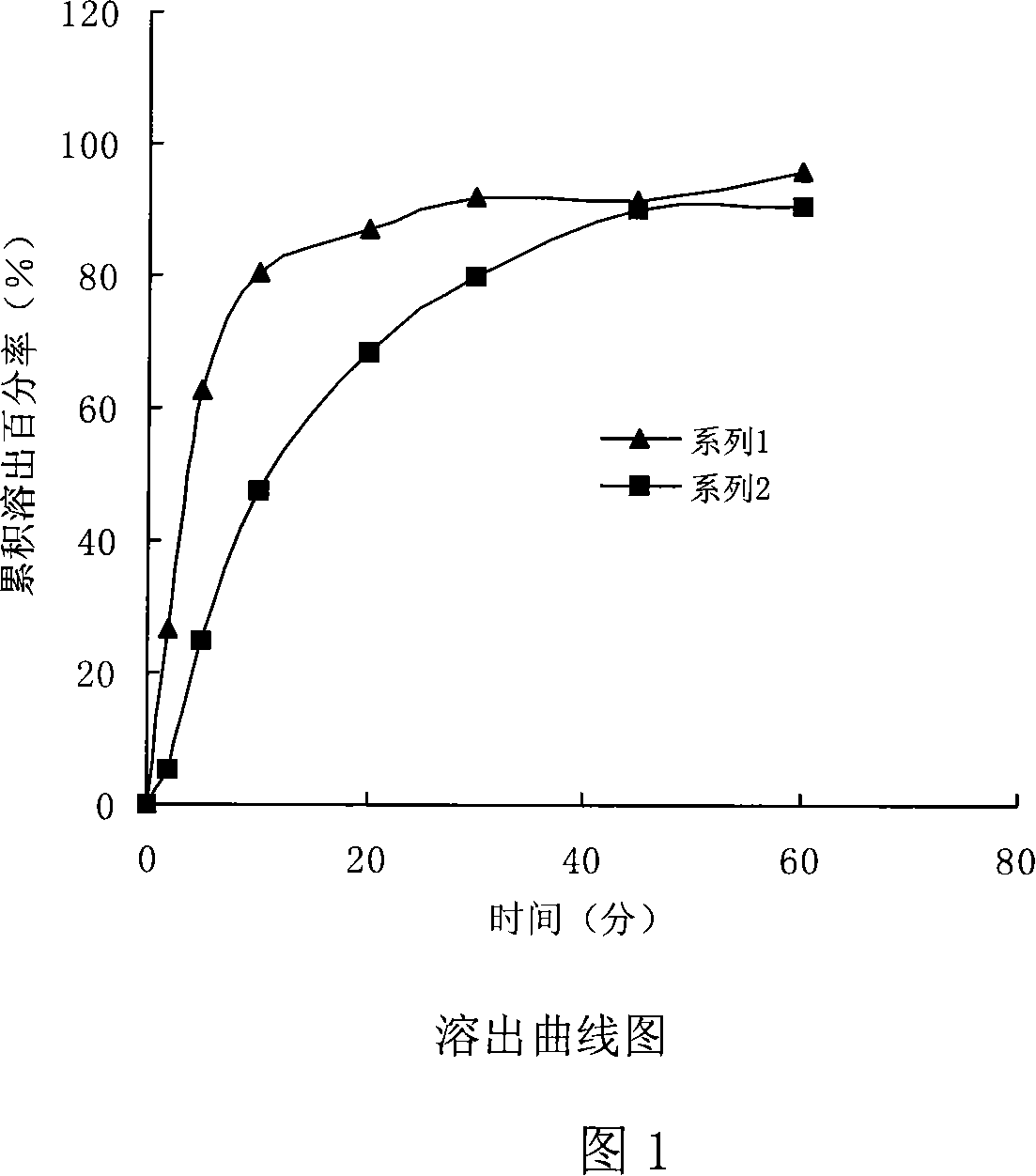
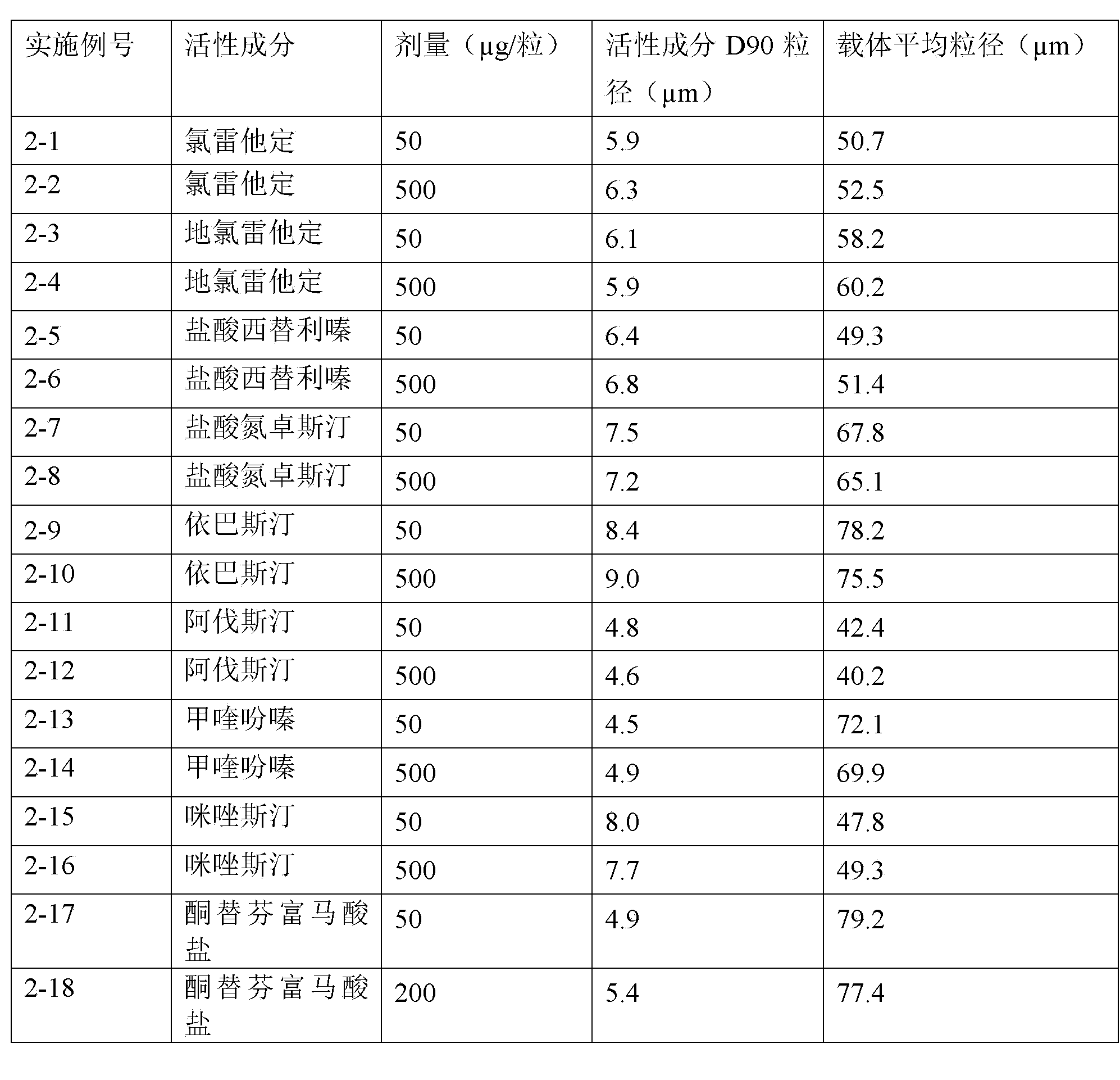
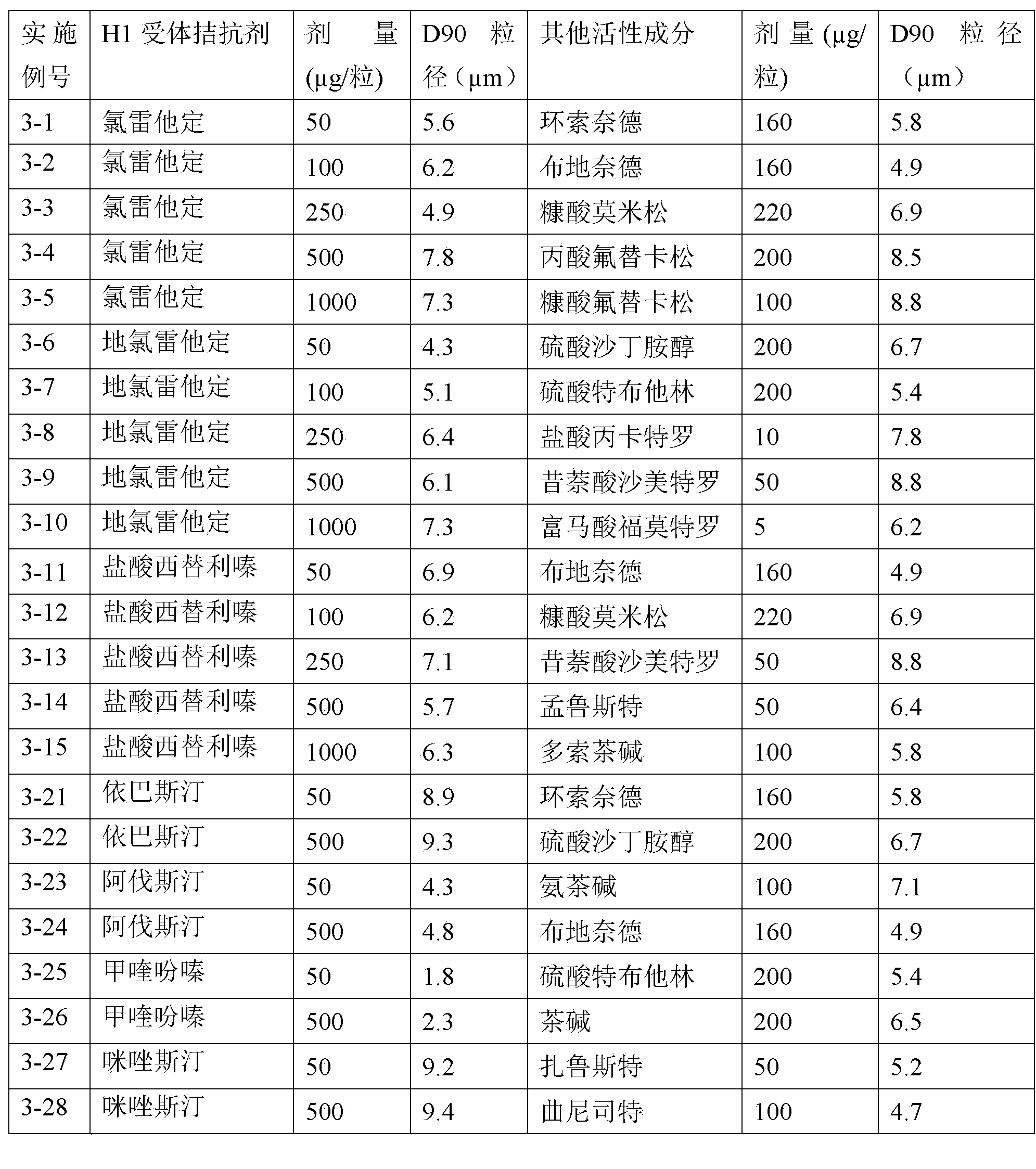
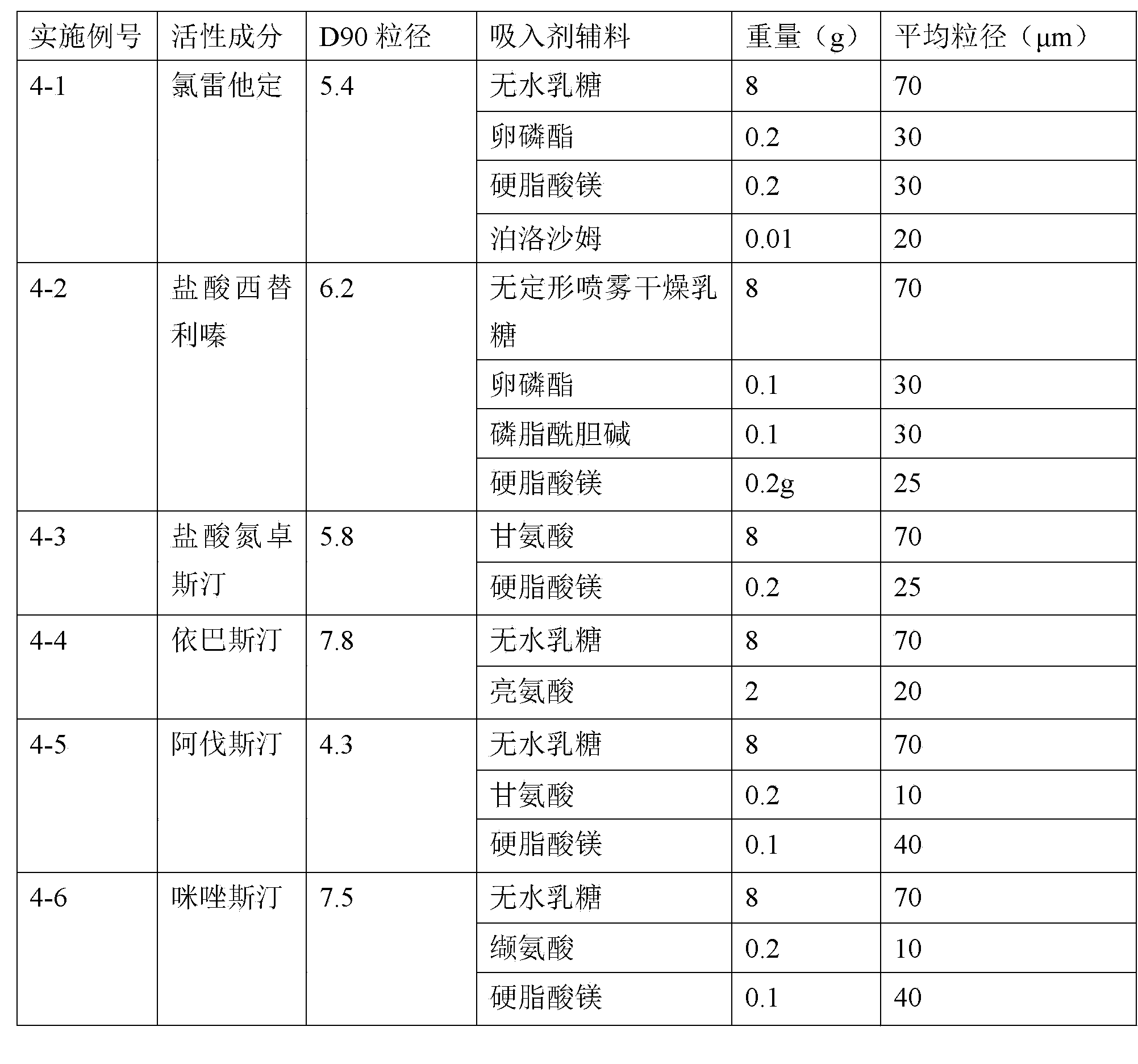
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Ebastine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ebastine is a H₁ antihistamine with low potential for causing drowsiness. It does not penetrate the blood–brain barrier to a significant amount and thus combines an effective block of the H₁ receptor in peripheral tissue with a low incidence of central side effects, i.e. seldom causing sedation or drowsiness.
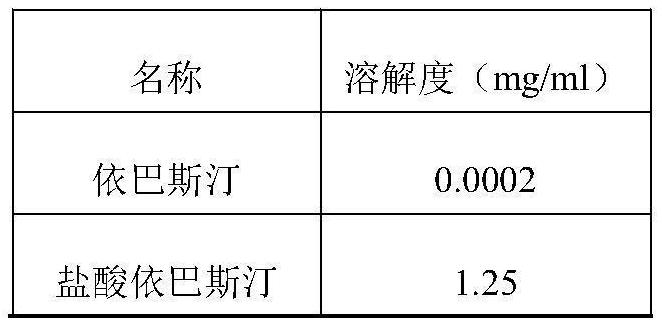
Nanoparticulate ebastine formulations
The invention is directed to compositions comprising at least one nanoparticulate H1-histamine receptor antagonist, such as ebastine or a salt or derivative thereof, having improved dissolution rate providing a faster onset of drug availability. The nanoparticulate H1-histamine receptor antagonist particles, such as ebastine, have an effective average particle size of less than about 2000 nm and are useful in the treatment of seasonal and perennial allergic rhinitis and related diseases.
