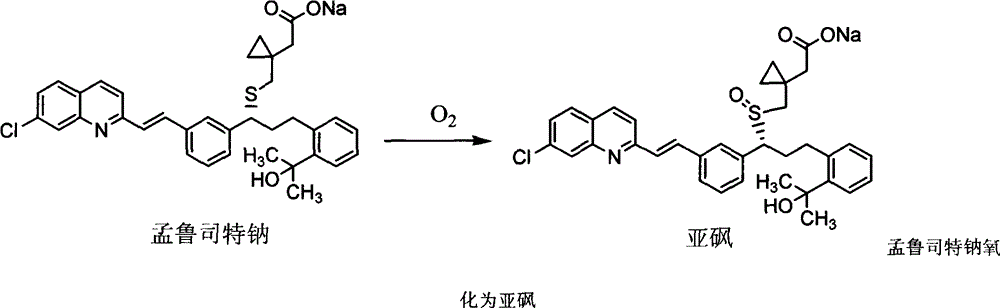
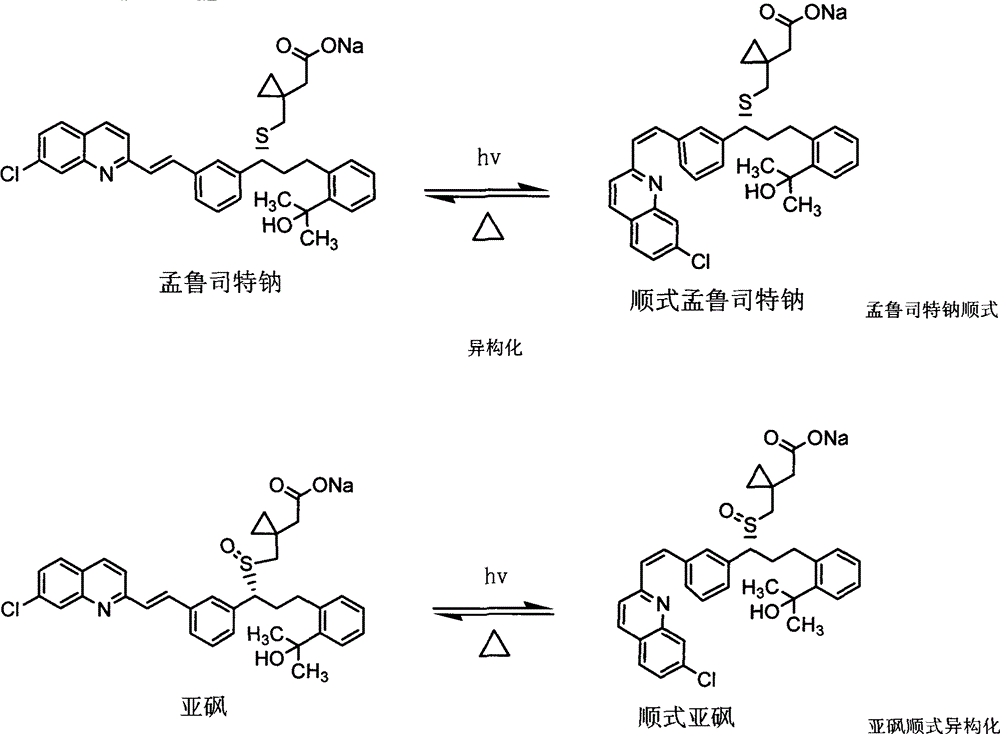
Stable montelukast sodium tablet and preparation method thereof
A stabilizer and stabilizing technology, applied in the field of pharmaceutical compositions containing montelukast or its salts, can solve the problems of wasteful tailings, poor friability, poor lubricity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
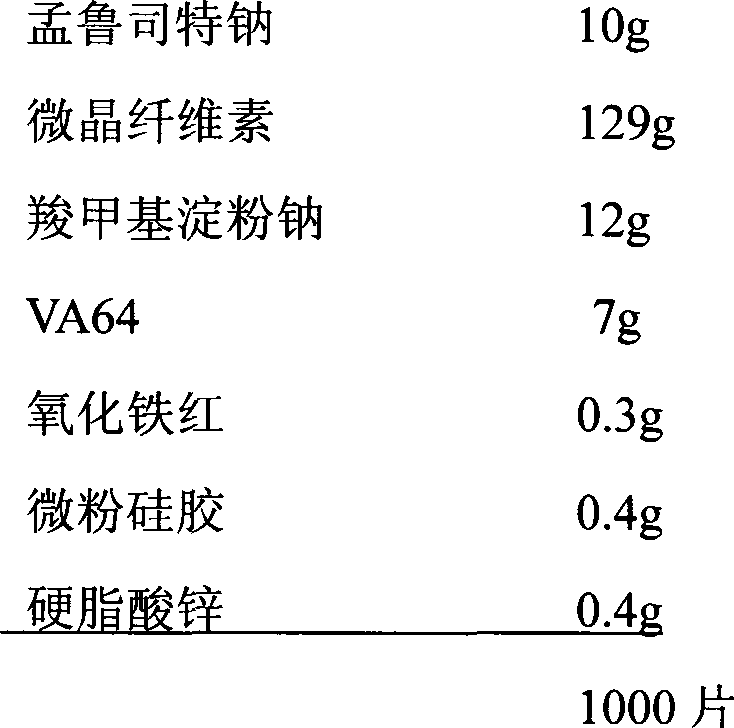
[0030] Preparation process: Montelukast sodium is passed through a 100-mesh sieve, iron oxide red is passed through a 80-mesh sieve, the prescribed amount of iron oxide red, microcrystalline cellulose, sodium carboxymethyl starch, and VA64 are weighed and mixed evenly, and then the prescribed amount is Montelukast sodium and the above mixture were added in equal amounts for 3 times and mixed evenly, passed through an 80-mesh sieve for dry granulation, plus the prescribed amount of micropowder silica gel and zinc stearate, mixed evenly, compressed into tablets, and coated.
[0031] Example 1
[0032]
[0033] Preparation Process
[0034] (1) Montelukast sodium is passed through a 100-mesh sieve, and magnesium metasilicate is passed through an 80-mesh sieve for subsequent use;
[0035] (2) Adhesive configuration: take the prescription amount of povidone K30, dissolve it in 40% ethanol solution, and prepare a 40% ethanol solution of 5% povidone K30;
[0036] (3) ...
Embodiment 2
[0042]
[0043] Preparation Process
[0044] (1) Montelukast sodium is passed through a 100-mesh sieve, and magnesium metasilicate is passed through an 80-mesh sieve for subsequent use;
[0045] (2) Adhesive configuration: take the prescription amount of povidone K30, dissolve it in 40% ethanol solution, and prepare a 40% ethanol solution of 5% povidone K30;
