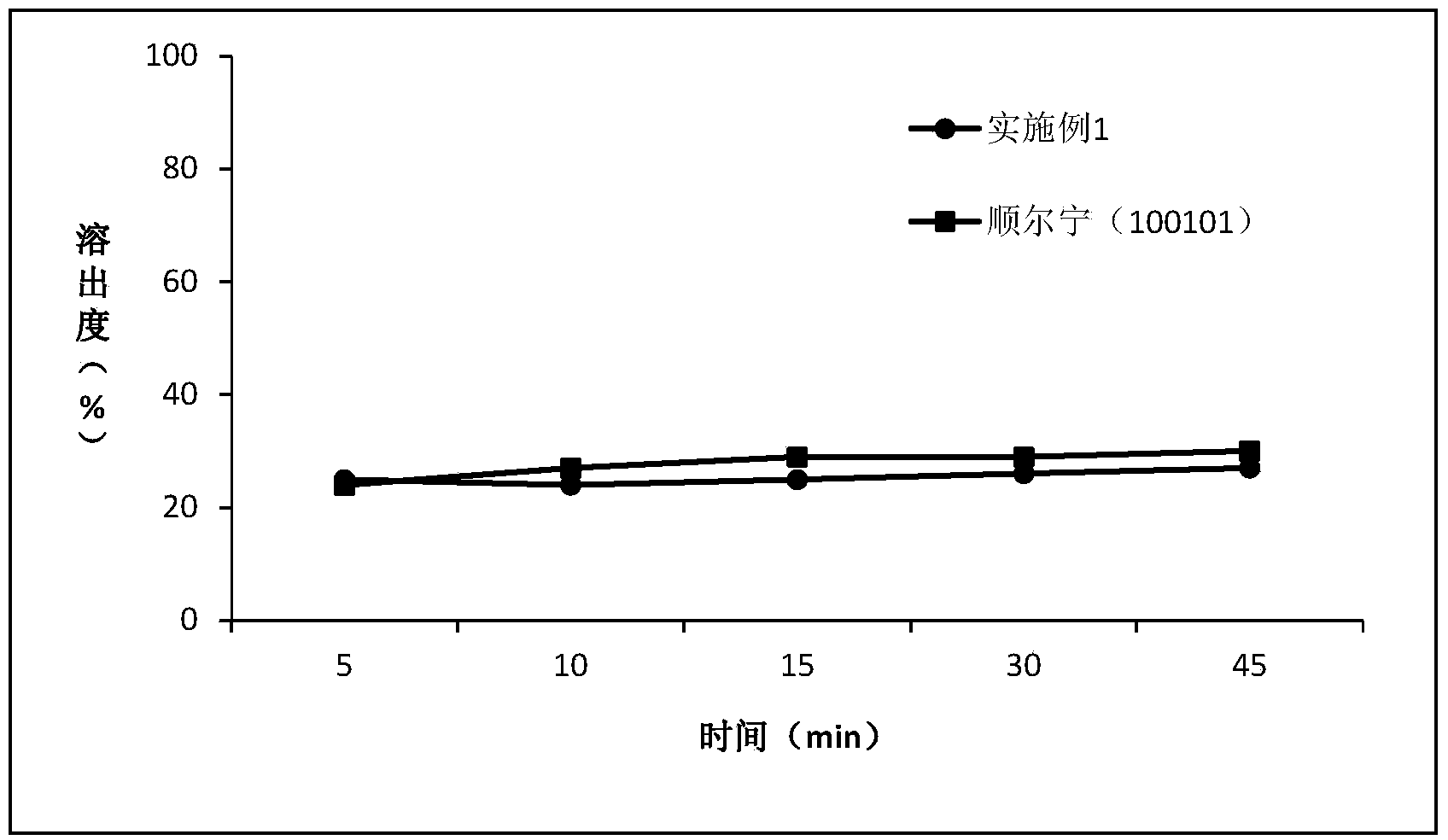
The invention relates to the field of medicinal preparations and particularly relates to a montelukast sodium chewable tablet, apreparation method and a determination method of a dissolution rate. Raw materials of the montelukast sodium chewable tablet comprise montelukast sodium, mannitol, microcrystalline cellulose, hydroxy propyl cellulose, crosslinking sodium carboxymethylcellulose, aspartame, ferric oxide, magnesium stearate and cherry essence. The hydroxy propyl cellulose is prepared into a solution with a mass percentage content being 6-8% through water being a solvent and a disintegrating agent is prepared through a wet process of an internal addition method. The montelukast sodium chewable tablet is significantly increased in disintegrating speed and in-vitro dissolution rate. By means of a dissolution curve detection method, quality differences among products in each batch can be effectively distinguished. Differences between products in each batch can be reduced better and a quality risk of the products can be controlled in a controllable range.
