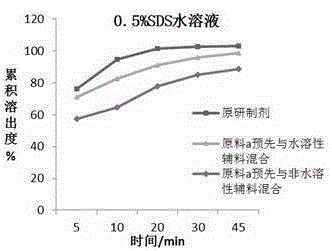
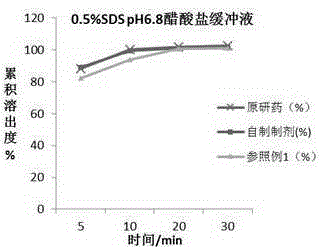
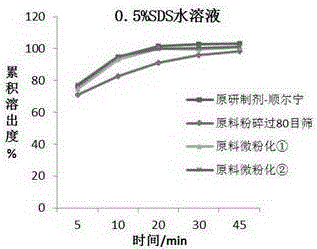
Chewable tablet containing montelukast sodium and preparation method of chewable tablet
A technology of montelukast sodium and chewable tablets, which is applied in the directions of active ingredients of heterocyclic compounds, pill delivery, respiratory system diseases, etc. High quality requirements for excipients, to achieve the effect of improving medication compliance, bright appearance, uniform and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1 montelukast sodium chewable tablet
[0064] Montelukast Sodium 5.19mg
[0065] Microcrystalline Cellulose PH101 (FMC) 65mg
[0066] Mannitol 215mg
[0067] Croscarmellose Sodium 6.0mg
[0068] Magnesium stearate 3.0mg
[0069] Allure Red Aluminum Tablets 0.4mg
[0070] Aspartame 0.6mg
[0071] Cherry essence 1.2mg
[0072] Proper amount of hydroxypropyl cellulose
[0073] Appropriate amount of absolute ethanol
[0074] Preparation process (avoid light during the process):
[0075] After the raw material of montelukast sodium is micronized, the raw material of montelukast sodium is added to mannitol, so that the raw material of montelukast sodium is evenly adsorbed and dispersed in mannitol to form a mannitol complex, and then microcrystalline Cellulose PH101 (FMC), cherry flavor, aspartame, allure red aluminum color tablets, mix well. To make soft material, granulate with 24-mesh sieve, and dry at 40°C-60°C. After granulating with 24-mesh sieve,...
Embodiment 2
[0077] Example 2 Montelukast Sodium Chewable Tablets
[0078] Montelukast Sodium 4.15mg
[0079] Microcrystalline Cellulose PH101 (FMC) 80mg
[0080] Mannitol 200mg
[0081] Croscarmellose Sodium 6.0mg
[0082] Magnesium stearate 3.0mg
[0083] Allure Red Aluminum Tablets 0.4mg
[0084] Aspartame 0.6mg
[0085] Cherry essence 1.2mg
[0086] Proper amount of hydroxypropyl cellulose
[0087] Appropriate amount of absolute ethanol
[0088] Preparation process (avoid light during the process):
[0089] After the raw material of montelukast sodium is micronized, the raw material of montelukast sodium is added to mannitol, so that the raw material of montelukast sodium is evenly adsorbed and dispersed in mannitol to form a mannitol complex, and then microcrystalline Cellulose PH101 (FMC), cherry flavor, aspartame, allure red aluminum color tablets, mix well. To make soft material, granulate with 24-mesh sieve, and dry at 40°C-60°C. After granulating with 24-mesh sieve, a...
Embodiment 3
[0090] Example 3 Montelukast Sodium Chewable Tablets
[0091] Montelukast Sodium 5.19mg
[0092] Microcrystalline Cellulose PH101 (FMC) 77mg
[0093] Mannitol 200mg
[0094] Croscarmellose Sodium 8.0mg
[0095] Magnesium stearate 3.5mg
[0096] Allure Red Aluminum Tablets 0.5mg
[0097] Aspartame 0.9mg
[0098] Cherry essence 1.2mg
[0099] Proper amount of hydroxypropyl cellulose
[0100] Appropriate amount of absolute ethanol
[0101] Preparation process (avoid light during the process):
[0102] After the raw material of montelukast sodium is micronized, the raw material of montelukast sodium is added to mannitol, so that the raw material of montelukast sodium is evenly adsorbed and dispersed in mannitol to form a mannitol-drug complex, and then added separately Microcrystalline cellulose PH101 (FMC), cherry flavor, aspartame, allure red aluminum color tablets, mix well. To make soft material, granulate with 24-mesh sieve, and dry at 40°C-60°C. After granulating...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com