Montelukast sodium chewable tablet, preparation method and determination method of dissolution rate
A technology of montelukast sodium and its determination method, which is applied in the field of montelukast sodium chewable tablets and its preparation, can solve the problems of decreased main content, high production environment requirements, and no improvement in disintegration speed, etc. Achieve the effect of increasing disintegration speed, improving in vitro dissolution rate and ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1- Embodiment 3
[0049] In embodiment 1-embodiment 3, montelukast sodium chewable tablet comprises raw material as follows:
[0050] Example 1 Example 2 Example 3 Montelukast Sodium 3g Montelukast Sodium 2g Montelukast Sodium 1g Mannitol 128g Mannitol 75.25g Mannitol 42.6g Microcrystalline cellulose 22g Microcrystalline Cellulose 14.75 Microcrystalline cellulose 7.5g Hydroxypropyl cellulose 2.33g Hydroxypropyl cellulose 1.5g Hydroxypropyl cellulose 0.8g Croscarmellose Sodium 9g Croscarmellose Sodium 6g Croscarmellose Sodium 3g Aspartame 0.3g Aspartame 0.2g Aspartame 0.1g Red iron oxide 0.9g Red iron oxide 0.9g Red iron oxide 0.3g Magnesium stearate 0.6g Magnesium stearate 0.6g Magnesium stearate 0.2g Cherry essence 0.45g Cherry essence 0.45g Cherry essence 0.15g
Embodiment 1
[0051] The preparation method of embodiment 1 montelukast sodium chewable tablet comprises the following steps:
[0052] (1) Under the condition of avoiding light, pass the montelukast sodium bulk drug, magnesium stearate, and red iron oxide through a 100-mesh sieve; mannitol, microcrystalline cellulose, croscarmellose sodium, and Spartame and cherry essence are passed through an 80-mesh sieve;