Owner:BENGBU BBCA MEDICINE SCI DEV
Montelukast orally disintegrating tablet and preparation method thereof
ActiveCN103655497APill deliveryPharmaceutical non-active ingredientsOrally disintegrating tabletMontelukast Sodium
The invention discloses a Montelukast orally disintegrating tablet and a preparation method thereof. The Montelukast orally disintegrating tablet comprises Montelukast, spraying and drying mannitol (particle diameters are controlled to be between 300 mu m to 500 mu m) and a lubricant. The Montelukast orally disintegrating tablet can be rapidly disintegrated in an oral cavity without adding a disintegrating agent, the dissolving is rapid, the tablet hardness is high, and the packaging, the transporting and the storing are easy; the invention further provides the preparation method of the Montelukast orally disintegrating tablet. Direct powder compression is adopted, the operation is convenient, influences of a drying process, dampness and heat on the product quality are avoided, the prescription has few varieties of auxiliary materials, a direct powder compression process is combined, and the stability is improved remarkably.
Owner:北京华禧联合科技发展有限公司
Preparation method for montelukast sodium intermediate
ActiveCN105330540AProcess environmental protectionSolve wasteOrganic compound preparationCarboxylic acid esters preparationHydrogenMontelukast Sodium
The invention discloses a preparation method for a montelukast sodium intermediate. The preparation method comprises the steps that a raw material methyl2-(3-(3-bromophenyl)-3-oxo-propyl)-methyl benzoate (II) is dissolved with solvent C under protection of inert gas, alkali B is added into the raw material, stirring is performed for 1 h-10 h, a catalyst A is added, stirring is continuously performed for 2 h-10 h, the inert gas is replaced by hydrogen, the pressure is increased to 2 atm-60 atm, stirring is performed under the pressure until the pressure keeps invariable, and then the montelukast sodium intermediate IV is obtained.
Process for the Preparation of Novel Amorphous Montelukast Sodium
A process for the preparation of novel amorphous montelukast sodium by dissolving montelukast free acid in an organic solvent, converting into its alkali salt followed by vacuum drying or spray drying the solution. Alternatively by dissolution of montelukast sodium in organic solvent followed by vacuum drying or spray drying the solution.
Chewable tablet containing montelukast sodium
ActiveCN101773481BImprove stabilityAvoid photolysisInorganic non-active ingredientsPill deliveryMedicineMontelukast Sodium
The invention belongs to the field of medicine preparations, which particularly relates to a chewable tablet of montelukast sodium. Because the production process of the chewable tablet of the montelukast sodium needs the light shielding operation at present, the mass production is inconvenient, and the chewable tablet of the montelukast sodium has the defect of poorer stability. In the invention, zinc stearate and an opacifier of iron oxide red, iron oxide yellow and titanium dioxide are added into the auxiliary materials of a chewable tablet of montelukast, so that the content of relevant substances can be lowered, and the stability of the chewable tablet is enhanced.