Owner:ELAN PHRMA INT LTD
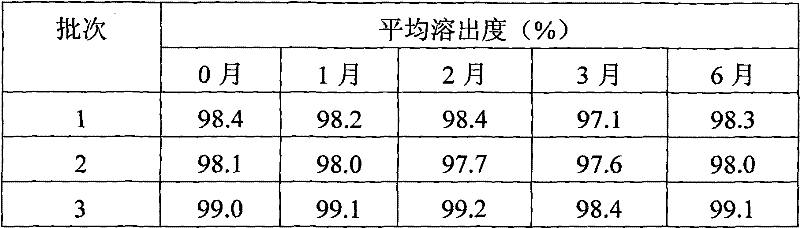
Ebstine solid oral preparation and its preparing method
ActiveCN101161233AImprove solubilityIncrease dissolution ratePill deliveryCapsule deliveryOrganic acidOrally disintegrating tablet
An ebastine solid oral preparation and preparation method are disclosed in the present invention. Made of ebastine, ethanol solution, cosolvent, diluent or filler, disintegrating agent, taste correcting agent, glidants or dispersing agent, effervescing agent, lubricant in proportion, its step is that firstly adding ebastine into ethanol solution, and then adding cosolvent organic acid, leading it to dissolve entirely; secondly mixing the diluent or filler, disintegrating agent, taste correcting agent, effervescing agent with dispersing agent, to obtain a mixer; third mixing the front two, preparing soft material, preparing granules, then adding disintegrating agent, effervescing agent, taste correcting agent, glidants and lubricant, tabletting or filling capsules, packing to obtain a solid oral preparation. The present invention preparing oral disintegrable tablet, dispersing tablet have short disintegrating time, good dispersing state. The obtained oral disintegrable tablet, dispersing tablet and chewing tablet have good taste and mouth feel, the obtained solid oral preparations dissolving out are all rapid. The method is easy, simple operation, without needing special equipment, and lower production cost.
Owner:HUBEI LIYI PHARM TECH CO LTD
Ebastine tablet and its prepn. method
InactiveCN1868474AQuality improvementNot easy to change colorPill deliveryImmunological disordersCurative effectLactose
A tablet of Ebastine is prepared from Ebastine, hydroxypropyl cellulose, microcrystalline cellulose, lactose, and adhesive through superfine pulverizing, proportional mixing, granulating, sieving, mixing and tabletting.
Owner:杭州仟源保灵药业有限公司
H1-receptor-antagonist-containing inhalation preparation
The invention relates to an H1-receptor-antagonist-containing inhalation preparation which contains an H1 receptor antagonist and one or more pharmaceutical auxiliary materials suitable for inhalation administration. The H1 receptor antagonist is one or more of loratadine, desloratadine, cetirizine, levocetirizine, astemizole, ketotifen, ebastine, fexofenadine, avastin, mequitazine, mizolastine and salts thereof, and preferably one or more of loratadine, desloratadine, cetirizine, levocetirizine, ebastine, mizolastine, avastin, mequitazine, ketotifen and hydrochlorides or fumarates thereof.
Owner:TIANJIN JINYAO GRP
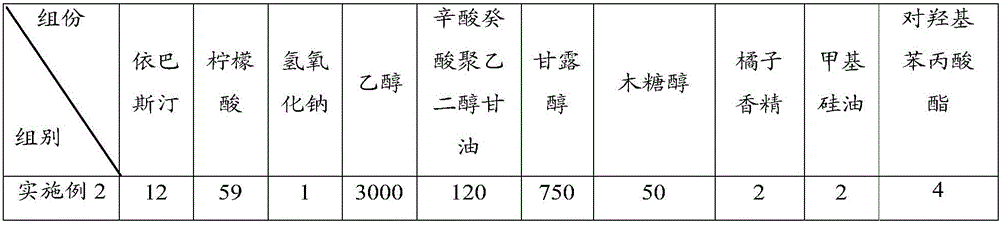
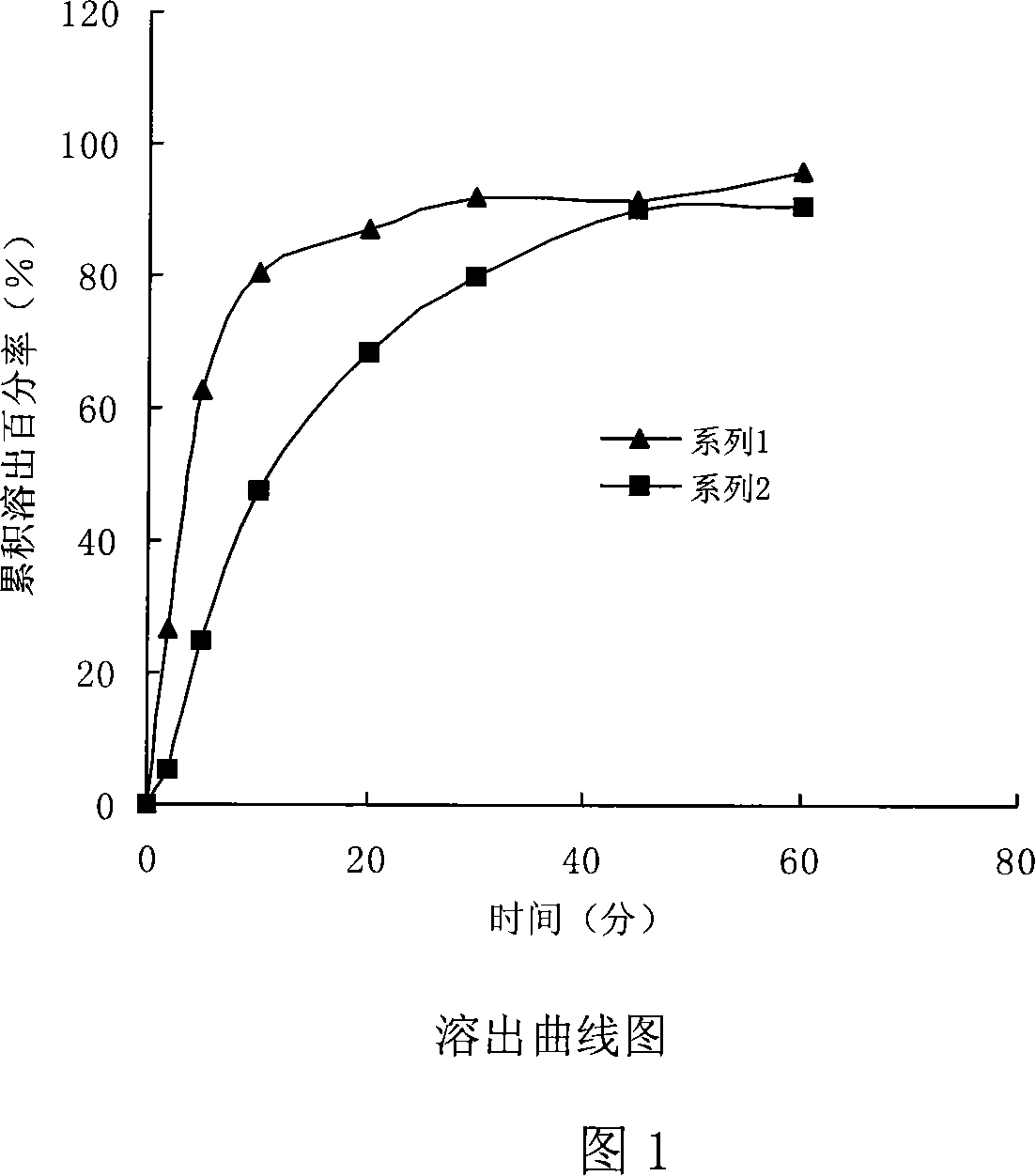
Ebastine solid dispersion and high-dissolubility ebastine tablet prepared by the same
The invention provides an ebastine solid dispersion which comprises ebastine, poloxamer F68 and / or polyvinylpyrrolidone k30. The invention also provides a preparation method of the ebastine solid dispersion, and a high-dissolubility ebastine tablet. The method of the invention adopts poloxamer F68, polyvinylpyrrolidone k30 or a mixture of the two as a carrier, and prepares the solid dispersion by the carrier and ebastine; and the solubility of ebastine in water in the ebastine tablet is significantly improved. After accelerated tests and long-term tests, the dissolubility is stable without obvious changes.