[0053] (2) Weigh hydroxypropyl cellulose and add purified water, stir until completely dissolved, and prepare 8% hydroxypropyl cellulose binder aqueous solution;
[0054] (3) Sifted montelukast sodium, mannitol, microcrystalline cellulose, and partially cross-linked carmellose sodium (by mass ratio, accounting for 60% of all cross-linked carmellose sodium) 1. Mix the red iron oxide evenly in the wet granulation equipment, add the 8% hydroxypropyl cellulose aqueous solution prepared in step (2), wet granulate, and granulate with a 30-mesh sieve;
[0055] (4) Put the wet granules prep...
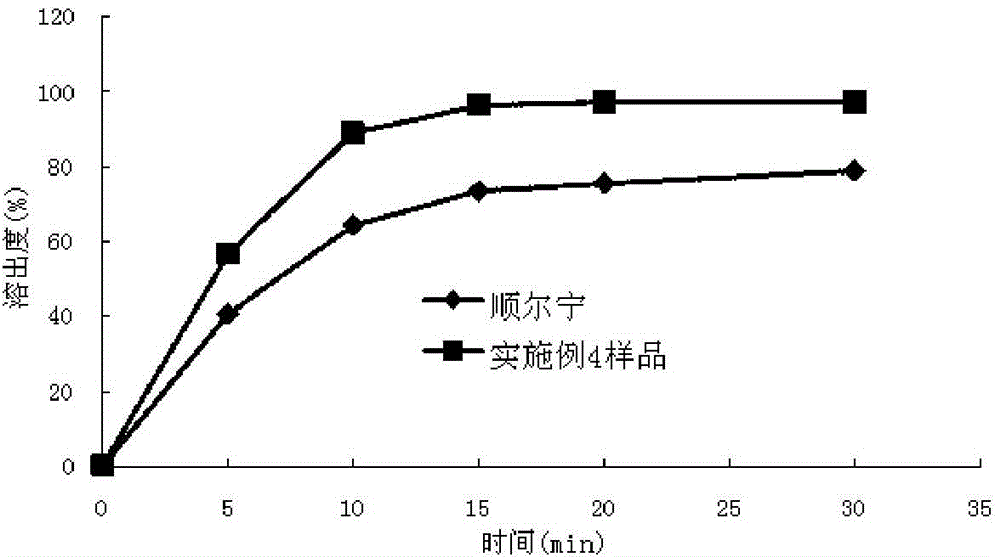
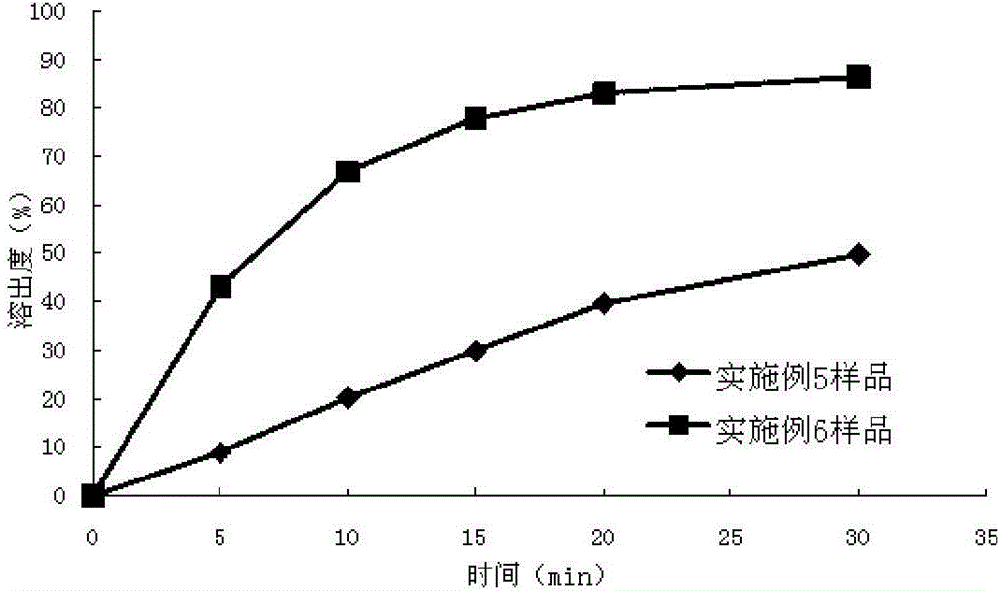
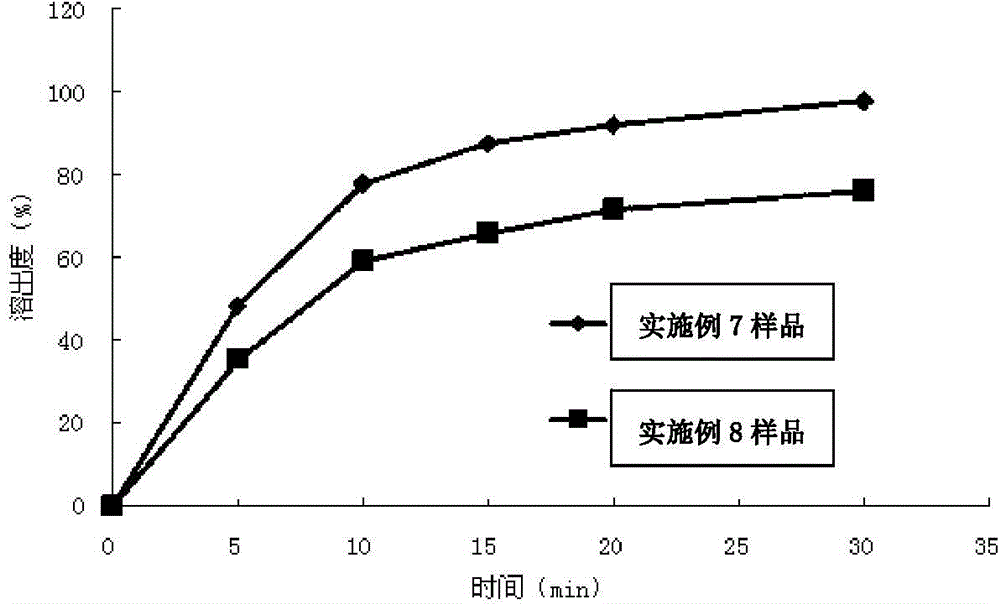
experiment example 1
[0060] This experimental example is a study on the dissolution profile of the montelukast sodium chewable tablets prepared in Example 1-Example 3 according to the dissolution testing method of the present invention.
[0061] The dissolution testing method of embodiment 1, comprises the following steps:
[0062] (1) According to Chinese Pharmacopoeia 2010 edition two appendices XC dissolution method second method, take one piece of chewable tablet (5mg / tablet) with 0.4% (mass volume ratio) sodium lauryl sulfate phosphate buffer solution (pH= 6.8) 900ml is the dissolution medium, and the rotating speed is 50 revolutions per minute. Take 10ml of samples at the 5th, 10th, 15th, 20th, and 30th minutes respectively, filter, and take the subsequent filtrate as the test solution. Dissolution medium with fresh temperature; take another 15mg of montelukast dicyclohexylamine salt, add methanol to dissolve and dilute to 50ml, shake well, take 2ml of the above solution, add 0.5% sodium lau...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com