Owner:LUNAN PHARMA GROUP CORPORATION
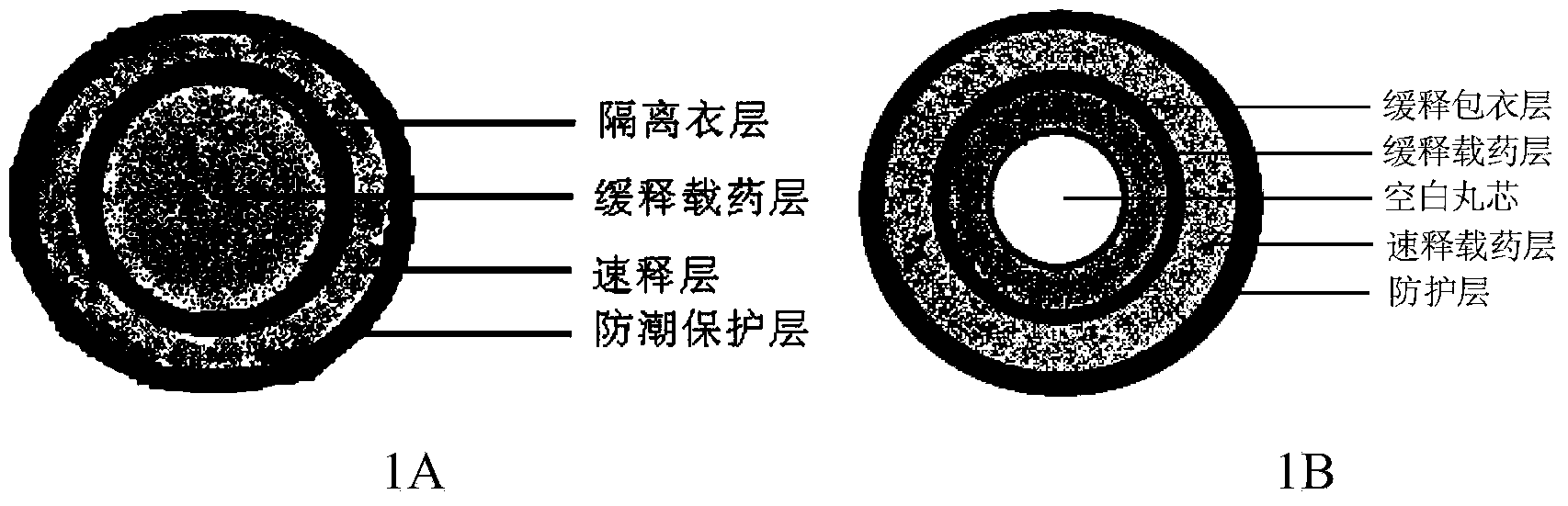
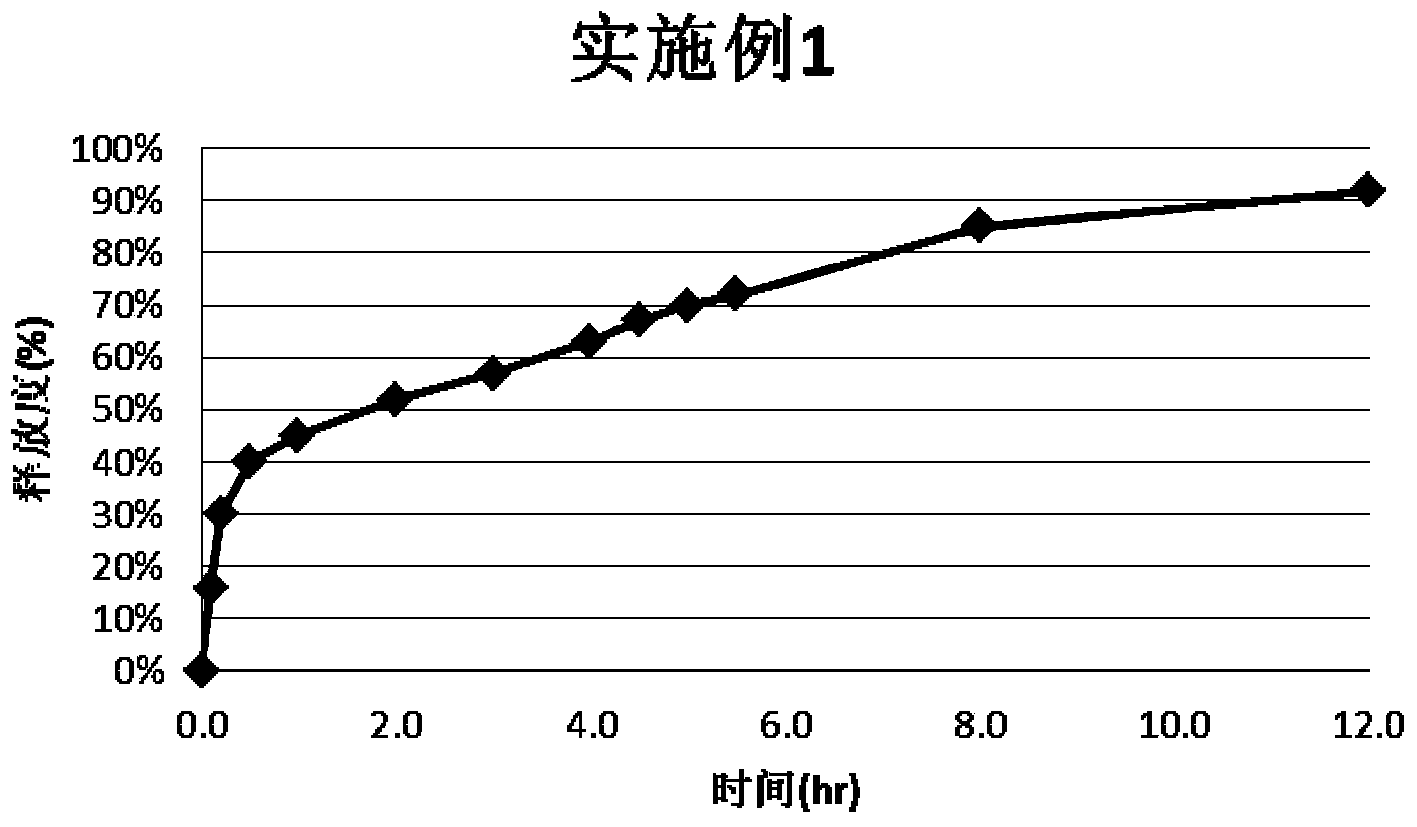
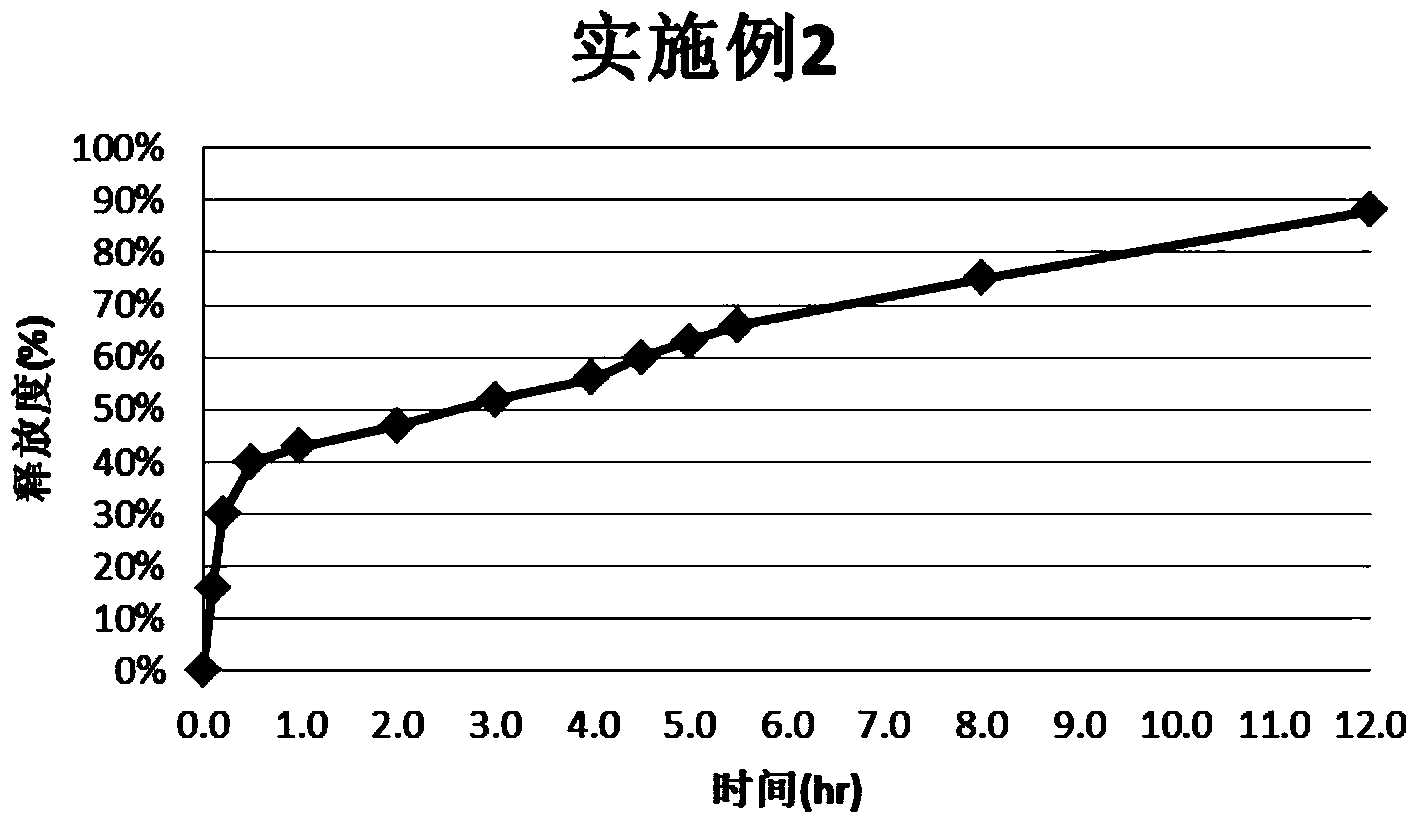
Montelukast sodium pulse release preparation
The invention relates to an oral solid drug dosage form containing montelukast sodium as a single active drug. The dosage form is prepared from a slow-release kernel and a quick-release outer layer, wherein the slow-release kernel can be prepared from blank-pellets coated by slow-release garments after loading a drug, also can be prepared from a main drug and other proper slow-release macromolecule after extrusion and spheronization, and then the quick-release outer layer is prepared on the slow-release kernel in a manner of loading the drug; the pellets can be filled into the capsules or tabletted. Compared with a common preparation, the novel pulsed release capsules disclosed by the invention achieve the peak plasma concentration at the