Owner:JIANGSU LIANHUAN PHARMA
Composition for treating respiratory and skin diseases, comprising at least one leukotriene antagonist and at least one antihistamine
A pharmaceutical composition useful in the treatment of sneezing, itching runny nose, nasal congestion, redness of the eye, tearing, itching of the ears or palate, shortness of breath, inflammation of the bronchial mucosa, reduced Forced Expiratory Volume In One Second (FEV1), coughs, rash, itchy skin, headaches, and aches and pains associated with seasonal allergic rhinitis, perennial allergic rhinitis, common colds, otitis, sinusitus, allergy, asthma, allergic asthma and / or inflammation, in a mammalian organism in need of such treatment. The composition comprises: i) an effective amount of at least one leukotriene antagonist selected from a) montelukast, b) 1-(((R)- (3-(2-(6,7- difluoro-2- quinolinyl)ethenyl) phenyl)-3-(2- (2-hydroxy-2- propyl)phenyl) thio)methylcyclopropaneacetic acid; c) 1-(((1(R)-3 (3-(2-(2,3- dichlorothieno[3, 2-b]pyridin-5-yl) -(E)-ethenyl)phenyl) -3-(2-(1-hydroxy-1- methylethyl) phenyl)propyl) thio)methyl) cyclopropaneacetic acid; d) pranlukast; or f) [2-[[2-(4-tert -butyl-2-thiazolyl) -5-benzofuranyl] oxymethyl]phenyl] acetic acid; or a pharmaceutically acceptable salt thereof; in admixture with ii) an effective amount of at least one antihistamine which is descarboethoxyloratidine, cetirizine, fexofenadine, ebastine, astemizole, norastemizole, epinastine, efletirizine or a pharmaceutically acceptable salt thereof.
Owner:SCHERING AG
Ebastine oral liquid preparation and preparation method thereof
InactiveCN105687128AMildThe effect is complete and comprehensiveInorganic non-active ingredientsPharmaceutical delivery mechanismMedicinePreservative
The invention provides an ebastine oral liquid preparation. The ebastine oral liquid preparation is mainly prepared from, by mass, 8-12 parts of ebastine, 90-120 parts of solubilizer, 0.8-2 parts of aromatic, 650-800 parts of sweetening agent, 1-4 parts of preservative, 0.8-2 parts of antifoaming agent, 2,000-3,000 parts of latent solvent, pH modifier adjusting pH to be 4-5 and a proper amount of water. The preparing method comprises the steps that solubilizer, latent solvent and a part of pH modifier are mixed and then water is added and stirred until the mixture is dissolved, then, ebastine, sweetening agent, aromatic and preservative are added and continue to be stirred until all the materials are dissolved, antifoaming agent and water are added, the residual pH modifier is added to adjust the pH value to be 4-5, and filtering, quality detecting and filling are added. The ebastine oral liquid preparation is absorbed by the human body easily, and is convenient to carry, wide in target user range and convenient to use.