[0046] (3) Take the sieved montelukast sodium and magnesium aluminosilicate, and mix them evenly; then weigh the microcrystalline cellulose, lactose, and croscarmellose sodium of the prescribed amount and mix them with the above mixed powder Uniform;
[0047] (4) Add adhesive to prepare soft material in (3) mixed powder, cross 18 mesh sieves to prepare wet granules;
[0048] (5) Start the blower to suck the material into the F-200B fluidized dryer. The air inlet temperature is set at 60°C±5°C, the outlet air temperature is set at 50°C±5°C, the material temperature is ≤55°C, and the drying time is about 25 minutes; ...
Embodiment 3
[0052]
[0053] Preparation Process
[0054] (1) Montelukast sodium is passed through a 100-mesh sieve, and magnesium metasilicate is passed through an 80-mesh sieve for subsequent use;
[0055] (2) Weigh the sieved montelukast sodium and magnesium aluminosilicate, and mix them evenly; then weigh the prescribed amount of microcrystalline cellulose, lactose, and croscarmellose sodium to mix with the above mixed powder Uniform; add magnesium stearate with general prescription quantity and mix with the above-mentioned mixed powder;
[0056] (3) Start the dry granulator, adjust the distance between the rollers to 0.5 mm, the feeding speed to 42 revolutions / min, the speed of the pressing wheel to 5 revolutions / min, and the screen mesh to be 10-18 mesh, and pour the above materials into the feeding hopper , receive material from the outlet.
[0057] (4) adding the magnesium stearate compressed tablet of prescription quantity;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com