moment that asthma easily breaks out before dawn; the target of enough time to treat can be met; the medication availability and security of an asthma patient are improved.
Owner:TIANYAO PHARMA TECH DEV SHANGHAI
Montelukast sodium granules and preparation method thereof
ActiveCN108057021AFast absorptionImprove solubilityPharmaceutical non-active ingredientsGranular deliveryAdhesiveLactose
The invention disclose montelukast sodium granules and a preparation method thereof, and relates to the field of a montelukast sodium preparation. The montelukast sodium granules provided by the invention are prepared from a raw material by dry granulation or fluidized bed granulation; the raw material comprises the following components in percentage by weight: 0.5% to 1.5% of montelukast sodium,35% to 65% of mannitol, 15% to 45% of lactose anhydrous, 10 to 30% of microcrystalline cellulose, 0% to 20% of adhesive used when a fluidized bed is adopted to carry out granulation, 0.5% to 3.5% of corrigent and 0.5% to 5% of lubricant. The montelukast sodium granules not only have the characteristics of rapid absorption, good dissoluvability, rapid response, convenience and comfort for taking, good taste and the like, but also have excellent stability.
Owner:南京康舟医药科技有限公司
Pharmaceutical composition containing montelukast sodium
ActiveCN104840427AImprove stabilityEasy to operatePharmaceutical non-active ingredientsGranular deliveryMedicineMontelukast Sodium
The invention belongs to the technical filed of a medicine, and particularly relates to a pharmaceutical composition containing montelukast sodium. The pharmaceutical composition contains montelukast sodium, sucrose fatty acid ester, and L-ascorbic acid. The pharmaceutical composition is good in stability and has excellent quality. The production operation of the pharmaceutical composition is simple and easy, and is suitable for industrial production.