Owner:HANGZHOU ZIQUAN BIOPHARMATEC CO LTD
Antiallergic nasal medicine composition with high moisture retention and preparation methods and applications thereof
InactiveCN109276715AUse lessImprove comfortPharmaceutical delivery mechanismPharmaceutical non-active ingredientsIrritationGlycerol
The invention provides an antiallergic nasal medicine composition with high moisture retention and preparation methods and applications thereof. The composition has high moisture retention and no irritation to a nasal mucosa. The composition comprises levocetirizine, azatadine, loratadine, ebastine, setastine, bilastine, clemastine, mizolastine, epinastine and moxifloxacin which are antiallergic medicine as main medicine, wherein the main medicine can be salts or free alkalis, sea salt as an osmotic pressure regulator, and a mixture of sodium hyaluronate and glycerin of different molecular weights as a moisturizing excipient. Plant essential oil can be contained in the composition. Preparations can be an aqueous solution, a cyclodextrin-coated suspension or a nanosuspension. Dosage forms include spray, aerosol and nose drops. Main indications include nasal dryness, runny nose, nasal itching, nasal obstruction and the like due to allergic rhinitis.
Owner:XIAN LIBANG PHARMA TECH
Solid oral forms of ebastine
The invention relates to compositions in the form of matrices consisting of solid ebastine dispersions in nonionic surfactants having a HLB of between 10 and 20 and a melting point of between 30° C. and 70° C. The invention also relates to solid oral pharmaceutical forms of ebastine containing said matrices, particularly tablets, and having good solubility and bioavailability properties and improved stability.
Owner:SIMBEC IBERICA SL
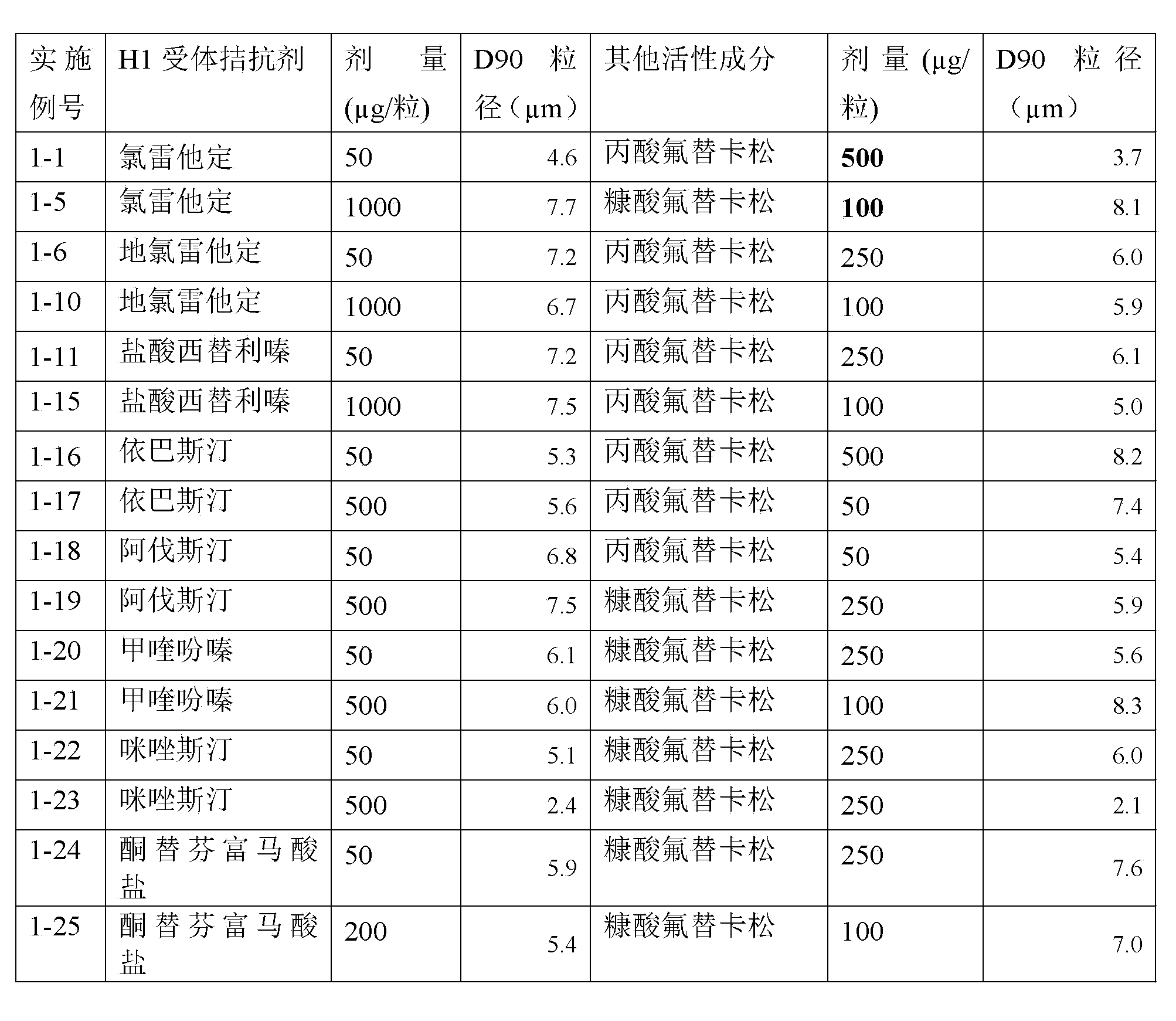
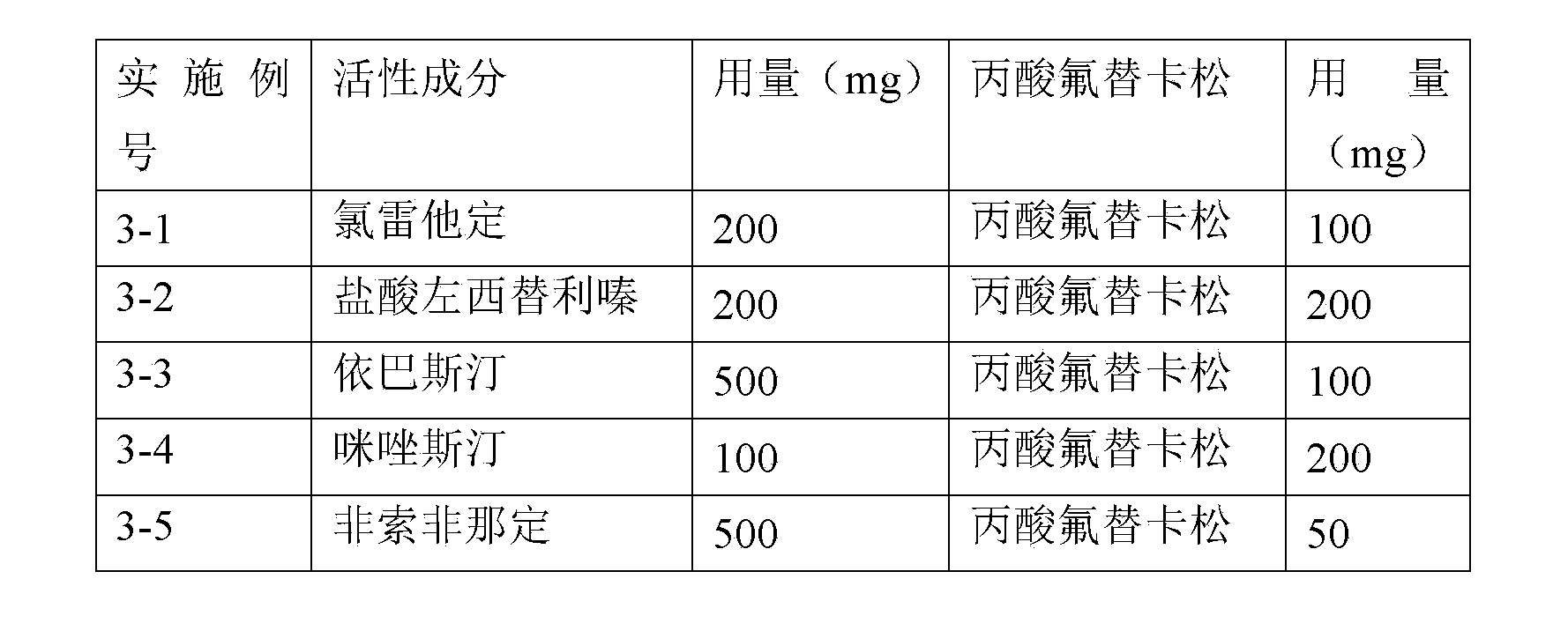
Pharmaceutical Compositions Comprising Ebastine and Fluticasone
A pharmaceutical composition comprises at least one antihistamine, at least one corticosteroid, and at least one pharmaceutical excipient, wherein the at least one antihistamine comprises ebastine or its pharmaceutically acceptable salt, solvate, ester or physiologically functional derivative thereof, and wherein the at least one corticosteroid comprises fluticasone or its pharmaceutically acceptable ester thereof.
Owner:CIPLA LTD
Prepn of ebastine
