Stable montelukast sodium chewable-tablets and preparation method thereof
A technology of montelukast sodium and chewable tablets, which is applied in the field of leukotriene receptor antagonist montelukast sodium chewable tablets and its preparation, which can solve the problem that the SDS dissolution medium does not have the ability to distinguish, and the research has no guiding significance, etc. problems, to achieve the effects of easy control of process parameters, short production time, and good process reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 montelukast sodium chewable tablet
[0044]
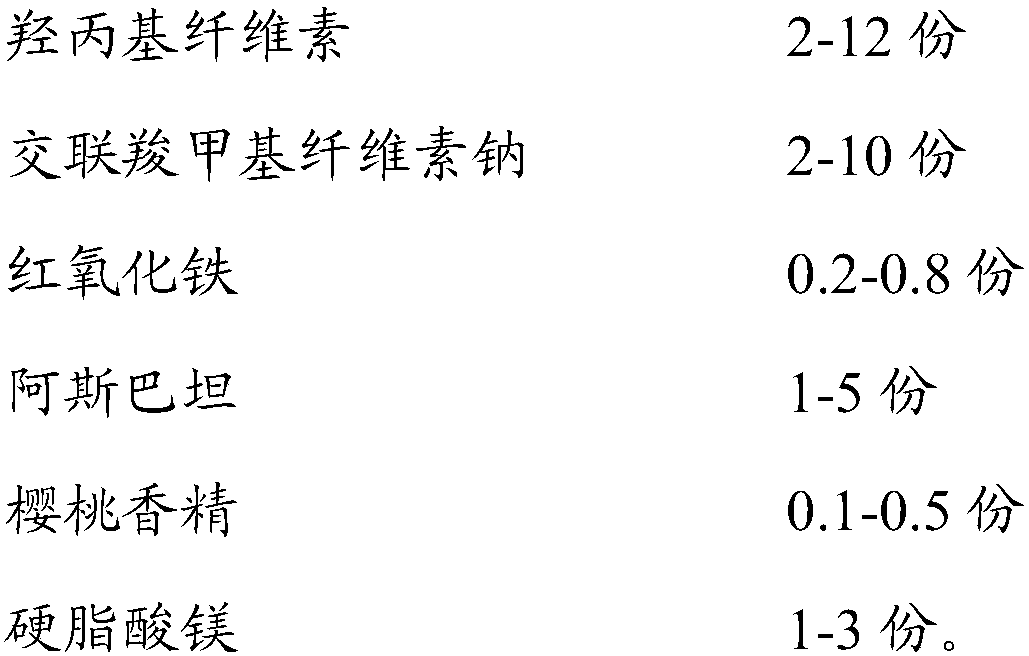
[0045] Take montelukast sodium, add 46.8g of purified water to prepare a 10% by weight aqueous solution, take hydroxypropyl cellulose, add 171g of purified water to prepare a 3.41% by weight aqueous solution, and mix the two. Take microcrystalline cellulose, mannitol, croscarmellose sodium, red iron oxide, aspartame, and cherry essence and add them to the mixer, mix well and transfer to the fluidized bed, spray montelukast sodium and The aqueous solution of hydroxypropyl cellulose is granulated, dried until the water content is less than 1.5% by weight, granulated with a 30-mesh sieve, added with magnesium stearate and mixed for 8 minutes, and compressed into tablets with a rotary tablet press. Dissolution test results are shown in Table 1-3 below:
[0046] Table 1 Cumulative dissolution % of each point of 0.01% Tween pH1.0
[0047] t / min
[0048] Table 2 Cumulative dissolution % of each point of ...
Embodiment 2
[0054] Example 2 Montelukast Sodium Chewable Tablets
[0055]
[0056] Take montelukast sodium, add 46.8g of purified water to prepare a 10% by weight aqueous solution, take hydroxypropyl cellulose, add 171g of purified water to prepare a 5% by weight aqueous solution, and mix the two. Take microcrystalline cellulose, mannitol, croscarmellose sodium, red iron oxide, aspartame, and cherry essence and add them to the mixer, mix well and transfer to the fluidized bed, spray montelukast sodium and The aqueous solution of hydroxypropyl cellulose is granulated, dried until the water content is less than 1.5% by weight, granulated with a 30-mesh sieve, added with magnesium stearate and mixed for 8 minutes, and compressed into tablets with a rotary tablet press. The dissolution test results are shown in Table 4-6 below:
[0057] Table 4 Cumulative dissolution % of each point of 0.01% Tween pH1.0
[0058] t / min
[0059] Table 5 Cumulative dissolution % of each point of 0...
Embodiment 3
[0064] Example 3 Montelukast Sodium Chewable Tablets
[0065]
[0066] Take montelukast sodium, add 46.8g of purified water to prepare a 10% by weight aqueous solution, take hydroxypropyl cellulose, add 171g of purified water to prepare a 6.56% by weight aqueous solution, and mix the two. Add microcrystalline cellulose, mannitol, croscarmellose sodium, red iron oxide, aspartame, and cherry essence into a mixer, mix well and transfer to a fluidized bed, spray montelukast sodium hydroxyl The propyl cellulose aqueous solution is granulated, dried until the water content is less than 1.5% by weight, sized with a 30-mesh sieve, added with magnesium stearate and mixed for 8 minutes, and compressed into tablets with a rotary tablet press. The dissolution test results are as follows in Table 7-9:
[0067] Table 7 Cumulative dissolution % of each point of 0.01% Tween pH1.0
[0068] t / min
[0069] Table 8 Cumulative dissolution % of each point of 0.01% Tween pH4.0
[007...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com