Owner:长春海悦药业股份有限公司
Montelukast sodium liposome solid preparation
InactiveCN102085187BWell mixedImprove stabilityPharmaceutical non-active ingredientsRespiratory disorderSide effectMANNITOL/SORBITOL
The invention provides a montelukast sodium liposome solid preparation which is prepared from raw and auxiliary materials comprising the following components in parts by weight: 5 parts of montelukast sodium, 15-30 parts of hydrogenated soybean phosphatidylcholine (HSPC), 8-20 parts of cholesterol, 4-12 parts of Tween 80, 20-50 parts of mannitol and 1-3 parts of soyasterol. The montelukast sodiumliposome solid preparation has high stability, is stable in light and heat environments, is convenient to store, has the advantages of simple preparation method, high encapsulation rate and uniform particle size and can be preserved in a body for a long time, thereby improving the product quality of the preparation and reducing the toxic side effects.
Owner:HAINAN MEIDA PHARMA
Montelukast sodium membrane-shape preparation
ActiveCN103393624AImprove aestheticsEnhance interestPharmaceutical non-active ingredientsRespiratory disorderMedicineMontelukast Sodium
The invention provides a Montelukast sodium film-like preparation, comprising a strip film belt and a treatment effective dose of an active medicine loratadine loaded on the strip film belt, wherein the strip film belt comprises acidic strip film belts and alkaline strip film belts, the acidic strip film belts and the alkaline strip film belts are connected to form one body and alternately arranged, each acidic strip film belt uses a highly water-soluble polymer film forming material as a main body and contains 1 to 20% of an acidic agent on the basis of the weight of the acidic strip film belt, and each alkaline strip film belt uses the highly water-soluble polymer film forming material as a main body and contains 1 to 20% by weight of an alkaline agent on the basis of the weight of the alkaline strip film belt. In the presence of water, the Montelukast sodium film-like preparation provided by the invention becomes an effervescent film, generates considerable bubbles, paralyzes olfactory sensation of people so as to mask odor and is accelerated in dissolving-out.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT +1
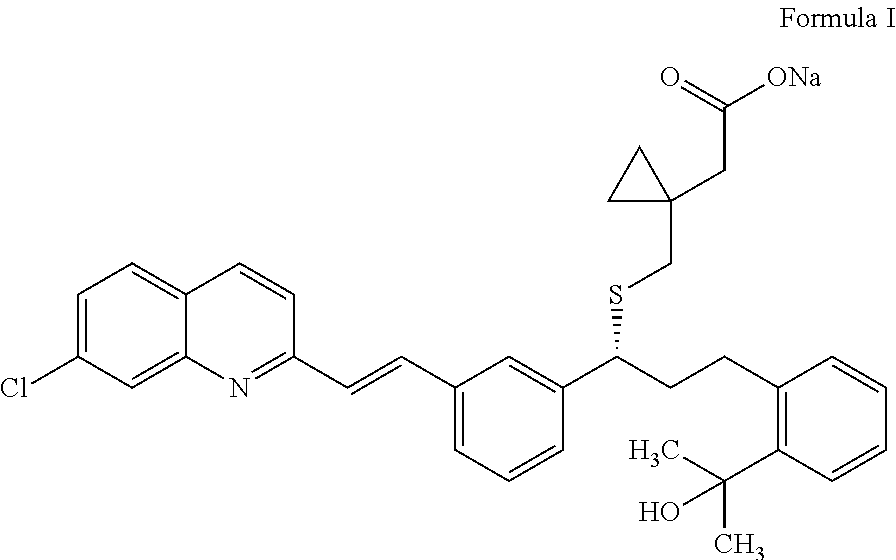
Process for preparation of montelukast sodium
ActiveUS20150299127A1High purityHigh yieldOrganic chemistryRespiratory disorderSulfonyl chloridePropanol
Disclosed is a process for the preparation of montelukast sodium. The process comprises a) reacting 2-(2-(3(S)-(3-(2-(7-chloro-2-quinolinyl)-ethenyl)phenyl)-3-hydroxypropyl)phenyl)-2-propanol with methane sulfonyl chloride and coupling the resultant mesylate compound with 1-(mercaptomethyl)cyclopropane acetic acid in presence of a base and free alkali source followed by saltification with an amine in a single step reaction and b) converting the montelukast amine salt to montelukast sodium salt.