The preparation process of ebastine includes the following steps: 1. condensation of 4-chlorobutyryl chloride and tert-butyl benzene to produce 4-chloro-1-[4-(1,1-dimethylethyl) phenyl]-1-butanone as intermediate I; 2. condensation of the intermediate I and 4-hydroxypiperidine to produce 1-[4-(1,1-dimethylethyl) phenyl]-4-[4-hydroxyl-1-piperidino] -butane ketone as intermediate II; 3. reflux condensation of methylisobutyl ketone ortoluene, chloro diphenylmethane and the intermediate II in the presence one small amount of phenylacetyl peroxide as reaction initiator, and 4. adding 2N hydrochloric acid in the same volume as the reactant after finishing the reaction, shaking, eliminating organic layer, adding 2N sodium hydroxide solution to neutralize to pH9, stirring to separate solid matter, filtering, water washing to neutral and obtain the ebastine product. The present invention has the features of complete reaction, high yield and other advantages.
Owner:杭州仟源保灵药业有限公司
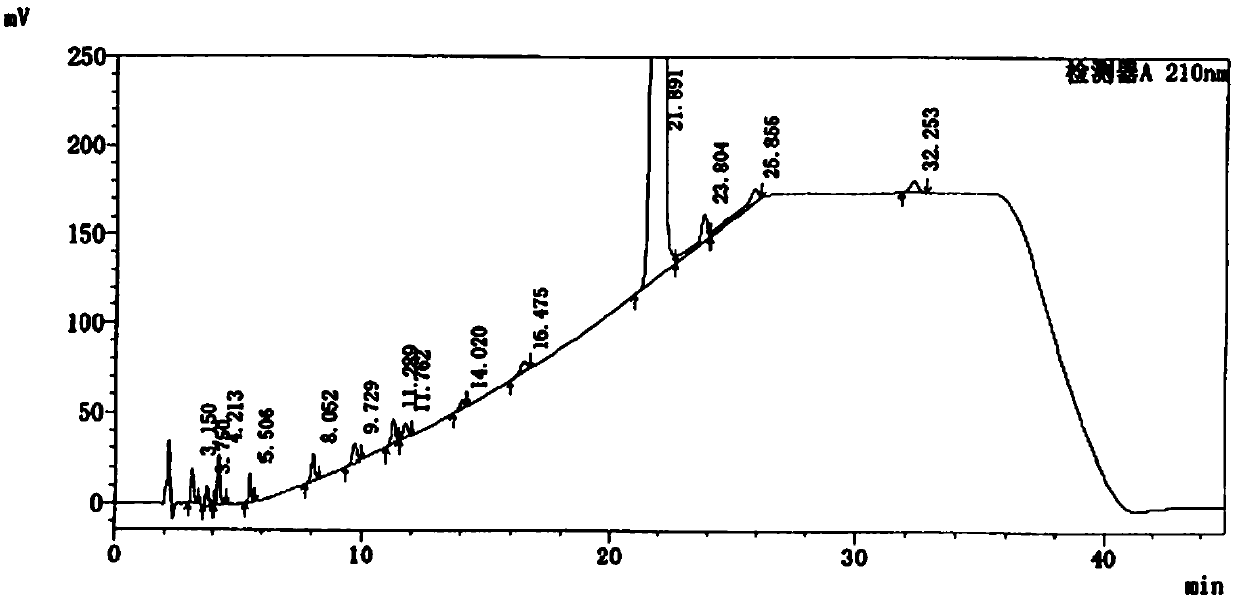
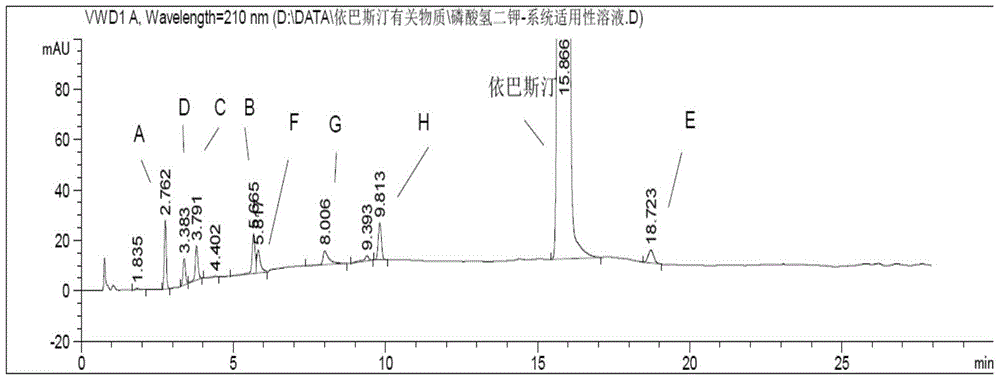
Method for detecting related substances in ebastine
ActiveCN110988215AEasy to analyzeEffective Detection SeparationComponent separationFluid phaseGradient elution
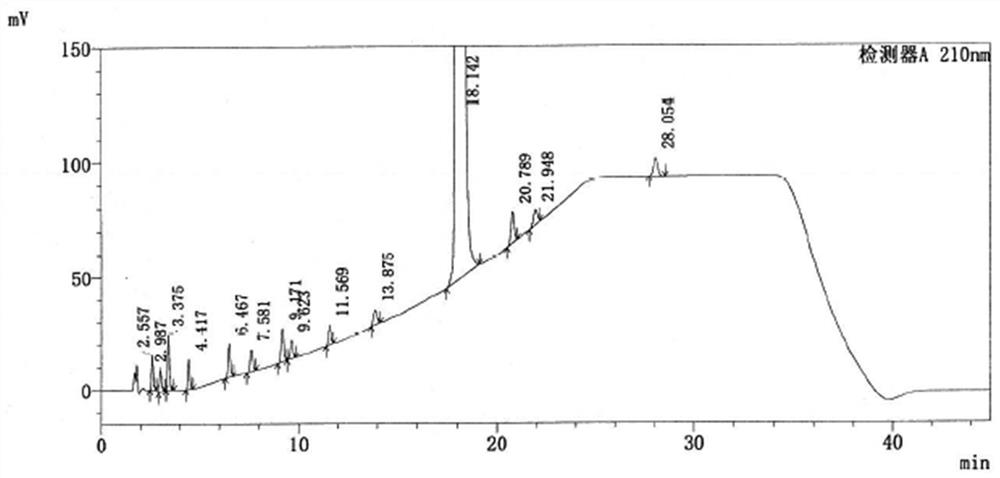
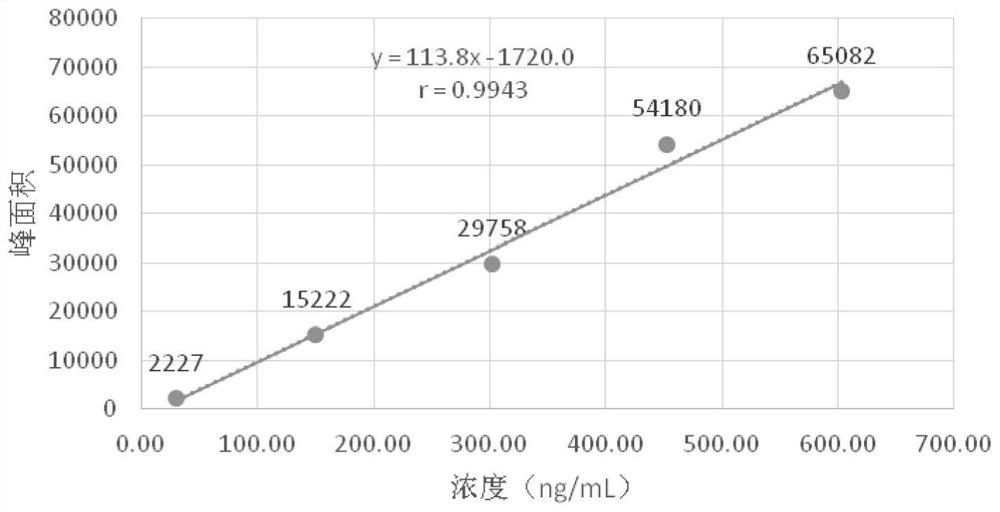
The application discloses a method for detecting related substances in ebastine. With high performance liquid chromatography, gradient elution is performed. When the detection method is used, each component in the ebastine bulk drug can be effectively detected and separated, the analysis time is proper, and the operation is simple and convenient.
Owner:HUNAN JIUDIAN PHARMA
Method for preparing ebastine
The invention provides a method for preparing ebastine. The method comprises the following steps: condensing an initial raw material benzophenone and 4-hydroxypiperidine to obtain 4-benzhydryloxypiperidine; condensing with 4-chloro-1-(4-tert-butylphenyl)-1-butanone to obtain ebastine. According to the method, benzophenone with low price is used as the initial raw material, has low cost and is simple and convenient to operate, only one reaction kettle is used, alkaline washing and rinsing are performed in the middle process, and the final product can be obtained by freezing and crystallizing with alcohol. The method has the advantages of good economic benefit, high safety, high yield, good purity and the like, and is suitable for industrial production.
Owner:JIANGSU LIANHUAN PHARMA
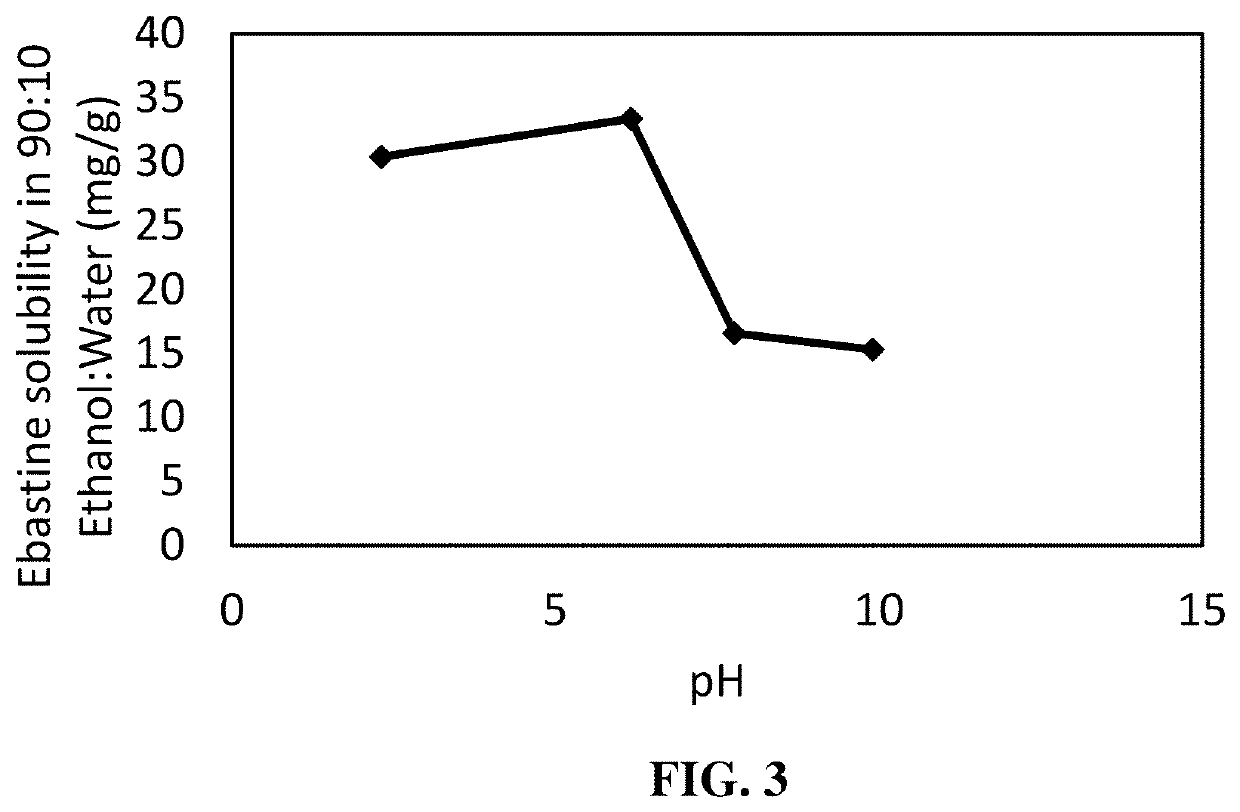
Ebastine topical composition
Provided herein is a topical composition for treatment of a hair-loss related condition or disease and related methods for making and using the topical composition. The topical composition generally comprises ebastine dissolved in a solvent. In some exemplary embodiments, the solvent comprises a monohydric aliphatic alcohol and an ester. In some exemplary embodiments, the solvent comprises a polyol.
