Montelukast sodium tablet and preparation method thereof
A technology of montelukast sodium tablet and montelukast sodium, which is applied in the field of pharmaceutical preparations, can solve the problems of long drying time, content drop, decomposition, etc., so as to avoid the increase of related substances, improve product stability, Avoid the effect of decreasing content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Tablet of Montelukast Sodium
[0024] Montelukast Sodium 100g
[0025] Microcrystalline Cellulose 1299g
[0026] Sodium carboxymethyl starch 120g
[0027] VA64 70g
[0028] Iron Oxide Red 3g
[0029] Micronized silica gel 4g
[0031] Preparation process: Montelukast sodium is passed through a 100-mesh sieve, iron oxide red is passed through a 80-mesh sieve, the prescribed amount of iron oxide red, microcrystalline cellulose, sodium carboxymethyl starch, and VA64 are weighed and mixed evenly, and then the prescribed amount is Montelukast sodium and the above mixture are added in equal amounts for 3 times and mixed evenly, passed through an 80-mesh sieve for dry granulation, plus the prescribed amount of micropowder silica gel and zinc stearate, mixed evenly, compressed into tablets, coated, and made 10,000 tablets in total .
Embodiment 2-9
[0032] Embodiment 2-9 montelukast sodium tablet
[0033] For the convenience of description, see Table 1 for the prescriptions of Montelukast Sodium Tablets in Examples 2-9. In the table, "Example 2" represents the prescription of Example 2, and "Example 3"-"Example 9" represent the prescriptions of "Example 3"-"Example 9" respectively.
[0034] Table 1 Example 2-9 Montelukast Sodium Tablet Prescription
[0035]
[0036] The preparation process was the same as in Example 1, and 10,000 tablets were produced in total.
Embodiment 10
[0037] Example 10 Tablet compression process of montelukast sodium tablet
[0038] 1. Equipment and materials
[0039] 1.1 Equipment
[0040] ZKG-5 type dry granulator (Zhejiang Jiangnan Pharmaceutical Machinery Co., Ltd.); ZDS three-way rotary vibrating screen (Jiangsu Zhangjiagang Yongda Chemical Machinery Factory); ZPS008 rotary tablet press (Shanghai Tianxiang-Jiantai Pharmaceutical Machinery Factory) ; LB-2B disintegration time limit tester (Shanghai Huanghai Drug Inspection Instrument Factory); 78X-2 tablet four-purpose tester (Shanghai Huanghai Pharmaceutical Factory); intelligent drug dissolution tester RCZ-5A (Tianjin University Precision Instrument Factory); 10A type high performance liquid chromatography.
[0041] 1.2 Materials
[0042] Granules prepared according to Example 1.
[0043] 2. Experimental methods and steps
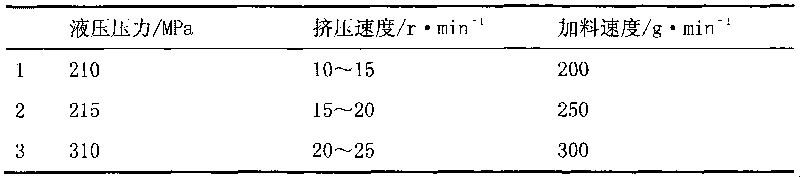
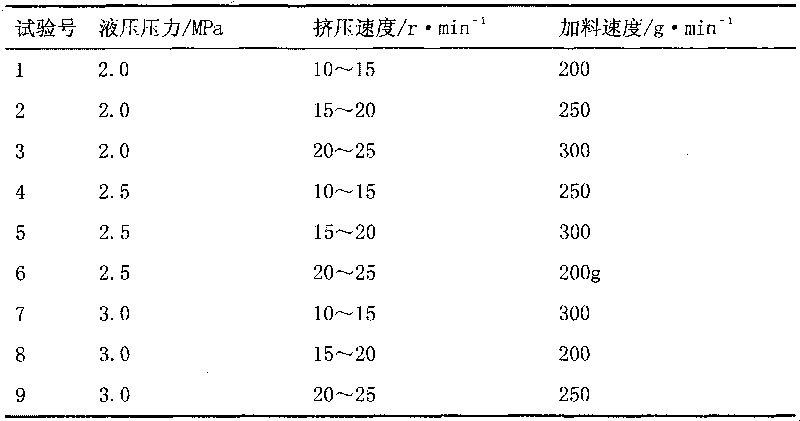
[0044] According to the pre-test results and equipment capabilities, three parameters of the ZPS008 rotary tablet press, hydraulic pressure, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com