Owner:LAURUS LABS
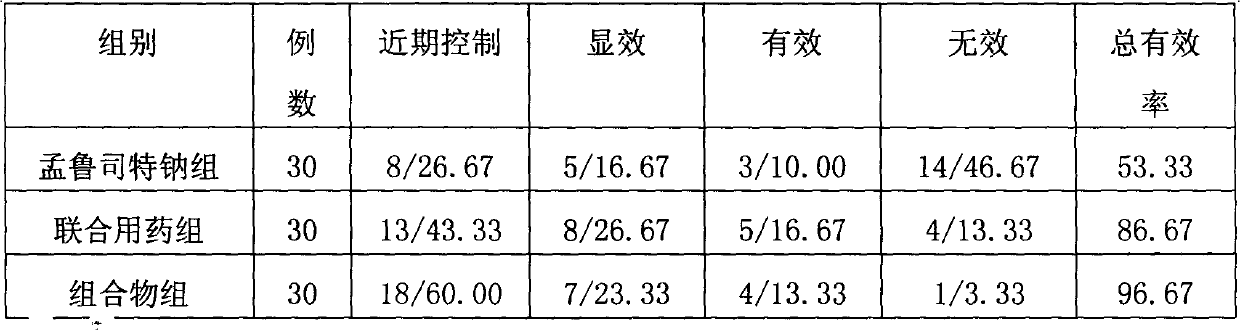
Pharmaceutical composition for treating asthma
InactiveCN102716128ARespiratory disorderHeterocyclic compound active ingredientsMontelukast SodiumBULK ACTIVE INGREDIENT
The invention discloses a pharmaceutical composition taking ambroxol hydrochloride and montelukast sodium as active ingredients and the application of the pharmaceutical composition. The composition can be made into oral preparations including tablets, capsules, granules, chewable tablets, oral solutions and dry suspensions. As the ambroxol hydrochloride and the montelukast sodium are synergistic, the composition has an unexpected effect of treating asthma and is very good in application prospect.
Owner:王学军
Stable montelukast sodium chewable-tablets and preparation method thereof
InactiveCN109833302APrevent crushingAvoid processing powerPill deliveryPharmaceutical non-active ingredientsAdhesiveDissolution
The invention discloses stable montelukast sodium chewable-tablets. The stable montelukast sodium chewable-tablets are composed of montelukast sodium and an adhesive. A preparation method of the stable montelukast sodium chewable-tablets comprises the following steps: blending the montelukast sodium and the adhesive so as to obtain a mixed solution; and then, carrying out granulation by adopting awet method. In addition, the invention further provides a preparation method of the stable montelukast sodium chewable-tablets. The preparation method provided by the invention is simple, controllable, easy to amplify, and good in reproducibility; moreover, the prepared montelukast sodium chewable-tablets are uniform in appearance, color and luster, small in inter-batch differences, and stable inin-vitro dissolution and release.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Chewable tablet containing montelukast sodium and preparation method of chewable tablet
InactiveCN105287413AOvercome stabilityOvercome consistencyPill deliveryPharmaceutical non-active ingredientsAdjuvantMannitol formation
The invention relates to a chewable tablet containing montelukast sodium and a preparation method of the chewable tablet. The chewable tablet consists of montelukast sodium and pharmaceutically acceptable adjuvants, and the chewable tablet is characterized in that a mannitol-pharmaceutical compound is formed from a montelukast sodium raw material which is micronized and from mannitol; and a microcrystalline cellulose source is optimized, so that the content of impurity sulfoxide and the content of total impurities are reduced, and moreover the preparation is good in dissolution consistency and uniformity in vitro and is greatly high in quality stability.
Owner:南京泽恒医药技术开发有限公司
Montelukast sodium chewable tablet and preparation method thereof
Owner:FUJIAN HUAHAI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com