Owner:BIOPHARMX
Pharmaceutical composition for treating urticaria and preparation method thereof
InactiveCN107982274AMaintain efficacyGood dissolution rateInorganic non-active ingredientsDermatological disorderSolventSolubility
The invention relates to the field of pharmaceutical preparations and specifically discloses a pharmaceutical composition for treating urticaria and a preparation method thereof. The pharmaceutical composition disclosed by the invention is prepared from the following raw materials in parts by weight: 44 to 48 parts of ebastine, 27 to 31 parts of compound glycyrrhizin, 3 to 5 parts of solubilizer,1 to 2 parts of cosolvent, 2 to 4 parts of suspending agent, 1 to 3 parts of extracting aid, 1 to 2 parts of flavoring agent, 1 to 2 parts of antioxidant and 11 to 12 parts of solvent. According to the pharmaceutical composition for treating urticaria disclosed by the invention, the two matters of the ebastine and the compound glycyrrhizin which are difficult to dissolve are dissolved together through the solvent; by means of adding the cosolvent, solubility of medicine in the solvent is improved; the solubilizer and the cosolvent are mutually matched to perform scientific compatibility to achieve the maximum solubility and the best treatment effect.
Owner:湖南博隽生物医药有限公司
Method for Determination of Related Substances of Ebastine by High Performance Liquid Chromatography
The invention provides a method for testing ebastine-related substances by high performance liquid chromatography. The method includes: a, preparing a sample solution for test: preparing the sample solution for test by mixing a to-be-tested sample with a solvent; b, testing: testing the sample solution at the test wavelength of 200-220 nanometers by a gradient elute method by means of utilizing octadecylsilane chemically bonded silica as a chromatographic column filler, utilizing mixed liquid of a phosphate buffered solution and an organic solvent as a moving phase A and utilizing the organic solvent as a moving phase B. The method for testing the ebastine-related substances by the high performance liquid chromatography has the advantage that related substances in ebastine active ingredients can be separated and tested quickly, effectively, accurately and reliably.
Owner:JIANGSU LIANHUAN PHARMA
Combined traditional Chinese and Western medicinal preparation for treating chronic simple moss and preparation method
InactiveCN106237004AImprove efficacyAchieve medicinal effectPill deliveryPteridophyta/filicophyta medical ingredientsPatriniaDelphinium brunonianum
The invention discloses combined traditional Chinese and Western medicinal preparation for treating chronic simple moss and a preparation method, and belongs to the technical field of medicines. The effective component of the combined traditional Chinese and Western medicinal preparation comprises the following raw materials: chlorpheniramine, vitamin C, tripelennamine, ebastine, cirsium lineare, campsis stem and leaf, kalopanax tree bark, vitex negundo leaf, baeckea frutescens, bidens biternata, polygonum senticosum, delphinium brunonianum royle, catalpa leaf, bupleurum candollei, cyperus rotundus, lindera angustifolia, patrinia scaniosaefolia and camptosorus sibiricus rupr. The combined traditional Chinese and Western medicinal preparation is prepared by combining a traditional Chinese medicine and a Western medicine, and has a better therapeutic effect than the single traditional Chinese medicine or the single Western medicine; the combined traditional Chinese and Western medicinal preparation can combine heat-clearing, inflammation-eliminating, dampness-eliminating, itching-stopping, stagnation-eliminating and stasis-removing efficacy of the traditional Chinese medicine with sedative, sterilizing and inflammation-eliminating efficacy of the Western medicine so as to improve the efficacy of the traditional Chinese medicine and the Western medicine to the maximum extent and play complementary and enhancing roles, so that the medicinal effect of treating the chronic simple moss is achieved.
Owner:闫倩
A kind of ebastine oral liquid preparation and preparation method thereof
InactiveCN105687128BMildThe effect is complete and comprehensiveInorganic non-active ingredientsPharmaceutical delivery mechanismPreservativeMedicine
The invention provides an ebastine oral liquid preparation. The ebastine oral liquid preparation is mainly prepared from, by mass, 8-12 parts of ebastine, 90-120 parts of solubilizer, 0.8-2 parts of aromatic, 650-800 parts of sweetening agent, 1-4 parts of preservative, 0.8-2 parts of antifoaming agent, 2,000-3,000 parts of latent solvent, pH modifier adjusting pH to be 4-5 and a proper amount of water. The preparing method comprises the steps that solubilizer, latent solvent and a part of pH modifier are mixed and then water is added and stirred until the mixture is dissolved, then, ebastine, sweetening agent, aromatic and preservative are added and continue to be stirred until all the materials are dissolved, antifoaming agent and water are added, the residual pH modifier is added to adjust the pH value to be 4-5, and filtering, quality detecting and filling are added. The ebastine oral liquid preparation is absorbed by the human body easily, and is convenient to carry, wide in target user range and convenient to use.
Owner:HANGZHOU ZIQUAN BIOPHARMATEC CO LTD
Ebstine solid oral preparation and its preparing method
ActiveCN101161233BImprove solubilityIncrease dissolution ratePill deliveryCapsule deliveryOrganic acidOrally disintegrating tablet
An ebastine solid oral preparation and preparation method are disclosed in the present invention. Made of ebastine, ethanol solution, cosolvent, diluent or filler, disintegrating agent, taste correcting agent, glidants or dispersing agent, effervescing agent, lubricant in proportion, its step is that firstly adding ebastine into ethanol solution, and then adding cosolvent organic acid, leading itto dissolve entirely; secondly mixing the diluent or filler, disintegrating agent, taste correcting agent, effervescing agent with dispersing agent, to obtain a mixer; third mixing the front two, preparing soft material, preparing granules, then adding disintegrating agent, effervescing agent, taste correcting agent, glidants and lubricant, tabletting or filling capsules, packing to obtain a solid oral preparation. The present invention preparing oral disintegrable tablet, dispersing tablet have short disintegrating time, good dispersing state. The obtained oral disintegrable tablet, dispersing tablet and chewing tablet have good taste and mouth feel, the obtained solid oral preparations dissolving out are all rapid. The method is easy, simple operation, without needing special equipment,and lower production cost.
Owner:HUBEI LIYI PHARM TECH CO LTD
Fluticasone and fluticasone ester/H1 receptor antagonist inhalant
InactiveCN103830729AOrganic active ingredientsPharmaceutical delivery mechanismFexofenadineLoratadine
The invention relates to a fluticasone and fluticasone ester / H1 receptor antagonist inhalant which contains fluticasone and fluticasone ester and one or more H1 receptor antagonists used as active components, and one or more pharmaceutical auxiliary materials suitable for inhalation administration. The H1 receptor antagonist is one or more of loratadine, desloratadine, cetirizine, levocetirizine, astemizole, ketotifen, ebastine, fexofenadine, avastin, mequitazine, mizolastine and salts thereof, and preferably one or more of loratadine, desloratadine, cetirizine, levocetirizine, ebastine, mizolastine, avastin, mequitazine, ketotifen and hydrochlorides or fumarates thereof.
Owner:TIANJIN JINYAO GRP
A method for detecting related substances in ebastine
ActiveCN110988215BEasy to analyzeEffective Detection SeparationComponent separationFluid phasePhysical chemistry
The application discloses a method for detecting related substances in ebastine, which uses high-performance liquid chromatography and performs gradient elution. By using the detection method, each component in the ebastine bulk drug can be effectively detected and separated, and the analysis time is suitable and the operation is simple and convenient.
Owner:HUNAN JIUDIAN PHARMA
Application of loratadine or ebastine in preparation of medicine for preventing and/or treating COVID-19 inflammation
InactiveCN113425724AReduce inflammationInhibit inflammatory factor stormAntiviralsRespiratory disorderInflammatory factorsLoratadine
The invention provides an application of loratadine or ebastine in preparation of a medicine for preventing and / or treating COVID-19 inflammation. In-vivo and in-vitro experiments prove that the loratadine or the ebastine can reduce the inflammatory reaction caused by SARS-CoV-2spike protein by inhibiting mast cell degranulation and inhibit inflammatory factor storm, and has certain protection on tissue damage. Therefore, the combination of the loratadine or the ebastine and an antiviral drug has important clinical application value in the aspect of treating the severe infection of the COVID-19.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Psoriasis eliminating powder
InactiveCN106266191AImprove itchingImprove microcirculation disorderAntimycoticsAnthropod material medical ingredientsDiseaseIrritation
The invention discloses psoriasis eliminating powder for treating psoriasis. The psoriasis eliminating powder is prepared from, by weight, 143 parts of milkvetch roots, 143 parts of dark plum fruit, 71 parts of fructus chebulae, 71 parts of radix rehmanniae, 71 parts of fructus tribuli, 71 parts of radix sophorae flavescentis, 71 parts of cortex dictamni, 71 parts of fructus kochiae, 50 parts of periostracum cicada, 50 parts of rhizoma atractylodis, 50 parts of lumbricus, 50 parts of radix scutellariae, 14 parts of herba asari, 28 parts of radix bupleuri, 28 parts of licorice roots and 14 parts of ebastine. The bulk herbs are pulverized and then mixed with the ebastine, and the mixed powder is contained in capsules. According to the psoriasis eliminating powder, all the medicines cooperatively and jointly achieve the effects of treating skin tinea, allergy, eczema, pruritus and chilblain according to the differential treatment theory of traditional Chinese medicine by taking the effects of clearing heat, removing toxicity, dispelling wind, eliminating dampness, promoting blood circulation to remove meridian obstruction and improving the disease resistance and immunocompetence of the skin as a formulating principle. By means of clinical practice and application, the formula is free of irritation, allergenicity and adverse skin reactions and has the obvious treatment effect on the tinea, the eczema, the allergy, the pruritus and the chilblain to promote skin health. Total 180 cases of patients are observed, and the total effective rate is 96%.
Owner:王立平
Analysis method of related substances in Ebastine
Owner:CHONGQING HUAPONT PHARMA
Compounds capable of inhibiting immunocyte-related allergic immune reactions
InactiveUS20060003988A1Inhibit productionAntibacterial agentsBiocideEpinastine HydrochlorideT cell migration
The present invention is based on novel discoveries relating to ebastine, carebastine, and epinastine hydrochloride. Specifically, the present invention relates to the use of ebastine and carebastine as an inhibitor of (A) T cell proliferation, (B) Th2 type cytokine production, (C) inflammatory cytokine production, and (D) T cell migration. In addition, it relates to the use of epinastine hydrochloride as an inhibitor of (A) T cell proliferation, (B) Th2 type cytokine production, and (C) Th1 type cytokine production.
Owner:YS THERAPEUTICS CO LTD
Method for detecting content of bromodiphenyl methane in ebastine medicine
PendingCN113466390AStrong specificityGood repeatabilityComponent separationGas liquid chromatographicSolvent
The invention relates to a method for detecting the content of bromodiphenyl methane in an ebastine medicine, which comprises the following steps of: 1, preparing a test solution by taking an ebastine raw material medicine and taking n-hexane as a solvent, and dissolving and diluting bromodiphenyl methane with n-hexane to prepare a reference solution; and 2, carrying out gas chromatographic analysis by taking a dimethyl polysiloxane column as a chromatographic column. According to the method for detecting the content of the bromodiphenyl methane in the ebastine medicine, gas chromatography is used for detection, and the method has the advantages of being good in specificity, high in sensitivity, good in repeatability and the like and can be widely used for detecting the content of the bromodiphenyl methane in the ebastine medicine.
Owner:杭州微源检测技术有限公司
Preparation method of high-purity ebastine
PendingCN114671802AMeet Pharmacopoeia Quality StandardsRaise quality standardsOrganic chemistryEthyl groupTosylphenylalanyl Chloromethyl Ketone
The invention provides a preparation method of high-purity ebastine, which comprises the following steps: condensing 4-chloro-1-(4-tert-butylphenyl)-1-butanone serving as an initial raw material with 4-hydroxypiperidine to obtain 4-chloro-1-(4-(1, 1-dimethyl ethyl) phenyl-4-(4-hydroxy-1-piperidyl)-butanone), adding benzhydrol into methyl isobutyl ketone or methylbenzene serving as a solvent, and reacting at the temperature of 60-80 DEG C for 2-3 hours to obtain the high-purity ebastine. Ebastine is obtained through condensation, the obtained ebastine is purified through salification and then free, the purity of the obtained ebastine finished product is larger than 99.9%, the single impurity content is smaller than 0.1%, and the ebastine finished product meets the quality standard of ebastine pharmacopeia.
Owner:JIANGSU LIANHUAN PHARMA
Compound inhalation medicine of ciclesonide and H1 receptor antagonist
InactiveCN103830728AOrganic active ingredientsPharmaceutical delivery mechanismFexofenadineNK1 receptor antagonist
The invention provides a compound inhalation medicine of ciclesonide and an H1 receptor antagonist. The medicine comprises ciclesonide serving as an active component and one or more H1 receptor antagonists, and one or more medical auxiliaries for inhalation administration, wherein the H1 receptor antagonist is one or more of loratadine, desloratadine, cetirizine, levocetirizine, astemizole, ketotifen, ebastine, fexofenadine, acrivastine, mequitazine, mizolastine and salt thereof, and preferably one or more of loratadine, desloratadine, cetirizine, levocetirizine, ebastine, mizolastine, acrivastine, mequitazine, ketotifen and hydrochloride or fumarate thereof.
Owner:TIANJIN JINYAO GRP
Ebastine salt tablet and preparation method thereof
PendingCN114712322AGood water solubilityImprove solubilityAntipyreticAnalgesicsCoated tabletsLactose
The invention provides a preparation method of an ebastine salt tablet. The preparation method comprises the following steps: S1, placing ebastine salt, lactose, starch and microcrystalline cellulose in a wet granulator to complete a granulation process, and then drying to obtain dry granules; s2, mixing the dry particles obtained in the S1 with magnesium stearate and croscarmellose sodium to prepare intermediate particles, and tabletting the intermediate particles to prepare intermediate tablets; s3, coating the intermediate tablet obtained in the step S2 to prepare an intermediate coated tablet, namely the tablet of the ebastine salt; in the step S1, the ebastine tablet comprises the following components in parts by mass: 5-10 parts of ebastine salt, 65-70 parts of lactose, 3-8 parts of microcrystalline cellulose and 10-20 parts of starch; in the step S2, the composition comprises the following components in parts by mass: 1-3 parts of croscarmellose sodium and 0.5-2.0 parts of magnesium stearate. According to the invention, ebastine and organic acid generate water-soluble salt, so that the solubility of the medicine is improved.
Owner:南京联智医药科技有限公司
Preparation method of Ebastine and fumarate thereof
ActiveCN112574097ASave solventLow costOrganic compound preparationCarboxylic acid salt preparationBenzeneEthyl group
The invention discloses a preparation method of Ebastine and fumarate of the Ebastine. The preparation method comprises the following steps: 1) mixing 4-(diphenylmethoxy)-piperidine, 4'-tert-butyl-4-chlorobutyryl benzene, an acid-binding agent and a first solvent to carry out a synthetic reaction, adding a second solvent and water after the reaction is finished, and stirring the reaction productsfor layering to obtain an organic layer and an inorganic layer; and 2) concentrating and drying the organic layer, adding a third solvent or the third solvent and fumaric acid, filtering and drying the mixture to obtain 1-[4-(1,1-dimethylethyl)phenyl]-4-[4-(Diphenylmethoxy)-1-piperidinyl]-1-butanone or fumarate thereof. By applying the technical scheme, solvent-free or low-solvent synthesis has the advantages of solvent saving, cost reduction, environmental friendliness and the like.
Owner:杭州仟源保灵药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com